Alemtuzumab, indicated for active relapsing-remitting multiple sclerosis (MS), produces lymphocyte depletion followed by a different pattern of T- and B-cell repopulation, increasing the risk of secondary autoimmune diseases.1 According to the published long-term safety data, thyroid disorders are the most common autoimmune adverse event.2 Myasthenia gravis (MG), an autoimmune antibody-mediated disorder, rarely coexists in patients with MS.3,4 To date, MG has not been reported in patients with MS exposed to alemtuzumab.
Case report
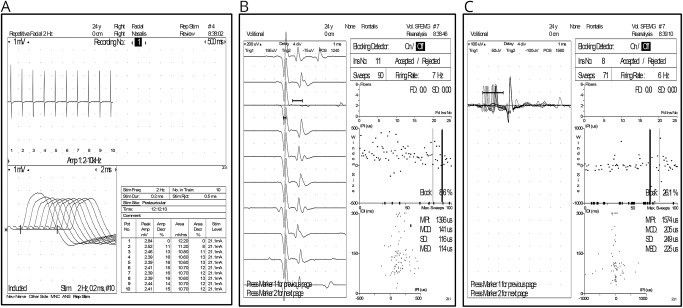
We present a 24-year-old woman with no medical history except for autoimmune hypothyroidism in the paternal grandmother. In July 2015, she presented with her first episode of neurologic dysfunction suggestive of left optic neuritis (visual acuity 0.6 left eye, 1.0 right eye). She was treated with IV methylprednisolone for 3 days (1 g/d) and completely recovered. A baseline brain MRI (August 2015) revealed typical inflammatory demyelinating lesions. A hyperintense signal was also observed in the intraorbital segment of the left optic nerve. After IV gadolinium administration, 2 subcortical lesions showed signs of inflammatory activity. Serologic and systemic autoimmune screenings were negative, and a lumbar puncture detected oligoclonal bands in the CSF. The diagnosis of MS was established, as per McDonald criteria, and an immunomodulatory treatment was recommended. A few days later, the patient developed a new episode of optic neuritis as well as altered coordination and fatigability of the left upper extremity. A new brain MRI showed more than 40 gadolinium-enhancing lesions in the context of a high lesion load (>100). The patient was treated again with corticosteroids with good clinical recovery. Given the highly active disease, we decided to start treatment with alemtuzumab. The patient received the first course from May 23 to 27, 2016. During the first year of treatment, the patient remained clinically stable. An MRI performed in July 2017 did not show new or active lesions. The patient received the second course of alemtuzumab from May 23 to 25, 2017. In August 2017, the patient developed fluctuating speech impairment and, in October, increasing difficulty chewing and occasional diplopia appeared. In November 2017, the patient had right ptosis, diplopia in lateral gaze with fatigability maneuvers, and weakness of neck flexion on neurologic examination. A brain MRI showed no new or active lesions, and EMG was consistent with neuromuscular junction involvement such that the nasalis muscle repetitive nerve stimulation performed at low frequencies (2 Hz) showed a pathologic decrement (16% and 13% compound muscle action potential amplitude and area decrease). In addition, frontal muscle single-fiber EMG during voluntary activation obtained with concentric needle confirmed an abnormal jitter in 85.7% of muscle fibers evaluated (6/7 fibers with jitter increase, mean jitter value 108 µs) with 57% of blockings (figure). Thoracic CT scan ruled out the presence of thymoma, and anti-acetylcholine receptor (AChR) antibodies in serum were positive with values of >20.00 nmol/L (0.00–1.00). Muscle-specific tyrosine kinase antibodies were negative. A retrospective determination of AChR antibodies in a blood sample extracted on the first day (course 1), before starting alemtuzumab treatment, yielded a value of 1.10 nmol/L.
Figure. Neurophysiologic tests.
(A) 2-Hz repetitive nerve stimulation of the nasalis muscle with 16% amplitude and 13% area decrement. (B, C) Abnormal jitter in the frontal muscle. (B) 141 μS mean consecutive difference (MCD) with blocking and (C) 205 μs MCD.
Upon diagnosing the patient with MG, symptomatic treatment was started in ascending doses of pyridostigmine up to 60 mg/4 hours and prednisone 60 mg/d. The patient showed full recovery at the end of December 2017 and has remained stable until the present. After completing 2 years of alemtuzumab therapy, we plan to continue with rituximab treatment, to cover both autoimmune pathologies.
Discussion
We report a well-studied case of a patient with MS treated with alemtuzumab who developed MG.
In addition to the widely described secondary autoimmune diseases, other autoimmune adverse effects, such us neutropenia, hemolytic anemia, agranulocytosis, and pancytopenia, were also described in the phase III clinical trials.1,2 During alemtuzumab postmarketing use, 1 case of type 1 diabetes,5 1 case of alopecia universalis,6 and 1 acquired hemophilia were described.7 All occurred after the second course of treatment, as in our case. As of May 2018, 18,000 patients with MS have received alemtuzumab, and the manufacturer has not received reports of other cases of MG to date (data on file).
Different hypotheses could explain the coexistence of MG and MS in our patient. The value of AChR antibodies in the sample taken immediately before alemtuzumab was first infused slightly exceeded the upper limit of normal. It is therefore difficult to completely rule out the presence of a subclinical form of MG. Common genetic and environmental factors exist in both autoimmune diseases (although the association is rare, several cases have been described).4 Alemtuzumab could have triggered MG in an already predisposed patient, but a more direct effect on MG causation remains probable. A few cases of MG in patients who received other immunomodulatory drugs (interferon-β and glatiramer acetate) also have been reported.8,9 Overall, the possibility of developing different autoimmune diseases in addition to those typically described in alemtuzumab-treated patients should be borne in mind.
Author contributions
L. Midaglia contributed to the concept and design of the work and acquisition, analysis, and interpretation of data for the work; participated in the drafting of the work and revised it for important intellectual content; and gave final approval of the version to be published. M. Gratacòs contributed to the acquisition, analysis, and interpretation of data for the work; revised it for important intellectual content; and gave final approval of the version to be published. E. Caronna contributed to the acquisition, analysis, and interpretation of data for the work; revised it for important intellectual content; and gave final approval of the version to be published. N. Raguer contributed to the acquisition, analysis, and interpretation of data for the work; revised it for important intellectual content; and gave final approval of the version to be published. J. Sastre-Garriga contributed to the acquisition, analysis, and interpretation of data for the work; participated in the drafting of the work and revised it for important intellectual content; and gave final approval of the version to be published. X. Montalban contributed to the concept and design of the work, acquisition, analysis, and interpretation of data for the work; participated in the drafting of the work and revised it for important intellectual content; and gave final approval of the version to be published. M. Tintore contributed to the concept and design of the work, acquisition, analysis, and interpretation of data for the work; participated in the drafting of the work and revised it for important intellectual content; and gave final approval of the version to be published.
Study funding
No targeted funding reported.
Disclosure
L. Midaglia, M. Gratacòs, E. Caronna, and N. Raguer report no disclosures relevant to the manuscript. J. Sastre-Garriga has received compensation for participating on advisory boards, speaking honoraria, and travel expenses for scientific meetings, consulting services, or research support from Celgene, Novartis, Biogen, Teva, Merck, Almirall, and Genzyme. X. Montalban has received speaking honoraria and travel expenses for participation in scientific meetings and has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past with Actelion, Amirall, Bayer, Biogen, Celgene, Genzyme, Hoffmann-La Roche, Novartis, Oryzon Genomics, Sanofi-Genzyme, and Teva Pharmaceutical. M. Tintore has received compensation for consulting services and speaking honoraria from Almirall, Bayer Schering Pharma, Biogen-Idec, Genzyme, Merck-Serono, Novartis, Roche, Sanofi-Aventis, and Teva Pharmaceuticals and is co-editor of Multiple Sclerosis Journal–ETC. Go to Neurology.org/N for full disclosures.
References
- 1.Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012;380:1829–1839. [DOI] [PubMed] [Google Scholar]
- 2.Coles AJ, Cohen JA, Fox EJ, et al. Alemtuzumab CARE-MS II 5-year follow-up: efficacy and safety findings. Neurology 2017;89:1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorenzoni PJ, Scola RH, Kay CSK, et al. Myasthenia gravis and multiple sclerosis: an uncommon presentation. Arq Neuro-Psiquiatr 2008;66:251–253. [DOI] [PubMed] [Google Scholar]
- 4.Marrie RA, Reider N, Cohen J, et al. A systematic review of the incidence and prevalence of autoimmune disease in multiple sclerosis. Mult Scler 2015;21:282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malmestrom C, Andersson BA, Lycke J. First reported case of diabetes mellitus type 1 as a possible secondary autoimmune disease following alemtuzumab treatment in MS. J Neurol 2014;261:2016–2018. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann J, Buhl T, Muller M. Alopecia universalis following alemtuzumab treatment in multiple sclerosis: a barely recognized manifestation of secondary autoimmunity: report of a case and review of the literature. Front Neurol 2017;8:569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCaughan G, Massey J, Sutton I, et al. Acquired haemophilia: a complicating alemtuzumab therapy for multiple sclerosis. BMJ Case Rep 2017:2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dionisiotis J, Zoukos Y, Thomaides T. Development of myasthenia gravis in two patients with multiple sclerosis following interferon beta treatment. J Neurol Neurosurg Psychiatry 2004;75:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frese A, Bethke F, Lüdemann P, Stögbauer F. Development of myasthenia gravis in a patient with multiple sclerosis during treatment with glatiramer acetate. J Neurol 2000;247:713. [DOI] [PubMed] [Google Scholar]