Abstract
Objective
To evaluate the relationship between deficit in digit span and genotype in nonsense mutation (nm) Duchenne muscular dystrophy (DMD) (nmDMD).
Methods
We investigated the relationship between normalized digit-span forward (d-sf) and digit-span backward (d-sb) scores to the location of nmDMD mutations in 169 participants ≥5 to ≤20 years who participated in a phase 2b clinical trial. Because alternative promoters are found upstream of DMD exons 30, 45, and 63, we correlated d-sf and d-sb to the specific nmDMD mutation location.
Results
Participants with nm downstream of exon 30, downstream of exon 45, and downstream of exon 63 had significantly lower normalized d-sf scores (p < 0.0001). Participants with nm downstream of exon 45 in addition had significantly lower normalized d-sb score (p < 0.04). There was no significant difference in the normalized d-sb score in participants with mutations upstream or downstream of DMD exon 30 or upstream or downstream of DMD exon 63.
Conclusion
Our data provide evidence that specific cognitive deficits correlate to genotype in individuals with nmDMD, highlighting the critical role of brain-specific dystrophin isoforms in the neurobiological manifestations of this disease.
Clinicaltrials.gov identifier
Duchenne muscular dystrophy (DMD) is the most common monogenic disorder caused by mutations in the dystrophin gene (DMD) located on the X-chromosome. Approximately 15% of DMD are due to a nonsense mutation (nm) that prevents translation of dystrophin mRNA into a functional protein.1 In addition to progressive skeletal and cardiac muscle manifestations, the higher incidence of attention-deficit/hyperactivity disorder, obsessive-compulsive disorder, and autism spectrum disorder posits the critical importance of dystrophin for brain development.2 The neurobiological manifestations in DMD are in part due to the loss of full-length dystrophin (dp427) and truncated dystrophin isoforms (dp260, dp140, and dp71) in the brain. These isoforms derive from alternative brain-specific promoters that use specific exons as the N-terminal domain and are as follows: exon 1 for dp427, exon 30 for dp260, exon 45 for dp140, and exon 63 for dp71. In nmDMD, based on the location of the mutation, full-length and truncated dystrophin will be absent.
A relationship between cognitive deficits and DMD genotype has been reported,3,4 with the lowest IQ in those with mutations distal to exon 635 and those with point mutations.6 A normal IQ can be misleading in DMD7; regardless of IQ, poor performance on digit span—a task that specifically tests working memory (WM)—is notable in this condition.8 Thus, WM deficit is a characteristic neuropsychological profile in DMD. The pervasive importance of WM in intellectual ability is because of its effect on attention regulation and executive function. As previous studies of cognition in DMD have focused exclusively on correlating IQ with DMD genotype, there is a gap in our knowledge regarding the relationship between digit span and DMD genotype.
Our primary objective was to evaluate the relationship between deficit in digit span and DMD genotype. We evaluated this hypothesis by correlating digit-span forward (d-sf) and digit-span backward (d-sb) to DMD genotype in 169 participants with nmDMD. Participants with mutations downstream of exon 45 have significantly lower normalized median d-sf and d-sb scores. Our data provide scientific evidence for a clear correlation between deficit in digit span and DMD genotype in nmDMD.
Methods
The data presented here were obtained from participants at their baseline study evaluation prior to enrollment in a prospective, phase 2b clinical trial.9
Standard protocol approvals, registrations, and patient consents
Institutional review boards/ethics committees approved the study protocol. The study was conducted in accordance with the Declaration of Helsinki (2000) and the principles of Good Clinical Practice according to the International Conference on Harmonization. This study is registered under Clinical trials.gov (NCT02090959).
Study participants
Ambulatory boys inclusive of ages 5–20 years with nmDMD diagnosis confirmed by gene sequencing were enrolled. Participants had been on stable dose of oral glucocorticoids for at least 6 months prior to study enrollment. All participants provided written consent to participate.
Study measures
d-sf and d-sb, which measure WM capacity and WM load, respectively, were assessed by a trained study team member. The tasks were performed as follows. A series of digits (0–9) were presented to the participant in an auditory format only. To evaluate both d-sf and d-sb, 3 digits were presented at the rate of 1 digit per second. The same digits were presented to all participants.
To assess d-sf, the participant was requested to repeat back the digits in the order they were presented; to assess d-sb, the participant was requested to reverse the order of presentation. A raw score of the total number of correct responses was converted to an age-scaled score using the appropriate normative values for the child's age and language.10
nmDMD mutation locus and participant grouping
We evaluated each participant based on individual nmDMD location and categorized participants into 2 groups based on whether their nm was located upstream or downstream of exon 30, upstream or downstream of exon 45, or upstream or downstream of exon 63.
Statistical analysis
A Wilcoxon rank-sum test was used to compare median normalized score between nmDMD locations. The correlation between digit span and DMD genotype was analyzed using linear regression with SAS software version 9.4 (SAS Institute, Cary, NC). All analyses were 2-sided, and the level of statistical significance was set at 0.05.
Data availability
Individual de-identified participant data (including related documents such as study protocols, statistical analysis plans, and data dictionaries) from this study, which was conducted between 2004 and 2010, will not be made available.
Results
The study enrolled 174 participants. The normalized d-sf was available in 170 participants, and d-sb was available in 169 participants. The median normalized d-sf score was 3 (range 1–15), and d-sb score was 1 (range 1–4). The mean age of study participants was 8.5 years (range 5–20 years; SD 2.6).
Comparison of digit span scores and nmDMD
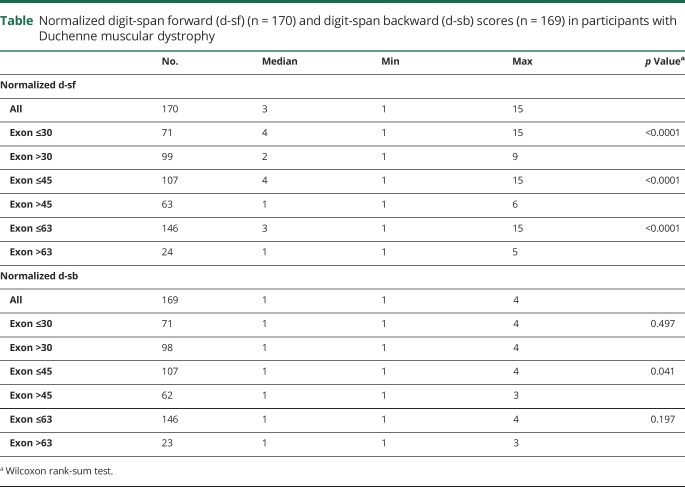
Participants with nm downstream of exon 30, downstream of exon 45, and downstream of exon 63 had significantly lower normalized d-sf scores (p < 0.0001) (table, upper panel). Participants with nm downstream of exon 45 also had significantly lower normalized d-sb (p < 0.04) score. There was no statistically significant difference in the normalized d-sb score in those with mutations upstream or downstream of DMD exon 30, or upstream or downstream of DMD exon 63 (table, lower panel).
Table.
Normalized digit-span forward (d-sf) (n = 170) and digit-span backward (d-sb) scores (n = 169) in participants with Duchenne muscular dystrophy
Correlation between digit span scores and nm location
Digit span scores were correlated to the location of nm. There was a negative correlation between the normalized d-sf score based on site of nm (figure, A). By contrast, there was no correlation between normalized d-sb score and nm either upstream or downstream of exon 45 and exon 63, but there was a correlation with nm downstream of exon 30 (figure, B).
Figure. Normalized digit-span forward (d-sf) score, normalized digit-span backward (d-sb) score, and Duchenne muscular dystrophy (DMD) mutation location.
(A) Normalized d-sf score. (B) Normalized d-sb score.
Discussion
Using a large cohort of participants with nmDMD, we provide evidence, for the first time to our knowledge, of the relationship between deficit in digit span and nmDMD, with those individuals with nm located downstream of exon 45 having deficits in WM load and capacity. The rationale to focus on digit span is that robust deficit in this measure is detected in individuals with DMD regardless of the level of intellectual capacity. Our finding that individuals with nm downstream of exon 45 have abnormalities in digit span—a specific measure of WM—supports the role of dystrophin isoforms in brain development in DMD. Consistent with existent literature, those with DMD mutations that affect dystrophin dp140 isoform have prominent cognitive deficits.4,11 The absence of brain-specific dystrophin isoforms not only has a functional consequence on cognitive skills, but also affects brain development. Smaller total and gray matter volume, and altered white matter microstructure, have been detected in individuals with mutations downstream of DMD exon 45.11 The loss of multiple brain-specific dystrophin isoforms in individuals with DMD may cumulatively increase the burden of cognitive deficits.12 Our data suggest that specific mutations in nmDMD may disrupt the physiologic development of neural networks, which may, in turn, affect WM and influence cognitive reserve.
There is probably a broader role for dystrophin in brain development and function. In a postmortem analysis of 13 brains in DMD, dendritic length and branching were abnormal, which suggests that dystrophin may be important for normal neuronal morphology.13 Glucose hypometabolism is seen in the cerebellum and temporal lobe as detected by PET imaging in individuals with DMD, supporting a functional role for dystrophin in normal brain function.14 Understanding the molecular basis of these changes will allow for pharmacologic approach to improve cognition in DMD.
Some limitations of our study include the cross-sectional evaluation of WM, which does not permit evaluation of intellectual gains (or decline) over time in this population. Second, we did not perform comprehensive psychometric testing in our participants. Acknowledging these limitations, our data lend support for a correlation between digit span and nmDMD. WM predicts early school achievements in mathematics, reading, and writing in children.15
This study also highlights the necessity to reappraise the unmet needs of these individuals. Current therapeutic focus in DMD continues to be exclusively centered on restoring motor function. As new therapeutic agents have been approved recently for DMD, and life expectancy has increased in this population, we need to address strategies to help these individuals meet their self-efficacy milestones of adulthood. Although fundamental questions regarding the role of dystrophin in synaptogenesis and in activity-dependent myelination are yet to be understood, the improved understanding of the neurobiology of cognitive deficits in DMD offers an opportunity to readdress pragmatic strategies towards cognitive rehabilitation in affected individuals.
Acknowledgment
The authors thank the study participants and their families for participation.
Glossary
- d-sb
digit-span backward
- d-sf
digit-span forward
- DMD
Duchenne muscular dystrophy
- nm
nonsense mutation
- WM
working memory
Contributor Information
Collaborators: Ataluren Phase 2b Study Group, Monique M. Ryan, Andrew J Kornberg, Victoria RodriguezCasero, Alison Wray, Kristi J. Jones, Kathryn North, Nathalie Goemans, Gunnar Marceo Buyse, Craig Campbell, Jean Mah, Harvey Sarnat, Kathryn Selby, Thomas Voit, Valerie Doppler, Denis De Castro, Brigitte Chabrol, Nicolas Levy, Cecile Halbert, Yann Pereon, Armelle Magot, Julie Perrier, Jean-Yves Mahe, Ulrike Schara, Soren Lutz, Melanie Busse, Adela Della Marina, Janbernd Kirschner, Angela Stanescu, Annette Pohl, Cornelia RensingZimmerman, Enrico Bertini, Adele D’Amico, Annamaria Kofler, Adelina Carlesi, Anna Maria Bonetti, Luigino Santecchia, Francesco Emma, Gianluca Bergami, Eugenio Maria Mercuri, Gessica Vasco, Flaviana Bianco, Elena Stacy Mazzone, Roberto De Sanctis, Paolo Alfieri, Marika Pane, Sonia Messina, Giacomo Pietro Comi, Francesca Magri, Valeria Lucchini, Stefania Paola Corti, Maurizio Gualtiero Moggio, Monica Sciacco, Nereo Bresolin, Alessandro Cesare Prelle, Roberta Magri, Roberta Virgilio, Costanza Lamperti, Yoram Nevo, Taia DorWollman, Juan Vilchez, Nuria Muelas, Teresa Sevilla, Patricia Smeyers, Alberto de la Osa, Jaume Colomer, Carlos Ignacio Ortez, Andres Nascimento, Ana Febrer, Julita Medina, Mar Tulinus, brynja Thorarinsdottir, Niklas Darin, Thomas Sejersen, Mia Hovmoller, Katherine Bushby, Volker Straub, Michela Guglieri, Anna Sarkozy, Tracey Willis, Michelle Eagle, Anna Mayhew, Francesco Muntoni, Sebahattin Cirak, Adnan Yousaf Manzur, Stephanie Ann Robb, Maria Kinali, Rosaline Christina Mary Quinlivan, Martin Richard Smith, Rajesh Pandey, Brenda Wong, James Collins, Richard Finkel, Cartsen Bonnemann, Michele Yang, Aileen Reghan Foley, Sabrina Yum, Jacinda Sampson, Mark Bromberg, Kathryn Swoboda, John Day, Peter Karachunski, Katherine Mathews, Daniel Bonthius, Karla Sue Laubenthal, Basil Darras, Peter Kang, Julie Parson, Richard Barohn, Majed Dasouki, Heather Anderson, Jeffrey Burns, Mazen Dimachkie, Mamatha Pasnoor, Yunxia Wang, Emma Ciafaloni, Chad Heatwole, Anne Connolly, Alan Pestronk, Muhammad Al-Lozi, Glenn Lopate, Paul Golumbek, Brian Sommerville, Leo Wang, Anna Wojcicka-Mitchell, Andrew Godbey, Matthew Harms, Arun Varadachary, Stanley Iyadurai, Luisa Rojas, Susan Iannacone, Chaiyos Khonghatithum, Douglas Sproule, Darryl De Vivo, Andre Constantinescu, Craig McDonald, Jay Han, Ben Renfroe, Barry Russman, Michael Sussman, Stephanie BurnsWechsler, Vern Juel, Lisa Hobson-Webb, and Edward Smith
Author contributions
M. Thangarajh and G.L. Elfring analyzed and interpreted the data. M. Thangarajh prepared the manuscript. All authors contributed to revising the manuscript.
Study funding
Dr. Mathula Thangarajh was supported by the American Brain Foundation/American Academy of Neurology Clinical Research Training Fellowship (2015–2017) and the American Association of Neuromuscular and Electrodiagnostic Medicine Development Grant (2017–2019). Editorial support was provided and funded by PTC Therapeutics, Inc.
Disclosure
M. Thangarajh has provided consultation services to PTC Therapeutics, Inc. G. Elfring is a full employee of PTC Therapeutics, Inc. P. Trifillis is a full employee of PTC Therapeutics, Inc. J. McIntosh is a full employee of PTC Therapeutics, Inc. S. Peltz is a full employee of PTC Therapeutics, Inc. Go to Neurology.org/N for full disclosures.
References
- 1.Pichavant C, Aartsma-Rus A, Clemens PR, et al. Current status of pharmaceutical and genetic therapeutic approaches to treat DMD. Mol Ther 2011;19:830–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hendriksen JG, Vles JS. Neuropsychiatric disorders in males with Duchenne muscular dystrophy: frequency rate of attention-deficit hyperactivity disorder (ADHD), autism spectrum disorder, and obsessive–compulsive disorder. J Child Neurol 2008;23:477–481. [DOI] [PubMed] [Google Scholar]
- 3.Bushby KM, Appleton R, Anderson LV, Welch JL, Kelly P, Gardner-Medwin D. Deletion status and intellectual impairment in Duchenne muscular dystrophy. Dev Med Child Neurol 1995;37:260–269. [DOI] [PubMed] [Google Scholar]
- 4.Felisari G, Martinelli Boneschi F, Bardoni A, et al. Loss of Dp140 dystrophin isoform and intellectual impairment in Duchenne dystrophy. Neurology 2000;55:559–564. [DOI] [PubMed] [Google Scholar]
- 5.Moizard MP, Toutain A, Fournier D, et al. Severe cognitive impairment in DMD: obvious clinical indication for Dp71 isoform point mutation screening. Eur J Hum Genet 2000. ;8:552–556. [DOI] [PubMed] [Google Scholar]
- 6.Magri F, Govoni A, D'Angelo MG, et al. Genotype and phenotype characterization in a large dystrophinopathic cohort with extended follow-up. J Neurol 2011;258:1610–1623. [DOI] [PubMed] [Google Scholar]
- 7.Hinton VJ, De Vivo DC, Nereo NE, Goldstein E, Stern Y. Poor verbal working memory across intellectual level in boys with Duchenne dystrophy. Neurology 2000;54:2127–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leaffer EB, Fee RJ, Hinton VJ. Digit span performance in children with dystrophinopathy: a verbal span or working memory contribution? J Int Neuropsychol Soc 2016;22:777–784. [DOI] [PubMed] [Google Scholar]
- 9.Bushby K, Finkel R, Wong B, et al. ; PTC124-GD-007-DMD Study Group. Ataluren treatment of patients with nonsense mutation dystrophinopathy. Muscle Nerve 2014;50:477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wechsler D. Wechsler Intelligence Scale for Children, 4th ed. San Antonio: The Psychological Corporation; 2003. [Google Scholar]
- 11.Doorenweerd N, Straathof CS, Dumas EM, et al. Reduced cerebral gray matter and altered white matter in boys with Duchenne muscular dystrophy. Ann Neurol 2014;76:403–411. [DOI] [PubMed] [Google Scholar]
- 12.Taylor PJ, Betts GA, Maroulis S, et al. Dystrophin gene mutation location and the risk of cognitive impairment in Duchenne muscular dystrophy. PLoS One 2010;5:e8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jagadha V, Becker LE. Brain morphology in Duchenne muscular dystrophy: a Golgi study. Pediatr Neurol 1988;4:87–92. [DOI] [PubMed] [Google Scholar]
- 14.Lee JS, Pfund Z, Juhász C, et al. Altered regional brain glucose metabolism in Duchenne muscular dystrophy: a pet study. Muscle Nerve 2002;26:506–512. [DOI] [PubMed] [Google Scholar]
- 15.Sedek G, Krejtz I, Rydzewska K, et al. Three functional aspects of working memory as strong predictors of early school achievements: the review and illustrative evidence. Polish Psychol Bull 2016;47:103–111. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual de-identified participant data (including related documents such as study protocols, statistical analysis plans, and data dictionaries) from this study, which was conducted between 2004 and 2010, will not be made available.