Abstract
Objective
To investigate the effect of including optic nerve involvement in dissemination in space (DIS) criteria for diagnosis of multiple sclerosis (MS) in patients with clinically isolated syndrome (CIS).
Methods
We studied 160 patients with CIS: 129 with optic neuritis (ON) and 31 with non-ON CIS. MRI brain/spinal cord was done at the time of presentation and a follow-up MRI brain after 3–12 months. We evaluated optic nerve involvement clinically or with visual evoked potentials (VEPs, n = 42). We investigated the performance of the McDonald 2017 DIS criteria and modified DIS criteria including optic nerve involvement for development of clinically definite MS after ∼15 years.
Results
In the ON group, including symptomatic optic nerve involvement identified an additional 15 patients with DIS. The modified DIS criteria that included optic nerve involvement were more sensitive (95% vs 83%) and more accurate (81% vs 78%) than the McDonald 2017 DIS criteria, but less specific (57% vs 68%). In combination with dissemination in time criteria, the modified DIS criteria remained more sensitive (83% vs 74%) and accurate (81% vs 75%), and the specificity was the same (77%). Including asymptomatic optic nerve involvement in DIS the non-ON group did not identify any additional patients and the performance of the McDonald 2017 criteria and the modified criteria was the same.
Conclusion
The inclusion of symptomatic optic nerve involvement in DIS in patients with ON improved the overall performance of MS diagnostic criteria. Including asymptomatic optic nerve involvement in DIS in patients with a non-ON CIS may be of limited value.
Classification of evidence
This study provides Class III evidence that for patients with suspected MS, inclusion of symptomatic optic nerve involvement in DIS criteria improves the overall performance of diagnostic criteria for MS.
Optic neuritis (ON) is a common presentation of multiple sclerosis (MS), accounting for 25%–30% of patients with a clinically isolated syndrome (CIS).1 Subclinical involvement of the optic nerve occurs in people with established MS without a history of ON, and less often in people with CIS or early MS.2 Involvement of the optic nerve in patients with CIS and MS can be determined clinically, or with abnormalities detected by MRI, visual evoked potentials (VEPs), and optical coherence tomography (OCT).3
The Magnetic Resonance Imaging in Multiple Sclerosis (MAGNIMS) group recently proposed the inclusion of optic nerve involvement in dissemination in space (DIS) criteria for MS, with no distinction between asymptomatic and symptomatic optic nerve lesions.3 The latter recommendation was based on expert opinion and evidence in patients with brainstem and spinal cord syndromes that inclusion of symptomatic lesions in DIS improves the performance of MS diagnostic criteria.4,5 A subsequent MAGNIMS multicenter study evaluating proposed changes to DIS criteria found that including optic nerve involvement (detected using VEPs or MRI) in DIS resulted in similar sensitivity but reduced specificity compared with the 2010 McDonald criteria.6 The International Panel on Diagnosis of Multiple Sclerosis considered whether optic nerve involvement should be included in DIS criteria, but concluded that there was insufficient evidence to support this proposal.7
Here we investigate the influence of including optic nerve involvement in DIS criteria in patients with CIS, both the inclusion of symptomatic optic nerve involvement in patients with ON and asymptomatic optic nerve involvement (detected clinically or with VEPs) in patients with a non-ON presentation (classification of evidence, Class III).
Methods
Standard protocol approvals, registrations, and patient consents
All patients provided written informed consent. The study was approved by the institutional ethics committee.
Patients
We studied 160 patients with CIS from a prospectively recruited CIS cohort.8 Inclusion criteria for the study were (1) a typical CIS suggestive of MS; (2) age 16–50 years; and (3) no history of neurologic symptoms. All patients underwent T2-weighted and postcontrast T1-weighted scans of the brain and spinal cord within 3 months of CIS and follow-up brain MRI after 3–12 months. The number, location, and activity of lesions was recorded by a single neuroradiologist (K.A.M.), blinded to the patient's clinical status.
At study entry, optic nerve involvement was evaluated by clinical assessment requiring objective evidence of an optic neuropathy, e.g., impaired best-corrected visual acuity, dyschromatopsia, relative afferent pupillary defect, and optic disc pallor/swelling. All patients with ON were examined by a single experienced neuro-ophthalmologist. A subset of patients (n = 42) had central field pattern reversal VEPs done as part of routine clinical care.
The patients were followed up for ∼15 years for the development of clinically definite MS (CDMS).9
Dissemination in space and time criteria
We retrospectively applied the McDonald 2017 DIS criteria requiring one or more T2-hyperintense lesions in at least 2 anatomical regions typically affected in MS (periventricular, cortical/juxtacortical, infratentorial, spinal cord).7 We then retrospectively applied modified criteria that included optic nerve involvement, both symptomatic and asymptomatic.
The McDonald 2017 MRI criteria for dissemination in time (DIT) were also applied, requiring the simultaneous presence of gadolinium-enhancing and nonenhancing lesions on a single MRI scan, or a new T2-hyperintense lesion (with or without gadolinium enhancement) on a follow-up MRI.7
Statistical analysis
We evaluated the performance of the DIS criteria separately in patients with ON and those with a non-ON CIS. We calculated the sensitivity, specificity, accuracy, positive predictive value, and negative predictive value (with 95% confidence intervals) of the McDonald 2017 DIS criteria and the modified DIS criteria that included optic nerve involvement alone, and in combination with DIT, for the development of CDMS. A McNemar test was performed to compare the performance of the McDonald 2017 criteria and the modified criteria, with significance reported at p < 0.05. Statistical analyses were done using STATA 14.2.
Data availability
Fully anonymized MRI data are available on request.
Results
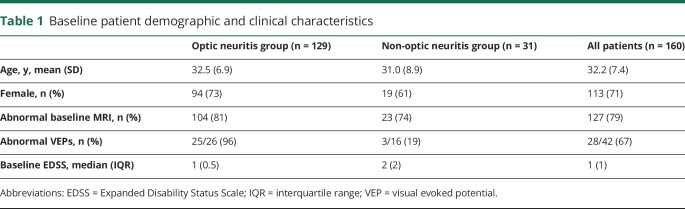
The demographic and clinical profile of the patients is shown in table 1. CDMS developed in 97 (61%) patients over a mean follow-up period of 14.9 years (range 5.1–19.7 years). One patient received disease-modifying treatment (DMT) prior to developing CDMS.
Table 1.
Baseline patient demographic and clinical characteristics
Optic neuritis group
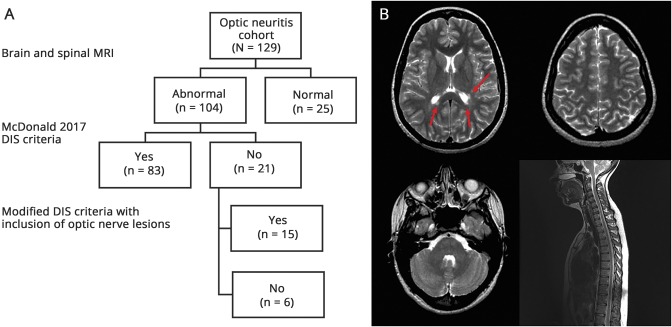
All of the patients in the ON group (n = 129) had objective clinical evidence of optic nerve involvement and 25/26 (96%) patients had abnormal VEPs. In the ON group, 104 (81%) patients had an abnormal MRI and 83 (64%) patients had evidence of DIS using the McDonald 2017 criteria. Among the 21 patients with ON with an abnormal MRI, but not meeting McDonald 2017 DIS criteria, an additional 15 patients were identified as having DIS when the modified criteria that included optic nerve involvement were applied (figure).
Figure. Effect of including symptomatic optic nerve involvement in dissemination in space (DIS).
(A) Flow chart shows the number of additional patients identified with evidence of DIS with inclusion of optic nerve involvement. (B) A 31-year-old woman with acute optic neuritis with multiple periventricular lesions (arrows) but no juxtacortical, infratentorial, or spinal cord lesions. The patient would satisfy the modified DIS criteria with inclusion of the symptomatic optic nerve lesion but not the McDonald 2017 DIS criteria.
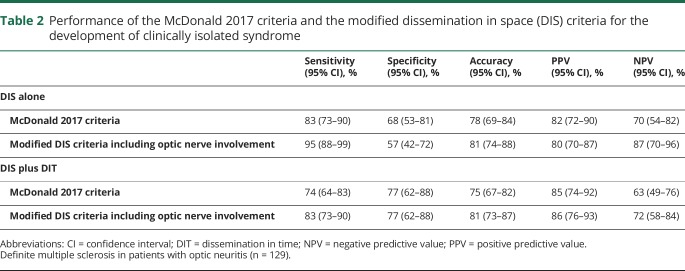
The performance of the McDonald 2017 DIS criteria and the modified DIS criteria is shown in table 2. The modified DIS criteria were more sensitive and slightly more accurate than the McDonald 2017 DIS criteria, but less specific. When the DIS criteria were considered along with MRI criteria for DIT, the modified criteria remained more sensitive and more accurate, and the specificity was the same as the McDonald DIS 2017 criteria. When compared using the McNemar test, the overall performance of modified criteria including optic nerve involvement was significantly better than the McDonald 2017 criteria (p = 0.016).
Table 2.
Performance of the McDonald 2017 criteria and the modified dissemination in space (DIS) criteria for the development of clinically isolated syndrome
Non-optic neuritis group
The non-ON group (n = 31) included 20 patients with a brainstem/cerebellar syndrome, 10 with partial myelopathy, and 1 with a hemispheric syndrome. Asymptomatic optic nerve involvement was found in 3 (10%) patients: 1/31 patients had optic disc pallor and 3/16 (19%) patients had abnormal VEPs (mean latency = 126.3 ms, mean amplitude = 7.5 µV). Inclusion of asymptomatic optic nerve involvement did not identify any additional patients with DIS. The performance of the McDonald 2017 DIS criteria and the modified criteria was the same.
Discussion
In patients with ON, the inclusion of symptomatic optic nerve involvement in DIS improves the sensitivity and accuracy of diagnostic criteria for MS, potentially facilitating earlier diagnosis and treatment in this group. We found no additional value in including the optic nerve in DIS in non-ON CIS patients. Very few patients in that group had optic nerve involvement, at least when assessed clinically or with VEPs (when available).
There has been limited previous investigation of how inclusion of optic nerve involvement in DIS influences the performance of MS diagnostic criteria. In the recent MAGNIMS multicenter study evaluating proposed changes to DIS criteria,3 241 patients (60% non-ON) had optic nerve assessment using VEPs or MRI.6 Inclusion of optic nerve involvement in DIS resulted in similar sensitivity but a decreased specificity compared with the McDonald 2010 criteria, even when combined with DIT.6 This contrasts with our findings of similar specificity for the McDonald 2017 and the modified DIS criteria (in combination with DIT), potentially reflecting the longer duration of follow-up in our study, and the low rate of DMT use prior to a second attack.
Several features of our study are worth noting. First, in the ON group, the diagnosis was confirmed by a single, experienced neuro-ophthalmologist. Acute ON is a clinical diagnosis made on the basis of a history of subacute unilateral visual loss associated with pain on eye movement and objective signs of an optic neuropathy (reduced visual acuity—particularly low-contrast vision, dyschromatopsia, relative afferent pupillary defect, optic disc pallor/swelling, but otherwise normal fundus examination).1 The diagnosis of ON may be supported by the results of paraclinical tests (MRI, VEPs, OCT). Careful clinical assessment is essential since the differential diagnosis of acute monocular visual loss is broad and diagnostic errors are common.1,10 Second, we investigated the modified DIS criteria in a group of young adults with a typical ON with a high pretest probability of MS. The McDonald criteria have been validated in this setting and should not be applied in ON patients with atypical clinical features (severe visual impairment, absence of pain or very severe pain, and bilateral involvement1), and applied with caution in younger children, older adults, and nonwhite populations. In these patient groups, other inflammatory (e.g., neuromyelitis optica spectrum disorder, neurosarcoidosis) and noninflammatory (e.g., ischemic optic neuropathy) disorders may be more likely. It is imperative that diagnostic criteria for MS are applied in appropriate clinical settings in order to avoid misdiagnosis.7 Finally, the subgroup of patients with a non-ON CIS was small and not all patients had VEPs to detect asymptomatic optic nerve involvement. However, previous studies have found that MRI or VEP-detected asymptomatic optic nerve involvement is relatively uncommon in CIS and early MS patients without a history of ON (∼20–25%).2 OCT frequently identifies subclinical retinal nerve fiber layer thinning in patients with established MS and may be more sensitive to the detection of asymptomatic optic nerve involvement in patients with CIS, but further studies are required.11
Our findings suggest that the inclusion of symptomatic optic nerve involvement in DIS in patients with ON may enhance the performance of diagnostic criteria for MS, when combined with DIT. This should be investigated further in CIS cohorts with complete evaluation of the optic nerve with MRI, VEPs, and OCT to guide future revisions to MS diagnostic criteria.
Glossary
- CDMS
clinically definite multiple sclerosis
- CIS
clinically isolated syndrome
- DIS
dissemination in space
- DIT
dissemination in time
- DMT
disease-modifying treatment
- MAGNIMS
Magnetic Resonance Imaging in Multiple Sclerosis
- MS
multiple sclerosis
- OCT
optical coherence tomography
- ON
optic neuritis
- VEP
visual evoked potential
Footnotes
Editorial page 545
Class of Evidence: NPub.org/coe
Author contributions
Dr. Brownlee contributed to study concept/design, analysis and interpretation of data, statistical analysis, and drafting the manuscript. Dr. Miszkiel contributed to data acquisition and manuscript revision. Dr. Tur contributed to statistical analysis and manuscript revision. Dr. Barkhof contributed to study concept/design and manuscript revision. Dr. Miller obtained funding and contributed to study concept/design and manuscript revision. Dr. Ciccarelli contributed to study concept/design and manuscript revision and provided study supervision.
Study funding
The study was supported by the United Kingdom MS Society (grant 995) and Neurological Foundation of New Zealand (grant 1207-CF). The Queen Square MS Centre is supported by the United Kingdom MS Society and the NIHR University College London Hospitals Biomedical Research Centre.
Disclosure
W. Brownlee has received speaker fees for educational activities from Merck Serono and Roche. K. Miszkiel reports no disclosures relevant to the manuscript. C. Tur received a postdoctoral ECTRIMS fellowship (2015). F. Barkhof acts as a consultant to Biogen-Idec, Janssen Alzheimer Immunotherapy, Bayer-Schering, Merck Serono, Roche, Novartis, Genzyme, and Sanofi-aventis. He has received sponsorship from EU-H2020, NWO, SMSR, EU-FP7, TEVA, Novartis, and Toshiba. He is on the editorial board of Radiology, Brain, Neuroradiology, Multiple Sclerosis Journal, and Neurology®. D. Miller has received honoraria through payments to UCL Institute of Neurology, for Advisory Committee and/or Consultancy advice from Novartis, Mitsubishi Pharma Europe, GlaxoSmithKline, and Bayer Schering Pharma, and compensation through payments to UCL Institute of Neurology for performing central MRI analysis of multiple sclerosis trials from Biogen Idec and Novartis. O. Ciccarelli is a consultant for Novartis, Roche, and Teva, and is an associate editor of Neurology. Go to Neurology.org/N for full disclosures.
References
- 1.Toosy AT, Mason DF, Miller DH. Optic neuritis. Lancet Neurol 2014;13:83–99. [DOI] [PubMed] [Google Scholar]
- 2.Miller DH, Newton MR, van der Poel JC, et al. Magnetic resonance imaging of the optic nerve in optic neuritis. Neurology 1988;38:175–179. [DOI] [PubMed] [Google Scholar]
- 3.Filippi M, Rocca MA, Ciccarelli O, et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol 2016;15:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brownlee WJ, Swanton JK, Miszkiel KA, Miller DH, Ciccarelli O. Should the symptomatic region be included in dissemination in space in MRI criteria for MS? Neurology 2016;87:680–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tintore M, Otero-Romero S, Rio J, et al. Contribution of the symptomatic lesion in establishing MS diagnosis and prognosis. Neurology 2016;87:1368–1374. [DOI] [PubMed] [Google Scholar]
- 6.Filippi M, Preziosa P, Meani A, et al. Prediction of a multiple sclerosis diagnosis in patients with clinically isolated syndrome using the 2016 MAGNIMS and 2010 McDonald criteria: a retrospective study. Lancet Neurol 2018;2:133–142. [DOI] [PubMed] [Google Scholar]
- 7.Thompson AJ, Banwell B, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018;2:162–173. [DOI] [PubMed] [Google Scholar]
- 8.Brownlee WJ, Swanton JK, Altmann DR, Ciccarelli O, Miller DH. Earlier and more frequent diagnosis of multiple sclerosis using the McDonald criteria. J Neurol Neurosurg Psychiatry 2015;86:584–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983;13:227–231. [DOI] [PubMed] [Google Scholar]
- 10.Stunkel L, Kung NH, Wilson B, McClelland CM, Van Stavern GP. Incidence and causes of overdiagnosis of optic neuritis. JAMA Ophthalmol 2018;136:76–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galetta SL, Balcer LJ. The optic nerve should be included as one of the typical CNS regions for establishing dissemination in space when diagnosing MS: yes. Mult Scler 2018;24:121–122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Fully anonymized MRI data are available on request.