Abstract
Objective
To determine the frequency and relative importance of symptoms experienced by adults with spinal muscular atrophy (SMA) and to identify factors that are associated with a higher burden of disease in this population.
Methods
We conducted a cross-sectional study of 359 adults with SMA using the International SMA Patient Registry. Participants provided input regarding 20 symptomatic themes and 207 symptoms that potentially affect adults with SMA. Participants were asked about the relative importance of each symptom, and analysis was conducted to determine how age, sex, SMA type, education, mobility, and employment status relate to symptom prevalence.
Results
Limitations with mobility or walking (98.6%) and the inability to do activities (98.6%) were the 2 themes with the highest prevalence in the study sample. Limitation with mobility or walking was the theme that was identified as having the greatest effect on the lives of adults with SMA. Employment status was associated with the prevalence of 4 of 20 themes and a reliance on an assistive device was associated with 7 of 20 themes. The prevalence of breathing difficulties, choking or swallowing difficulties, and communication difficulties differed among those with different SMA types.
Conclusions
There are many symptomatic themes that affect the lives of adults with SMA. These themes vary in prevalence and relative importance in the adult SMA population.
Spinal muscular atrophy (SMA) is a neuromuscular disorder caused by a homozygous mutation of the SMN1 gene on chromosome 5.1 SMA has an estimated incidence of 1 in 11,000 live births, making it one of the more common genetically inherited autosomal recessive disorders.2 The onset of symptoms is variable in patients with SMA. Historically, SMA classification was tied to the age of symptom onset and the achievement of motor milestones. Type 1 patients develop symptoms within the first 6 months of life, patients with type 2 develop symptoms between 6 and 18 months of age, patients with type 3 develop symptoms in early childhood after 18 months of age, and type 4 patients develop symptoms in adulthood.3–5 Collectively, the adult SMA population consists of patients with an early onset of disease in their childhood who live beyond 18 years of age and those with a milder disease phenotype who develop symptoms later in life.
As novel therapeutics are developed for SMA, it is crucial to understand the symptoms and issues that have the greatest effect on adults with SMA. In our group's prior study, we identified the issues and symptoms that matter most to adults with SMA in a sample of 15 participants.6 Using qualitative interviews, we analyzed 1,045 quotes regarding the issues that have the greatest effect on the daily lives of adults with SMA. While prior studies have explored the disease burden of children and the parents of children with SMA, a large-scale cross-sectional study to explore what is of greatest importance to adults with SMA has not been previously attempted.7,8
Herein, we present the results of the Patient Reported Impact of Symptoms in SMA (PRISM-SMA) study. We designed this international, cross-sectional evaluation of adults with SMA to determine the relative importance and prevalence of the most significant symptoms in the adult SMA population and simultaneously identify the factors that are associated with a higher level of disease burden in this population.
Methods
Study participants
Eligible participants included those aged 18 years or older with a diagnosis of SMA. All participants were registered and consenting members of the International SMA Patient Registry.9 Every member of the registry that met inclusion criteria was contacted by mail. All study activities were fully approved by the University of Rochester institutional review board. All potential participants were sent and provided a detailed information letter regarding the study prior to their completion of the study survey. Surveys were sent in February of 2016 and received up until June of 2016.
Generation of survey
We generated a survey that included questions about the symptoms and themes previously identified by patients with SMA as having a potentially high level of importance in their lives.6 Our survey also included supplemental questions on symptoms identified as important by other neuromuscular disease populations.10–12 Altogether, we inquired about 207 individual symptoms and 20 symptomatic themes. Participants were allowed to use a proxy if they had physical limitations preventing them from filling out the survey.
For each symptom, participants were asked “How much does the following impact your life now?” and response options were (1) I don't experience this; (2) I experience this but it does not affect my life; (3) It affects my life a little; (4) It affects my life moderately; (5) It affects my life very much; and (6) It affects my life severely. Participants were also provided space to write in any symptoms that were important to them but not mentioned during the survey. Lastly, participants reported their sex, age, race, ethnicity, SMA type, level of education, employment status, age at onset of symptoms, country and state of residence, and ambulatory status. Ambulatory status was queried by asking “Which of the following best describes how you get around?” The response options were: “I walk independently without assistance”; “I primarily use a cane”; “I primarily use a walker”; and “I primarily use a wheelchair or motorized scooter.”
Statistical analysis
We determined the frequency (prevalence) of each symptom and theme in our sample. We calculated the relative importance of each symptom in the participants that reported that they experienced the individual symptom. For this metric, each participant response was assigned a numerical value, as follows: I experience the symptom but it does not affect my life = 0; it affects my life a little = 1; it affects my life moderately = 2; it affects my life very much = 3; and it affects my life severely = 4. Average life impact scores were calculated for each symptom and theme by summing the impact scores for each and dividing by the number of participants that reported experiencing that symptom or theme. This methodology has been previously utilized for other neuromuscular diseases.10–12
Participants' responses were categorized into subgroups by SMA type, employment status, sex, age (divided into 2 groups based on the median age of the sample), education level (those with a college degree or graduate degree vs those with a high school or technical degree), and ambulation status (those who could walk independently vs those who could not walk or required assistive devices). We compared the prevalence of symptoms and themes across subgroups using Fisher exact tests. Pairwise comparisons were performed between SMA types. The Benjamini-Hochberg procedure was used to correct for multiple comparisons using a false discovery rate of 0.05 and 220 test statistics. In this method, the 220 p values are ranked from smallest to largest (p(1) ≤ p(2) ≤ … ≤ p(220)), and the largest value of i such that p(i) ≤ 0.05 i/220 is determined. The hypotheses associated with the p values p(1), …, p(i) are rejected, resulting in i “discoveries.”
Data availability
Anonymized data will be shared by request from any qualified investigator.
Results
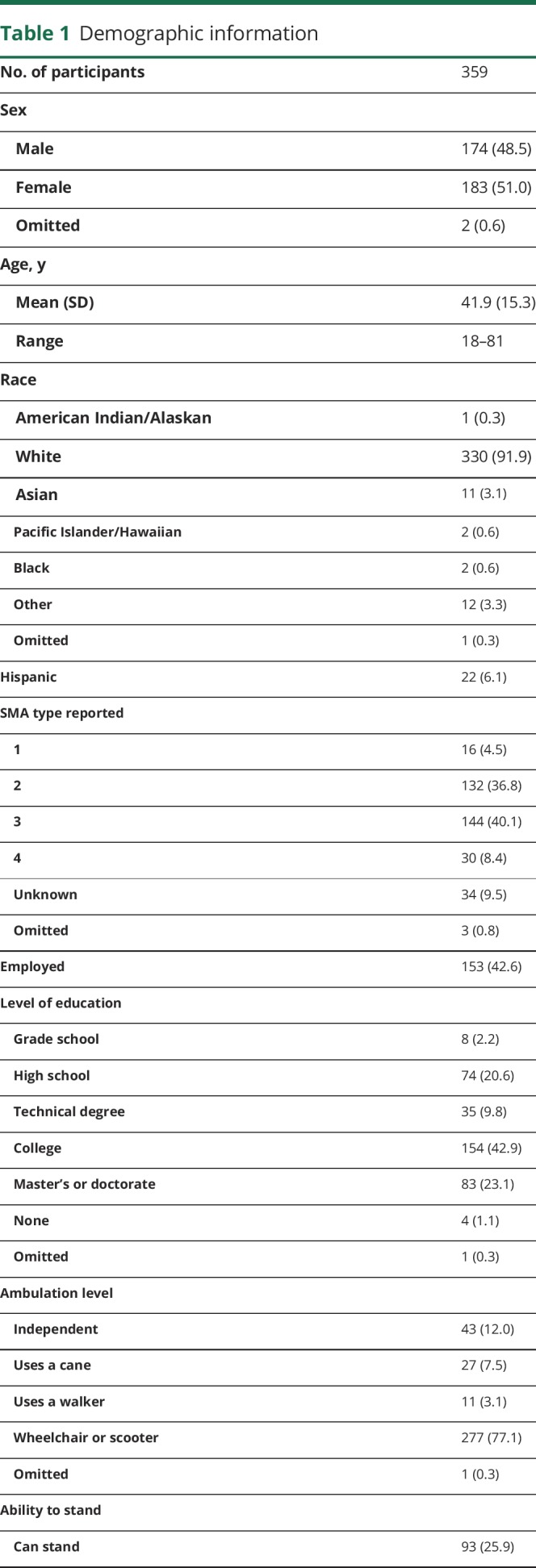
Our survey was sent to 1,438 adults worldwide who were registered in the International SMA Patient Registry. We received survey responses from 359 adults (25% response rate), representing 43 states in the United States and 34 countries worldwide. The respondents were 51% women with an overall age range of 18–81 years (mean age of 41.9). Participants answered 97.8% of the questions (79,758 of 81,493 questions) regarding the 20 symptomatic themes and 207 symptoms. Table 1 provides additional demographic information for our sample.
Table 1.
Demographic information

Prevalence of symptoms/themes
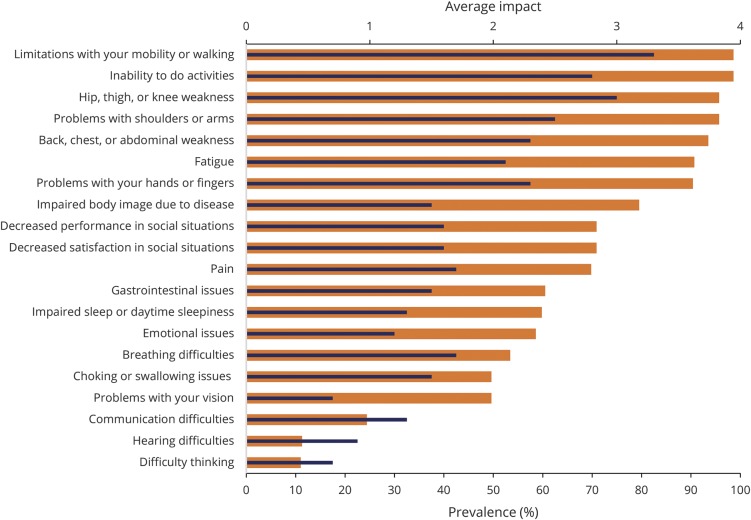
Fifteen of the 20 themes had a prevalence of greater than 50% in our sample (figure). The 2 most prevalent themes were limitations with mobility or walking, and inability to do activities, each of which were experienced by 98.6% of our study participants. Other symptomatic themes with greater than 90% prevalence included hip, thigh or knee weakness; problems with shoulders or arms; back, chest or abdominal weakness; problems with hands or fingers; and fatigue. The most prevalent of the 207 individual symptoms were limitations physically on what you can do (98%), difficulty lifting objects (97.7%), impaired ability to exercise (97.7%), and limited activities due to weakness (97.5%).
Figure. Prevalence and average impact of symptomatic themes, with prevalence (%) on the lower x-axis (wide bars) and average impact ranging from 0 to 4 on the upper x-axis (thin bars).
Themes are listed in order of their prevalence.
Average impact of symptoms/themes
The average impact of each theme is highlighted as it compares with the prevalence in the figure. Limitations in mobility or walking was the theme with the greatest effect on participant's lives (average impact score: 3.3). Of the 207 symptoms, difficulty with stairs, and difficulty getting up from the floor/ground were reported to have the greatest impact on participants' lives with average impact scores of 3.6 each.
Subgroup analysis
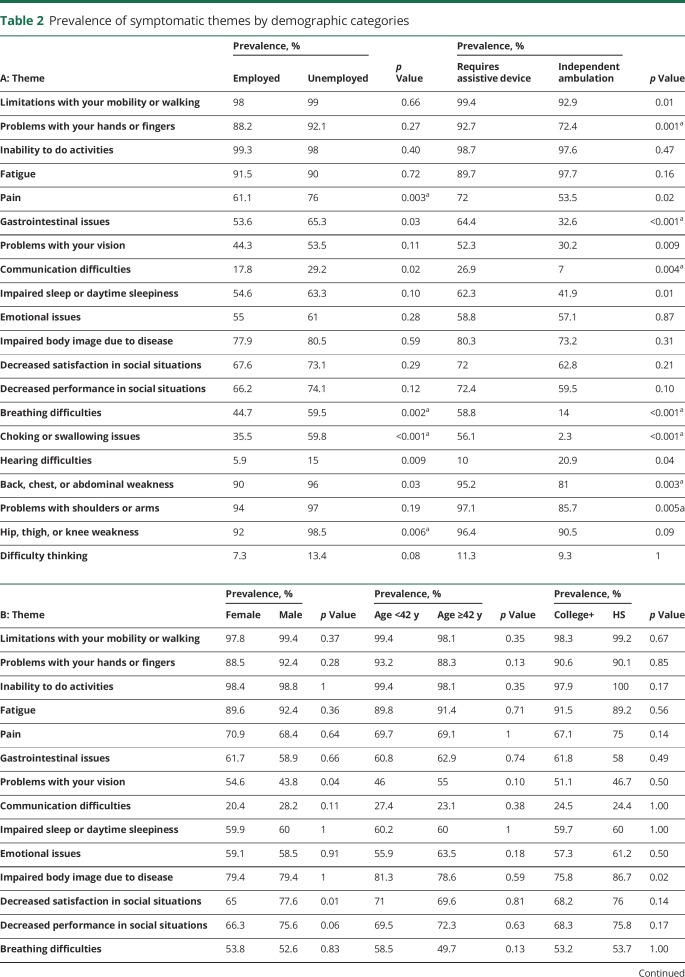
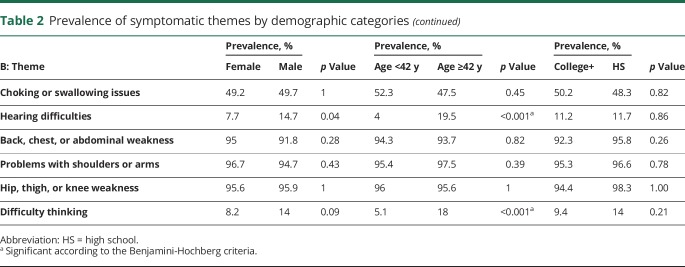
Four of the 20 symptomatic themes were less prevalent in employed individuals compared to the unemployed study participants, as shown in table 2A. The largest difference in prevalence was seen with choking or swallowing issues, which had a prevalence of 35.5% in the employed and 59.8% in the unemployed participants (p < 0.001). Age was associated with a difference in the prevalence of difficulty hearing (p < 0.001) and difficulty thinking (p = 0.002). There were no significant differences in the prevalence of any themes based on education status or sex (table 2B).
Table 2.
Prevalence of symptomatic themes by demographic categories
Ambulation status was associated with the prevalence of 7 symptomatic themes. For example, those who walked independently had a 72.4% prevalence of problems with hands or fingers, compared to 92.7% for those who could not walk or required assistive devices (p = 0.001). Problems with shoulders or arms were also noted in 97.1% of the nonambulatory group, while being noted in only 85.7% of those who walked independently (p = 0.005). Gastrointestinal issues were reported in 64.4% of those who could not walk or required assistive devices but only in 32.6% of those who walked independently (p < 0.001). Breathing difficulties and choking or swallowing issues were significantly less prevalent in the group that walked independently (p < 0.001). Additional themes that differed based on ambulation status included communication difficulties (p = 0.004) and back, chest, or abdominal weakness (p = 0.003).
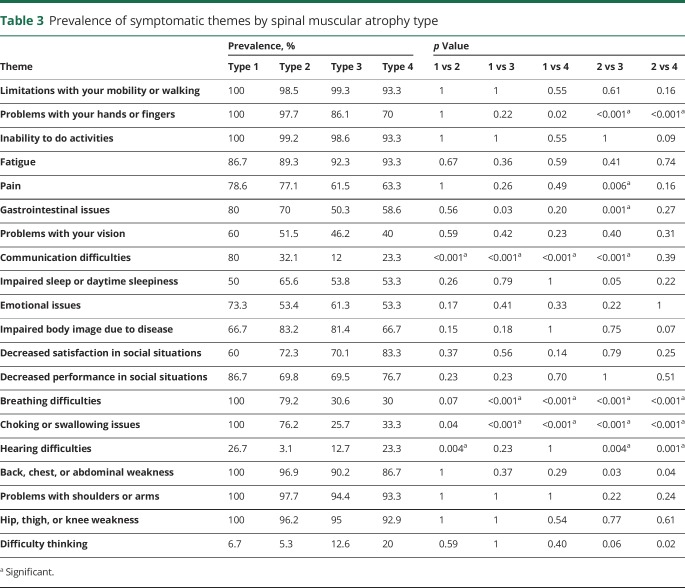
The prevalence of symptomatic themes was associated with participant reported SMA type (table 3). SMA type 1 had a higher prevalence of communication difficulties (p < 0.001 compared to all other SMA types), choking and swallowing issues (p < 0.001 compared to types 3 and 4), and breathing difficulties (p < 0.001 compared to types 3 and 4). Problems with hands or fingers (p < 0.001), pain (p = 0.006), gastrointestinal issues (p = 0.001), communication difficulties (p < 0.001), breathing difficulties (p < 0.001), choking or swallowing issues (p < 0.001), and hearing difficulties (p = 0.004) all differed between SMA type 2 and type 3. Similarly, problems with hands or fingers (p < 0.001), breathing difficulties (p < 0.001), choking or swallowing issues (p < 0.001), and hearing difficulties (p = 0.001) differed between SMA type 2 and type 4. In these instances, except for hearing difficulties, the prevalence of the theme was greater in SMA type 2 than in SMA type 3 or type 4. There was no significant difference in the prevalence of any theme between SMA type 3 and SMA type 4 (data not shown).
Table 3.
Prevalence of symptomatic themes by spinal muscular atrophy type
Open field responses
One hundred forty-three participants utilized the write-in spaces to report additional symptoms. These represented a variety of topics, many of which were expansions or specifications of a previous survey item. For example, difficulty lifting objects from the ground was mentioned in the write-in section, though difficulty lifting objects was included in the survey. Some respondents also mentioned other medical issues that affected their life, such as renal disease, cancer, or headaches. Other issues included difficulty with transportation services, financial difficulties, difficulties with personal care assistants, difficulty pressing buttons on a card payment machine, public restroom accessibility, problems transferring into a dental chair for teeth cleaning, and a feeling of being left out of SMA drug trials.
Discussion
There are many symptoms and themes that generate disease burden in adults with SMA. These symptoms have a variable prevalence and importance in this population. Herein, we document results from one of the largest international studies designed to identify the phenotypic profile and relative importance of individual symptoms in an adult SMA sample. A thorough knowledge of SMA symptomatic burden is important as clinicians strive to better monitor and treat adults with SMA. In addition, our baseline data provide researchers with useful information to plan future clinical trials focused on the symptomatic issues that are most important to adults with SMA.
We found that the prevalence of many symptomatic themes was quite high in adult SMA. Seven of the 20 principal symptomatic SMA themes had a prevalence of 90% or greater in our sample. Despite this high level of symptomatic burden, 42.6% of our study participants were employed at the time of the study and more than 66% had previously obtained a college degree, master's degree, or graduate degree demonstrating a high level of academic and educational resilience in this sample.
Results of our subgroup analyses provided insight into how symptom prevalence differs depending on patient characteristics. Four symptomatic themes were associated with employment status. Of these themes, 2 were related to bulbar function (breathing difficulties, choking or swallowing issues) while one theme (pain) could potentially be amenable to therapeutic intervention. Of note, neither limitations with mobility or walking nor problems with hands or fingers (2 cardinal symptomatic issues in SMA) were associated with employment status. The lack of association of employment with these 2 issues highlights the SMA population's ability to compensate and maintain employment even in the context of significant physical limitations. In the US population, with community and workplace accessibility standards in place as a result of the Americans with Disabilities Act, employment in the setting of physical limitations is more possible. However, some of the issues shared in the open field responses by participants included accessibility in public restrooms, on airplanes, and at hotels, and concerns about employment discrimination. This suggests that societal interventions aimed at improving accessibility to those with physical disabilities may still be needed.
Hearing and cognitive function were the only themes whose prevalence worsened with age in our SMA sample. We suspect that the increased prevalence of dysfunction in these 2 areas with age is largely attributable to the general aging process and not specifically to the pathomechanisms of SMA. The lack of worsening prevalence of other symptomatic themes with age may suggest that many of the cardinal symptoms of adults with SMA occur before age 18 and do not become more common after this age.
As expected, we found that symptomatic burden varies based on patient-reported SMA type. Participants who reported that they had SMA type 1 also reported a higher prevalence of communication difficulties, breathing difficulties, and choking or swallowing issues. In addition, there were differences in 7 symptomatic themes between SMA type 2 and type 3 participants. The most notable differences by group occurred in breathing difficulties and choking or swallowing issues, which were reported by all participants with SMA type 1, more than 75% of participants with SMA type 2, and less than 34% of participants with SMA type 3 or type 4.
We found that the inability to walk independently, that is, without any assistive device, had the most widespread association with symptomatic prevalence in our sample. Seven of our 20 symptomatic themes were associated with this characteristic. Ambulation status is an important marker of disease severity in the adult SMA population and thus strongly relates to the level of disease burden in adults with SMA. Ambulation status is a continuum with a wide range of what could be considered independent mobility. The goal of this specific analysis was to compare those participants with the least burden of disease, i.e., the respondents with complete independence with walking were compared to the rest of the sample. Interventions that improve ambulation status may have a large effect on overall disease burden reported by adults with SMA.
The most prevalent SMA symptoms were not always the ones that were most important to participants. Of the 207 studied symptoms, difficulty with stairs and difficulty getting up from the floor/ground were the symptoms that were identified by participants as having the greatest effect on their lives; however, neither was the most prevalent symptom in the sample. Of note, both of these issues could potentially be addressed by a mechanical or societal intervention. Similarly, the ratio of the impact of a theme to the prevalence of a symptomatic theme varied based on the individual issue. Two themes, pain and breathing difficulties, ranked in the top half of average impact scores but were in the bottom half when ranked by prevalence. In contrast, impaired body image was experienced by nearly 80% of participants, but the impact score was ranked in the lower half of the symptomatic themes.
We acknowledge that our sample may not be representative of the general population of adults with SMA. Our survey was sent to members of a large international registry. No registry member was financially (or otherwise) incentivized to participate. The survey was sent only in English. As expected, our response rate was lower than what we have experienced in our other similar studies using isolated English-speaking North American samples and registries.10–12 We suspect that the reasons for the lower response rate were multifactorial. It is likely that some international participants did not complete the survey because they were unable to read English or gain access to someone who could translate the survey. It is also likely that some registry participants did not complete the survey because of death or a move. We also recognize that answering more than 200 questions requires some degree of attention and concentration, and although there was no strict time limit, it may have appeared too daunting a task for some individuals. Despite the length of the survey, among study participants, there was a 98.7% completion rate for the first 5 symptom questions of the survey, similar to the 98.6% completion rate for the last 5 questions. Overall, this demonstrates that participants were not quitting and submitting the survey before completion. Adults with SMA who were too sick to participate in this registry and those not interested in clinical research may also have been underrepresented in our sample. Most of participants were from the United States (61%). Therefore, our data must be interpreted in this context.
Our sample of participants with SMA was highly educated, with 23.1% having an advanced degree (master's or doctorate) and 66% having either a college degree or an advanced degree. In 2015 US population data, only 12% hold an advanced degree, with 42% having an associate's degree or more.13 In a comparable study evaluating the impact of symptoms in patients with myotonic dystrophy type 1, the proportion of survey respondents with advanced degrees was only 15.5%, again highlighting the academic achievement of adults with SMA even in the context of significant physical limitations.10 Although it is unlikely that our registry-based SMA sample is a perfect representation of the greater SMA population, we suspect that our group is representative of a subset of adults with SMA that will be willing and able to participate in future clinical studies. Lastly, it should be noted that all demographic information was provided directly by participants in a deidentified fashion. Consequently, it is possible that some participants reported their SMA type incorrectly based on misunderstanding, the variation in SMA classification protocols over time, or potentially misclassification by their treating physicians.
PRISM-SMA adds to available knowledge regarding the total symptomatic burden that adults with neuromuscular diseases face. This study again demonstrates that while some overlap exists, the specific symptomatic profile and relative importance of individual symptoms vary based on the specific neuromuscular disease population.9–11 This research highlights a large-scale, patient-centric approach to defining the complex disease burden associated with adult SMA and may prove useful in the future management of these patients, development and validation of disease-specific, patient-reported outcome measures, and the planning and implementation of future SMA therapeutics research.
Acknowledgment
The authors acknowledge the thoughtful insight provided by all of the SMA study participants associated with this research.
Glossary
- PRISM-SMA
Patient Reported Impact of Symptoms in Spinal Muscular Atrophy
- SMA
spinal muscular atrophy
Footnotes
Editorial page 585
Author contributions
Phillip Mongiovi, MD: analysis and interpretation of data, manuscript preparation. Nuran Dilek, MS: analysis and interpretation of data. Connie Garland, BS, CCRP: acquisition of data. Michael Hunter, MD: study concept and design. John T. Kissel, MD: study concept and design. Elizabeth Luebbe, MS: study concept and design. Michael P. McDermott, PhD: analysis and interpretation of data. Nicholas Johnson, MD: study concept and design. Chad Heatwole, MD, MS-CI: study concept and design, manuscript preparation, analysis and interpretation of data.
Study funding
Support for this research was provided by CURE SMA, the Spinal Muscular Atrophy Research Team, the Nathan Goldberg Foundation, the New York State Empire Clinical Research Investigator Program, and the International SMA Patient Registry (smaregistry.iu.edu/).
Disclosure
P. Mongiovi, N. Dilek, C. Garland, M. Hunter, J. Kissel, E. Luebbe, and M. McDermott report no disclosures relevant to the manuscript. N. Johnson serves as an associate editor for Neurology® Genetics. He is funded by the NIH, grant 1K23NS091511-01, and CDC (1U01DD001108-01). He has received research support from the Muscular Dystrophy Association, Myotonic Dystrophy Foundation, Valerion Therapeutics, Ionis Pharmaceuticals, and Biogen Idec. He receives royalties for the CMT-HI. C. Heatwole has received grant funding from the NIH, FDA, and Cure SMA Foundation. He is the founder and CEO of the Neuromuscular Quality of Life Institute. He receives royalties for the Myotonic Dystrophy Health Index, the FSHD-HI, the CMT-HI, and the SMA-HI. He has provided expert testimony for neuromuscular cases unrelated to this research. He has provided consultation to Biogen, Ionis, aTyr, AMO, The Marigold Foundation, Regeneron, Cytokinetics, and Acceleron Pharma. Go to Neurology.org/N for full disclosures.
References
- 1.Melki J, Abdelhak S, Sheth P, et al. Gene for chronic proximal spinal muscular atrophies maps to chromosome 5q. Nature 1990;344:767–768. [DOI] [PubMed] [Google Scholar]
- 2.Sugarman EA, Nagan N, Zhu H, et al. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: clinical laboratory analysis of >72,400 specimens. Eur J Hum Genet 2012;20:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munsat TL, Davies KE. International SMA consortium meeting (26–28 June 1992, Bonn, Germany). Neuromuscul Disord 1992;2:423–428. [DOI] [PubMed] [Google Scholar]
- 4.Piepers S, van den Berg LH, Brugman F, et al. A natural history study of late onset spinal muscular atrophy types 3b and 4. J Neurol 2008;255:1400–1404. [DOI] [PubMed] [Google Scholar]
- 5.Zerres K, Rudnik-Schoneborn S. Natural history in proximal spinal muscular atrophy: clinical analysis of 445 patients and suggestions for a modification of existing classifications. Arch Neurol 1995;52:518–523. [DOI] [PubMed] [Google Scholar]
- 6.Hunter M, Heatwole C, Luebbe E, Johnson NE. What matters most: a perspective from adult spinal muscular atrophy patients. J Neuromuscul Dis 2016;3:425–429. [DOI] [PubMed] [Google Scholar]
- 7.Qian Y, McGraw S, Henne J, Jarecki J, Hobby K, Yeh WS. Understanding the experiences and needs of individuals with spinal muscular atrophy and their parents: a qualitative study. BMC Neurol 2015;15:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iannaccone ST, Hynan LS, Morton A, Buchanan R, Limbers CA, Varni JW. The PedsQL™ in pediatric patients with spinal muscular atrophy: feasibility, reliability, and validity of the Pediatric Quality of Life Inventory™ generic core scales and neuromuscular module. Neuromuscul Disord 2009;19:805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International SMA Patient Registry. Indiana University. Available at: smaregistry.iu.edu/. Accessed August 29, 2015.
- 10.Heatwole C, Bode R, Johnson N, et al. Patient-Reported Impact of Symptoms in Myotonic Dystrophy Type 1 (PRISM-1). Neurology 2012;79:348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heatwole C, Johnson N, Bode R, et al. Patient-Reported Impact of Symptoms in Myotonic Dystrophy Type 2 (PRISM-2). Neurology 2015;85:2136–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson NE, Heatwole CR, Dilek N, et al. Quality-of-life in Charcot-Marie-Tooth disease: the patient's perspective. Neuromuscul Disord 2014;24:1018–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan CL, Bauman K; U.S. Census Bureau. Educational attainment in the United States: 2015. Curr Popul Rep 2016;20. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.