Abstract
Introduction:
Encapsulation of pancreatic islets or beta cells is a promising strategy for treatment of type 1 diabetes by providing an immune isolated environment and allowing for transplantation in a different location than the liver. However, islets used for encapsulation often show lower functionality due to the damaging of islet endothelial cells during the isolation procedure. Factors produced by endothelial cells have great impact on beta cell insulin secretion. Therefore, mutual signaling between endothelial cells and beta cells should be considered for the development of encapsulation systems to achieve high insulin secretion and maintain beta cell viability. Here, we investigate whether co-culture of beta cells with endothelial cells could improve beta cell function within encapsulation devices.
Materials and methods:
Mouse insulinoma MIN6 cells and human umbilical vein endothelial cells were used for creating composite aggregates on agarose microwell platform. The composite aggregates were encapsulated within flat poly(ether sulfone)/polyvinylpyrrolidone device. Their functionality was assessed by glucose-induced insulin secretion test and compared to non-encapsulated free-floating aggregates.
Results:
We created composite aggregates of 80–100 µm in diameter, closely mimicking pancreatic islets. Upon glucose stimulation, their insulin secretion is improved in comparison to aggregates consisting of only MIN6 cells. Moreover, the composite aggregates encapsulated within a device secrete more insulin than aggregates consisting of only MIN6 cells.
Conclusion:
Composite aggregates of MIN6 cells with human umbilical vein endothelial cells have improved insulin secretion in comparison to MIN6 aggregates showing that the interaction of beta cell and endothelial cell is crucial for a functional encapsulation system.
Keywords: Bioartificial pancreas, encapsulation, composite aggregates, human umbilical vein endothelial cell, beta cells
Introduction
The bioartificial pancreas represents a viable solution for the treatment of type 1 diabetes. The encapsulation of pancreatic islets or beta cells in a semipermeable membrane can allow for nutrient, glucose, and insulin exchange and provide necessary immunoisolation to avoid the administration of immunosuppressive drugs.1 However, reproducing a natural insulin release profile, while applying a membrane as an immune barrier, requires using of highly functional islets or beta cells and maintaining their viability.2
The native pancreatic islet is highly vascularized with an extensive capillary network.3 However, the isolation of islets by enzymatic digestion disrupts the islet vascular connection contributing to lower islet viability and loss of function.4 In the pancreas, islet vasculature provides nutrients and oxygen to the endocrine cells and transports the hormones to the peripheral circulation.3 Therefore, it is important to provide encapsulated islets with close proximity to blood vessels. In addition, islet endothelial cells (ECs), which form capillaries, are an important source of signals that enhance survival and function of the islet beta cells.3 In fact, each beta cell in the native islet is surrounded by at least one EC, and therefore, these cells by necessity are exposed to each other’s products.5
After the isolation process, human islets suffer from hypoxia and express high levels of vascular endothelial growth factor (VEGF).6 In the islet, the beta cell is a major source of VEGF production which is required to maintain EC viability and promotes EC proliferation. It has been shown that beta cell-specific reduction of vascular endothelial growth factor-A (VEGF-A) expression in mice results in islet capillary loss and decreased insulin release in vivo.7 Insulin is also a major signal for EC function. It is, for example, required for phosphorylation (activation) of endothelial nitric oxide synthase (NOS3), which catalyzes production of the vasodilator nitric oxide (NO).3 Thus, signals produced by the beta cell influence the islet EC, contributing to the overall islet health. Importantly, ECs signal back and contribute to the maintenance of beta cell viability and function.8,9 Johansson et al. examined the effects of multiple endothelial-derived molecules on insulin release in vitro. This study was based on a model where exposure of whole islets to factors secreted from cultured islet ECs resulted in no change in basal insulin release, but significantly enhanced glucose-stimulated insulin release and increased insulin content,10 similar to findings of another very recent study.11 Besides, there are indications that beta cells, in contrast to exocrine pancreatic cells, do not form a basement membrane. Instead, using VEGF-A, they attract ECs which form a vascular basement membrane containing laminins next to beta cells.12 Exposure of beta cells to various laminin isoforms can increase insulin gene transcription and insulin release, enhancing beta cell function.10,11 This effect is at least partly dependent on integrins, a family of heterodimeric cell-surface receptors with broad specificity for extracellular matrix (ECM) molecules (e.g. laminins, collagens, and fibronectin), some of which are expressed by the beta cells.13 Sebara and Vermette have also studied the influence of separation distance between beta cells and ECs on insulin secretion. They have shown that the insulin release of rat insulinoma cells (INS-1) was significantly increased when the cells were co-cultured in close proximity (100 µm) to human umbilical vein ECs in comparison to INS-1 cells cultured alone.14 All the above show the importance of the presence of ECs in close proximity to beta cells. Hence, this should be considered during the development of islet encapsulation systems in order to achieve high insulin secretion, after encapsulation, and maintain beta cell viability.
In this study, we hypothesize that the encapsulation of beta cells co-cultured with ECs would be beneficial for improved insulin secretion upon glucose stimulation due to possible signaling between these two cell types. We create stable composite aggregates consisting of MIN6 cells co-cultured with human umbilical vein ECs (HUVECs) using a non-adherent agarose microwell platform. These aggregates are encapsulated within poly (ether sulfone)/polyvinylpyrrolidone (PES/PVP) microwell device15 and their functionality, assessed by glucose-induced insulin secretion test (GIIST), is compared to encapsulated MIN6 aggregates without HUVECs. Mouse insulinoma MIN6 cell line is often used as a model for primary beta cells as it closely resembles native beta cells and reflects physiological conditions, while HUVECs have been employed in many studies as an EC model for experiments attempting to achieve micro-vessel formation and vascular remodeling.16,17
Materials and methods
Cell culture and labeling
MIN6-B1 mouse insulinoma cells (kindly provided by Dr P. Halban, University Medical Center, Geneva, Switzerland) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco) supplemented with 10% (v/v) fetal bovine serum (FBS; Lonza), 100 U/mL penicillin and 100 mg/mL streptomycin (Gibco), and 70 µM freshly added beta-mercaptoethanol (Gibco) at 37°C and 5% CO2. HUVECs (CC2519A; Lonza) were cultured in endothelial growth medium-2 (ΕGΜ™-2 BulletKit™; Lonza). HUVECs used for the experiments had passage numbers lower than six. In order to distinguish HUVECs from MIN6 cells, HUVECs were labeled with Dil (red) solution, according to the manufacturer’s protocol (Life Technologies).
Aggregate formation
Non-adherent agarose microwell chips containing 2865 wells were fabricated, as described previously.18 In short, polydimethylsiloxane (PDMS) negative molds carrying 200 µm pillars were sterilized with 70% ethanol. A volume of 3% UltraPure™ agarose (Gibco) was dissolved in phosphate-buffered saline (PBS). The solution was heated to 100°C in a microwave oven. Molds were placed inside a six-well plate and filled with 8 mL of 3% agarose solution. The plates were centrifuged at 300g for 1 min to remove air bubbles and stored at 4°C for at least 30 min. After the gel was formed, the molds were gently removed from the agarose using a sterile spatula. Using a sterile punching device, chips were punched out leaving a thin agarose wall on all sides to fit into a 12-well plate. Prior to cell seeding, the agarose chips were incubated in medium prepared for MIN6 cells (for MIN6 aggregates) or mixture of this medium and endothelial cell growth medium-2 (EGM-2) in ratio 1:1 (for composite aggregates consisting of MIN6 cells and HUVECs).
Stable cell aggregates were prepared based on the work of Hilderink et al.19 MIN6 cells were seeded onto the agarose chips (250 cells/aggregate – 1 aggregate/well). The plates were centrifuged at 150g for 1 min, and 2 mL of medium was carefully added to the chips. In order to prepare composite aggregates, the suspension of HUVECs (1500 cells/aggregate – 1 aggregate/well, the number of HUVECs was based on the work of Buitinga et.al.20) was added to the MIN6 aggregates after 1 day of culture. The plates were centrifuged at 150g for 30 s, and then, every 10 min, plates were stirred on the shaker for 5 min during the first hour of co-culture. MIN6 aggregates were cultured in DMEM medium described earlier, and composite aggregates were cultured in mixture of DMEM medium and EGM-2 medium in ratio 1:1. The medium was refreshed 24 h after seeding. After 48 h at 37°C, aggregates were flushed out of the chips (2865 aggregates from one chip). The suspension of 150 aggregates was used for a functionality experiment with free-floating aggregates as well as for an encapsulation experiment, both described in the following section.
Free-floating aggregate functionality
After 1 day of culture, a GIIST was performed with 150 free-floating MIN6 aggregates as a control and 150 free-floating composite aggregates using a commercial transwell system (Millipore). Modified Krebs buffer (115 mM NaCl, 5 mM KCl, and 24 mM NaHCO3; Sigma-Aldrich) supplemented with 2.2 mM CaCl2, 20 mM hydroxyethyl piperazineethanesulfonic acid (HEPES; Gibco), 30% bovine serum albumin, 1 mM MgCl2, and 0.1 mM Theophylline (Sigma-Aldrich) was prepared at pH 7.421 and was used to prepare low (1.67 mM) and high (16.7 mM) glucose concentration solutions. The free-floating aggregates were washed three times (5 min) in the Krebs buffer, followed by a pre-incubation of 90 min in the low-glucose concentration buffer. All samples were then incubated for 60 min in subsequent low-, high-, and low-glucose concentration buffer with three times, 5 min, washing in the Krebs buffer between each high- and low-glucose incubation. Samples were taken after each incubation time, spun down (300g, 3 min), and the supernatant was stored at –20°C. Samples were analyzed using insulin mouse enzyme-linked immunosorbent assay (ELISA; Mercodia) according to the manufacturer’s instructions. The functionality of the aggregates was assessed by determining the amount of insulin secreted after glucose stimulation and displayed as the glucose-induced insulin stimulation index. For the calculation of the stimulation index, the insulin secretion of all samples was normalized to the insulin secreted during the first low-glucose incubation (1.7 mM glucose), in agreement with literature.15,21
Encapsulation device preparation
The encapsulation device was prepared based on Skrzypek et al.15 In short, a polymer blend of 15 wt% PES (E6020 P; Ultrason®) and 5 wt% PVP (molecular weight (MW) = 40,000 g/mol; Sigma-Aldrich) in N-Methyl-pyrrolidone (NMP; Acros Organics) was used for casting on a custom-made, micropatterned mold with spatially organized dome-like structures of 500 µm height and 500 µm in diameter. The casting thickness was 100 µm. After casting followed by immersion into water coagulation bath, the polymer solution precipitated and the membranes were removed from the mold. In order to increase the membrane porosity, the membranes were treated with 4000 ppm sodium hypochlorite aqueous solution (NaClO, Fluka™) for 24 h. Subsequently, the membranes were washed and stored in demineralized water.
The microwell membrane and the flat lid membrane (Sterlitech Corporation) with diameter of 10 mm were sealed (90°C, 10 s) on the edges using a custom-made sealing device, leaving open the middle part and a small inlet for cell seeding. The sealed device was sterilized with 70% ethanol, washed in PBS, and pre-incubated in culture medium overnight.
Functionality of encapsulated aggregates
150 MIN6 aggregates or 150 composite aggregates in 10 µL of medium were seeded inside the device via the small inlet, which was closed after seeding using sterile, surgical staples (Ligating clips, HORIZON™; Teleflex Medical). The sealed device with encapsulated aggregates was placed in the culture medium. After 1 day of culture, the functionality of encapsulated aggregates was determined following the GIIST procedure earlier described for free-floating aggregates (section “Free-floating aggregate functionality”).
Statistical analysis
Results are presented as the mean ± standard deviation. Statistical analyses were performed using two-tailed analysis of variance (ANOVA) using SPSS Statistics software (version 24; IBM Corporation) to compare the insulin concentration and stimulation indexes upon glucose stimulation for MIN6 aggregates and composite aggregate used as free-floating as well as encapsulated within PES/PVP microwell device. Statistical significance was considered at p values <0.05.
Results
Formation of multicellular aggregates
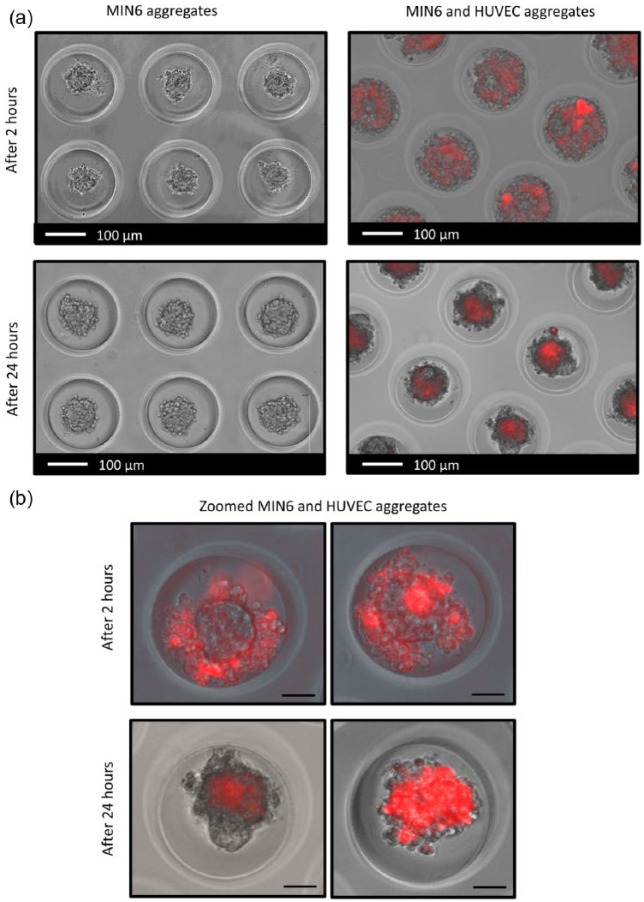
Agarose microwell chips with wells of 200 µm were used for controlled cell aggregation. Figure 1(a) shows the images of MIN6 aggregates and composite aggregates consisting of MIN6 cells co-cultured with HUVECs. MIN6 single cells cultured on non-adherent agarose chips clustered over time, resulting in stable size, rounded aggregates of 80–100 µm in diameter (Figure 1 (left)). In order to prepare composite aggregates, the red-labeled HUVEC suspension was added to the MIN6 aggregates. Over time, HUVECs attached to the MIN6 aggregates and upon culturing become more uniformly distributed over the aggregates (Figure 1 (right)). After 24 h, aggregates consisting of only MIN6 cells as well as composite aggregates were similar in size; however, not all of the composite aggregates were spheroidal shaped.
Figure 1.
Aggregate formation. (a) MIN6 aggregates and composite aggregates consisting of MIN6 cells and HUVECs (red) in agarose chips after 2 and 24 h of culture. (b) Zoomed composite aggregates consisting of MIN6 cells and HUVECs (red) after 2 and 24 h (scale bars: 50 µm).
Figure 1(b) shows higher magnification images of the formed composite aggregates over time. Initially, HUVECs (red labeled) surrounded MIN6 aggregates and attached to them, as well as to each other (Figure 2(a)). After 24 h, we obtained the composite aggregates where HUVECs were either uniformly integrated with MIN6 aggregates (Figure 2(b) (right)) or HUVECs formed clusters attached to the MIN6 aggregate (Figure 2(b) (left)).
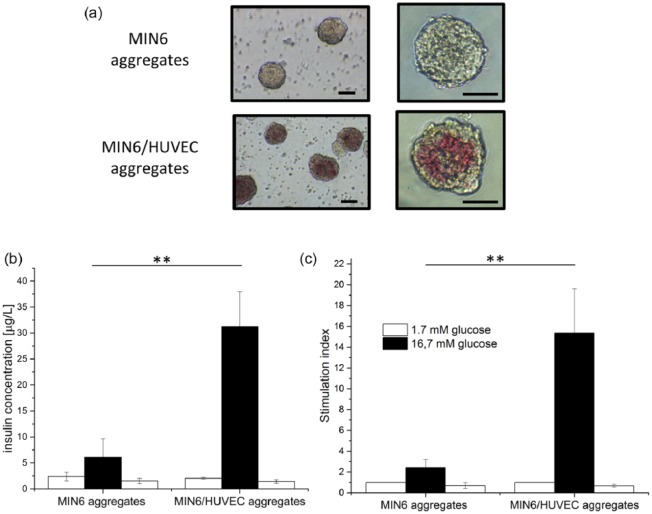
Figure 2.
Free-floating aggregates. (a) After flushing from the agarose chips (scale bars: 50 µm). (b) and (c) Functionality after 1 day of culture, where (b) insulin concentration obtained after glucose stimulation and (c) insulin secretion is normalized to the first low-glucose stimulation and presented as a stimulation index. Error bars indicate standard deviation (n = 3; **p < 0.05).
Functionality of free-floating aggregates
In order to perform a functionality test (GIIST) on free-floating aggregates, the MIN6 and composite aggregates were flushed out of the agarose chips. Figure 2(a) shows that both types of aggregates remained intact, and cell integration was preserved during and after harvesting from the chips. Figure 2(b) and (c) compares the functionality of free-floating MIN6 aggregates and of the composite aggregates displayed as insulin concentration after glucose stimulation and stimulation index. Both secreted insulin in response to glucose concentration changes. However, we observed a significant increase in the insulin concentration (five times more) after high glucose stimulation for the composite aggregates in comparison to the MIN6 aggregates used as positive control. In fact, the HUVECs-MIN6 composite aggregates have stimulation index of 15 ± 4, six times higher than the index of control MIN6 aggregates, clearly indicating better functionality of the composite aggregates.
Functionality of encapsulated aggregates
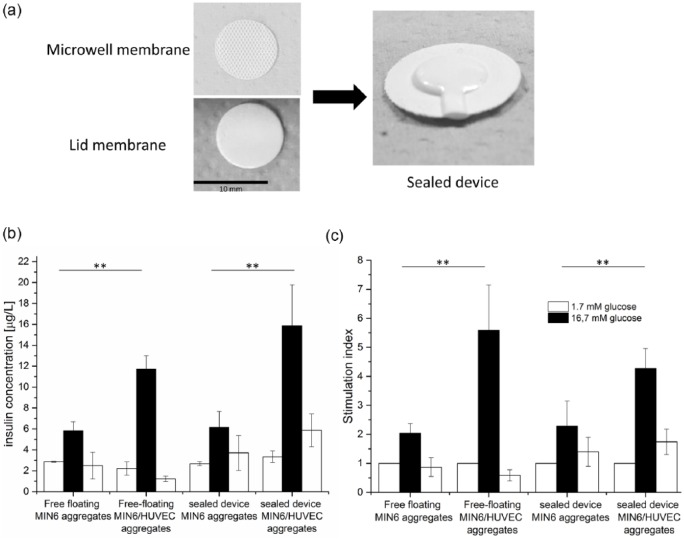
The MIN6 aggregates and MIN6/HUVEC composite aggregates were further encapsulated within our microwell device (Figure 3(a)) and their functionality was studied after 1 day of culture. Figure 3(b) compares the insulin concentration after glucose stimulation obtained for both types of aggregates, when used as free-floating controls and when encapsulated within our device. In all cases, the aggregates function well and respond clearly to glucose concentration changes, indicating also their viability. Moreover, the composite aggregates secrete a significantly higher amount of insulin after high glucose stimulation in comparison to MIN6 aggregates, what is attributed to the addition of ECs within the beta cell aggregate.
Figure 3.
Aggregate encapsulation. (a) Images of microwell and lid membrane used for preparation of sealed device. (b) and (c) Functionality of aggregates encapsulated within sealed flat device in comparison to free-floating aggregates after 1 day of culture, where (b) insulin concentration is obtained after glucose stimulation and (c) insulin secretion is normalized to the first low-glucose stimulation and presented as a stimulation index. Error bars indicate standard deviation (n = 3; **p < 0.05).
The free-floating composite aggregates performed better (stimulation index of 6) than our positive-control MIN6 aggregates (stimulation index of 2) in terms of insulin secretion upon high glucose stimulation (Figure 3(c)), as previously observed. MIN6 aggregates remained functional after encapsulation within our device and responded to glucose concentration changes in a similar manner to free-floating positive controls. Moreover, we observed a clear increase in insulin concentration and the stimulation index when composite aggregates were encapsulated, confirming the positive effect of the addition of HUVECs to MIN6 aggregates on their insulin secretion also after encapsulation.
Discussion
ECs play an important role in maintaining pancreatic islet viability and enhancing their function. However, the isolation procedure disrupts the islet’s own vasculature, negatively affecting the beta cell connection with the islet ECs, which is required for proper cell signaling and promoting insulin secretion.3,4 Therefore, to improve islet transplantation outcomes using islet encapsulation devices, it is important to provide islets with close proximity to host vasculature. However, the revascularization of large implants still needs a prolonged time period, while the lack of factors produced by ECs contributes to insufficient insulin release and loss of beta cell function.22 Kaufman-Francis et al. cultured mouse islets together with HUVECs and human foreskin fibroblast on highly porous and biodegradable porous poly(l-lactide)/poly(lactide-co-glycolide) (PLLA/PLGA) scaffolds. Their results show that ECs promote upregulation of ECM-associated genes in islet culture, improving islet survival and function in vitro, as well as in vivo.23 Therefore, we can hypothesize that the encapsulation of islets with supportive ECs would allow for cell–cell contact and necessary signaling within the encapsulation device, improving beta cell function.
In this study, we investigated whether the co-culture of beta cells with ECs could improve beta cell function within membrane-based encapsulation devices. Johansson et al.10 reported that insulin release as well as insulin content is enhanced in islets exposed to endothelium-conditioned culture medium. However, in terms of encapsulation devices, which are developed with the aim of implantation, we cannot rely only on conditioned medium which will not be present in in vivo conditions. Therefore, here we incorporated ECs within beta cell aggregate used for the encapsulation.
We used mouse insulinoma MIN6 cells as a beta cell model, as they closely resemble primary beta cells and reflect physiological conditions.16 Since islet beta cells require cell–cell contact to survive and properly function in vitro,24 we created stable MIN6 cell aggregates mimicking pancreatic islets and used them as our positive controls. The viability and functionality of MIN6 cells have been shown to improve in three-dimensional (3D) cell aggregates compared to two-dimensional monolayer culture, due to enhanced cell–cell contact.25
We obtained MIN6 aggregates of 80–100 µm in diameter using a non-adhesive agarose microwell platform based on the work of Hilderink et al.19 The agarose chips served us also as a platform to create MIN6/HUVECs composite aggregates, where HUVECs, as an EC model, were attached and incorporated with the MIN6 aggregates. Buitinga et al.20 in their study used a similar agarose platform to create composite human islets with proangiogenic support cells for improvement of islet revascularization at the subcutaneous transplantation site. Our composite aggregates were similar in size to MIN6 aggregates; however, not all of them were spheroidal shaped as MIN6 aggregate controls. In fact, their shape was similar to that of the native islets.26 The islets of Langerhans are 3D structures which contain insulin-producing beta cells in direct contact with islet ECs. Therefore, recreating a more native structure of islet beta cells by the formation of the beta cell aggregates co-cultured with ECs is an important tool for the study of beta cell physiology in a 3D conformation.27 We chose mouse insulinoma beta cell line for the preparation of composite aggregates which is responsive to glucose stimulation in contrast to available human beta cell lines, which often show lack of this important characteristic of native beta cells.16 In addition, rodent beta cell lines have been already used in co-culture with human ECs to study the effect of various EC factors on beta cell insulin secretion.14,28
Our sealed encapsulation device15 was used for the encapsulation of the cell aggregates. The device consists of a microwell membrane, a selective layer which does not allow for cell infiltration, and flat lid membrane with 0.45 µm pore size, in agreement with other studies, which have shown that this pore size does not allow host cells to permeate to the device providing protection to allogeneic and xenogeneic transplants.29–31 The functionality of MIN6 cell aggregates encapsulated within this device was compared with encapsulated composite aggregates. As expected, after 1 day of culture, free-floating MIN6 aggregates showed a response to glucose concentration changes. In the case of the free-floating composite aggregates, we observed a significant increase in insulin secretion after high glucose stimulation (stimulation index six times higher than for the MIN6 aggregates), which can be associated to the presence of HUVECs in co-culture with MIN6 aggregates. Moreover, both types of aggregates function after encapsulation, and importantly, an increase in insulin secretion was observed for the composite aggregates in comparison to encapsulated MIN6 aggregates, indicating the positive effect of HUVEC addition on MIN6 cells functionality. Therefore, we believe that interface beta cell/EC within the cluster allows for the cell interaction significantly improving insulin content and beta cell function. Kusamori et al.32 in their study also observed improved insulin secretion when multicellular spheroids were created consisting of MIN6 cells co-cultured with aortic vascular ECs. Although factors produced by ECs have been shown to significantly enhance glucose-stimulated insulin release,3,10,28 their beneficial effect on beta cell functionality within encapsulation devices, presented in this work, has not been previously studied and represents a novel finding in the area of bioartificial pancreas.
One of the important considerations during the development of beta cell aggregates used for encapsulation is their size and the number of cells used for their preparation, as beta cells are sensitive to hypoxia which often occurs after encapsulation and causes cell apoptosis and loss of function. Although hypoxic conditions often have a negative effect on the viability of cell clusters, in case of MIN6 aggregates, it has been shown that oxygen stress does not cause a pronounced drop in viability; however, it is clearly impacting MIN6 aggregation and function as measured by glucose-stimulated insulin secretion.33 In our study, we show that prepared aggregates function after encapsulation; therefore, we expect that the oxygen supply was sufficient within our macroencapsulation device. Moreover, both the MIN6 aggregates and composite aggregates (80–100 µm) are smaller than 150 µm, which is the size of the islets recommended for transplantation due to their higher viability, higher functionality, and greater oxygen consumption.34 In addition, our composite aggregates perform very well in terms of insulin secretion and show higher insulin content than pure MIN6 aggregates, indicating also their viability, although they consist of a higher number of cells (approximately 1750 cells). Ichihara et al.35 showed that pseudo-islets prepared using 4600 cells had less than 2% of apoptotic cells, as expected mainly in the central core. As our composite aggregates have a lower number of cells in comparison to their study, we do not expect hypoxic conditions to affect the viability of the prepared aggregates.
Conclusion
In this study, we created composite cell aggregates consisting of co-cultured MIN6 cells and HUVECs, which mimic the beta cell relation with ECs in native islets. By addition of HUVECs, we achieved improved MIN6 aggregate functionality in terms of glucose-stimulated insulin secretion. Importantly, these composite aggregates maintain their function after encapsulation within our membrane-based sealed device and show better insulin release than encapsulated pure MIN6 aggregates, indicating that providing beta cells connection with ECs within an encapsulation device is beneficial in terms of improved cell functionality and better device performance.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was financially supported by Juvenile Diabetes Research Foundation (Grant title: New islet encapsulation method for ideal mass transport and immune-protection. Grant key: 17-2013-303). In addition, this work was partially funded by Deutscher Akademischer Austauschdienst DAAD grant to Y. Brito Barrera.
References
- 1. Iacovacci V, Ricotti L, Menciassi A, et al. The bioartificial pancreas (BAP): biological, chemical and engineering challenges. Biochem Pharmacol 2016; 100: 12–27. [DOI] [PubMed] [Google Scholar]
- 2. Barkai U, Rotem A, de Vos P. Survival of encapsulated islets: more than a membrane story. World J Transplant 2016; 6(1): 69–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hogan MF, Hull RL. The islet endothelial cell: a novel contributor to beta cell secretory dysfunction in diabetes. Diabetologia 2017; 60(6): 952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Menger MD, Yamauchi J, Vollmar B. Revascularization and microcirculation of freely grafted islets of Langerhans. World J Surg 2001; 25(4): 509–115. [DOI] [PubMed] [Google Scholar]
- 5. Bonner-Weir S. Morphological evidence for pancreatic polarity of beta-cell within islets of Langerhans. Diabetes 1988; 37(5): 616–621. [DOI] [PubMed] [Google Scholar]
- 6. Johansson U, Olsson A, Gabrielsson S, et al. Inflammatory mediators expressed in human islets of Langerhans: implications for islet transplantation. Biochem Biophys Res Commun 2003; 308(3): 474–149. [DOI] [PubMed] [Google Scholar]
- 7. Brissova M, Shostak A, Shiota M, et al. Pancreatic islet production of vascular endothelial growth factor-A is essential for islet vascularization, revascularization, and function. Diabetes 2006; 55(11): 2974–1285. [DOI] [PubMed] [Google Scholar]
- 8. Olsson R, Carlsson PO. The pancreatic islet endothelial cell: emerging roles in islet function and disease. Int J Biochem Cell Biol 2006; 38(5–6): 710–174. [DOI] [PubMed] [Google Scholar]
- 9. Peiris H, Bonder CS, Coates PTH, et al. The β-cell/EC axis: how do islet cells talk to each other? Diabetes 2014; 63(1): 3–11. [DOI] [PubMed] [Google Scholar]
- 10. Johansson A, Lau J, Sandberg M, et al. Endothelial cell signalling supports pancreatic beta cell function in the rat. Diabetologia 2009; 52(11): 2385–1294. [DOI] [PubMed] [Google Scholar]
- 11. Hogan MF, Liu AW, Peters MJ, et al. Markers of islet endothelial dysfunction occur in male B6.BKS(D)-Leprdb/J mice and may contribute to reduced insulin release. Endocrinology 2017; 158(2): 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nikolova G, Jabs N, Konstantinova I, et al. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell 2006; 10(3): 397–405. [DOI] [PubMed] [Google Scholar]
- 13. Jiang FX, Naselli G, Harrison LC. Distinct distribution of laminin and its integrin receptors in the pancreas. J Histochem Cytochem 2002; 50(12): 1625–1632. [DOI] [PubMed] [Google Scholar]
- 14. Sabra G, Vermette P. A 3D cell culture system: separation distance between INS-1 cell and endothelial cell monolayers co-cultured in fibrin influences INS-1 cells insulin secretion. Biotechnol Bioeng 2013; 110(2): 619–127. [DOI] [PubMed] [Google Scholar]
- 15. Skrzypek K, Groot Nibbelink M, van Lente J, et al. Pancreatic islet macroencapsulation using microwell porous membranes. Sci Rep 2017; 7(1): 9186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Skelin M, Rupnik M, Cencic A. Pancreatic beta cell lines and their applications in diabetes mellitus research. ALTEX 2010; 27(2): 105–113. [DOI] [PubMed] [Google Scholar]
- 17. Schechner JS, Nath AK, Zheng L, et al. In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc Natl Acad Sci U S A 2000; 97(16): 9191–9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rivron NC, Le GS, van den BA, et al. Tissue deformation spatially modulates VEGF signaling and angiogenesis. Proc Natl Acad Sci U S A 2012; 109(18): 6886–6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hilderink J, Spijker S, Carlotti F, et al. Controlled aggregation of primary human pancreatic islet cells leads to glucose-responsive pseudoislets comparable to native islets. J Cell Mol Med 2015; 19(8): 1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buitinga M, Janeczek Portalska K, Cornelissen DJ, et al. Coculturing human islets with proangiogenic support cells to improve islet revascularization at the subcutaneous transplantation site. Tissue Eng Part A 2016; 22(3–4): 375–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Groot Nibbelink M, Marchioli G, Moroni L, et al. A protocol to enhance INS1E and MIN6 functionality—the use of theophylline. International Journal of Molecular Sciences 2016; 17(9): 1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Laschke MW, Menger MD. Prevascularization in tissue engineering: current concepts and future directions. Biotechnol Adv 2016; 34(2): 112–121. [DOI] [PubMed] [Google Scholar]
- 23. Kaufman-Francis K, Koffler J, Weinberg N, et al. Engineered vascular beds provide key signals to pancreatic hormone-producing cells. PLoS ONE 2012; 7(7): e40741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wojtusciszyn A, Armanet M, Morel P, et al. Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts. Diabetologia 2008; 51(10): 1843–1152. [DOI] [PubMed] [Google Scholar]
- 25. Brereton HC, Carvell MJ, Asare-Anane H, et al. Homotypic cell contact enhances insulin but not glucagon secretion. Biochem Biophys Res Commun 2006; 344(3): 995–1000. [DOI] [PubMed] [Google Scholar]
- 26. Kilimnik G, Jo J, Periwal V, et al. Quantification of islet size and architecture. Islets 2012; 4(2): 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spelios MG, Kenna LA, Wall B, et al. In vitro formation of β cell pseudoislets using islet-derived endothelial cells. PLoS ONE 2013; 8(8): e72260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paget MB, Murray HE, Bailey CJ, et al. Rotational co-culture of clonal β-cells with endothelial cells: effect of PPAR-gamma agonism in vitro on insulin and VEGF secretion. Diabetes Obes Metab 2011; 13(7): 662–168. [DOI] [PubMed] [Google Scholar]
- 29. Barkai U, Weir GC, Colton CK, et al. Enhanced oxygen supply improves islet viability in a new bioartificial pancreas. Cell Transplant 2013; 22(8): 1463–1176. [DOI] [PubMed] [Google Scholar]
- 30. Elliott RB, Escobar L, Calafiore R, et al. Transplantation of micro- and macroencapsulated piglet islets into mice and monkeys. Transplant Proc 2005; 37(1): 466–469. [DOI] [PubMed] [Google Scholar]
- 31. Loudovaris T, Jacobs S, Young S, et al. Correction of diabetic nod mice with insulinomas implanted within Baxter immunoisolation devices. J Mol Med 1999; 77(1): 219–222. [DOI] [PubMed] [Google Scholar]
- 32. Kusamori K, Nishikawa M, Mizuno N, et al. Increased insulin secretion from insulin-secreting cells by construction of mixed multicellular spheroids. Pharm Res 2016; 33(1): 247–256. [DOI] [PubMed] [Google Scholar]
- 33. Skiles ML, Sahai S, Blanchette JO. Tracking hypoxic signaling within encapsulated cell aggregates. J Vis Exp 2011; 58: 3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krishnan R, Ko D. Strategies to combat hypoxia in encapsulated islet transplantation. Surg: Curr Res 2016; 6: 259. [Google Scholar]
- 35. Ichihara Y, Utoh R, Yamada M, et al. Size effect of engineered islets prepared using microfabricated wells on islet cell function and arrangement. Heliyon 2016; 2(6): e00129. [DOI] [PMC free article] [PubMed] [Google Scholar]