Abstract
Background
Previous studies have shown that differences in marital status contribute to different prognoses for certain cancers, but the relationship between marital status and the prognosis of chondrosarcoma has not been reported previously.
Material/Methods
In this study, we selected 4502 eligible cases through the Surveillance, Epidemiology, and End Results (SEER) database from 1977 to 2014 to analyze the impact of marital status on chondrosarcoma cancer-specific survival (CSS) by Kaplan-Meier method and Cox regression model.
Results
The sex, age, histotype, pathological grade, tumor location, tumor size, SEER stage, socioeconomic status, marital status, and treatment were identified as independent prognostic factors for chondrosarcoma CSS. Widowed patients presented the worst CSS compared with their married, divorced, and single counterparts (P<0.001). Subgroup analyses showed widowed patients also had a significantly higher risk of cancer-specific mortality compared with married patients in localized stage (HR: 1.971, 95% CI: 1.298–2.994, P=0.001), regional stage (HR: 1.535, 95% CI: 1.094–2.154, P=0.013), low pathological grade (HR: 1.866, 95% CI: 1.332–2.613, P<0.001), and high pathological grade (HR: 1.662, 95% CI: 1.139–2.426, P=0.008).
Conclusions
Marital status was first identified as an independent prognostic factor for chondrosarcoma CSS, and widowhood was always associated with a high risk of cancer-specific mortality. It is necessary to provide timely psychological treatment for widowed patients in clinical practice, which can improve the survival of chondrosarcoma patients.
MeSH Keywords: Chondrosarcoma, Marital Status, Prognosis, SEER Program, Survival Analysis
Background
In 2015, approximately 28 000 and 3000 new cases of bone tumors were diagnosed in China and the United States, respectively, leading to about 20 000 and 1500 deaths, respectively, in these 2 countries [1,2]. Chondrosarcoma is the second most frequently diagnosed malignancy of bone, with a 5-year survival rate of 70% [3,4]. However, high-grade chondrosarcoma is prone to local recurrence or metastasis and is always associated with poor outcome [5]. Unlike other primary bone tumors, chondrosarcoma is relatively resistant to chemotherapy and radiotherapy due to abundant extracellular matrix, fewer dividing cells, and slow growth [6,7]. Surgical resection remains the primary treatment option for chondrosarcoma, and en bloc excision is usually needed for aggressive tumors [8]. Treatment can be challenging, especially for spinal tumors, because of the complex structures and the need for spine stabilization [9]. For these reasons, we need to further study the prognostic factors to develop more holistic therapeutic strategies and improve the outcome of chondrosarcoma.
Previous studies of the prognostic factors of chondrosarcoma mainly focused on the pathological grade, tumor extension, TNM stage, and therapeutic regimen [9–11]. The impact of socioeconomic determinants on cancer survival has recently attracted the attention of academia. Many studies have shown that marital status differences contribute to different prognosis for certain cancers. A large population-based study reported that among the 10 leading death-related cancers, unmarried patients were at higher risk of cancer metastasis, undertreatment, and cancer-specific death than married patients [12]. Some studies have also demonstrated the protective effect of marriage on survival of patients with thyroid cancer [13], cutaneous melanoma [14], soft tissue sarcomas [15], and gallbladder cancer [16]. Other studies indicated that the influence of marital status on cancer survival was mixed [17,18] or non-significant [19]. However, to the best of our knowledge, the relationship between marital status and the prognosis of chondrosarcoma has not been previously reported. As such, it is important for us to address the impact of marital status on the survival of chondrosarcoma patients and to explore the potential mechanisms, which may help to develop more individual and holistic approaches to treatment and to improve patient outcomes.
In the present study, we extracted data from the Surveillance, Epidemiology, and End Results (SEER) cancer registry database to assess the effects of marital status on chondrosarcoma cancer-specific survival (CSS) and to explore the underlying mechanisms between them.
Material and Methods
Data source
The SEER database, sponsored by the National Cancer Institute, consists of 18 population-based cancer registries, accounting for about 28% of the population in the United States. The current database we used was Incidence-SEER 18 Registries Custom Data (with additional treatment fields), Nov 2016 Sub (1973–2014 varying), which collects clinicopathological data of cancer patients, including demographics, tumor histology, tumor morphology, cancer stage at diagnosis, treatment regimen, and survival from 1973 to 2014.
Patient selection
We used the SEER*Stat software (version 8.3.4) to identify the chondrosarcoma patients diagnosed between 1973 and 2014. The histologic ICD-0–3 codes (International Classification of Diseases for Oncology, Third Edition) were employed to identify the following subtypes: chondrosarcoma not otherwise specified (ICD-O-3 code 9220), juxtacortical chondrosarcoma (ICD-O-3 code 9221), chondroblastoma malignant (ICD-O-3 code 9230), myxoid chondrosarcoma (ICD-O-3 code 9231), mesenchymal chondrosarcoma (ICD-O-3 code 9240), clear cell chondrosarcoma (ICD-O-3 code 9242), and dedifferentiated chondrosarcoma (ICD-O-3 code 9243).
The exclusion criteria were as follows: (1) the diagnosis was not histologically confirmed; (2) more than one primary cancer was diagnosed but chondrosarcoma was not the first one; (3) the marital status was unknown; (4) the cause of death was unknown; (5) the survival time was unknown or less than one month; and (6) the age at diagnosis was younger than 18 years old.
Study variables
Variables about demographics of patients, including age, race, sex, and marital status, were extracted from the database. To simplify the statistical analysis, the patients were categorized into 2 age groups: <60 years old and ≥60 years old. Patient race was classified as white, black, or other. Marital status was classified as married, single, separated, divorced, and widowed in the database. In the present study, we assigned separated and divorced into the divorced group.
Variables about tumor histology and morphology were extracted from the database, including pathological grade, tumor location and size, SEER stage, and therapy regimen. The pathological grade was divided into 3 categories: (1) low-grade, including well-differentiated and moderately-differentiated; (2) high-grade, including undifferentiated, anaplastic, and poorly-differentiated; and (3) unknown stage. The tumor location was classified as 3 categories: (1) extremities, including bones of the upper and lower extremities; (2) axial skeleton, including pelvic bones, sacrum, coccyx, ribs, sternum, clavicle, and vertebral columns; and (3) other location, including bones of skull and face, mandible, and other atypical locations. The patients were divided into 3 groups according to the tumor size: ≤8 cm group, >8 cm group, and unknown group. In the SEER program, the stage was coded as localized, regional, distant, and unstaged. The surgery therapy and radiation therapy were both classified into performed and not performed.
We extracted 3 standard 2000 US Census variables from the database, including median family income, the percentage of persons who have less than high school education, and the percentage of persons with income below the poverty level. The 3 socioeconomic status (SES) variables were used to create a composite SES variable, as described in previous studies [20–22]. The composite SES variable was further classified as low-SES (composite SES score <5) and high-SES (composite SES score ≥5).
Chondrosarcoma CSS was the primary outcome of this study. As described in previous studies [23,24], the deaths attributed to chondrosarcoma were considered as events, while deaths from other causes or the alive individuals were considered as censored observations.
Statistical analysis
The baseline data of demographics and clinicopathological characteristics of different marital status groups was compared by chi-squared tests. The Kaplan-Meier method was performed to estimate CSS. The univariate analysis was conducted by using the log-rank test, and multivariate analysis was performed using the Cox regression model. All data were analyzed using SPSS statistics software, version 20 (IBM, SPSS, Inc., Chicago, IL, USA). All P values were 2-sided, and P<0.05 was considered as statistically significant.
Results
Baseline patient characteristics
A total of 4502 eligible cases (2510 males and 1992 females) were included in this study through the SEER database from 1977 to 2014. Of these cases, 2830 (62.9%) were married, 392 were divorced (8.7%), 975 were single (21.7%), and 305 (6.7%) were widowed. Chi-square tests showed significant differences in most variables, including sex (P<0.001), age (P<0.001), race (P<0.001), histotype (P<0.001), SEER stage (P=0.003), socioeconomic status (P=0.002), and treatment (P<0.001). Among these comparisons, the widowed group had the highest proportion of women (76.4%), elderly patients (87.5%), undifferentiated chondrosarcoma (8.9%), tumor at the stage of regional (40.6%) and distant (10.9%), non-treatment (12.1%), and cancer-specific deaths (38.4%). The percentage of high-grade tumors and large tumors (≥8 cm) in the widowed group was also the highest, but the differences were not significant. The baseline demographics and clinicopathological features of this study are shown in Table 1.
Table 1.
Baseline demographics and clinicopathological characteristics of chondrosarcoma patients in SEER database.
| Characteristic | Total | Married | Divorced | Single | Widowed | P value |
|---|---|---|---|---|---|---|
| n=4502 | n=2830 N(%) |
n=392 N(%) |
n=975 N(%) |
n=305 N(%) |
||
| Sex | <0.001 | |||||
| Male | 2510 | 1678 (59.3%) | 183 (46.7%) | 577 (59.2%) | 72 (23.6%) | |
| Female | 1992 | 1152 (40.7%) | 209 (53.3%) | 398 (40.8%) | 233 (76.4%) | |
| Age | <0.001 | |||||
| <60 | 2965 | 1827 (64.6%) | 263 (67.1%) | 837 (85.8%) | 38 (12.5%) | |
| ≥60 | 1537 | 1003 (35.4%) | 129 (32.9%) | 138 (14.2%) | 267 (87.5%) | |
| Race | <0.001 | |||||
| White | 3949 | 2543 (89.9%) | 344 (87.8%) | 792 (81.2%) | 270 (88.5%) | |
| Black | 315 | 137 (4.8%) | 33 (8.4%) | 126 (12.9%) | 19 (6.2%) | |
| Other | 238 | 150 (5.3%) | 15 (3.8%) | 57 (5.9%) | 16 (5.3%) | |
| Histotype | <0.001 | |||||
| Chondrosarcoma, NOS | 3483 | 2191 (77.4%) | 315 (80.4%) | 746 (76.5%) | 234 (76.7%) | |
| Myxoid | 561 | 378 (13.4%) | 42 (10.7%) | 106 (10.9%) | 35 (11.5%) | |
| Mesenchymal | 158 | 80 (2.8%) | 8 (2.0%) | 63 (6.5%) | 7 (2.3%) | |
| Dedifferentiated | 195 | 124 (4.4%) | 19 (4.9%) | 25 (2.6%) | 27 (8.9%) | |
| Other | 102 | 57 (2.0%) | 8 (2.0%) | 35 (3.5%) | 2 (0.6%) | |
| Pathological grade | 0.064 | |||||
| Low-grade | 2985 | 1903 (67.2%) | 259 (66.1%) | 645 (66.2%) | 178 (58.4%) | |
| High-grade | 689 | 420 (14.8%) | 66 (16.8%) | 142 (14.6%) | 61 (20.0%) | |
| Unknown | 828 | 507 (18.0%) | 67 (17.1%) | 188 (19.2%) | 66 (21.6%) | |
| Tumor location | 0.170 | |||||
| Extremities | 1904 | 1173 (41.4%) | 176 (44.9%) | 406 (41.6%) | 149 (48.9%) | |
| Axial | 1413 | 910 (32.2%) | 117 (29.8%) | 309 (31.7) | 77 (25.2%) | |
| Other | 1185 | 747 (26.4%) | 99 (25.3%) | 260 (26.7%) | 79 (25.9%) | |
| Tumor size | 0.058 | |||||
| <8 cm | 1892 | 1211 (42.8%) | 169 (43.1%) | 410 (42.1%) | 102 (33.4%) | |
| ≥8 cm | 1226 | 747 (26.4%) | 106 (27.0%) | 280 (28.7%) | 93 (30.5%) | |
| Unknown | 1384 | 872 (30.8%) | 117 (29.9%) | 285 (29.2%) | 110 (36.1%) | |
| SEER stage | 0.003 | |||||
| Localized | 2272 | 1465 (51.8%) | 206 (52.6%) | 485 (49.7%) | 116 (38.0%) | |
| Regional | 1492 | 903 (31.9%) | 130 (33.2%) | 335 (34.4%) | 124 (40.6%) | |
| Distant | 413 | 262 (9.3%) | 33 (8.4%) | 85 (8.7%) | 33 (10.9%) | |
| Unstaged | 325 | 200 (7.0%) | 23 (5.8%) | 70 (7.2%) | 32 (10.5%) | |
| Socioeconomic status | 0.002 | |||||
| Low-SES | 2275 | 1367 (48.3%) | 210 (53.6%) | 533 (55.6%) | 165 (54.1%) | |
| High-SES | 2227 | 1463 (51.7%) | 182 (46.4%) | 442 (43.3%) | 140 (45.9%) | |
| Treatment | <0.001 | |||||
| Surgery and radiation | 650 | 404 (14.3%) | 54 (13.8%) | 144 (14.8%) | 48 (15.7%) | |
| Only surgery | 3385 | 2164 (76.4%) | 299 (76.3%) | 724 (74.2%) | 198 (64.9%) | |
| Only radiation | 141 | 78 (2.8%) | 15 (3.8%) | 26 (2.7%) | 22 (7.3%) | |
| None | 326 | 184 (6.5%) | 24 (6.1%) | 81 (8.3%) | 37 (12.1%) | |
| Cancer-specific death | <0.001 | |||||
| Events | 1046 | 638 (22.5%) | 99 (25.3%) | 192 (19.7%) | 117 (38.4%) | |
| Censored | 3456 | 2192 (77.5%) | 293 (74.7%) | 783 (80.3%) | 188 (61.6%) |
SEER – Surveillance, Epidemiology, and End Results; NOS – not otherwise specified; SES – socioeconomic status.
Effect of marital status on chondrosarcoma CSS
The Kaplan-Meier method and univariate analysis were performed to determine the chondrosarcoma CSS. As shown in Table 2, significant differences were observed in the survival among the different marital statuses (P<0.001). The 5-year CSS of widowed patients was significantly worse than that of married, divorced, and single patients (Figure 1). In addition, male patients (P<0.001), old patients (P<0.001), and patients with mesenchymal and dedifferentiated histotype (P<0.001), high-grade (P<0.001), axial location (P<0.001), large tumor (P<0.001), regional and distant SEER stage (P<0.001), low socioeconomic status (P=0.001), only radiotherapy, and non-treatment (P<0.001) had worse 5-year CSS. By multivariate analysis, these variables were also determined as independent risk factors for chondrosarcoma CSS. Divorced (HR 1.367, 95% CI 1.103–1.693, P=0.004) or widowed patients (HR 1.516, 95% CI 1.222–1.880, P<0.001) had a significantly higher risk of cancer-specific death compared to married patients, after controlling for sex, age, histotype, pathological grade, tumor location, tumor size, SEER stage, socioeconomic status, and treatment.
Table 2.
Univariate and multivariate survival analysis for evaluating the effect of marital status on chondrosarcoma CSS in SEER database.
| Variable | 5-year CSS | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| Log rank χ2 test | P | HR (95% CI) | P | ||
| Sex | 37.283 | <0.001 | |||
| Male | 76.9% | Reference | |||
| Female | 83.3% | 0.659 (0.577–0.753) | <0.001 | ||
| Age | 189.183 | <0.001 | |||
| <60 | 85.1% | Reference | |||
| ≥60 | 69.0% | 1.743 (1.520–1.998) | <0.001 | ||
| Race | 1.452 | 0.484 | NI | ||
| White | 79.7% | ||||
| Black | 79.2% | ||||
| Other | 80.6% | ||||
| Histotype | 576.205 | <0.001 | |||
| Chondrosarcoma, NOS | 83.3% | Reference | |||
| Myxoid | 79.0% | 0.989 (0.806–1.214) | 0.919 | ||
| Mesenchymal | 51.9% | 2.031 (1.560–2.644) | <0.001 | ||
| Dedifferentiated | 28.1% | 2.376 (1.892–2.984) | <0.001 | ||
| Other | 91.9% | 0.524 (0.280–0.983) | 0.044 | ||
| Pathological grade | 621.672 | <0.001 | |||
| Low-grade | 88.5% | Reference | |||
| High-grade | 50.6% | 2.719 (2.300–3.215) | <0.001 | ||
| Unknown | 71.4% | 1.845 (1.563–2.179) | <0.001 | ||
| Tumor location | 40.407 | <0.001 | |||
| Extremities | 82.6% | Reference | |||
| Axial | 74.6% | 1.176 (1.015–1.362) | 0.031 | ||
| Other | 81.1% | 0.760 (0.631–0.915) | 0.004 | ||
| Tumor size | 195.597 | <0.001 | |||
| <8 cm | 87.7% | Reference | |||
| ≥8 cm | 67.8% | 1.594 (1.353–1.879) | <0.001 | ||
| Unknown | 79.0% | 1.474 (1.245–1.745) | <0.001 | ||
| SEER stage | 1006.710 | <0.001 | |||
| Localized | 90.2% | Reference | |||
| Regional | 76.0% | 1.930 (1.647–2.263) | <0.001 | ||
| Distant | 35.4% | 4.847 (4.011–5.856) | <0.001 | ||
| Unstaged | 79.1% | 1.460 (1.119–1.904) | 0.005 | ||
| Socioeconomic status | 11.734 | 0.001 | |||
| Low-SES | 77.6% | Reference | |||
| High-SES | 81.9% | 0.863 (0.762–0.978) | 0.021 | ||
| Marital status | 67.442 | <0.001 | |||
| Married | 80.3% | Reference | |||
| Divorced | 78.4% | 1.367 (1.103–1.693) | 0.004 | ||
| Single | 84.0% | 0.892 (0.754–1.056) | 0.185 | ||
| Widowed | 62.3% | 1.516 (1.222–1.880) | <0.001 | ||
| Treatment | 625.543 | <0.001 | |||
| Surgery and radiation | 71.6% | Reference | |||
| Only surgery | 85.2% | 0.661 (0.560–0.780) | <0.001 | ||
| Only radiation | 23.3% | 2.264 (1.755–2.921) | <0.001 | ||
| None | 60.0% | 1.461 (1.155–1.848) | 0.002 | ||
SEER – Surveillance, Epidemiology, and End Results; CSS – cancer-specific survival; HR – hazard ratio; CI – confidence interval; NOS – not otherwise specified; SES – socioeconomic status; NI – not included in the multivariate survival analysis.
Figure 1.
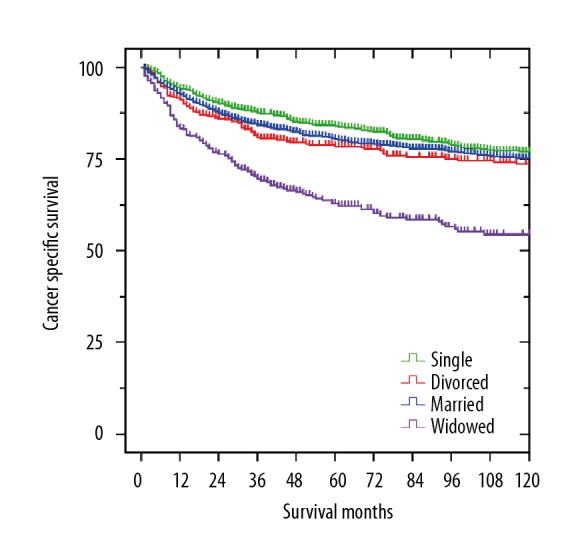
Survival curves of chondrosarcoma patients according to marital status (married, divorced, single, and widowed). χ2=67.442, P<0.001.
Subgroup analysis of the effects of marital status by SEER stage
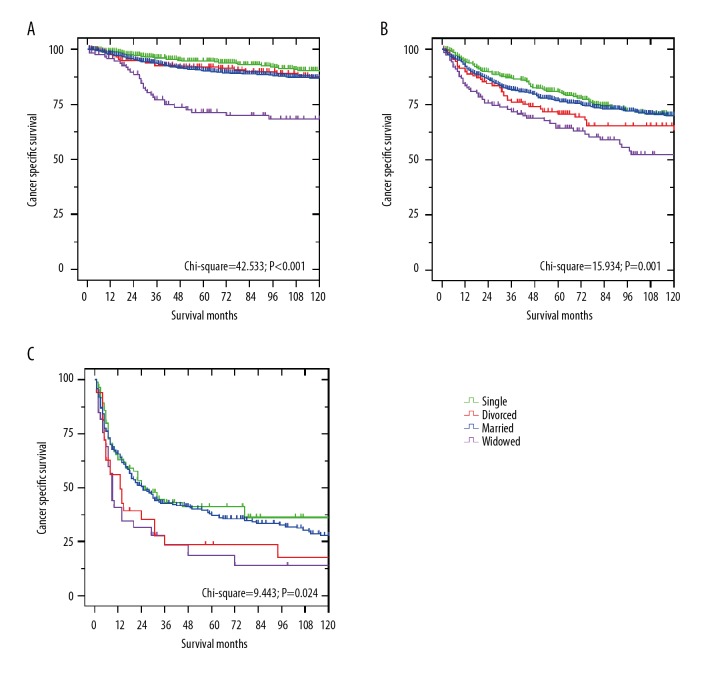
We next evaluated the effects of marital status on CSS regarding the SEER stage. As shown in Table 3, widowed patients always had the worst 5-year CSS compared to other patients in the localized and regional group (Figure 2A, 2B, P<0.05). After adjusting for sex, age, race, histotype, pathological grade, tumor location, tumor size, socioeconomic status, and treatment, Cox regression analyses identified widowhood as an independent risk factor of chondrosarcoma CSS (P<0.05). For the distant group, the 5-year CSS of widowed patients was only about one-third that of the married patients (Figure 2C, 14.0% vs. 37.3%, P=0.024), but the risk of cancer-specific death in widowed patients was not significantly higher than in other patients, according to the multivariate analysis (HR 1.270, 95% CI 0.834–1.932, P=0.265). Differences between married patients and single patients were not significant at any SEER stage (P>0.05).
Table 3.
Univariate and multivariate survival analysis for marital status on chondrosarcoma cause-specific survival based on different SEER stages.
| SEER stage | 5-year CSS | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| Log rank χ2 test | P | HR (95% CI) | P | ||
| Localized | 42.533 | <0.001 | |||
| Married | 90.1% | Reference | |||
| Divorced | 91.2% | 1.203 (0.797–1.815) | 0.380 | ||
| Single | 94.2% | 0.807 (0.560–1.162) | 0.249 | ||
| Widowed | 69.8% | 1.971 (1.298–2.994) | 0.001 | ||
| Regional | 15.934 | 0.001 | |||
| Married | 76.6% | Reference | |||
| Divorced | 70.7% | 1.440 (1.034–2.007) | 0.031 | ||
| Single | 80.7% | 1.047 (0.811–1.351) | 0.724 | ||
| Widowed | 64.3% | 1.535 (1.094–2.154) | 0.013 | ||
| Distant | 9.443 | 0.024 | |||
| Married | 37.3% | Reference | |||
| Divorced | 20.1% | 1.396 (0.901–2.162) | 0.135 | ||
| Single | 36.2% | 0.745 (0.539–1.031) | 0.076 | ||
| Widowed | 14.0% | 1.270 (0.834–1.932) | 0.265 | ||
SEER – Surveillance, Epidemiology, and End Results; CSS – cancer-specific survival; HR – hazard ratio; CI – confidence interval.
Figure 2.
Survival curves of chondrosarcoma patients according to marital status in different SEER stages. (A) localized stage: χ2=42.533, P<0.001; (B) regional stage: χ2=15.934, P= 0.001; (C) distant stage: χ2=9.443, P=0.024.
Subgroup analysis of the effects of marital status by pathological grade
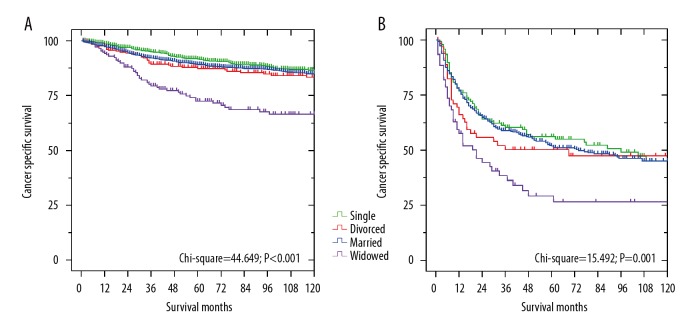
We further assessed the effects of marital status on CSS at each grade. As shown in Table 4, marital status was also determined to be an independent prognostic factor of CSS among patients with low- or high-grade chondrosarcoma according to the log-rank tests and Cox regression analyses (Figure 3A, 3B, P<0.05). Widowed patients always had the worst 5-year CSS. Compared with married patients, widowed patients had a significant reduction in survival rate in the low-grade group (71.5% vs. 89.1%, P<0.001; HR 1.866, 95% CI 1.332–2.613, P<0.001) and high-grade group (26.6% vs. 51.8%, P=0.001; HR 1.662, 95% CI 1.139–2.426, P=0.008). In contrast, the survival differences were not significant between married patients and divorced or single patients (P>0.05).
Table 4.
Univariate and multivariate survival analysis for marital status on chondrosarcoma cause-specific survival based on different pathological grades.
| Pathological grade | 5-year CSS | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| Log rank χ2 test | P | HR (95% CI) | P | ||
| Low-grade | 44.649 | <0.001 | |||
| Married | 89.1% | Reference | |||
| Divorced | 87.3% | 1.527 (1.111–2.100) | 0.009 | ||
| Single | 91.6% | 1.043 (0.803–1.354) | 0.751 | ||
| Widowed | 71.5% | 1.866 (1.332–2.613) | <0.001 | ||
| High-grade | 15.492 | 0.001 | |||
| Married | 51.8% | Reference | |||
| Divorced | 47.4% | 1.236 (0.840–1.817) | 0.282 | ||
| Single | 55.0% | 0.938 (0.689–1.276) | 0.682 | ||
| Widowed | 26.6% | 1.662 (1.139–2.426) | 0.008 | ||
CSS – cancer-specific survival; HR – hazard ratio; CI – confidence interval.
Figure 3.
Survival curves of chondrosarcoma patients according to marital status in different grades. (A) low-grade: χ2=44.649, P<0.001; (B) high-grade: χ2=15.492, P=0.001.
Discussion
The influence of marital status on prognosis of various tumors is not the same. Some studies found that marital status was an independent prognostic factor for cancer survival [13–15], while other studies suggested that this effect was mixed [17,18] or not significant [19]. To find more individual and holistic approaches to the treatment of chondrosarcoma, it is necessary to clarify the impact of marital status on survival. The present study is the first to identify marital status as an independent prognostic factor for chondrosarcoma CCS after controlling for age, histotype, pathological grade, tumor location, tumor size, SEER stage, socioeconomic status, and treatment in multivariate analyses. In addition, we found that in both the univariate and multivariate survival analyses, widowed patients always had the worst CCS compared with their married, divorced, and single counterparts.
Previous studies on Hodgkin lymphoma [25], colorectal cancer [26], and breast cancer [27] also indicated results similar to those reported here. Several hypotheses are proposed to explain the poor prognosis for widowed patients. First, delayed diagnosis may be one reason for this phenomenon. Tumor size, tumor grade, and tumor stage have been identified as important factors in predicting the survival of chondrosarcoma patients [4,9]. In the present study, we found that the widowed patients had the highest percentage of large, high-grade, regional, and distant tumors, which may contribute to the poor survival rate. Second, similar to the results of our study, low-SES was also determined to be a risk factor of survival in patients with multiple myeloma [28] and testicular germ cell tumors [21]. Thus, the relatively high percentage of low-SES status among widowed patients could be another reason for the poor survival. However, the proportion of widowed patients who received surgery was the lowest of all groups, suggesting that undertreatment can also cause poor prognosis in the widowed group.
The high percentage of delayed diagnosis and undertreatment among widowed patients were also observed in other studies. Shi et al. found that the widowed patients with differentiated thyroid cancer had the highest prevalence rates of advanced-stage tumors and distant metastasis [13]. Zhou et al. noticed that widowed patients with gastric cancer had the lowest rates of surgery [29]. Delayed diagnosis and undertreatment can be attributed to lack of spousal support and financial assistance. Spousal support can increase the convenience of medical screening, adherence to the prescribed regimen, and the possibility of receiving more aggressive treatments [30–32]. Additionally, the financial assistance can relieve non-medical-related stress and make it possible for patients to access more advanced health facilities, as well as having a better lifestyle and higher standard of living [33]. Due to the lack of these advantages, widowed patients are prone to delayed diagnosis and undertreatment.
In the same condition of lacking spousal support and financial assistance, the 5-year CSS of single patients was significantly better than that of widowed patients, which means that, in addition to the above hypotheses, other underlying etiologies may have an influence on the prognosis of widowed patients with chondrosarcoma. The relationship between poor survival and widowhood can be explained hypothetically by psychosocial factors. Because of the need to transit and adapt to new social roles, the death of a spouse is very stressful for the surviving companion [34]. Thus, widowed patients are associated with high risk of psychological disorders. Carr et al. found that the incidence of depression with clinical symptoms was about 15~30% within the first year of widowhood, and subclinical depression was much more common [34]. van Grootheest et al. found that, compared with married people, widowed people maintain a higher level of depressive symptoms for many years, although the symptoms tend to diminish over time [35]. It has been reported that depression and other psychosocial distresses are detrimental to the immune and endocrine systems [36]. The immune dysfunction may result in a low level of natural-killer cell cytotoxicity [37], which can lead to cancer progression. The endocrine dysfunction results in secretion disorders of various endocrine hormones, including catecholamines and cortisol [37,38], which contribute to cancer development and metastasis [39]. Furthermore, clinical depression can lead to a significant decline in medical compliance, thereby increasing the risk of tumor progression and mortality [40]. Goodwin et al. showed that women with depression were less likely to undergo surgery and thus had worse survival after being diagnosed with breast cancer [41]. Therefore, physicians should pay special attention to widowed patients with chondrosarcoma. If necessary, psychiatric intervention and daily community health system care are needed. Timely psychotherapy and support can minimize the impact of mental illness on patients and improve their compliance with surgical treatment, thereby increasing the survival rate of widowed chondrosarcoma patients.
In the subgroup analysis according to SEER stage and pathological grade, the survival of divorced and single patients was similar to that of married patients, while widowhood independently predicted poor survival in almost all subgroups.
These results further indicate that psychosocial distress caused by the death of a spouse was the primary reason for the poor prognosis of widowed chondrosarcoma patients. In addition, when considering chondrosarcoma with distant stage, we found the marital status was a significant prognostic factor in univariate analysis, and widowed patients presented the worst survival, but this was not found in multivariate analysis. We believe that the small number (only 33 cases) of patients with distant chondrosarcoma in the widowed group makes the influence of marital status on survival difficult to identify and quantify.
The significance of marital status in cancer survival has been identified in numerous cancer types, including lung, colorectal, breast, pancreatic, prostate, liver, ovarian [12], and thyroid cancer [13]. The present study also showed the significance of marital status in the survival of patients with chondrosarcoma. Marriage has a positive effect on cancer prognosis, which may be independent of cancer types. Married patients are more likely to receive timely detection; thus, they are more likely to be diagnosed at an early stage. Additionally, married patients are more compliant with proper therapy and medication under the support and encouragement from their spouses and family [30–32]. Contrary to widowhood, marriage also has a positive impact on cardiovascular and endocrine function, cortisol level, and immune function, which may improve the effects of treatment [36–38].
However, a few studies suggested that marital status might have no significant effect on cancer prognosis. Goodwin et al. indicated that, due to the limitations of the database, the SES could not be controlled for in the analyses, so their conclusion about the relationship between epithelial cancer and marital status might be unreliable [17]. Jatoi et al. used a database from the Mayo Clinic and demonstrated that the survival differences between married, single, divorced, and widowed persons with non-small cell lung cancer were not significant, which was at odds with previous studies [12,42]. The authors suggested that strong social support and high SES of the patients diagnosed in the Mayo Clinic biased the data, which explain the conflicting results [19]. In the present study, we extracted 3 standard 2000 US Census variables to create a composite SES variable, and we determined that marital status was an independent prognostic factor after SES and other factors were controlled for. Therefore, the benefit of marriage on different cancer types still needs verification in further studies.
Some limitations have to be considered in our study. First, the SEER database only provides the marital status at diagnosis, but marital status may change during the therapeutic process. For example, few widowed patients get married after diagnosis, which may affect our findings. Second, in the SEER database, it is difficult to distinguish between single and cohabitation, and patients defined as single may actually have been cohabitating. Finally, we believe that psychosocial distresses are the primary reason for the poor prognosis of widowed patients, but we cannot carry out rigorous psychosocial tests to validate this hypothesis. Therefore, further clinical and psychological studies are needed to confirm our findings.
Conclusions
Our study is the first to identify marital status as an independent prognostic factor for chondrosarcoma CSS, and widowhood was long known to be associated with the highest risk of cancer mortality. Psychosocial distresses may be the primary reason for the poor prognosis of widowed patients, so it is necessary to provide timely psychological treatment and adequate social support for widowed patients in clinical practice, which can improve the survival of chondrosarcoma patients.
Footnotes
Competing interests
None.
Source of support: This study was funded by the National Natural Science Foundation of China (81571209)
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. Cancer J Clin. 2016;66(2):115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Larsson SE, Lorentzon R. The geographic variation of the incidence of malignant primary bone tumors in Sweden. J Bone Joint Surg Am. 1974;56(3):592–600. [PubMed] [Google Scholar]
- 4.Giuffrida AY, Burgueno JE, Koniaris LG, et al. Chondrosarcoma in the United States (1973 to 2003): An analysis of 2890 cases from the SEER database. J Bone Joint Surg Am. 2009;91(5):1063–72. doi: 10.2106/JBJS.H.00416. [DOI] [PubMed] [Google Scholar]
- 5.Angelini A, Guerra G, Mavrogenis AF, et al. Clinical outcome of central conventional chondrosarcoma. J Surg Oncol. 2012;106(8):929–37. doi: 10.1002/jso.23173. [DOI] [PubMed] [Google Scholar]
- 6.Bovee JV, Cleton-Jansen AM, Taminiau AH, Hogendoorn PC. Emerging pathways in the development of chondrosarcoma of bone and implications for targeted treatment. Lancet Oncol. 2005;6(8):599–607. doi: 10.1016/S1470-2045(05)70282-5. [DOI] [PubMed] [Google Scholar]
- 7.Delaney TF, Kepka L, Goldberg SI, et al. Radiation therapy for control of soft-tissue sarcomas resected with positive margins. Int J Radiat Oncol Biol Phys. 2007;67(5):1460–69. doi: 10.1016/j.ijrobp.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 8.Chen JC, Fong YC, Tang CH. Novel strategies for the treatment of chondrosarcomas: targeting integrins. Biomed Res Int. 2013;2013 doi: 10.1155/2013/396839. 396839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arshi A, Sharim J, Park DY, et al. Chondrosarcoma of the osseous spine: An analysis of epidemiology, patient outcomes, and prognostic factors using the SEER registry from 1973 to 2012. Spine (Phila Pa 1976) 2017;42(9):644–52. doi: 10.1097/BRS.0000000000001870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andreou D, Ruppin S, Fehlberg S, et al. Survival and prognostic factors in chondrosarcoma: Results in 115 patients with long-term follow-up. Acta Orthop. 2011;82(6):749–55. doi: 10.3109/17453674.2011.636668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukherjee D, Chaichana KL, Adogwa O, et al. Association of extent of local tumor invasion and survival in patients with malignant primary osseous spinal neoplasms from the surveillance, epidemiology, and end results (SEER) database. World Neurosurg. 2011;76(6):580–85. doi: 10.1016/j.wneu.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869–76. doi: 10.1200/JCO.2013.49.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi RL, Qu N, Lu ZW, et al. The impact of marital status at diagnosis on cancer survival in patients with differentiated thyroid cancer. Cancer Med. 2016;5(8):2145–54. doi: 10.1002/cam4.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLaughlin JM, Fisher JL, Paskett ED. Marital status and stage at diagnosis of cutaneous melanoma: Results from the Surveillance Epidemiology and End Results (SEER) program, 1973–2006. Cancer. 2011;117(9):1984–93. doi: 10.1002/cncr.25726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alamanda VK, Song Y, Holt GE. Effect of marital status on treatment and survival of extremity soft tissue sarcoma. Ann Oncol. 2014;25(3):725–29. doi: 10.1093/annonc/mdt583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Liu Y, Wang Y, et al. The influence of marital status on survival of gallbladder cancer patients: A population-based study. Sci Rep. 2017;7(1):5322. doi: 10.1038/s41598-017-05545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodwin JS, Hunt WC, Key CR, Samet JM. The effect of marital status on stage, treatment, and survival of cancer patients. JAMA. 1987;258(21):3125–30. [PubMed] [Google Scholar]
- 18.Nelles JL, Joseph SA, Konety BR. The impact of marriage on bladder cancer mortality. Urol Oncol. 2009;27(3):263–67. doi: 10.1016/j.urolonc.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Jatoi A, Novotny P, Cassivi S, et al. Does marital status impact survival and quality of life in patients with non-small cell lung cancer? Observations from the mayo clinic lung cancer cohort. Oncologist. 2007;12(12):1456–63. doi: 10.1634/theoncologist.12-12-1456. [DOI] [PubMed] [Google Scholar]
- 20.Du XL, Fang S, Coker AL, et al. Racial disparity and socioeconomic status in association with survival in older men with local/regional stage prostate carcinoma: Findings from a large community-based cohort. Cancer. 2006;106(6):1276–85. doi: 10.1002/cncr.21732. [DOI] [PubMed] [Google Scholar]
- 21.Sun M, Abdollah F, Liberman D, et al. Racial disparities and socioeconomic status in men diagnosed with testicular germ cell tumors: A survival analysis. Cancer. 2011;117(18):4277–85. doi: 10.1002/cncr.25969. [DOI] [PubMed] [Google Scholar]
- 22.Robert SA, Strombom I, Trentham-Dietz A, et al. Socioeconomic risk factors for breast cancer: Distinguishing individual- and community-level effects. Epidemiology. 2004;15(4):442–50. doi: 10.1097/01.ede.0000129512.61698.03. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Li S, Li Y, et al. Prognostic factors for survival among patients with primary bone sarcomas of small bones. Cancer Manag Res. 2018;10:1191–99. doi: 10.2147/CMAR.S163229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guller U, Tarantino I, Cerny T, et al. Population-based SEER trend analysis of overall and cancer-specific survival in 5138 patients with gastrointestinal stromal tumor. BMC Cancer. 2015;15:557. doi: 10.1186/s12885-015-1554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang FF, Xie XY, Yang XM, et al. The influence of marital status on the survival of patients with Hodgkin lymphoma. Oncotarget. 2017;8(31):51016–23. doi: 10.18632/oncotarget.16879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q, Gan L, Liang L, et al. The influence of marital status on stage at diagnosis and survival of patients with colorectal cancer. Oncotarget. 2015;6(9):7339–47. doi: 10.18632/oncotarget.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghazali SM, Othman Z, Cheong KC, et al. Non-practice of breast self examination and marital status are associated with delayed presentation with breast cancer. Asian Pac J Cancer Prev. 2013;14(2):1141–45. doi: 10.7314/apjcp.2013.14.2.1141. [DOI] [PubMed] [Google Scholar]
- 28.Fiala MA, Finney JD, Liu J, et al. Socioeconomic status is independently associated with overall survival in patients with multiple myeloma. Leuk Lymphoma. 2015;56(9):2643–49. doi: 10.3109/10428194.2015.1011156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou R, Yan S, Li J. Influence of marital status on the survival of patients with gastric cancer. J Gastroenterol Hepatol. 2016;31(4):768–75. doi: 10.1111/jgh.13217. [DOI] [PubMed] [Google Scholar]
- 30.Haley WE. Family caregivers of elderly patients with cancer: Understanding and minimizing the burden of care. J Support Oncol. 2003;1(4 Suppl 2):25–29. [PubMed] [Google Scholar]
- 31.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–23. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwashyna TJ, Christakis NA. Marriage, widowhood, and health-care use. Soc Sci Med. 2003;57(11):2137–47. doi: 10.1016/s0277-9536(02)00546-4. [DOI] [PubMed] [Google Scholar]
- 33.Baine M, Sahak F, Lin C, et al. Marital status and survival in pancreatic cancer patients: A SEER based analysis. PLoS One. 2011;6(6):e21052. doi: 10.1371/journal.pone.0021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carr D, Utz R. Late-life widowhood in the United States: New directions in research and theory. Ageing International. 2001;27(1):65–88. [Google Scholar]
- 35.van Grootheest DS, Beekman AT, Broese van, et al. Sex differences in depression after widowhood. Do men suffer more? Soc Psychiatry Psychiatr Epidemiol. 1999;34(7):391–98. doi: 10.1007/s001270050160. [DOI] [PubMed] [Google Scholar]
- 36.Garssen B, Goodkin K. On the role of immunological factors as mediators between psychosocial factors and cancer progression. Psychiatry Res. 1999;85(1):51–61. doi: 10.1016/s0165-1781(99)00008-6. [DOI] [PubMed] [Google Scholar]
- 37.Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumour biology: Pathways and mechanisms. Nat Rev Cancer. 2006;6(3):240–48. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moreno-Smith M, Lutgendorf SK, Sood AK. Impact of stress on cancer metastasis. Future Oncol. 2010;6(12):1863–81. doi: 10.2217/fon.10.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McEwen BS, Biron CA, Brunson KW, et al. The role of adrenocorticoids as modulators of immune function in health and disease: Neural, endocrine and immune interactions. Brain Res Brain Res Rev. 1997;23(1–2):79–133. doi: 10.1016/s0165-0173(96)00012-4. [DOI] [PubMed] [Google Scholar]
- 40.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–7. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 41.Goodwin JS, Zhang DD, Ostir GV. Effect of depression on diagnosis, treatment, and survival of older women with breast cancer. J Am Geriatr Soc. 2004;52(1):106–11. doi: 10.1111/j.1532-5415.2004.52018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. Lung carcinoma symptoms – an independent predictor of survival and an important mediator of African-American disparity in survival. Cancer. 2004;101(7):1655–63. doi: 10.1002/cncr.20547. [DOI] [PubMed] [Google Scholar]