Abstract
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is an x-linked recessive genetic disorder with mutation in the G6PD gene. Defect in the enzyme G6PD causes red blood cells (RBCs) to breakdown prematurely causing hemolytic anemia. Hemolytic anemia is also a known hematological complication associated with viral hepatitis. In such patients, hemolysis may be more severe if there is any secondary injury to RBC in the form of membrane defect, oxidative stress, or enzyme deficiency like in G6PD deficiency. Here, we present a case of an adult, not previously diagnosed with G6PD deficiency, who presented with viral hepatitis, severe hemolysis, and multiorgan failure.
Keywords: Glucose-6-phosphate dehydrogenase deficiency, hemolytic anemia, hemoperfusion, hepatitis A, methemoglobinemia, multiorgan failure
INTRODUCTION
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is an x-linked recessive genetic disorder with mutation in the G6PD gene. Defect in the enzyme G6PD causes red blood cells (RBCs) to breakdown prematurely causing hemolytic anemia. Mostly, patients with G6PD deficiency remain asymptomatic, but following any trigger, symptoms of hemolysis may develop. It is more prevalent in the African, Asian, Mediterranean, and middle-east regions.[1,2,3,4,5]
Hemolytic anemia is also a known hematological complication associated with viral hepatitis. In such patients, hemolysis may be more severe if there is any secondary injury to RBC in the form of membrane defect, oxidative stress, or enzyme deficiency like in patients with G6PD deficiency.[6,7,8] Here, we present a case, not previously diagnosed with G6PD deficiency, who presented with viral hepatitis, severe hemolysis, and multiorgan failure.
CASE REPORT
A 39-year-old male presented with a history of high-grade fever of 15 days and yellowish discoloration of eyes and urine of 10-day duration. Associated features included dull aching pain in the right hypochondrium and epigastric region, dyspnea at rest, decreased urine output, and vomiting. He also had a history of use of analgesics such as paracetamol (up to 3 g/day), tramadol, and diclofenac for his back pain for the past 15 days.
On examination, the patient was drowsy, and had a heart rate of 100/min, blood pressure of 136/82 mmHg, respiratory rate of 36/min, and was maintaining saturation SpO2 of 88%–92% on 15 L of oxygen through nonrebreathing mask. Icterus was present, but there was no flapping tremor, pedal edema, or lymphadenopathy. The patient was anuria. Basic investigations are shown in Table 1.
Table 1.
Basic laboratory investigations during the Intensive Care Unit stay
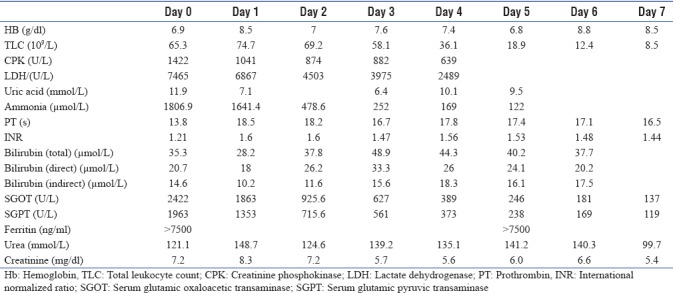
Over the next few hours, the patient continued to deteriorate with worsening of sensorium and hypotension with increasing vasopressor requirement and need for noninvasive ventilation. One unit packed RBCs were transfused, and hemodialysis plus hemoperfusion was done using ammonia (Jaffron HA330-II) and bilirubin (Jaffron BS330) filters in view of worsening sensorium, life-threatening multiorgan failure, and high bilirubin and ammonia levels [Figures 1 and 2].
Figure 1.

Serial ammonia, serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase levels, and effect of ammonia filters
Figure 2.

Serial bilirubin and prothrombin levels and effect of bilirubin filter
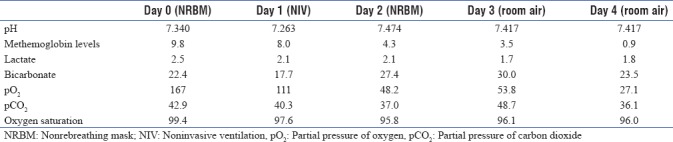
Arterial blood gases showed disparity between SpO2 (86%) and SaO2 (99.9%), and hence, Co-oximetry was done which showed raised levels of methemoglobin (9.8%) [Table 2]. High-performance liquid chromatography G6PD levels were sent which came out to be very low 2.5 u/ghb (normal range: 7–20 u/ghb).
Table 2.
Arterial blood gases parameters over the first 4 days
Workup for fever was done, including for tropical fevers, which was negative, but hepatitis A virus antigen was positive. Peripheral blood smear showed microspherocytes and fragmented RBCs. Hence, the diagnosis of acute liver failure, secondary to hepatitis A infection, with G6PD deficiency, acute severe hemolysis, and multiorgan dysfunction was made. The possibility of need for liver transplantation was also assessed. However, after 48 h of aggressive management, the patient started to show clinical recovery with the improvement in sensorium, normalization of blood pressure, and reduced oxygen requirement. Liver and kidney function tests continued to remain deranged, and hemodialysis was continued. Multiple blood transfusions were given, as and when indicated. He was shifted out of the intensive care unit after day 6 and was discharged from the hospital after 11 days of hospitalization. However, he remained anuria and required intermittent hemodialysis.
DISCUSSION
According to the World Health Organization report,[9] the prevalence of G6PD deficiency in India ranges between 0% and 10% which is population specific and is found to be higher among tribals.[10] In the present case, severe hemolysis was induced by hepatitis A infection, with a background G6PD deficiency. This might have been further complicated by prolonged and chronic use of paracetamol leading to severe liver dysfunction. Early aggressive care, including the use of hemodialysis and hemoperfusion filters, might have improved the outcome in our case.
Infection or exposure to certain drugs and chemicals may precipitate severe hemolytic anemia in G6PD deficiency patients. Infections such as viral hepatitis, typhoid fever, and pneumonia, as well as upper respiratory and gastrointestinal infections are well-known triggers of hemolytic episodes in these patients.[11] As hepatitis A is generally a self-limiting disease, it may get complicated only if there is a secondary stress on RBCs. Severe hemolysis may be precipitated in patients with acute hepatitis A, especially in the background of G6PD deficiency. The initial clinical severity of hepatitis may also be worse in patients with underlying G6PD deficiency.[12] However, only a few such cases have been described in India.[13,14]
Our patient presented with multiorgan failure and hepatic encephalopathy with very high ammonia levels. Previous reports have suggested that patients with hepatic coma have worse clinical outcomes.[14,15] Hence, it may seem prudent to treat these patients aggressively. Extracorporeal therapy, other than hemodialysis, has been tried in such cases, with good results.[16]
Plasmapheresis has also been used previously in patients with hepatitis A infection with G6PD deficiency in the background of autoimmune hemolysis, with good results.[17] However, ours is the first report of using hemodialysis with hemoperfusion in such patients.
Liver support system using hemoperfusion cartridge was applied in our case in view of severe hyperbilirubinemia, multiorgan failure, and worsening sensorium. Nonbioartificial liver support may be indicated in patients with acute liver failure as these may have good short-term efficacy and reduce the level of hepatic encephalopathy, reduce serum bilirubin levels, improve prothrombin activity or international normalized ratio, and may also improve other laboratory parameters, including blood ammonia and endotoxins.[18,19] In our case too, it helped in reducing bilirubin and ammonia levels and tide over the acute crisis.
Our case is unique in several aspects. First, our patient was an adult without any previous history of hemolysis secondary to G6PD deficiency. Then, our patient presented with severe hemolysis and multiorgan failure. His clinical course was further complicated by the development of methemoglobinemia. In the end, early aggressive care, with the use of hemoperfusion filters, might have helped in achieving favorable outcome in our patient.
CONCLUSION
Sometimes, even generally mild diseases such as hepatitis A may get complicated in the presence of underlying occult diseases such as G6PD deficiency, and patients may present with multiorgan failure and may be associated with high mortality rates. Hence, it may seem prudent to look for any such previously undiagnosed medical conditions if the patient presents with any unexpectedly severe clinical symptoms.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet. 2008;371:64–74. doi: 10.1016/S0140-6736(08)60073-2. [DOI] [PubMed] [Google Scholar]
- 2.Frank JE. Diagnosis and management of G6PD deficiency. Am Fam Physician. 2005;72:1277–82. [PubMed] [Google Scholar]
- 3.Luzzatto L, Nannelli C, Notaro R. Glucose-6-phosphate dehydrogenase deficiency. Hematol Oncol Clin North Am. 2016;30:373–93. doi: 10.1016/j.hoc.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Manganelli G, Masullo U, Passarelli S, Filosa S. Glucose-6-phosphate dehydrogenase deficiency: Disadvantages and possible benefits. Cardiovasc Hematol Disord Drug Targets. 2013;13:73–82. doi: 10.2174/1871529x11313010008. [DOI] [PubMed] [Google Scholar]
- 5.Nkhoma ET, Poole C, Vannappagari V, Hall SA, Beutler E. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: A systematic review and meta-analysis. Blood Cells Mol Dis. 2009;42:267–78. doi: 10.1016/j.bcmd.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Chan TK, Todd D. Haemolysis complicating viral hepatitis in patients with glucose-6-phosphate dehydrogenase deficiency. Br Med J. 1975;1:131–3. doi: 10.1136/bmj.1.5950.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salen G, Goldstein F, Haurani F, Wirts CW. Acute hemolytic anemia complicating viral hepatitis in patients with glucose-6-phosphate dehydrogenase deficiency. Ann Intern Med. 1966;65:1210–20. doi: 10.7326/0003-4819-65-6-1210. [DOI] [PubMed] [Google Scholar]
- 8.Hargrove MD. Marked increase in serum bilirubin in sickle anemia: A report of six cases. Am J Dig Dis. 1970;15:437. doi: 10.1007/BF02283871. [DOI] [PubMed] [Google Scholar]
- 9.WHO Working Group. Glucose-6-phosphate dehydrogenase deficiency. WHO working group. Bull World Health Organ. 1989;67:601–11. [PMC free article] [PubMed] [Google Scholar]
- 10.Tripathy V, Reddy BM. Present status of understanding on the G6PD deficiency and natural selection. J Postgrad Med. 2007;53:193–202. doi: 10.4103/0022-3859.33867. [DOI] [PubMed] [Google Scholar]
- 11.Ho L, John RM. Understanding and managing glucose-6-phosphate dehydrogenase deficiency. J Nurse Pract. 2015;11:443–50. [Google Scholar]
- 12.Gotsman I, Muszkat M. Glucose-6-phosphate dehydrogenase deficiency is associated with increased initial clinical severity of acute viral hepatitis A. J Gastroenterol Hepatol. 2001;16:1239–43. doi: 10.1046/j.1440-1746.2001.02611.x. [DOI] [PubMed] [Google Scholar]
- 13.Charan VD, Desai N, Choudhury VP. Hyperbilirubinemia following hepatitis A in a patient with G6pD deficiency. Indian J Gastroenterol. 1993;12:99. [PubMed] [Google Scholar]
- 14.Agarwal RK, Moudgil A, Kishore K, Srivastava RN, Tandon RK. Acute viral hepatitis, intravascular haemolysis, severe hyperbilirubinaemia and renal failure in glucose-6-phosphate dehydrogenase deficient patients. Postgrad Med J. 1985;61:971–5. doi: 10.1136/pgmj.61.721.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M, Wang Z, Wang Y, Du C, Li S, Shi Z, et al. Part of plasmapheresis with plasma filtration adsorption combined with continuous hemodiafiltration in the treatment of severe acute liver failure. Exp Ther Med. 2016;12:2582–4. doi: 10.3892/etm.2016.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huo TI, Wu JC, Chiu CF, Lee SD. Severe hyperbilirubinemia due to acute hepatitis A superimposed on a chronic hepatitis B carrier with glucose-6-phosphate dehydrogenase deficiency. Am J Gastroenterol. 1996;91:158–9. [PubMed] [Google Scholar]
- 17.Hosnut FO, Ozcay F, Bayrakci US, Avci Z, Ozbek N. Etiology of hemolysis in two patients with hepatitis A infection: Glucose-6-phosphate dehydrogenase deficiency or autoimmune hemolytic anemia. Eur J Pediatr. 2008;167:1435–9. doi: 10.1007/s00431-008-0694-1. [DOI] [PubMed] [Google Scholar]
- 18.Lanjuan L. Liver Failure and Artificial Liver Group; Society of Infectious Diseases; Chinese Medical Association. Guidelines for non-bioartificial liver support system in treatment of liver failure: 2016 edition. Chin J Clin Infect Dis. 2016;9:97–109. [Google Scholar]
- 19.Li M, Sun J, Li J, Shi Z, Xu J, Lu B, et al. Clinical observation on the treatment of acute liver failure by combined non-biological artificial liver. Exp Ther Med. 2016;12:3873–6. doi: 10.3892/etm.2016.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]


