Abstract
Background:
The biomimetic self-assembling peptide technology is a paradigm for dental hard tissue regeneration.
Aim:
To investigate the efficacy of biomimetic self-assembling peptide (P11-4) on enamel remineralization compared to casein phosphopeptide-amorphous calcium phosphate fluoride (CPP-ACPF) and fluoride-based delivery systems.
Materials and Methods:
Artificial enamel lesions were created on buccal surfaces of 40 extracted human molars. Specimens were randomly assigned to four groups (n = 10) according to the remineralizing agent used: G1 – control: artificial saliva, G2 – fluoride varnish, G3 – CPP-ACPF varnish, G4 – self-assembling peptide agent. All products were applied according to the manufacturer's instructions and the specimens were stored in daily renewed artificial saliva. Surface microhardness (SMH) was assessed at baseline, after demineralization, after 1 week and after 4 weeks storage. SMH values were analyzed using ANOVA and Tukey's post hoc test.
Results:
Self-assembling peptide showed the highest statistically significant mean SMH followed by fluoride and CPP-ACPF while the lowest mean SMH was found in artificial saliva. However, no statistically significant difference was found between fluoride and CPP-ACPF. Higher statistically significant mean SMH was found after 4 weeks compared to 1 week remineralization in all groups.
Conclusions:
Self-assembling peptide confers the highest remineralizing efficacy compared to fluoride and CPP-ACPF, showing a promising, noninvasive regeneration potential. Furthermore, extended period of time helped attain more benefits from the remineralizing regimens applied.
Keywords: Biomimetic remineralization, enamel regeneration, self-assembling peptides, surface microhardness
INTRODUCTION
Dental caries, a disease affecting hard dental tissues, is still considered a major oral health challenge. By understanding the nature of the disease, being a reversible dynamic process, caries detection, prevention and treatment modalities have changed over time. Modern dentistry advocates the noninvasive approach for the management of noncavitated, subsurface lesions via remineralization. Through remineralization, these lesions can be reversed noninvasively without following the traditional surgical approach of drilling and filling.[1]
Remineralization is defined as the natural repair process for noncavitated lesions relying on calcium and phosphate ions assisted by fluoride to rebuild a new surface on existing crystal remnants in subsurface lesions remaining after demineralization.[2] Many materials for remineralization are available. Although fluoride is the most well-known and commonly used remineralizing agent, many limitations exist in its usage. Among its limitations is the fact that fluoride (particularly high concentrations) has been shown to give rise to predominantly surface remineralization at the expense of the lesion body, making full remineralization difficult to achieve.[3]
The limitations of fluoride urged manufacturers to introduce calcium phosphate-based remineralizing systems, of which many are now commercially available such as casein phosphopeptide-amorphous calcium phosphate (CPP-ACP), functionalized tricalcium phosphate and bioactive glass containing calcium sodium phosphosilicate.[4]
Dental enamel, unlike bone, its extracellular organic matrix is degraded and removed from the tissue before tooth eruption and thus is unable to regenerate during the tooth's lifespan. During enamel development, enamel matrix proteins are known to form self-assembling structures that control the nucleation and growth of hydroxyapatite crystals.[5]
Recently, new bioactive materials were introduced acting as biomimetic scaffolds to induce mineral deposition in situ and promote guided tissue regeneration. The innovative self-assembling peptide (P11-4) is a candidate for this biomimetic enamel regeneration approach. P11-4 is a rationally designed β-sheet-forming peptide that under certain environmental triggers, self-assembles into three-dimensional fibrillar scaffolds and mimics the action of the extracellular enamel matrix proteins found during tooth development.[5,6] Thus, it recapitulates histogenesis, by attracting, binding and stabilizing calcium ions inducing de novo hydroxyapatite precipitation. These self-assembling peptides offer a potentially useful route to the rise of smart dental biomaterials for the modulation of mineral behavior during in situ dental tissue engineering.[5,6]
In an attempt to clarify the remineralization potential of the novel self-assembly peptide technology, this study was conducted to evaluate the remineralizing efficacy of self-assembling peptide compared to CPP-ACP fluoride (CPP-ACPF) and fluoride-based delivery systems using surface microhardness (SMH) assessment.
The null hypotheses tested are as follows: first, there is no difference in the remineralization potential between self-assembling peptide, CPP-ACPF, fluoride and artificial saliva in terms of microhardness testing. Second, time does not affect the remineralization potential of all the tested agents.
MATERIALS AND METHODS
Materials
Three remineralizing agents were tested in this study: (a) Bifluorid 10®(VOCO, Cuxhaven, Germany) as fluoride-based varnish containing 22,600 ppm fluoride. (b) MI varnish™(GC Corporation, Tokyo, Japan) as CPP-ACPF-based varnish containing 5% CPP-ACP and 22,600 ppm fluoride. (c) CURODONT Repair™(Credentis AG, Windisch, Switzerland) that incorporates the self-assembling peptide (P11-4) based Curolox™ technology.
Specimen preparation
A total of 40 sound freshly extracted molar teeth were used. The roots were removed 2 mm below the cementoenamel junction using a microtome (Leica 1600 saw microtome, Wetzlar, Germany). The selected teeth were cleaned using scalers, ultrasonic scaling tips, rubber cup/pumice prophylaxis and then stored in a saturated thymol solution for 2 weeks.[7] The teeth were inspected under a stereomicroscope (Leica S8 APO, Wetzlar, Germany) at × 40 magnification to exclude teeth with enamel defects, cracks, stains or caries. Each tooth crown was embedded in self-cured acrylic resin mold with the buccal surface facing upward. The buccal surfaces were polished using polishing disks (Sof-Lex Pop-On Disks 3M ESPE, St Paul, MN, USA) in progressively finer grits (coarse, medium, fine and superfine) on a slow-speed contra-angle hand-piece (T1 Line series, Sirona, Germany).[8] The buccal surface of each specimen was coated with an acid-resistant nail varnish (Bourjois, Paris) leaving four equal windows of exposed enamel 2 mm × 2 mm each. The first window was coated with nail varnish to act as control for baseline.
Demineralization
Artificial caries-like enamel lesions were created by immersing the specimens in a demineralizing solution. The solution contained 2.2 mM calcium chloride (CaCl2), 2.2 mM sodium dihydrogen orthophosphate dehydrate (NaH2PO4) and 0.05 M acetic acid; the pH was adjusted to 4.4 with 1 M potassium hydroxide. Each specimen was immersed separately in a daily renewed demineralizing solution for 4 consecutive days (96 h) until a uniform white spot lesion was created.[9] Specimens were then rinsed carefully and stored in distilled deionized water. The second window was coated with nail varnish to act as control for demineralization.
Remineralization
Artificial saliva was prepared according to the formulation of ten Cate and Duijsters[10] and contained 1.5 mM CaCl2, 0.9 mM NaH2PO4 and 0.15 M potassium chloride at pH 7.0. Specimens were randomly divided into four groups (n = 10) according to the treatment employed:
G1 (control group): no treatment was applied and the specimens were stored in daily renewed artificial saliva
G2 (fluoride group): The specimens were dried and a thin, uniform layer of the varnish was applied in a single stroke painting motion. The varnish was allowed to be absorbed for 20 s and then air-dried. The specimens were stored in daily renewed artificial saliva
G3 ( CPP-ACPF group): The specimens were dried and a thin, uniform layer of the varnish was applied in a single stroke painting motion. The varnish was also left undisturbed for 20 s. The specimens were stored in daily renewed artificial saliva
G4 (self-assembling peptide group): The material was supplied in powdered form, in glass vials. Just before application, it was reconstituted with 50 μl distilled water. The agent was applied and left undisturbed for 5 min (till disappearance) to allow diffusion and self-assembly. The specimens were stored in daily renewed artificial saliva.
For groups G2 and G3, after 6 h of immersion in artificial saliva, the remaining varnish was removed using a No. 15 scalpel blade followed by cleaning off the surface with cotton tip immersed in deionized water. After treatment, each specimen was stored separately in a daily renewed artificial saliva till testing.[7] The third window was covered with nail varnish after 1 week remineralization. Following 4 weeks remineralization, the nail varnish was peeled off carefully and the specimens were tested using SMH assessment.
Assessment of surface microhardness
SMH was measured at baseline of sound enamel, after demineralization, after 1 week and after 4 weeks remineralization. SMH was performed using a Vickers Microhardness Tester (Buehler Wilson Hardness Tester, Lake Bluff, USA) with a Vickers diamond indenter. Each measurement was carried out by applying 100 g load for 5 s oriented perpendicularly to the enamel surface. All readings were performed by the same examiner using the same calibrated machine. In each reading, three indentations were made and their average was taken to represent the specimen's hardness value.
Statistical analyses
Statistical analysis was performed with IBM® SPSS® (version 20) software (SPSS Inc., IBM Corporation, NY, USA). The significance level was set at P ≤ 0.05. Data are presented as means and standard deviation (SD) values that were calculated for each group. Two-way ANOVA analysis was used to determine the effect of different variables on mean microhardness values. One-way ANOVA followed by Tukey's post hoc test was used to compare between more than two groups.
RESULTS
Enamel surface microhardness
The results of two-way ANOVA analysis for the effect of different variables on mean SMH are shown in Table 1. The remineralizing agents and the storage period had a statistically significant effect on mean SMH. The interaction between the two variables had a statistically significant effect on mean SMH.
Table 1.
Two-way analysis of variance results
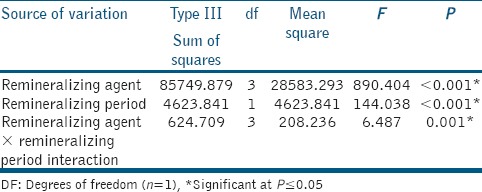
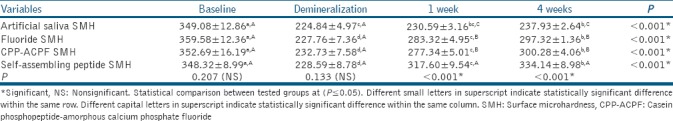
The mean (±SD) SMH values of all groups at different testing periods are shown in Table 2. The enamel baseline showed the highest statistically significant SMH values (P ≤ 0.05). Demineralized enamel showed a significant reduction in SMH values (P ≤ 0.05). After remineralization, 4 weeks remineralization showed higher statistically significant SMH values than 1 week remineralization in all groups (P ≤ 0.05). For artificial salvia, no statistically significant difference was found either between demineralization and 1 week remineralization or between 1 week and 4 weeks remineralization at P = 0.29 and P = 0.12, respectively. The highest statistically SMH values were found in self-assembling peptide followed by fluoride and CPP-ACPF while the lowest values were found in artificial saliva (P ≤ 0.05). However, no statistically significant difference was found between fluoride and CPP-ACPF at P = 0.96.
Table 2.
Mean±standard deviation of all tested groups at baseline, after demineralization, 1 week and 4 weeks remineralization
DISCUSSION
The emerging goal of modern dentistry is to manage noncavitated carious lesions noninvasively through remineralization in an effort to prevent disease progression and improve strength, esthetics and function of teeth. A critical element fundamental to this therapeutic philosophy is the need for new and highly efficacious technologies for enamel remineralization, which resulted in the evolution of various contemporary remineralizing agents.[4]
Many methods are used to provide evidence of mineral loss or gain. SMH analyses have been widely used to assess demineralization and remineralization changes occurring in enamel. SMH evaluations are simple, fast and easy to measure nondestructively. This method, measuring the resistance of materials against plastic deformation from a standard source, allows repeated measurements of the same specimen over a period of time, reducing the experimental variation, reinforcing that SMH evaluations are a feasible choice to estimate mineral changes.[11]
The findings indicated that all three treatment regimens significantly promoted the remineralization of enamel lesions and increased enamel microhardness compared to artificial saliva. Thus, the first null hypothesis has to be rejected. The highest mean SMH was found in self-assembling peptide followed by fluoride and CPP-ACPF while the lowest mean SMH was found in artificial saliva. However, no statistically significant difference was found between fluoride and CPP-ACPF.
Self-assembling peptides showed the highest remineralizing efficacy which is attributed to the fact that P11-4 is a rationally designed self-assembling peptide that undergoes, in response to specific environmental factors, a hierarchically predetermined process of self-assembly forming three-dimensional fibrillar scaffolds. Once assembled, these scaffolds serve as ideal templates for hydroxyapatite nucleation promoting guided enamel regeneration.[5,6,12] They mirror biological macromolecules found in extracellular matrices, including those of the mammalian skeleton, which are known to control the deposition, morphology and growth of hydroxyapatite crystals.[5]
The responsiveness of the peptide to different external environmental triggers serves as a property for potential indications, offering the possibility for its application in a fluid state and subsequent in situ-triggered gelation inside areas of tissue porosity such as caries lesions and exposed dentin.[5,13] At neutral pH, P11-4 exists in a low viscosity, liquid monomeric form. When the pH is decreased (pH < 7.4), P11-4 changes into bioactive gel scaffolds that binds to tooth surface inducing de novo hydroxyapatite crystallization.
Triggering the process of self-assembly and gelation after its application is attributed to the low pH, the presence of cations and the high ionic strength; conditions presumed to be found within a caries lesion.[6] P11-4 spontaneously switches and self-assembles to an elastomeric nematic 3D gels that show high affinity to tooth mineral, based on matching distances of Ca-binding sites on P11-4 and Ca spacing in the crystal lattice of hydroxyapatite.[5,6,13] This was confirmed by a study conducted by Kirkham et al.[5] where the incubation of P11-4 in mineralizing solutions for 7 days resulted in the presence of needle-like electron-dense deposits within the scaffold itself, suggested to be crystalline hydroxyapatite.
The results obtained in this study are in agreement with studies done by Kirkham et al., Brunton et al., Schmidlin et al., Takahashi et al., Bröseler et al., Schlee et al. and Ceci et al. where they also found P11-4 able to induce biomimetic regeneration of early caries lesions.[5,6,12,13,14,15,16]
Its enamel rehardening and high SMH values are suggested to be due to its potential for subsurface remineralization which was concluded in studies investigating its remineralizing potential in deep lesions.[12,15] This is attributed to the fact that the peptide is designed to be in monomeric liquid form when originally applied to the lesion's surface. This allows for diffusion thanks to its very low viscosity that ensures deep penetration into the subsurface lesion body micropores followed by a rapidly driven self-assembly promoting in-depth biomimetic remineralization.[12,15]
Fluoride's remineralizing potential showed a significant increase in the mean SMH compared to artificial saliva. The increase in microhardness values is due to its ability to form fluorapatite crystals where the incorporated fluoride attracts calcium and phosphate ions increasing mineral content. However, there are shortcomings to fluoride as its surface remineralization at the expense of the body of the lesion resulting in lesion arrest without its full remineralization.[4]
From calcium phosphate-based agents is CPP-ACPF, which was used in this study and showed a significant increase in mean SMH compared to artificial saliva. CPP-ACP is a bioactive agent formulated from two parts: CPPs and ACP. CPP is a milk-derived phosphoprotein that has a remarkable ability to stabilize high concentrations of soluble ACP compounds, preventing their growth to the critical size required for nucleation and precipitation. CPP-ACP nanocomplexes bind onto tooth surfaces and dental plaque providing a reservoir of nonstructurally bound, high level of calcium and phosphate ions which favor remineralization.[17]
In this study, the mean SMH among the remineralizing agents used revealed no statistically significant difference between fluoride and CPP-ACPF. These results are in agreement with studies done by Shetty et al., Memarpour et al. and Mohd Said et al.[18,19,20]
However, these results were in disagreement with studies by Zhang et al. and Peric et al.[21,22] which found CPP-ACPF superior to fluoride. It seemed logical to have conflicting results with these studies due to the fact that they used 0.05% sodium fluoride (NaF) solutions compared to 5% NaF varnish used in this study. This indicates a much higher concentration of fluoride available promoting remineralization and for a much longer period of time due to adherent nature of a varnish compared to solutions.
Other studies found that fluoride shows higher remineralizing potential than CPP-ACPF. These results were employed by Lata et al.,[23] who stated that CPP-ACPF crème is not as effective as fluoride in remineralizing early enamel caries. This could be attributed to the fact that the study compared a fluoride varnish to a CPP-ACPF tooth crème that might have been easily washed away and so will not have the same contact time as fluoride varnish.
The net remineralization gain produced by artificial saliva was small and a slow process, due to the low ion concentration gradient from saliva into the lesion, precipitating only superficially and preventing the remineralization process from occurring in the body of the lesion.[7,12] Thus, the control group showed the least values of remineralization as saliva fails to initiate the process of increasing the levels of calcium and phosphate delivery compared to the remineralizing regimens applied[19] which goes in accordance with studies done by Zhang et al. and Somani et al.[21,24] In addition, the formula of the artificial saliva used in the present study did not contain any fluoride which could explain the limited remineralization by the control group.[7,19]
Regarding the storage period of this study, the results revealed that the remineralization potential of all the tested materials increased significantly over time. Thus, the second null hypothesis is also rejected. Higher SMH values were obtained after 4 weeks period with a significant difference between 1-week and 4-week measurements, which is in accordance with studies done by Elkassas and Arafa, Oliveira et al. and Vyavhare et al.[7,11,25] Remineralizing therapies act either by releasing or by attracting calcium and phosphate ions that will penetrate the demineralized surface, initiating a regeneration process of ion deposition into crystal voids with subsequent crystal growth rebuilding the lost hydroxyapatite latticework structure. This denotes that remineralization is a time-dependent process.
CONCLUSIONS
Under the limitations of the present study, it can be concluded that self-assembling peptide confers the highest remineralizing efficacy compared to fluoride and CPP-ACPF, showing a promising, noninvasive enamel regeneration potential. Furthermore, extended period of time had helped attain more benefits from the remineralization regimens applied.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Pretty IA, Ellwood RP. The caries continuum: Opportunities to detect, treat and monitor the re-mineralization of early caries lesions. J Dent. 2013;41(Suppl 2):S12–21. doi: 10.1016/j.jdent.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Featherstone JD. Dental caries: A dynamic disease process. Aust Dent J. 2008;53:286–91. doi: 10.1111/j.1834-7819.2008.00064.x. [DOI] [PubMed] [Google Scholar]
- 3.Goswami M, Saha S, Chaitra TR. Latest developments in non-fluoridated remineralizing technologies. J Indian Soc Pedod Prev Dent. 2012;30:2–6. doi: 10.4103/0970-4388.95561. [DOI] [PubMed] [Google Scholar]
- 4.Amaechi BT. Remineralization therapies for initial caries lesions. Curr Oral Health Rep. 2015;2:95–101. [Google Scholar]
- 5.Kirkham J, Firth A, Vernals D, Boden N, Robinson C, Shore RC, et al. Self-assembling peptide scaffolds promote enamel remineralization. J Dent Res. 2007;86:426–30. doi: 10.1177/154405910708600507. [DOI] [PubMed] [Google Scholar]
- 6.Brunton PA, Davies RP, Burke JL, Smith A, Aggeli A, Brookes SJ, et al. Treatment of early caries lesions using biomimetic self-assembling peptides – a clinical safety trial. Br Dent J. 2013;215:E6. doi: 10.1038/sj.bdj.2013.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elkassas D, Arafa A. Remineralizing efficacy of different calcium-phosphate and fluoride based delivery vehicles on artificial caries like enamel lesions. J Dent. 2014;42:466–74. doi: 10.1016/j.jdent.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Asl-Aminabadi N, Najafpour E, Samiei M, Erfanparast L, Anoush S, Jamali Z, et al. Laser-casein phosphopeptide effect on remineralization of early enamel lesions in primary teeth. J Clin Exp Dent. 2015;7:e261–7. doi: 10.4317/jced.52165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itthagarun A, Verma S, Lalloo R, King NM, Wefel JS, Nair RG. Effects of fluoridated milk on artificial enamel carious lesions: A pH cycling study. J Dent. 2011;39:817–24. doi: 10.1016/j.jdent.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 10.ten Cate JM, Duijsters PP. Alternating demineralization and remineralization of artificial enamel lesions. Caries Res. 1982;16:201–10. doi: 10.1159/000260599. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira GM, Ritter AV, Heymann HO, Swift E, Jr, Donovan T, Brock G, et al. Remineralization effect of CPP-ACP and fluoride for white spot lesions in vitro. J Dent. 2014;42:1592–602. doi: 10.1016/j.jdent.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidlin P, Zobrist K, Attin T, Wegehaupt F. In vitro re-hardening of artificial enamel caries lesions using enamel matrix proteins or self-assembling peptides. J Appl Oral Sci. 2016;24:31–6. doi: 10.1590/1678-775720150352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi F, Kurokawa H, Shibasaki S, Kawamoto R, Murayama R, Miyazaki M. Ultrasonic assessment of the effects of self-assembling peptide scaffolds on preventing enamel demineralization. Acta Odontol Scand. 2016;74:142–7. doi: 10.3109/00016357.2015.1066850. [DOI] [PubMed] [Google Scholar]
- 14.Bröseler F, Tiemann C, Schleich R, Drechsel T, Bommer C. Effect of curodont ™ repair in patients with buccal carious lesions: A mono-centre, single-blinded, randomised, controlled, split-mouth study-intermediate report. Clin Oral Investig. 2013;17:1055. [Google Scholar]
- 15.Schlee M, Rathe F, Bommer C. Effect of curodont TM repair in patients with proximal carious lesions: Uncontrolled, non-interventional study-interim report. Clin Oral Investig. 2013;17:1046–7. [Google Scholar]
- 16.Ceci M, Mirando M, Beltrami R, Chiesa M, Colombo M, Poggio C. Effect of self-assembling peptide P11 -4 on enamel erosion: AFM and SEM studies. Scanning. 2016;38:344–51. doi: 10.1002/sca.21276. [DOI] [PubMed] [Google Scholar]
- 17.Reema SD, Lahiri PK, Roy SS. Review of casein phosphopeptides-amorphous calcium phosphate. Chin J Dent Res. 2014;17:7–14. [PubMed] [Google Scholar]
- 18.Shetty S, Hegde MN, Bopanna TP. Enamel remineralization assessment after treatment with three different remineralizing agents using surface microhardness: An in vitro study. J Conserv Dent. 2014;17:49–52. doi: 10.4103/0972-0707.124136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Memarpour M, Fakhraei E, Dadaein S, Vossoughi M. Efficacy of fluoride varnish and casein phosphopeptide-amorphous calcium phosphate for remineralization of primary teeth: A randomized clinical trial. Med Princ Pract. 2015;24:231–7. doi: 10.1159/000379750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohd Said SN, Ekambaram M, Yiu CK. Effect of different fluoride varnishes on remineralization of artificial enamel carious lesions. Int J Paediatr Dent. 2017;27:163–73. doi: 10.1111/ipd.12243. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Zou J, Yang R, Zhou X. Remineralization effects of casein phosphopeptide-amorphous calcium phosphate crème on artificial early enamel lesions of primary teeth. Int J Paediatr Dent. 2011;21:374–81. doi: 10.1111/j.1365-263X.2011.01135.x. [DOI] [PubMed] [Google Scholar]
- 22.Peric TO, Markovic DL, Radojevic VJ, Heinemann RM, Petrovic BB, Lamovec JS. Influence of pastes containing casein phosphopeptide- amorphous calcium phosphate on surface of demineralized enamel. J Appl Biomater Funct Mater. 2014;12:234–9. doi: 10.5301/jabfm.5000194. [DOI] [PubMed] [Google Scholar]
- 23.Lata S, Varghese NO, Varughese JM. Remineralization potential of fluoride and amorphous calcium phosphate-casein phospho peptide on enamel lesions: An in vitro comparative evaluation. J Conserv Dent. 2010;13:42–6. doi: 10.4103/0972-0707.62634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Somani R, Jaidka S, Singh DJ, Arora V. Remineralizing potential of various agents on dental erosion. J Oral Biol Craniofac Res. 2014;4:104–8. doi: 10.1016/j.jobcr.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vyavhare S, Sharma DS, Kulkarni VK. Effect of three different pastes on remineralization of initial enamel lesion: An in vitro study. J Clin Pediatr Dent. 2015;39:149–60. doi: 10.17796/jcpd.39.2.yn2r54nw24l03741. [DOI] [PubMed] [Google Scholar]


