Abstract
Background:
Excessive extravascular lung water (EVLW) is associated with increased morbidity and mortality. We compared three lung-ultrasound (L-US) techniques against the reference-standard transpulmonary thermodilution (TPTD) technique to access EVLW.
Materials and Methods:
This was a prospective, single-blind, cross-sectional study. Forty-four septic patients were enrolled. EVLW index was measured by the TPTD method, and an index of ≥10 mL/kg was considered diagnostic of pulmonary edema. EVLW index was then compared to three established bedside L-US protocols that evaluate sonographic B-lines: (1) a 28-zone protocol (total B-line score [TBS]) (2) a scanning 8-region examination, and (3) a 4-point examination.
Results:
Eighty-nine comparisons were obtained. A statistically significant positive correlation was found between L-US TBS and an EVLW index ≥10 mL/kg (r = 0.668,P < 0.001). The 28-zone protocol score ≥39 has a sensitivity of 81.6% and a specificity of 76.5% to define EVLW index ≥10 mL/kg. In contrast, the positive 4-point examination and scanning 8-regions showed low sensitivity (23.7% and 50.0%, respectively) but high specificity (96.1% and 88.2%, respectively). Ten patients with a total of 21 comparisons met criteria for acute respiratory distress syndrome (ARDS). In this subgroup, only the TBS had statistically significant positive correlation to EVLW (r = 0.488,P = 0.025).
Conclusion:
L-US is feasible in patients with severe sepsis. In addition, L-US 28-zone protocol demonstrated high specificity and better sensitivity than abbreviated 4- and 8-zone protocols. In ARDS, the L-US 28-zone protocol was more accurate than the 4- and 8-zone protocols in predicting EVLW. Consideration of limitations of the latter protocols may prevent clinicians from reaching premature conclusions regarding the prediction of EVLW.
Trial Registration:
ISRCTN11419081. Registered 4 February 2015 retrospectively.
Keywords: Acute interstitial syndrome, B-lines, extravascular lung water, lung ultrasound, transpulmonary thermodilution
INTRODUCTION
Following sepsis, defined as life-threatening organ dysfunction caused by a dysregulated host response to infection, fluid resuscitation can be acutely lifesaving.[1] However, excessive extravascular lung water (EVLW) is associated with subsequent organ dysfunction and increased mortality.[2] Accordingly, clinicians need to be able to detect excessive EVLW if they are to achieve clinical balance in order to maximize the likelihood of patient rescue. Several techniques exist, but all have limitations. For example, chest X-ray and physical examination are limited by their inability to detect EVLW during early stages[3] and because of substantial interobserver variability.[4] Transpulmonary thermodilution (TPTD),[5] the current reference standard, detects water accumulated in the interstitium, lymphatics, and alveoli, and an extravascular lung water index (EVLWI) of ≥10 mL/kg suggests pulmonary edema.[6,7] Elevated EVLW by TPTD has been shown to be predictive of 28-day mortality in septic shock patients after initial fluid resuscitation.[8] However, TPTD is costly, requires proprietary equipment, and is invasive. Lung-ultrasound (L-US) offers the putative advantages of being noninvasive, repeatable, portable, and low cost.[9,10,11,12] However, there are several protocols (see below). To date, there is insufficient data to recommend a single L-US technique, or whether an algorithmic L-US approach could be employed, or whether EVLW determination is feasible in a low-resource setting.
Sonographic evaluation of lung parenchyma for interstitial edema is based on visualizing B-lines. These are discrete, laser-like vertical hyperechoic reverberation artifacts. B-lines arise from the pleura, extend to the bottom of the image without fading, and move synchronously with respiration.[13] Sonographic B-lines are biophysically correlated to EVLW and their number correlates with the degree of pulmonary interstitial edema.[11,14,15] Studies have compared L-US and determination of lung water by chest X-ray,[14,16] chest computed tomography (CT),[17] and pulmonary capillary wedge pressure.[11,18] L-US has also been compared to TPTD in a variety of studies[9,11,18,19] and it demonstrates strong positive correlation with the amount of EVLW. Enghard et al. demonstrated a strong correlation between a 4-zone L-US protocol and EVLW by TPTD in a heterogeneous group of critically ill patients.[9] Volpicelli et al. compared an 8-zone qualitative L-US protocol to EVLW by TPTD and found that patients with an elevated EVLWI (≥10 ml/kg) almost invariably also displayed a B-line-dominant pattern.[18] Both Enghard and Volpicelli included patients with sepsis and septic shock, respectively, but did not perform predefined subgroup analyses. To our knowledge, ours is the first study which compares three common L-US techniques against TPTD and focuses on intensive care unit (ICU) patients with sepsis.[9,11,20] This study would add more information on the sensitivity and specificity of each method of L-US in detecting pulmonary edema. This would aid the intensivists to select the appropriate method for the patients.
MATERIALS AND METHODS
This prospective, single-blind, cross-sectional study of medical ICU sepsis patients compares a TPTD-derived measurement of EVLWI against three L-US techniques. Between May 2014 and November 2015, we enrolled patients admitted to the medical ICU of a tertiary care hospital who met the following criteria for diagnosis of sepsis: as defined by the third international consensus definitions of sepsis and septic shock[21] and requiring fluid boluses and continuous blood pressure monitoring. Due to its prevalence in sepsis patients, acute respiratory distress syndrome (ARDS) was not an exclusion criterion. However, patients with conditions that can lead to inaccurate EVLW measurement by either L-US or TPTD were excluded from the study. These included pulmonary embolism, prior lung resection, interstitial lung diseases, and pleural diseases. For the sample size calculation, we were expecting a population correlation between EVLWI <10 ml/kg and ≥10 ml/kg of around 0.66, a sample size of forty patients generating approximately eighty comparisons between L-US and TPTD will give us approximately 90% power (alpha = 0.05, one sided) to reject the null hypothesis of zero correlation. Ethical approval was obtained from the Institutional Review Board of Royal Thai Army Medical Department (R032 h/56), and consent was obtained from the patients or the patients' next of kin. This study was conducted according to the principles of the Declaration of Helsinki.
L-US examinations (LOGIQ e ultrasound, GE Healthcare, China) were performed using a 2.5–3.5 MHz phased array probe. We utilized this probe because it is commonly used by bedside clinicians. We also turned off tissue harmonics in order to maximize the visualization of lung artifacts. Current evidence-based recommendations for L-US suggest that, although a microconvex probe is preferred, linear and phased array probes are acceptable alternatives with no difference in visualization in the number of B-lines.[11,12] All patients were studied supine and with the US transducer-oriented cephalad. The pleural line was identified (between and deep to two rib shadows) and used as an indicator of the visceral and parietal pleural layers.
The B-line was defined by seven features: a comet-tail artifact, arising from the pleural line, hyperechoic, well defined, spreading out indefinitely, erasing A-lines, and moving in concert with lung sliding when present [Figure 1]. Per rib space, the number of B-lines ranged from 0 to 10, with 10 designated as “white lung” and three or more indicative of subpleural interstitial edema. According to 2012 international L-US recommendations,[10] there are three methods for assessing interstitial syndrome. We performed each of these methods on each patient, each day, and until hemodynamic support was discontinued. As the focus of this study was on quantification and correlation between B-lines and EVLW, we did not record pleural- or other parenchymal-based abnormalities. B-line determination was made with strict adherence to criteria previously mentioned. The absence of an independent reviewer for US images also reflected resource constraints. Instead, one experienced investigator scored all scans.
Figure 1.
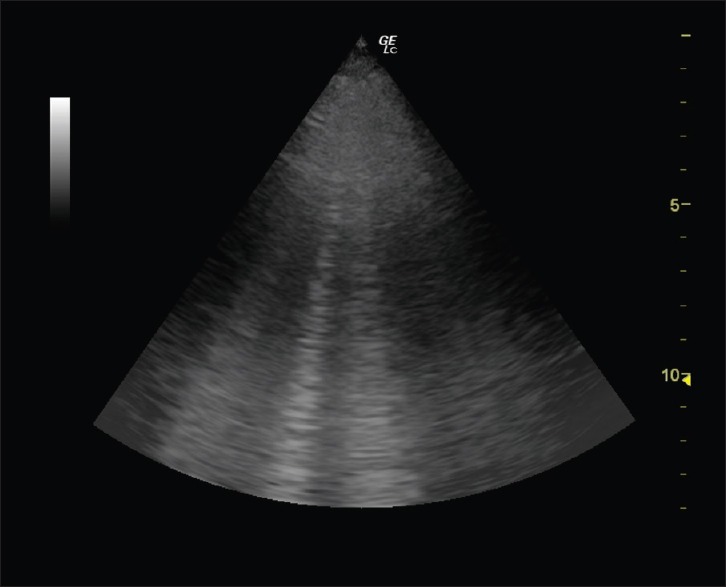
B-lines detected by lung-ultrasound
Total B-line scores
The anterior and lateral chest was scanned on the left from the second to the fourth intercostal space (and on the right side from the second to the fifth), plus along the parasternal, mid-clavicular, anterior-axillary, and mid-axillary lines. The total number of B-lines was counted and recorded at each of the 28 sites, from which a total B-line score (TBS) is determined.[14]
4-point examination
Four sites were scanned, namely, the upper and lower points of each hemithorax. Placement was determined using the clinician's hands: the fifth finger of the upper hand touches the lower border of the patient's clavicle and fingertips touch the midline. The lower hand was applied below, and both thumbs were excluded. The upper point is in the middle of the hand (the root of the middle and ring fingers). The lower point is in the middle of the palm of the lower hand. This method avoids the cardiac window. A scan with at least three artifacts at each point is considered abnormal.[4,22]
Scanning 8-regions
The chest wall is divided into eight areas (four from each hemithorax: two anterior and two lateral), and one scan is obtained from each area.[16] The anterior chest wall is delineated from the sternum to the anterior axillary line and subdivided into upper and lower halves (approximately from the clavicle to the second–third intercostal spaces, and from the third space to the diaphragm). The lateral zone is between the anterior and posterior axillary lines and is subdivided into upper and basal halves. An abnormal pattern has all three of the following features: (1) multiple artifacts per scan (at least three), (2) positive findings in more than one scan per side, and (3) positive findings bilaterally.
Extravascular lung water measurement methods
The EV1000 (VolumeView Set, EV1000, Edwards Lifesciences, Irvine, CA, USA) measures cardiac output (CO), preload, and lung water. It computes CO utilizing an arterial pulse contour analysis algorithm and a TPTD method. In all patients, a 5F thermistor-tipped catheter was placed into the right or left femoral artery, and then connected to the EV1000 System and calibrated. Arterial input impedance to arterial pressure is calculated by determining the area under the systolic portion of the arterial pulse wave. A 15–20 mL bolus of cold 0.9% saline was injected through a central venous catheter, and the thermodilution curve was evaluated with the arterial catheter in the femoral artery. The mean of three consecutive boluses was used to obtain the intrathoracic thermal and intrathoracic blood volume. From the difference of these two parameters, the device could determine the EVLW. Normally, EVLWI is <10 mL/kg, whereas an EVLWI of ≥10 mL/kg suggests pulmonary edema.[6,7] EVLW measurements were done promptly after L-US by the principal investigator. The results of EVLW were blinded to the investigator who did L-US.
Statistical analysis
Data were expressed as mean value ± standard deviation, median (interquartile range), or percentages. The correlations between TBS, 4-point examination, scanning 8-regions, and EVLWI were analyzed by the Spearman's correlation coefficient analysis (r) and coefficient of determination (r2). Receiver operating characteristic (ROC) curves were constructed to evaluate the predictive value of each method of L-US using one-sided P value. The ROC curves were compared using proportion tests. All tests were two sided and P < 0.05 was considered statistically significant. Similar subgroup analysis was done in the patients meeting ARDS criteria according to the Berlin definition.[23] All data were analyzed and graphs were generated with Stata/IC 13.0 (StataCorp, TX, USA).
RESULTS
Eighty-nine comparisons between L-US and TPTD were obtained from 44 patients (28 men and 16 women, 67.3 ± 17.4 years of age). Baseline patient characteristics are displayed in Table 1. The median TBS was 36 (22–67) and the mean EVLWI was 9.1 ± 2.6 ml/kg. Intraobserver variability of TBS was 3.5%.
Table 1.
Baseline characteristics of the study population
There was a statistically significant positive linear correlation between TBS and EVLWI (r = 0.668, P < 0.001) [Figure 2], 4-point examination, and EVLWI (r = 0.558, P < 0.001) and also between scanning 8-regions and EVLWI (r = 0.640, P < 0.001). The coefficient of determination between TBS, 4-point examination, scanning 8-regions, and EVLWI was statistically significant at 0.408 (P < 0.001), 0.254 (P < 0.001), and 0.396 (P < 0.001), respectively. ROC curves of each L-US-based method (TBS, 4-point examination, and scanning 8-regions) for the prediction of EVLWI ≥10 ml/kg were 0.844, 0.731, and 0.785, respectively [Figure 3]. A one-sided P value of the ROC curve comparison between TBS and 4-point examination was 0.008, between TBS and scanning 8-regions was 0.111, and between 4-point examination and scanning 8-regions was 0.087. For the TBS US method, using ≥39 as cutoff point had a sensitivity of 81.6% and a specificity of 76.5% for predicting EVLWI ≥10 ml/kg. The presence of ≥3 B-lines in all 4-point examination points and any two regions on each side of the 8-regions method exhibited sensitivities of 23.7% and 50.0% and specificities of 96.1% and 88.2% in predicting EVLWI ≥10 ml/kg, respectively.
Figure 2.
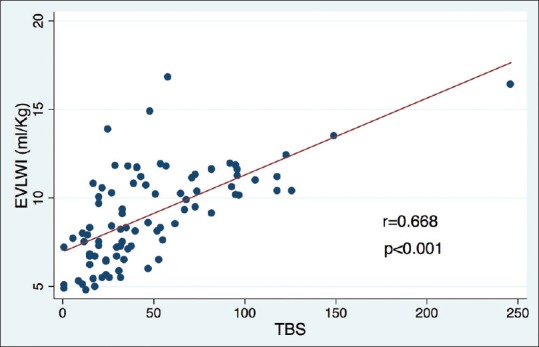
Significant positive linear correlation between total B-line scores and extravascular lung water index determined with the transpulmonary thermodilution technique (EV1000 System)
Figure 3.
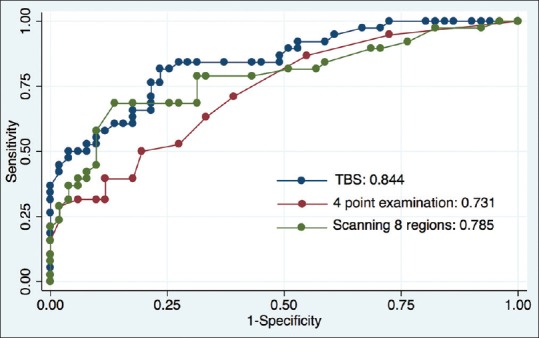
Receiver operating characteristic curves for prediction of extravascular lung water index ≥10 ml/kg. (blue line = total B-line scores; red line = 4-point examination; green line = scanning 8-regions)
Ten patients with a total of 21 comparisons met criteria for ARDS. In this subgroup, the correlation coefficients between TBS, 4-point examination, scanning 8-regions, and EVLWI were 0.488, 0.209, and 0.312, respectively, with only the TBS reaching statistical significance (P = 0.025, P = 0.362, and P = 0.168, respectively).
DISCUSSION
Despite limitations (see below), our study confirms the feasibility and potential clinical utility of L-US in ICU patients with sepsis and in a low-resource setting. We demonstrated a strong correlation between TBS and EVLWI (r = 0.668, P < 0.001). Using a cutoff point of TBS ≥39 yielded a sensitivity of over 80% and a specificity of over 75% for predicting EVLWI ≥10 ml/kg. Our findings duplicate the strong correlation between L-US and EVLW shown in other studies.[9,11,18] Next, the 4-zone and 8-zone scanning methods demonstrated both low sensitivities (23.7% and 50.0%, respectively) but high specificities (96.1% and 88.2%, respectively). This suggests that the abbreviated 4- and 8-zone protocols may be more suitable to rule in fluid overload rather than rule it out. If higher sensitivity and specificity is required or more quantitative information, then our pilot data help to justify a more time-consuming 28-zone protocol.
Our subgroup analysis of ten patients with ARDS revealed findings that contrasted with the overall study results. In this analysis, the correlation of L-US to EVLW was poor for the 4- and 8-point examination and only reached statistical significance in the TBS protocol. This disparity in L-US protocols may be a consequence of the characteristic heterogeneous topographical distribution of ARDS that may vary with inciting pathology, time, mechanical ventilation, and patient position.[24,25] The propensity of alveolar collapse, atelectasis, and consolidation to occur in a gravitationally dependent manner in the dependent (dorsal) lung areas in ARDS may limit accurate assessment by an abbreviated, predominantly ventral, L-US protocol.[24,25] The TBS protocol, by way of its comprehensiveness, may better account for both mixed topographical distribution and ventral-to-dorsal loss of parenchymal density.[17] Indeed, a study by Zhao et al. found that in a group of ARDS patients, a 12-region method of LUS demonstrated a strong positive correlation between L-US and EVLWI (r2 = 0.906) and sensitivity and specificity of 84.6% and 91.8%, respectively, by area under the curve analysis of a ROC.[26] Notably, the L-US examinations described in our study were strictly designed to assess B-line prevalence and were not designed to account for the full range of pleural and parenchymal abnormalities seen in ARDS including lack of lung sliding, subpleural consolidation, and consolidation.[27,28] Given the limitations of our subgroup analysis, including the small number of patients, and limited available literature that examines L-US in ARDS, further study is warranted.
Increased EVLW is associated with high morbidity (fewer ventilator-free days and longer hospital admission) as well as with increased mortality.[20,29,30] Chest X-ray does not typically detect EVLW until it is two to three times normal.[3] As a result, more sensitive EVLW measurements are needed. In vivo methods have also been validated and include quantitative CT, positron emission tomography scanning, magnetic resonance imaging,[31] and EVLW by TPTD.[20,32] Unfortunately, all these methods are costly, time-consuming, invasive, and impractical at the bedside, or limited to a single static time point. L-US, in contrast, is appealing because it is noninvasive, nonionizing, repeatable, and has a high concordance rate even after limited training.[13] Previous research comparing L-US scoring to EVLW by TPTD has largely focused on cardiogenic pulmonary edema,[18,33,34] and an increased number of US B-lines has been used to approximate its severity. Notably, there is scant literature comparing the performance of L-US to EVLW in patients with sepsis, one of the most common conditions in ICU. Moreover, there is scant literature examining the challenges and feasibility of determining EVLW in low-resource settings.
This study has limitations, including the relatively small number of patients and single-center enrollment. This reflects the fact that the study was a pragmatic investigation of real-world L-US in a resource-limited setting. We also used a higher cutoff point than previously described in outpatients with systolic heart failure.[33] Notably, using a single operator would eliminate intra- and inter-observer variability, which has been reported as 3.1% and 4.4%, respectively, in B-line scoring (TBS protocol).[11] We believe that our developing world location, rather than being an impediment, helps to demonstrate the feasibility of L-US as a bedside test.
Using EV1000 as a reference standard for evaluating EVLW was another limitation. Currently, the PiCCO (Pulsion system) is the gold standard. More importantly, there is no human validation study for EV1000. This might lead to some error of EVLW measurement.
Our findings differ from those of Enghard et al.[9] who found that a novel 4-zone protocol had a strong and significant correlation with EVLW values by TPTD and could predict severely increased EVLW (>15 ml/kg), with a sensitivity of 92.3% and a specificity of 94.6%. This may reflect their heterogeneous cohort of critically ill patients integrating patients suffering from heart failure. Unfortunately, it is difficult to know exactly why given that they did not perform a predefined subgroup analysis for sepsis patients and we did not include all-comers. We chose to study sepsis in this pilot work because it is characterized by inflammatory cytokines that should enhance fluid leakage. In addition to future studies that encompass the widest range of ICU pathologies, we intend not only to see how it might supplement simple modalities such as physical examination and chest X-ray, but also to examine where its use is associated with rational changes in clinical management.
CONCLUSION
Our research demonstrates the clinical applicability of three distinct L-US protocols for noninvasive detection of EVLW in patients with sepsis. More extensive 28-zone protocols demonstrate high specificity and better sensitivity than abbreviated 4- and 8-zone protocols. In patients with ARDS, our subgroup analysis suggests that the TBS protocol is more accurate than the abbreviated 4- and 8-zone protocols. Consideration of limitations of abbreviated 4- and 8-zone protocols may prevent clinician from reaching premature conclusions regarding the prediction of EVLW.
Financial support and sponsorship
The study was supported by Phramongkutklao Hospital Foundation under Her Royal Highness Princess MahaChakriSirindhorn Patronage, Bangkok, Thailand.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank Dr. Pichaya Tantiyavarong, M.D., Ph.D., Division of Clinical Epidemiology and Clinical Statistics, Faculty of Medicine, Thammasat University, for helping us with the statistical analysis of this study.
REFERENCES
- 1.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 2.Sakka SG, Klein M, Reinhart K, Meier-Hellmann A. Prognostic value of extravascular lung water in critically ill patients. Chest. 2002;122:2080–6. doi: 10.1378/chest.122.6.2080. [DOI] [PubMed] [Google Scholar]
- 3.Bongard FS, Matthay M, Mackersie RC, Lewis FR. Morphologic and physiologic correlates of increased extravascular lung water. Surgery. 1984;96:395–403. [PubMed] [Google Scholar]
- 4.Lichtenstein DA. Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4:1. doi: 10.1186/2110-5820-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jozwiak M, Silva S, Persichini R, Anguel N, Osman D, Richard C, et al. Extravascular lung water is an independent prognostic factor in patients with acute respiratory distress syndrome. Crit Care Med. 2013;41:472–80. doi: 10.1097/CCM.0b013e31826ab377. [DOI] [PubMed] [Google Scholar]
- 6.Sibbald WJ, Warshawski FJ, Short AK, Harris J, Lefcoe MS, Holliday RL, et al. Clinical studies of measuring extravascular lung water by the thermal dye technique in critically ill patients. Chest. 1983;83:725–31. doi: 10.1378/chest.83.5.725. [DOI] [PubMed] [Google Scholar]
- 7.Tagami T, Sawabe M, Kushimoto S, Marik PE, Mieno MN, Kawaguchi T, et al. Quantitative diagnosis of diffuse alveolar damage using extravascular lung water. Crit Care Med. 2013;41:2144–50. doi: 10.1097/CCM.0b013e31828a4643. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Cui N, Su L, Long Y, Wang X, Zhou X, et al. Prognostic value of extravascular lung water and its potential role in guiding fluid therapy in septic shock after initial resuscitation. J Crit Care. 2016;33:106–13. doi: 10.1016/j.jcrc.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Enghard P, Rademacher S, Nee J, Hasper D, Engert U, Jörres A, et al. Simplified lung ultrasound protocol shows excellent prediction of extravascular lung water in ventilated intensive care patients. Crit Care. 2015;19:36. doi: 10.1186/s13054-015-0756-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–91. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 11.Agricola E, Bove T, Oppizzi M, Marino G, Zangrillo A, Margonato A, et al. “Ultrasound comet-tail images”: A marker of pulmonary edema: A comparative study with wedge pressure and extravascular lung water. Chest. 2005;127:1690–5. doi: 10.1378/chest.127.5.1690. [DOI] [PubMed] [Google Scholar]
- 12.Picano E, Pellikka PA. Ultrasound of extravascular lung water: A new standard for pulmonary congestion. Eur Heart J. 2016;37:2097–104. doi: 10.1093/eurheartj/ehw164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lichtenstein D. Lung ultrasound in acute respiratory failure an introduction to the BLUE-protocol. Minerva Anestesiol. 2009;75:313–7. [PubMed] [Google Scholar]
- 14.Jambrik Z, Monti S, Coppola V, Agricola E, Mottola G, Miniati M, et al. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am J Cardiol. 2004;93:1265–70. doi: 10.1016/j.amjcard.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Soldati G, Copetti R, Sher S. Sonographic interstitial syndrome: The sound of lung water. J Ultrasound Med. 2009;28:163–74. doi: 10.7863/jum.2009.28.2.163. [DOI] [PubMed] [Google Scholar]
- 16.Volpicelli G, Mussa A, Garofalo G, Cardinale L, Casoli G, Perotto F, et al. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med. 2006;24:689–96. doi: 10.1016/j.ajem.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Baldi G, Gargani L, Abramo A, D'Errico L, Caramella D, Picano E, et al. Lung water assessment by lung ultrasonography in intensive care: A pilot study. Intensive Care Med. 2013;39:74–84. doi: 10.1007/s00134-012-2694-x. [DOI] [PubMed] [Google Scholar]
- 18.Volpicelli G, Skurzak S, Boero E, Carpinteri G, Tengattini M, Stefanone V, et al. Lung ultrasound predicts well extravascular lung water but is of limited usefulness in the prediction of wedge pressure. Anesthesiology. 2014;121:320–7. doi: 10.1097/ALN.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 19.Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med. 1997;156:1640–6. doi: 10.1164/ajrccm.156.5.96-07096. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Lu B, Ni H. Prognostic value of extravascular lung water index in critically ill patients: A systematic review of the literature. J Crit Care. 2012;27:420, e1–8. doi: 10.1016/j.jcrc.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: The BLUE protocol. Chest. 2008;134:117–25. doi: 10.1378/chest.07-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: The Berlin definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 24.Gattinoni L, Caironi P, Pelosi P, Goodman LR. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med. 2001;164:1701–11. doi: 10.1164/ajrccm.164.9.2103121. [DOI] [PubMed] [Google Scholar]
- 25.Pelosi P, Crotti S, Brazzi L, Gattinoni L. Computed tomography in adult respiratory distress syndrome: What has it taught us? Eur Respir J. 1996;9:1055–62. doi: 10.1183/09031936.96.09051055. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Z, Jiang L, Xi X, Jiang Q, Zhu B, Wang M, et al. Prognostic value of extravascular lung water assessed with lung ultrasound score by chest sonography in patients with acute respiratory distress syndrome. BMC Pulm Med. 2015;15:98. doi: 10.1186/s12890-015-0091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Copetti R, Soldati G, Copetti P. Chest sonography: A useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6:16. doi: 10.1186/1476-7120-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corradi F, Brusasco C, Pelosi P. Chest ultrasound in acute respiratory distress syndrome. Curr Opin Crit Care. 2014;20:98–103. doi: 10.1097/MCC.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 29.Valentine SL, Sapru A, Higgerson RA, Spinella PC, Flori HR, Graham DA, et al. Fluid balance in critically ill children with acute lung injury. Crit Care Med. 2012;40:2883–9. doi: 10.1097/CCM.0b013e31825bc54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–75. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 31.Lange NR, Schuster DP. The measurement of lung water. Crit Care. 1999;3:R19–R24. doi: 10.1186/cc342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakka SG, Reuter DA, Perel A. The transpulmonary thermodilution technique. J Clin Monit Comput. 2012;26:347–53. doi: 10.1007/s10877-012-9378-5. [DOI] [PubMed] [Google Scholar]
- 33.Miglioranza MH, Gargani L, Sant'Anna RT, Rover MM, Martins VM, Mantovani A, et al. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: A comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging. 2013;6:1141–51. doi: 10.1016/j.jcmg.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Volpicelli G, Caramello V, Cardinale L, Mussa A, Bar F, Frascisco MF, et al. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med. 2008;26:585–91. doi: 10.1016/j.ajem.2007.09.014. [DOI] [PubMed] [Google Scholar]


