Abstract
Objective
To determine the association left atrial diameter (LAD) and vascular brain injury on brain MRI.
Methods
We analyzed data from the Cardiovascular Health Study (CHS), a prospective cohort of community-dwelling adults ≥65 years old. LAD was measured from 2-dimensional transthoracic echocardiograms. Among CHS participants who underwent brain MRI, we examined associations of LAD with brain infarcts and leukoaraiosis. Primary outcomes (number for analysis) were prevalent infarcts (2,327) and degree of leukoaraiosis on initial MRI (2,315). Secondary outcomes were prevalent nonlacunar infarcts (2,327), incident infarcts (939), incident nonlacunar infarcts (1,185), and degree of leukoaraiosis on follow-up MRI adjusted for initial MRI (1,158). Relative risk (RR) and linear regression models were adjusted for demographics, vascular risk factors, and potential confounders.
Results
Mean age of the 2,335 participants with initial brain MRI was 72.0 ± 4.8 years; 38.7% were men; and 29.0% participants had prevalent infarcts. In multivariable, fully adjusted models, LAD was associated with prevalent infarcts (RR 1.20, 95% confidence interval [CI] 1.08–1.34) and prevalent nonlacunar infarcts (RR 1.28, 95% CI 1.06–1.54) but not with leukoaraiosis (−0.08, 95% CI −0.17 to 0.07), incident infarcts (RR 1.00, 95% CI 0.78–1.29), nonlacunar infarcts (RR 0.98, 95% CI 0.67–1.42), or worsening leukoaraiosis (−0.04, 95% CI −0.10 to 0.02).
Conclusion
LAD is independently associated with prevalent brain infarcts, particularly nonlacunar infarcts, but not leukoaraiosis. Larger studies are needed to determine associations with incident infarct risk and whether this risk in patients with left atrial enlargement can be reduced with anticoagulant agents.
Left atrial enlargement (LAE) is associated with paroxysmal atrial fibrillation (AF) in the general population,1,2 as well as ischemic stroke.3,4 In addition, recent evidence suggests that LAE is specifically associated with ischemic stroke subtypes related to embolism: cardioembolic strokes or cryptogenic stroke subtypes.5 Several gaps in knowledge persist, however, because less is known about the association between left atrial size and MRI-defined vascular brain injury in the elderly, specifically brain infarcts and white matter hyperintensities, namely leukoaraiosis.6 While 1 study showed an association between left atrial volume (LAV) and subclinical vascular brain injury on brain MRI,6 this study was limited by the inclusion of patients with both lacunar and nonlacunar infarcts and thus failed to provide evidence of a mechanistic relationship between LAV and stroke. Nonlacunar infarcts are more likely to have an embolic etiology than lacunar infarcts, which may simply share risk factors with cardiac disease. Understanding the association between LAE and brain injury, particularly nonlacunar infarcts, may therefore improve stroke prevention strategies and add to the evidence supporting the notion that atrial cardiopathy, or atrial structural or functional abnormalities that predispose to clot formation, can cause stroke even in the absence of AF.7
We hypothesized that left atrial diameter (LAD) would be associated with vascular brain injury on brain MRI.
Methods
Design
The Cardiovascular Health Study (CHS) enrolled 5,888 community-dwelling men and women ≥65 years of age in 2 waves. Participants were randomly selected from Medicare eligibility lists in 4 counties from California, Pennsylvania, Maryland, and North Carolina. The first cohort was enrolled in 1989 to 1990, and the second, a predominantly black cohort, was enrolled in 1992 to 1993. Participants were followed up through 1998 to 1999 via annual clinic visits and semiannual telephone calls and were linked to Medicare claims data, continuing thereafter with 6-month phone calls and Medicare linkage.8
Standard protocol approvals, registrations, and patient consents
Institutional review boards at the field centers and coordinating center approved the study, and all study participants provided written informed consent.
Echocardiographic measurements
Two-dimensional transthoracic echocardiograms (TTEs) were performed as part of the study protocol in 1989 to 1990 in the first cohort and 1994 to 1995 in both cohorts. Measurements were made in accordance with guidelines from the American Society of Echocardiography.9 The primary exposure variable, left atrial anteroposterior diameter, was obtained from the parasternal long-axis view at the level of the aortic valve. The secondary predictor, LAV, was obtained only in 1994 to 1995 and was calculated with a length ellipsoid method, which used 3 orthogonal planes obtained from 2-dimensional TTE images.10 Another echocardiographic covariate was left ventricular hypertrophy (LVH). To define LVH, left ventricular mass was indexed to sex, height, and weight with a regression equation developed in a subset of healthy CHS participants, and a threshold was applied that was based at the 95th percentile of the healthy population.
Outcome
The initial MRI scan was performed between 1991 and 1994, and a follow-up scan was performed ≈5 years later between 1997 and 1999.11–14 Primary outcomes were prevalent infarcts (covert and overt) and leukoaraiosis on the first MRI after TTE: initial MRI for cohort 1 and follow-up MRI for cohort 2. Neuroradiologists blinded to clinical data and following standard protocols evaluated all brain MRI scans, as detailed elsewhere.12 An infarct was defined as an area of abnormal signal ≥3 mm in diameter within 1 vascular distribution without associated mass effect. Lacunar infarct was defined as a subcortical infarct 3 to 20 mm in size.14 Nonlacunar infarcts were defined as any infarct that did not meet the criteria for a lacunar infarct. Leukoaraiosis was graded semiquantitatively with a 10-point scale with the white matter grade (WMG) ranging from 0, the least, to 9, the most severe leukoaraiosis, as previously described.12,13
Secondary outcomes were nonlacunar infarcts on the initial MRI; incident infarcts (covert or overt) and nonlacunar infarcts on the follow-up MRI, excluding those with an infarct on the initial MRI; and the degree of leukoaraiosis on the follow-up MRI from a side-by-side reread, after adjustment for leukoaraiosis on the initial MRI.11,12
Covariates
To determine the associations between LAD and the MRI findings, we adjusted for demographics (self-reported age, sex, and race-ethnicity) and the following vascular risk factors at the time of the TTE: systolic blood pressure, antihypertensive drug therapy, low-density lipoprotein (LDL), high-density lipoprotein (HDL), diabetes mellitus (defined as fasting glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, or insulin or oral hypoglycemic use), smoking, body surface area, AF (based on ECG, hospitalization records, and inpatient, outpatient, or carrier claims records from Medicare claims data), coronary heart disease (myocardial infarction, angina, or report of angioplasty or bypass surgery), heart failure (HF), oral anticoagulant use, LVH, and time between TTE and the MRI outcome. Coronary heart disease and HF were based on self-report at baseline and adjudicated incident events before the TTE for the second cohort. LDL and HDL were carried forward from study baseline (1992–1993) for the second cohort.
Study population
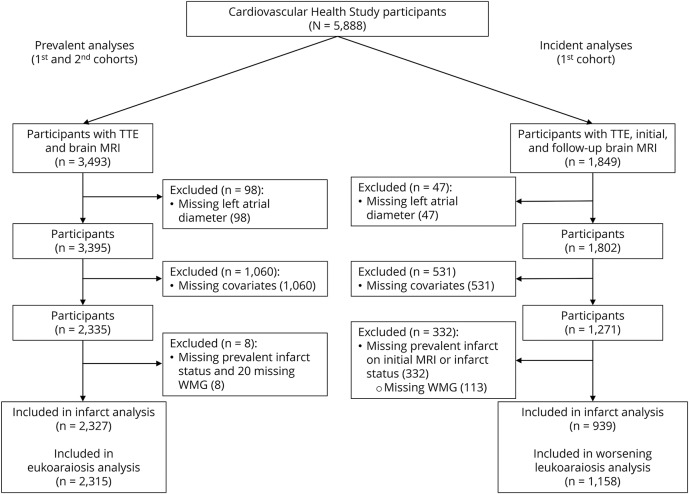
Details about how analytical samples were formed are provided in the figure. For the prevalent vascular brain injury analysis, CHS participants who underwent TTE and initial brain MRI (first cohort) or follow-up brain MRI (second cohort) (n = 3,493) were eligible. We excluded participants who lacked a measurement of LAD on the 2-dimensional TTE (from 1989 to 1990 for the first cohort and 1994 to 1995 for the second cohort) (n = 98) and other potential confounders (n = 1,060). The main reason for exclusion due to missing confounders was missing data on LVH on TTE. The final sample included 2,327 participants eligible for the prevalent infarct analysis and 2,315 eligible for the leukoaraiosis analysis.
Figure. Study flowchart.
Prevalent analyses included participants from the first cohort (echocardiogram obtained between 1989 and 1990 and brain MRI obtained between 1991 and 1994) and the second cohort (echocardiogram obtained between 1994 and 1995 and brain MRI obtained between 1997 and 1999). Incident analyses included participants from the first cohort only (echocardiogram obtained between 1989 and 1990, first brain MRI obtained between 1991 and 1994, and follow-up brain MRI obtained between 1997 and 1999). TTE = transthoracic echocardiogram; WMG = white matter grade.
For the incident vascular brain injury analysis, CHS participants from the first cohort who underwent TTE and an initial and follow-up brain MRI (n = 1849) were eligible. Because the second cohort only had 1 MRI scan after their TTE, they were not included in the incident infarct analyses. We excluded participants who lacked a measurement of LAD on the TTE (n = 47) and potential confounders (n = 531). An additional exclusion criterion for the incident infarct analysis was a prevalent infarct on the initial MRI (n = 330) to ensure that any infarcts on the follow-up MRI were actually incident infarcts. An additional exclusion criterion for the worsening leukoaraiosis analysis was the extreme WMG of 9 on the initial MRI (n = 1). The final sample included 939 participants eligible for the incident infarct analysis and 1,158 eligible for the worsening leukoaraiosis analysis.
Statistical analysis
We used relative risk (RR) regression models15 to determine the association of LAD as a continuous variable with each of prevalent and incident infarcts, as well as nonlacunar infarcts specifically. We used linear regression to determine the association of LAD with WMG on the initial MRI scan and on the follow-up MRI scan, adjusting for the grade on the initial MRI. Estimates were obtained with the following models selected a priori: model 1 adjusted for age, sex, race-ethnicity, body surface area, and time between TTE and brain MRI and model 2 adjusted as in model 1 plus systolic blood pressure, LDL, HDL, diabetes mellitus, coronary heart disease, smoking status, AF, HF, oral anticoagulants, LVH, and antihypertensive medications. We also performed further analyses adjusting for markers of atrial cardiopathy, including P-wave terminal force in lead V1 (PTFV1) measured on ECG,16 and log-transformed serum N-terminal pro-B-type natriuretic peptide (NT-proBNP). In addition, we performed analyses to study the association between the MRI findings and LAE categories based on LAD: normal (for women ≤38 mm, for men ≤40 mm), mild enlargement (for women 39–42 mm, for men 41–46 mm), and moderate/severe enlargement (for women ≥43 mm, for men ≥47 mm).17 An exploratory analysis examined the association of LAV measured in 1994 to 1995 with MRI outcomes from 1997 to 1999 similar to the prevalent analyses. Sensitivity analyses examined the effect of excluding participants with an overt clinically defined stroke and of removing LVH from the models because it was so often missing. Furthermore, sensitivity analyses were performed with incident AF and carotid stenosis (<50%, ≥50%) added to model 2. Statistical analyses were performed with Stata 12.1 (StataCorp, College Station, TX), and a 2-tailed value of p < 0.05 was considered statistically significant.
Data availability
CHS routinely deposits data into the NIH public data repository (BioLINCC, https://chs-nhlbi.org/CHS_PublicData). Researchers who obtain CHS data must sign an agreement agreeing to this deposit.
Results
Characteristics of study sample
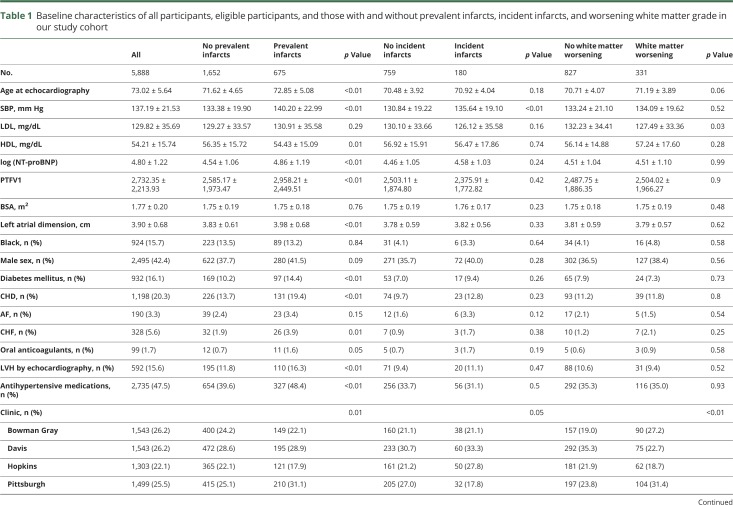
The mean age of participants with LAD, initial MRI, and covariate data available (n = 2,335) was 72.0 ± 4.8 years, and 38.7% were men. The baseline characteristics of all participants, eligible participants, and those with and without prevalent infarcts, incident infarcts, and worsening WMG in our study cohort are shown in table 1. Twenty-nine percent had prevalent infarcts (87.0% covert), and 19.2% of those eligible for the incidence analyses had incident infarcts (88.9% covert). The baseline characteristics of eligible patients in each analysis are similar and are shown in table e-1 (links.lww.com/WNL/A680).
Table 1.
Baseline characteristics of all participants, eligible participants, and those with and without prevalent infarcts, incident infarcts, and worsening white matter grade in our study cohort
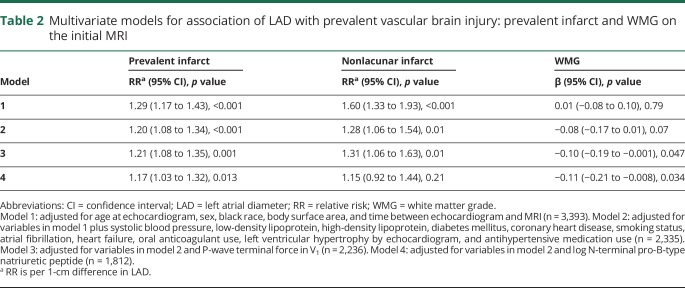
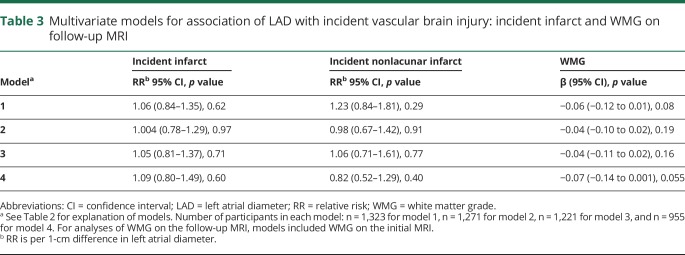
The main results are detailed in tables 2 and 3. Briefly, in minimally and fully adjusted models (table 2), LAD was significantly associated with prevalent infarcts and prevalent nonlacunar infarcts but not with leukoaraiosis. In analyses further adjusted for PTFV1 and log-transformed NT-proBNP, the effect size of LAD on infarct prevalence and leukoaraiosis remained similar (table 2). In minimally and fully adjusted models (table 3), LAD was not significantly associated with incident infarcts, incident nonlacunar infarcts, or leukoaraiosis on the follow-up MRI. In analyses further adjusted for PTFV1 and log-transformed NT-proBNP, the effect size of LAD on incident infarct and leukoaraiosis on the follow-up MRI remained largely unchanged (table 3).
Table 2.
Multivariate models for association of LAD with prevalent vascular brain injury: prevalent infarct and WMG on the initial MRI
Table 3.
Multivariate models for association of LAD with incident vascular brain injury: incident infarct and WMG on follow-up MRI
Considering LAE in categories with no LAE as the reference (tables e-2 and e-3, links.lww.com/WNL/A680), moderate/severe LAE was significantly associated with prevalent infarcts (RR 1.22, 95% confidence interval [CI] 1.04–1.44, p = 0.02) after adjustment for demographics and potential confounders (model 2). The effect size for the association between moderate/severe LAE and nonlacunar infarcts was similar, but the association was not significant (RR 1.19, 95% CI 0.85–1.68, p = 0.32). In addition, compared to no LAE, mild LAE was inversely associated with leukoaraiosis in the prevalent analysis (β = −0.13, 95% CI −0.26 to −0.003, p = 0.04), and moderate/severe LAE was inversely associated with leukoaraiosis on the follow-up MRI (β = −0.09, 95% CI −0.18 to −0.003, p = 0.04).
LAV was obtained for 762 participants, of whom 240 were eligible for the analysis of volume. After adjustment for demographics and potential confounders (model 2), LAV was significantly associated with prevalent infarcts (RR per 1-cm3 difference 1.02, 95% CI 1.01–1.03, p = 0.003) but not with leukoaraiosis (β = −0.01, 95% CI −0.02 to 0.01, p = 0.57) (table e-3, links.lww.com/WNL/A680).
Sensitivity analyses
To determine the association between LAD and subclinical or covert brain infarcts, we performed sensitivity analyses excluding participants with a prior overt clinically defined stroke before the MRI (122 participants for prevalent analysis and 74 participants for incident analysis). Results were similar to the main analyses. With model 2, LAD remained significantly associated with prevalent infarcts (RR per 1-cm difference 1.23, 95% CI 1.09–1.39, p < 0.001) and prevalent nonlacunar infarcts (RR per 1-cm difference 1.40, 95% CI 1.11–1.77, p = 0.005) but not with leukoaraiosis (β = −0.07, 95% CI −0.16 to 0.03, p = 0.16). Associations were not significant between LAD and incident infarcts, incident nonlacunar infarcts, and leukoaraiosis on follow-up MRI.
Furthermore, because of the relatively large number of patients (n = 966 for prevalent analyses and n = 484 for incident analyses) missing the LVH variable, we performed sensitivity analyses excluding LVH from model 2. Again, results were similar to the main analyses. LAD remained significantly associated with prevalent infarcts (RR per 1-cm difference 1.12, 95% CI 1.03–1.21, p = 0.008) and prevalent nonlacunar infarcts (RR per 1-cm difference 1.24, 95% CI 1.07–1.43, p = 0.005) but not with leukoaraiosis (β = −0.03, 95% CI −0.11 to 0.05, p = 0.42). Associations were not significant between LAD and incident infarcts, incident nonlacunar infarcts, and leukoaraiosis on follow-up MRI.
There were 62 individuals who were in our analysis and had history of AF before their first TTE. Moreover, there were 91 cases of incident AF before the MRI for the prevalent analysis and 129 for the incident analysis. Sensitivity analyses were performed including incident AF before the MRI of interest and then internal carotid stenosis dichotomized (<50% stenosis and ≥50% stenosis) in model 2. In these analyses, there was an association between LAD and brain infarcts and nonlacunar infarcts but not WMG. Furthermore, there was no association between LAD and incident infarcts, incident nonlacunar infarcts, or WMG on follow-up brain MRI. These results are consistent with our main findings.
Discussion
In this population-based, elderly cohort, we showed an association between LAD and both MRI-defined prevalent brain infarcts and nonlacunar infarcts. The results persisted even after adjustment for potential confounders such as AF and other recently described cardiac biomarkers of atrial cardiopathy, including serum NT-proBNP and PTFV1.18 We could not confirm an association between LAD and incident brain infarcts, likely related to a smaller sample size or the exclusion of participants with prevalent infarcts, who may be at higher risk of additional infarcts compared to those without prevalent infarcts. Our study showed an inverse relationship between LAD and leukoaraiosis, suggesting that they are not mechanistically related.
The association between LAD and both prevalent brain infarcts and nonlacunar infarcts could be due to several causes. First, this association may be at least partially mediated by undiagnosed AF, especially because LAD and AF are associated. Although we adjusted for history of AF, our ascertainment of AF is imperfect. AF may occur intermittently, and long-term monitoring to search for incident AF was not performed in this cohort. Another potential explanation is that both left atrial size and vascular brain injury share vascular risk factors, which are themselves the cause of brain infarcts. Within our study, however, the association between LAD and brain infarcts was independent of most known vascular risk factors, arguing against this being the explanation for our findings. Nonetheless, we cannot exclude residual confounding as an explanation. Another explanation is that an increase in LAD causes reduced flow velocity in the left atrium,19 therefore contributing to stasis and predisposing to clot formation. This possibility is supported by transesophageal echocardiographic data suggesting an association between embolic events and both left atrial dilatation and spontaneous echocardiographic contrast.3
LAE is a likely a biomarker of atrial cardiopathy, and besides LAE, other biomarkers of atrial cardiopathy such as NT-proBNP and PTFV1 have been shown to be associated with ischemic stroke, particularly of embolic subtypes.18 In addition, PTFV1 has been associated with brain infarcts.16 These biomarkers may help identify a population who benefits from anticoagulation therapy for stroke prevention; nevertheless, the utility of individual biomarkers and cutoff levels associated with heightened risk of embolic events remains undetermined. A post hoc analysis of the Warfarin-Aspirin Recurrent Stroke Study showed that warfarin was superior to aspirin in reducing the risk of stroke and death among patients with NT-proBNP >750 pg/m.20 This observation may lead to clinical trials aiming to improve primary and secondary stroke prevention strategies in patients with biomarkers of atrial cardiopathy such as LAE. The Atrial Cardiopathy and Antithrombotic Drugs in Prevention After Cryptogenic Stroke (ARCADIA) trial is an ongoing clinical trial designed to enroll 1,100 patients with cryptogenic stroke and evidence of atrial cardiopathy—as suggested by at least 1 of 3 biomarkers (LAE, NT-proBNP, and PTFV1)—to test the efficacy of apixaban vs aspirin for secondary stroke prevention (ClinicalTrials.gov identifier NCT03192215).
Finally, our study has several limitations, including low power to detect an association between LAD and the incident vascular brain injury and variable time intervals between TTE and MRI. Our study cohort tended to be younger and healthier than the overall CHS cohort and thus is less generalizable. In addition, because routine ECG monitoring was not performed in our cohort, there is incomplete ascertainment of AF; therefore, it remains unclear whether LAE predicts ischemic stroke independently of AF or whether LAE is a marker of subsequent AF, which is a more direct contributor to future ischemic stroke. We used LAD in our main analysis even though LAV is the preferred tool to assess LAE. However, analyses with LAV are underpowered, and LAD has shown association with outcomes and expected clinical phenotypes in the CHS cohort. Moreover, the fact that nearly 90% of the infarcts (incident and prevalent) in this cohort were clinically covert is another limitation of this study because the cost-effectiveness and risk-to-benefit ratio of stroke prevention strategies in preventing covert brain infarcts remain undetermined. Finally, there have been improvements in imaging modalities from the time these studies were performed in our cohort; however, most of the findings on MRI have been confirmed in prior studies.
Our study also had important strengths such as a large, diverse cohort with both MRI and TTE performed, the availability of other biomarkers of atrial cardiopathy, and standardized measures of infarcts and leukoaraiosis on MRI.
LAE is associated with prevalent brain infarcts, particularly with nonlacunar infarcts, but not with white matter disease. These findings support a mechanistic relationship between LAE and vascular brain injury. Further research in larger cohorts and using different modes of assessing left atrial size such as LAV may be needed to detect associations with incident infarcts and to determine whether the risk of stroke associated with LAE can be reduced with anticoagulants.
Glossary
- AF
atrial fibrillation
- ARCADIA
Atrial Cardiopathy and Antithrombotic Drugs in Prevention After Cryptogenic Stroke
- CHS
Cardiovascular Health Study
- CI
confidence interval
- HDL
high-density lipoprotein
- HF
heart failure
- LAD
left atrial diameter
- LAE
left atrial enlargement
- LAV
left atrial volume
- LDL
low-density lipoprotein
- LVH
left ventricular hypertrophy
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- PTFV1
P-wave terminal force in lead V1
- RR
relative risk
- TTE
transthoracic echocardiogram
- WMG
white matter grade
Author contributions
Dr. Yaghi: study concept and design and drafting of the manuscript. Ms. Bartz and Dr. Kronmal: analysis and interpretation. Dr. Kamel, Dr. Gottdiener, Dr. Longstreth, and Dr. Elkind: study concept and design and critical revision of the manuscript for important intellectual content.
Study funding
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, N01HC85084, N01HC35129, and N01HC15103 and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurologic Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging. A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Shadi Yaghi received funding from the New York Stroke Trials Network of Columbia and Cornell (NINDS U10NS086728).
Disclosure
S. Yaghi, T. Bartz, R. Kronmal, H. Kamel, J. Gottdiener, and W. Longstreth, Jr., report no disclosures relevant to the manuscript. M. Elkind discloses receiving personal compensation for serving on advisory boards and consulting from BioTelemetry/Cardionet, Boehringer-Ingelheim, Inc, BMS-Pfizer Alliance, Daiichi-Sankyo, and Janssen Pharmaceuticals. Go to Neurology.org/N for full disclosures.
References
- 1.Wozakowska-Kaplon B. Changes in left atrial size in patients with persistent atrial fibrillation: a prospective echocardiographic study with a 5-year follow-up period. Int J Cardiol 2005;101:47–52. [DOI] [PubMed] [Google Scholar]
- 2.Mattioli AV, Sansoni S, Lucchi GR, Mattioli G. Serial evaluation of left atrial dimension after cardioversion for atrial fibrillation and relation to atrial function. Am J Cardiol 2000;85:832–836. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death: the Framingham Heart Study. Circulation 1995;92:835–841. [DOI] [PubMed] [Google Scholar]
- 4.Di Tullio MR, Sacco RL, Sciacca RR, Homma S. Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke 1999;30:2019–2024. [DOI] [PubMed] [Google Scholar]
- 5.Yaghi S, Moon YP, Mora-McLaughlin C, et al. Left atrial enlargement and stroke recurrence: the Northern Manhattan Stroke Study. Stroke 2015;46:1488–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russo C, Jin Z, Liu R, et al. LA volumes and reservoir function are associated with subclinical cerebrovascular disease: the CABL (Cardiovascular Abnormalities and Brain Lesions) Study. JACC Cardiovasc Imaging 2013;6:313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamel H, Okin PM, Elkind MS, Iadecola C. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke 2016;47:895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–276. [DOI] [PubMed] [Google Scholar]
- 9.Picard MH, Adams D, Bierig SM, et al. American Society of Echocardiography recommendations for quality echocardiography laboratory operations. J Am Soc Echocardiogr 2011;24:1–10. [DOI] [PubMed] [Google Scholar]
- 10.Gottdiener JS, Kitzman DW, Aurigemma GP, Arnold AM, Manolio TA. Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons > or = 65 years of age (the Cardiovascular Health Study). Am J Cardiol 2006;97:83–89. [DOI] [PubMed] [Google Scholar]
- 11.Longstreth WT Jr, Arnold AM, Beauchamp NJ Jr, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke 2005;36:56–61. [DOI] [PubMed] [Google Scholar]
- 12.Longstreth WT Jr, Dulberg C, Manolio TA, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke 2002;33:2376–2382. [DOI] [PubMed] [Google Scholar]
- 13.Longstreth WT Jr, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people: the Cardiovascular Health Study. Stroke 1996;27:1274–1282. [DOI] [PubMed] [Google Scholar]
- 14.Longstreth WT Jr, Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol 1998;55:1217–1225. [DOI] [PubMed] [Google Scholar]
- 15.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–706. [DOI] [PubMed] [Google Scholar]
- 16.Kamel H, Bartz TM, Longstreth WT Jr, et al. Association between left atrial abnormality on ECG and vascular brain injury on MRI in the Cardiovascular Health Study. Stroke 2015;46:711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr 2006;7:79–108. [DOI] [PubMed] [Google Scholar]
- 18.Yaghi S, Boehme AK, Hazan R, et al. Atrial cardiopathy and cryptogenic stroke: a cross-sectional pilot study. J Stroke Cerebrovasc Dis 2016;25:110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tabata T, Oki T, Fukuda N, et al. Influence of left atrial pressure on left atrial appendage flow velocity patterns in patients in sinus rhythm. J Am Soc Echocardiogr 1996;9:857–864. [DOI] [PubMed] [Google Scholar]
- 20.Longstreth WT Jr, Kronmal RA, Thompson JL, et al. Amino terminal pro-B-type natriuretic peptide, secondary stroke prevention, and choice of antithrombotic therapy. Stroke 2013;44:714–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
CHS routinely deposits data into the NIH public data repository (BioLINCC, https://chs-nhlbi.org/CHS_PublicData). Researchers who obtain CHS data must sign an agreement agreeing to this deposit.