Abstract
Objective:
The present study was aimed to establish a threshold value for cardiac troponin I (cTnI) for nonacute coronary syndrome (ACS) participants from the local population and also to determine the importance of serial time point estimation of cTnI in acute myocardial infarction (AMI), non-ST-elevated MI (NSTEMI), and unstable angina cases.
Methods:
The present study included 194 cases, admitted in ICCU with the complaint of anginal pain; 31 were diagnosed with AMI with typical electrocardiography (ECG) changes; whereas, 48 cases were diagnosed with NSTEMI. The latter group of cases was selected for the time point study of cTnI release at 0–4 h, 6–12 h, 72 h, and 144 h of admission. cTnI levels were assessed using the Abbott ARCHITECT i1000SR system.
Results:
ACS was clinically ruled out in 98 cases, and cTnI level for them was used to decide cTnI threshold for the non-ACS group. cTnI level was checked in 17 cases of unstable angina. The threshold value of cTnI for non-ACS participants was 0.1 ng/ml and can be considered as cut-off value for the regional population. The data suggested that the peak of cTnI levels in most of the AMI cases reached during 6–12 h. The cTnI levels were lower than 0.1 ng/ml, and no significant change in ECG was noticed in 17 cases of unstable angina.
Conclusion:
The present study suggested that the repeat of cTnI assay after 4–6 h of admission is required if the initial value is <3 ng/ml.
Keywords: Acute coronary syndrome, acute myocardial infarction, cardiac troponin I, non-ST-elevated MI, unstable angina
INTRODUCTION
”Cardiac troponin I” (cTnI) is a highly sensitive and specific myocardial injury marker. cTnI is very useful for evaluating ischemic chest pain wherein biochemical data are not confirmative. Electrocardiography (ECG) changes may not be obvious in all cases of acute myocardial infarction (AMI). Hence, the Joint European Cardiology/American College of Cardiology Committee and the National Academy of Clinical Biochemistry proposed cTnI and cTnT as the appropriate markers for a definitive AMI diagnosis.[1,2] The higher cardiac specificity of cTnI confers superiority over cTnT.[3,4] Clinical study results have demonstrated that elevated serum levels of cTnI are detectable within 4–6 h after the onset of chest pain, reach peak concentration in approximately 12 h and remain elevated for 3–10 days following AMI.[5] Since patients may present with the rise in cTnI at varying times following the onset of symptoms, it is necessary to obtain serial measurements for optimal diagnostic accuracy.[6]
The researchers also recommend that each institution establishes their own release patterns of cardiac biomarkers.[7] However, there have been no established data on cTnI values of the Indian population. Further, published data on serial sampling of cTnI for the Indian population are lacking. Besides, there are no published data for the threshold values of cTnI in nonacute coronary syndrome (ACS) cases in India. Although cTnI is now accepted as a standard marker for definitive AMI diagnosis, creatinine phosphokinase (CPK) and CPK-MB were not included for comparison.[1,2] Further, the aim of the study is to establish a threshold value for cTnI for non-ACS participants from the local population and also to determine the importance of serial time point estimation of cTnI in AMI, non-ST-elevated MI (NSTEMI), and unstable angina cases.
METHODS
The study was a prospective study from January 2014 to June 2015 at Choithram Hospital and Research Centre, Indore. One hundred and ninety-four cases were referred to the center with the complaint of anginal pain. The age group ranged from 30 to 70 years, and 68% of them were male. Thirty-one of them had ST elevation in ECG with ischemic symptoms and diagnosed with acute MI. These cases were not included for cTnI levels since cardiac marker was not required for the diagnosis. Ninety-eight of them had normal ECG and follow-up observation for 3 days did not show any suspicion of ACS and were considered as non-ACS group, and the basal level of cTnI for these participants was used to establish the normal threshold value for population.
Forty-eight cases of AMI admitted to our ICCU were included in the cTnI study. These cases were in agreement with the recent definition of MI based on the guideline of American College of Cardiology (ACC) and European Society of Cardiology (ESC)[8] with a cTnI value above 0.1 ng/ml but had not shown characteristic ST-segment elevation at the time of admission. Two cases had cTnI value <0.03 ng/ml at the time of admission but had Q-wave change without ST elevation and after 6 h had shown elevated cTnI level. Blood samples were collected at admission, 6–12 h, 72 h, and 144 h of admission for cTnI estimation.
The cTnI levels were measured using the ARCHITECT i1000SR Kit from MS Abbott (USA) on ARCHITECT i1000SR immunoanalyzer. The ARCHITECT i1000SR is a microparticle enzyme immunoassay (MEIA) and highly sensitive for the quantitative determination of cTnI in human serum or plasma on the ARCHITECT i1000SR System.[9,10]
Of 194 cases, 17 had anginal pain, but their cTnI levels were <0.1 ng/ml; no ECG changes found out at the time of admission; and the cTnI level was reassessed after 4–6 h.
RESULTS
The mean value of cTnI level in 98 non-ACS participants was 0.034 with the standard deviation (SD) value of ± 0.022. Mean + 3 SD value was 0.1 ng/ml and was accepted as threshold value of cTnI in non-ACS participants.
The rest of the 96 cases were included in this study, as ACS cases. Of these 96 cases, 26 underwent angiographic assessment. The angiographic findings showed a triple vessel block in 8 patients, a double vessel in 13 patients, and 5 patients had a single vessel block. Of these 8 triple vessel blocked patients, 5 underwent angioplasty.
Data for 34 patients of 48 patients are displayed in Figure 1. Initial values ≤0.03 ng/ml were seen in two cases who had severe angina chest pain and Q-wave abnormalities. The subsequent value of cTnI was quite high for these two cases. The peak value of the cTnI was noted for 11 cases in the first 4 h of admission while in 20 cases, the peak value reached during 6–12 h, and only 2 cases had peak level at 72 h of admission. Fourteen cases had peak level not >2.4 ng/ml [not shown in Figure 1].
Figure 1.
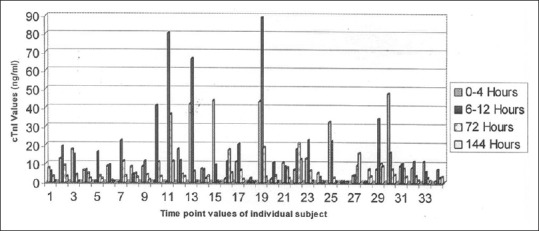
Individual data presentation of cardiac troponin I in different time (n = 34)
The mean values for cTnI at various time points are shown in the bar diagram in Figure 2. The mean value was raised maximum at 6–12 h and dropped subsequently. At 6 days, the cTnI levels dropped to mean of 3.31 ng/ml.
Figure 2.
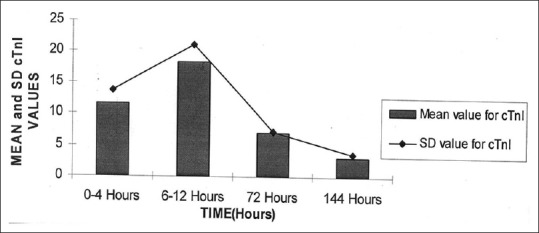
Mean value of cardiac troponin I in different time (n = 34)
In all 17 unstable angina cases, cTnI values did not exceed the basal level during admission as well as in 4–6 h reassessment. There were no ECG changes seen, but the response to the clinical treatment observed.
DISCUSSION
The threshold value of 0.1 ng/ml in the present study was based on the mean +3 SD for 98 non-ACS participants. The threshold appears to be more meaningful than the values in the normal nonsymptomatic patients. The value of ≥0.25 ng/ml is accepted as significant by some laboratories,[9,10] but the threshold based on local non-ACS participants was 0.1 ng/ml and has been suggested by Misson and Calzolari.[11]
The serial time point study of cTnI levels among the ACS patients is unique in India. In the present time point study, 11 of 34 cases of AMI showed a peak value of cTnI in the first 4 h, whereas the majority (20/34) had shown the peak at 6–12 h, and only two cases had a late peak of cTnI value, at 72 h. The gross findings are similar to those referred to other studies.[12] The present study observations were noted that the level of cTnI value in the first 4 h may not be high and is impo'rtantly pointed out the need of repeat of cTnI evaluation during 6–12 h. It is not a very regular practice till now in India, to carry out a repeat estimation of cTnI, after a negative serum cTnI value during hospital admission and may miss some cases of ACS.
The levels of cTnI for unstable angina cases were often lower than 0.1 ng/ml and did not show a notable rise in 6–12 h. Musso et al.[13] described only 3/32 cases of unstable angina to have elevated cTnI while Janorkar et al.[14] observed cTnI level >0.6 ng/ml in 43% of the cases. A general consensus appears that the serum cTnI values are much lower in AMI cases and less frequently elevated. However, the elevated cTnI is associated with severity of coronary disease and increased risk of subsequent cardiac events.[12,15]
The method of cTnI needs to be sensitive to detect 0.05 ng/ml, and the time for reporting should be <1 h for faster management of the ACS. For conveniences and rapidity, immunochromatographic methods are introduced by several diagnostic firms. It needed to be mentioned that almost dozen diagnostic kits available in Indian markets were checked at our end, and none of the immunochromatographic method gave consistent detection of the cTnI levels ≤3 ng/ml (unpublished observation). The criticism was for the rapid detection by qualitative/semiqualitative tests and not for quantitative methods. The detection of the methodology Abbott ARCHITECT i1000SR Troponin I assay is an MEIA for the quantitative determination in human serum, or plasma on the ARCHITECT i1000SR immunoanalyzer was used in the present study.[16] The recent studies recommend that serial time point patient studies are most useful diagnostically.[17]
CONCLUSION
The threshold value for cTnI derived in the present study is 0.1 ng/ml, and the peak of the cTnI level in AMI cases is usually noted in the first 12 h after the onset of AMI and elevated levels above the threshold persisted for more than 144 h. It was observed in the present study that the cTnI level is often lower than 2.5 ng/ml for NSTEMI. If the initial cTnI level is less 3 ng/ml, a repeat cTnI estimation is suggested during 6–12 h after the onset of AMI.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined – A consensus document of the joint european society of cardiology/American college of cardiology committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–69. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 2.Wu AH, Apple FS, Gibler WB, Jesse RL, Warshaw MM, Valdes R, Jr, et al. National academy of clinical biochemistry standards of laboratory practice: Recommendations for the use of cardiac markers in coronary artery diseases. Clin Chem. 1999;45:1104–21. [PubMed] [Google Scholar]
- 3.Adams JE. Utility of cardiac troponins in patients with suspected cardiac trauma or after cardiac surgery. Clin Lab Med. 1997;17:613–23. [PubMed] [Google Scholar]
- 4.Apple FS, Sharkey SW, Hoeft P, Skeate R, Voss E, Dahlmeier BA, et al. Prognostic value of serum cardiac troponin I and T in chronic dialysis patients: A 1-year outcomes study. Am J Kidney Dis. 1997;29:399–403. doi: 10.1016/s0272-6386(97)90201-8. [DOI] [PubMed] [Google Scholar]
- 5.Mair J, Wagner I, Puschendorf B, Mair P, Lechleitner P, Dienstl F, et al. Cardiac troponin I to diagnose myocardial injury. Lancet. 1993;341:838–9. doi: 10.1016/0140-6736(93)90622-n. [DOI] [PubMed] [Google Scholar]
- 6.Adams JE, 3rd, Miracle VA. Cardiac biomarkers: Past, present, and future. Am J Crit Care. 1998;7:418–23. [PubMed] [Google Scholar]
- 7.Christenson RH. National Academy of Clinical Biochemistry. National academy of clinical biochemistry laboratory medicine practice guidelines for utilization of biochemical markers in acute coronary syndromes and heart failure. Clin Chem. 2007;53:545–6. doi: 10.1373/clinchem.2006.079749. [DOI] [PubMed] [Google Scholar]
- 8.Schreiber D, Brenner BE. Cardiac Markers. Heart.org (Medscape) 2017. Jan, Available from: https://emedicine.medscape.com/article/811905-overview .
- 9.National Institute of Health and Care Excellance. Diagnostic adoption support: Myocardial infarction (acute): Early Rule Out Using High-Sensitivity Troponin Tests (Elecsys Troponin T high-sensitive, ARCHITECT STAT High Sensitive Troponin-I and AccuTnI+3 assays) Health Technologies Adoption Programme. 2017. Available from: https://www.nice.org.uk/guidance/dg15/evidence/review-decision-april-2018-pdf-4841038189 .
- 10.Fonarow GC, Middlekauff H, Tillisch J, Demer L, Morgan M, Hoffman J, et al. Troponin I Diagnostic Module. 2000 [Google Scholar]
- 11.Misson E, Calzolari C. Elevated cardiac troponin I in some patients with severe congestive heart failure [abstract] J Mol Cell Cardio. 1995;27:A405. [Google Scholar]
- 12.Hollander JE. The Future of Cardiac Biomarkers, New Concepts and Emerging Technology for Emergency Physicians. Philadelphia, Pennsylvania: Emergency Medicine Cardiac Research and Education Group; 2005. Department of Emergency Medicine Hospital of the University of Pennsylvania; p. 4. [Google Scholar]
- 13.Musso P, Vernocchi A, Pasquino M, Crippa A, Ottello B, Panteghini M, et al. Cardiac troponin I and T in unstable angina: Incidence, correlation, kinetics of release and prognostic value. G Ital Cardiol. 1996;26:1013–23. [PubMed] [Google Scholar]
- 14.Janorkar S, Koning R, Eltchaninoff H, Andres H, Lavoinne A, Cribier A, et al. Relation between serum cardiac troponin I values and severity of clinical, electrocardiographic and quantitative angiographic features in unstable angina. Indian Heart J. 1999;51:31–4. [PubMed] [Google Scholar]
- 15.Benamer H, Steg PG, Benessiano J, Vicaut E, Gaultier CJ, Aubry P, et al. Elevated cardiac troponin I predicts a high-risk angiographic anatomy of the culprit lesion in unstable angina. Am Heart J. 1999;137:815–20. doi: 10.1016/s0002-8703(99)70404-7. [DOI] [PubMed] [Google Scholar]
- 16.Saadeddin SM, Habbab MA, Siddieg HH, Al Seeni MN, Tahery AB, Dafterdar RM, et al. Evaluation of 6 cardiac troponin assays in patients with acute coronary syndrome. Saudi Med J. 2003;24:1092–7. [PubMed] [Google Scholar]
- 17.Cembrowski GS, Cembrowski AR, Kunst AN, Holmes DT, Apple FS. Short term variation important in evaluating goodness of troponin assays for diagnosing myocardial damage. Clin Chem. 2014;60:S223. [Google Scholar]


