Abstract
Despite advances in surgical technique, postoperative complications may lead to refractory cutaneous sinus tracts or tunnels. Negative pressure wound therapy is difficult to apply in longer tracts with a narrow diameter opening and conservative treatment failures ultimately necessitate surgical revisions. The aim of this pilot study was a clinical utility assessment of two different commercial placental membrane products for refractory cutaneous sinus tracts of surgical origin. Patients were treated with viable cryopreserved placental membrane (vCPM, n = 6) or devitalized dehydrated amnion/chorion membrane (dHACM, n = 6). The primary outcome measurement was the proportion of complete sinus tract depth resolution without exudate. Secondary endpoints included 4-week percent reduction in sinus tract probing depth and peri-tract wound surface area, days and number of grafts to resolution, number of wound-related infections, and 1-year recurrence rate for closed sinus tracts. All vCPM patients demonstrated complete sinus tract resolution compared to zero closures in the dHACM group (p = 0.00216). The vCPM group achieved greater percent reduction in probing depth (73.3 ± 21.9 versus −4.4 ± 91.3) and surrounding wound surface area (34.8 ± 86.8 versus −279.3 ± 454.9) at 4 weeks than dHACM. The use of viable intact cryopreserved placental membrane has demonstrated positive clinical outcomes for the treatment for refractory exudative sinus tracts and may be an alternative to repeat surgical intervention.
Keywords: Tunneling wound, Surgical sinus tract, Placental membrane, Pilot study
Introduction
Despite advances in general, orthopedic or neurological surgical techniques, postoperative complications—including infection, retained foreign body, seroma or hematoma formation, as well as skin and fat necrosis—may lead to the development of a refractory sinus tract.1 A sinus tract, also referred to as wound tunneling, is a cutaneous opening with a narrow passageway that tracks in one direction underneath the tissues and is oftentimes blind ended or accompanied by drainage.2, 3 Treatment failure of conservative wound care ultimately necessitates surgical revision such as a simple to wide excision of the tissue defect with either primary closure, secondary intention healing, or tissue flaps.4, 5, 6, 7, 8 Post-operative complication and the need for secondary operative procedures contributes to increased patient morbidity and prolonged recovery times.1, 9, 10 Over two-thirds of non-healing fistulas result from prior abdominal surgery, of these, 89% will require secondary surgeries for definitive closure.11 Post-operative complications can increase patient re-admittance rates and length of stay while doubling financial resources used by the hospital.12, 13
Radiographic examination via sinogram (water-soluble contrast material injected into the external cutaneous communication) provides adequate anatomical evaluation of the tract when blind ended.14 Over 40% of sinus tracts are inflammatory in origin, 31.4% are directly linked to a postsurgical complication, and the remainder are either post-traumatic (16.1%), post-radio-therapy (5.1%), neoplastic (2.2%), or of unknown etiology (2.2%).14 Histologic evaluation of excised sinus tracts reveal that the majority are lined with granulation tissues containing chronic inflammatory cells, while 10% are lined with stratified squamous epithelium.15 Both tissue types contribute to development of tracts that are recalcitrant in nature, thereby rendering them clinically indistinguishable; however, the removal of an epithelial lining has been shown to reduce recurrence.9, 15
The initial treatment approach for exudative tracts is conservative, historically involving gauze or gauze ribbon packing. This technique has been proven to contribute to prolonged treatment and sinus tract persistence.10 Negative pressure wound therapy (NPWT) provides a nonsurgical treatment approach however it can be difficult to apply in longer tracts with a narrow diameter opening. A review of the literature indicates that limited research has been conducted on the nonsurgical and non-NPWT management of cutaneous sinus tracts that are unrelated to pilonidal, hidradenitis suppurativa, or odontogenic origins. Several studies have described alternative treatment formulations and delivery methods for introducing living cells, tissue scaffolds, enzymatic solutions, or other collagen-containing amalgams into nonhealing wound tunnels. However, depending on the depth and accessibility to the tract, the alternative utilization of such allografts, advanced bioengineered or other tissue products has also proved challenging.
As a versatile biologic dressing, human placental membrane (HPM) has been investigated both clinically and experimentally in chronic ulcers, contaminated surgical wounds, undermined wounds, burns, and traumatic soft-tissue wounds with positive outcomes.16, 17, 18, 19 Various methods of tissue processing and preservation have led to HPM commercialization and widespread clinical use. Although fresh HPM contains a 3D collagen rich extracellular matrix (ECM), biologic factors, as well as viable fibroblasts and mesenchymal stem cells, most methods of tissue processing alter the placental matrix and growth factors while destroying viable cells— resulting in a loss or decrease in functionality after processing.20, 21, 22, 23, 24, 25, 26 In addition to summarizing previous attempts on nonsurgical and non-NPWT approaches to nonhealing tunnels as reflected in the literature, the aim of this pilot study was to evaluate and compare the clinical outcomes associated with two differently processed HPM products when used in the management of refractory exudative sinus tracts.
Materials and Methods
Inclusion Criteria for Literature Search
A literature search of published studies highlighting sinus tract and/or tunneling wound management—without the use of surgery or NPWT— was conducted using key words and Medical Subject Headings of the U.S. National Library of Medicine's (MeSH) in three online electronic databases: PubMed/MEDLINE, Ovid MEDLINE, and EBSCO CINAHL. Search dates were January 1950 to June 2017. To be considered, articles were required to have:
-
(1)
Description of the advanced treatment and its method of delivery to the sinus tract/tunnel;
-
(2)
Number of subjects treated with or without comparator;
-
(3)
Data for at least one of the following: progress and timeframe for tract depth reduction, proportion of patients with complete sinus tract/tunneling resolution, and/or the number of treatments required to achieve complete sinus tract/tunneling resolution.
All relevant prospective and retrospective types of clinical studies, systemic reviews, case reports, case series, and secondary database analyses were considered. After selection, studies were reviewed and a summary was prepared.
Pilot Study Design
This study was conducted at Bozeman Health Deaconess Hospital Wound and Hyperbaric Center after review and approval by the internal board. The aim was to evaluate closure of exudative sinus tracts of surgical etiology with the adjunct of two commercial placental products: a viable cryopreserved chorion membrane (vCPM: Grafix CORE®; Osiris Therapeutics, Inc., Columbia, MD) and devitalized dehydrated amnion/chorion (dHACM: EpiFix®; MiMedx Group Inc., Marietta, GA). All de-identified data were prepared in a manner consistent with the Health Insurance Portability and Accountability Act of 1996 (HIPAA).
Informed patient consent was obtained prior to initiation of treatment. Six patients were treated with dHACM in Jan–Dec 2014, and six patients were treated with vCPM in Jan–Dec 2015. The primary clinical analysis was the rate of complete sinus tract depth resolution without exudate. Secondary endpoints included 4-week percent reduction in both sinus tract depth and the peri-tract wound surface area; days and number of grafts to achieve tract resolution; the number of wound-related infections after initiation of HPM use; and the 1-year recurrence rate for closed sinus tracts.
Population
A total of 12 subjects treated with vCPM (n = 6) or dHACM (n = 6) were included in this study (5 male; 7 female; age range 27–86 years; mean age 59 years, SD ± 16.9). All subjects had undergone prior orthopedic, general or neurosurgery and developed a sinus tract as a secondary postoperative complication. Location of sinus tracts in the vCPM and dHACM groups, respectively, were as follows: abdominal: 3 vs. 2; over a major joint or orthopedic hardware: 2 vs. 2; posterior trunk/spinal: 1 vs. 2. These full-thickness sinus tracts were characterized by persistent measurable probing depth and exudate, resulting in chronic partial-thickness breakdown of the surrounding tissues. Each patient failed standard medical management, including previous NPWT, the elimination of any underlying infection or iatrogenic cause, i.e. retained foreign body; bony involvement, i.e. osteomyelitis; and a long-term use of standard dressing options, such as alginates, hydrogels, ribbon gauze, and foams. Prior to an advanced therapy regimen with HPM, active infection was ruled out by routine wound culture with sensitivity to antibiotics. Diagnostic examinations demonstrated that tracts did not communicate with an ongoing abscess, infected cavity, or infected osseous structure.
Treatment and Data Collection
Prior to treatment with vCPM or dHACM, patients received protocol-based medical management of their postoperative surgical sinus tract for a mean duration of 6.8 months. During the study period from January 2014 to December 2015 patients were treated according to a protocol consisting of:
-
(1)
Site preparation: tunnel irrigation with NS 0.9% solution and curette-based debridement of the accessible sinus tract depth and surrounding wound bed for removal of devitalized tissues.
-
(2)
Application of placental allograft sheets into tracts using a semi-flexible sterile probe and onto the exposed peri-tract wound bed.
-
(3)
Appropriate dressings: primary foam (Allevyn; Smith & Nephew, London, UK) or a non-adherent contact layer (Adaptic™; Acelity, San Antonio, TX) with a secondary gauze bolster, depending on amount of exudate.
Dressings changes were done weekly (7 ± 2 days) with planned biweekly graft application. Clinical response was monitored by serial measurements of sinus tract depth and peri-tract wound size. The option to discontinue applications of the HPM were available if, after 28 days of treatment, sinus tract depth reduction was ≤50.0%. One year follow up was used to collect long-term outcomes for both closed and non-closed sinus tracts.
Statistical Analysis
Categorical variables are expressed as percentages and continuous variables are expressed as mean values ± SD. Sinus tract probing depth (mm) and wound surface area (cm2) were calculated at 4 weeks and compared to baseline measurements to determine the percentage of reduction. Qualitative data analysis compared two treatment cohorts using a 2 × 2-test or Fisher's exact test while quantitative data analysis included a Wilcoxon rank-sum test. Statistical comparisons between control and treatment groups were made and significance was defined as a p value ≤ 0.05.
Results
Review of the Literature
Based on predefined search criteria, a total of 9 studies discussing treatment for a total of 104 surgical, trauma, and chronic sinus tracts were identified.27, 28, 29, 30, 31, 32, 33, 34, 35 Previously evaluated treatments included topical phenytoin powder, plasmin and hyaluronidase, dehydrated micronized decellularized cadaveric dermis, autologous platelet-rich plasma combined with bovine collagen, or cross-linked bovine collagens. Described delivery materials included daily instillation of solutions, reconstitution of micronized xenograft and allograft tissues into a flowable matrix, an autologous platelet-rich plasma combined with bovine type I collagen as topical gel, and sub-dermal needle injections of autologous bone marrow or devitalized micronized amnion in amniotic fluid suspensions. No studies described the use of HPM in sheet form for sinus tract management. Results of the literature search are summarized in Table 1.
Table 1.
Summary of Literature Search on Nonsurgical Treatment Options for Sinus Tracts/Tunnels.
| Study, year | Subjects with tract, n | Etiology | Treatment | Method of preparation for application | Depth reduction, % (weeks)a | Proportion of complete tract closure, % (weeks)a | No. treatments to tract closurea |
|---|---|---|---|---|---|---|---|
| Clifton et al., 1956 | 9 | Post-surgical | Plasmin and hyaluronidase enzyme solution | Mixed into solution for daily instillation | Not stated | 100.0 (3.7) | 16.8 |
| Anstead et al., 1996 | 1 | Pressure-related | Phenytoin powder | Topical placement of powder | Not stated | 100.0 (7.7) | 108 |
| Banta et al., 2003 | 2 | Post-surgical | Dehydrated, micronized decellularized cadaveric dermal matrix | Reconstituted into flowable form | 41.5 (2.6) | 100.0 (5.0) | 2 |
| Brigado et al., 2009 | 12 | Chronic lower extremity | Dehydrated, micronized acellular human dermal replacement scaffold | Reconstituted into flowable form | 50.0 (2.0) | 83.3 (7.8) | 1 |
| de Leon et al., 2011 | 28 | Chronic wounds and post-surgical | Autologous platelet-rich plasma combined with a fibrin matrix | Mixed into topical gel formation | 49.3 (1.8) | Not stated | Not stated |
| Allam & Partain, 2011 | 12 | Chronic wounds and post-surgical | Autologous platelet-rich plasma combined with bovine collagen | Mixed into topical gel formation | Not stated | 75.0 (Not stated) | 1 |
| Williams et al., 2013 | 3 | Chronic lower extremity | Dehydrated, micronized acellular human dermal replacement scaffold | Reconstituted into flowable form | Not stated | 100.0 (8.0) | 1 |
| Werber & Martin, 2013 | 19 | Chronic lower extremity | Devitalized cryopreserved micronized amnion in amniotic fluid | Needle injected into tissues | Not stated | 95.0 (Not stated) | 1.9 |
| Campitiello et al., 2015 | 18 | Post-surgical, post-traumatic, neuropathic | Granulated cross-linked bovine tendon collagen and glycosaminoglycan and suture approximation of wound | Saline hydrated into flowable form | Not stated | 88.9 (6.4) | 1.2 |
Represented by mean study values.
Pilot Study
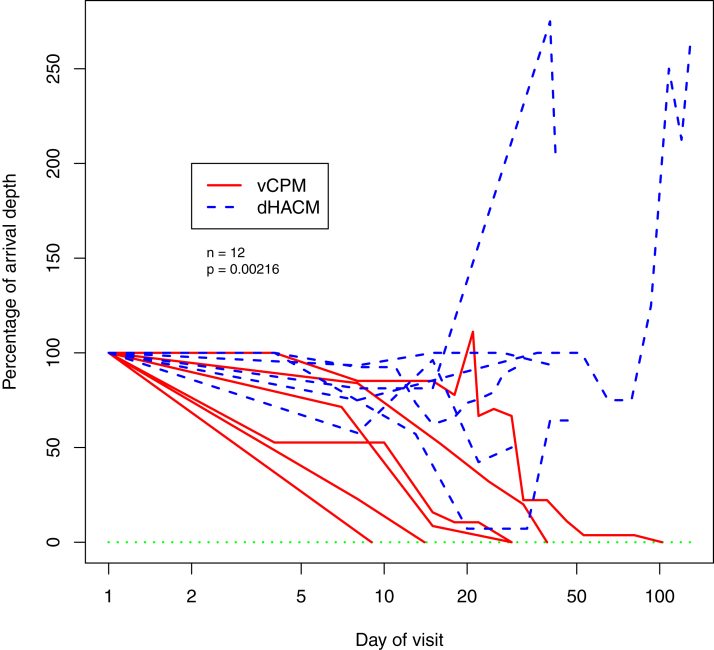
Baseline patient and wound characteristics are shown in Table 2. There are no statistically significant differences between treatment cohorts. During the study period, all 6 patients in the vCPM cohort achieved complete sinus tract and surrounding wound resolution versus 0 patients in the dHACM cohort. The association between the use of vCPM and the primary outcome measure of complete sinus tract depth reduction was statistically significant (p = 0.00216) (Fig. 1). Analysis of additional clinical endpoints demonstrated that patients in the vCPM group reached a greater percent depth reduction (73.3 ± 21.9 vs. −4.4 ± 91.3) and surrounding wound surface area (34.8 ± 86.8 vs. −279.3 ± 454.9) at 4 weeks when compared to the dHACM group. The vCPM group achieved closure in 37.0 ± 33.7 days. In comparison, after 52.5 ± 37.7 days, treatment in the dHACM was discontinued due to non-closure. (Table 2). The number of vCPM applications to achieve sinus depth and wound resolution was 1.7 ± 0.8 grafts. There were no graft-related infections observed in either group.
Table 2.
Study Summary.
| vCPM | dHACM | p value | |
|---|---|---|---|
| Baseline patient and wound characteristics | |||
| Patients, n | 6 | 6 | |
| Sex, M/F | 1M/5F | 4M/2F | 0.242 |
| Age, years | 62.0 ± 12.8 | 56.0 ± 21.0 | 0.589 |
| BMI, kg/m2 | 31.3 ± 2.9 | 26.5 ± 6.0 | 0.310 |
| Wounds, n | 6 | 6 | |
| Orthopedic surgery | 2 | 2 | |
| Abdominal surgery | 3 | 2 | |
| Posterior trunk/spinal surgery | 1 | 2 | |
| Previous duration, days | 136.0 ± 151.8 | 246.7 ± 123.5 | 0.132 |
| Tract/tunnel, probing depth (mm) | 28.0 ± 6.0 | 22.0 ± 1.6 | 0.179 |
| Peri-tract/tunnel wound, surface area (cm2) | 10.7 ± 25.2 | 2.6 ± 6.0 | 0.394 |
| Study outcomes | |||
| Complete tract/tunnel resolution, n (%)a | 6 (100) | 0 (0) | 0.00216 |
| Complete surrounding wound closure, n (%)b | 6 (100) | 0 (0) | 0.00216 |
| 4-week tract/tunnel probing depth reduction, % | 73.3 ± 21.9 | −4.4 ± 91.3 | 0.114 |
| 4-week surrounding surface area reduction, % | 34.8 ± 86.8 | −279.3 ± 454.9 | 0.172 |
| Graft applications,# | 1.7 ± 0.8 | 1.8 ± 0.4 | 0.589 |
| Treatment duration, days | 37.0 ± 33.7 | 52.5 ± 37.7 | 0.113 |
| Wound related infection, n | 0 (0) | 0 (0) | N/A |
Data presented as mean ± SD or (%) when indicated.
Sinus tract depth of 0 mm.
Peri-sinus tract wound surface area of 0 cm2.
Figure 1.
Reduction in Probing Depth of Sinus Tract vs. Treatment Time: For each subject, and for each visit day after the arrival, the depth of the sinus tract is shown as a percentage of the depth at the time of their arrival.
All six patients in the dHACM group exited treatment after tract depth remained unchanged or had increased after >28-days. After discontinuation of treatment with the dHACM, 2 patients closed their sinus tracts with SOC in a mean of 278.5 ± 67.2 days; the remaining 4 patients were lost to follow up after a mean 120.5 ± 98.5 days of failure to close with continued SOC. After completion of treatment, all 6 sinus tracts in the vCPM group remained closed without recurrence for 1 year or longer follow-up.
Discussion
This is the first report on the use of two commercial HPM products, differing in their compositions, in the management of refractory post-surgical sinus tracts. Various methods of commercial HPM tissue processing that range from freezing and cryopreservation to dehydration and decellularization have been shown to alter the structural and cellular integrity of the membrane and impact angiogenic, anti-inflammatory, antimicrobial, anti-adhesion, and antioxidant activities.22, 23, 24, 36 Unaltered HPM components, including naturally occurring cells, growth factors, cytokines, and structural ECM are preserved in a viable intact cryopreserved placental membrane.22, 23, 24 In a dehydrated devitalized amnion/chorion membrane, the ECM and growth factors are altered, tissue resident cells are destroyed and the trophoblast layer remains attached. Comparative effectiveness cohort studies have also indicated that differing preparations of HPM may manifest in statistically different outcomes with regards to overall rates of closure when compared clinically in head-to-toe wound management.37
In vivo studies on the use of nonviable cryopreserved HPM have demonstrated promising outcomes in the repair of challenging narrow and exudative cutaneous defects, including oronasal fistulas in pigs.38 When compared to a collagen-based dermal substitute (Integra® Regeneration Template, Integra Life Sciences, Plainsboro, NJ, USA) epithelial-lined fistular tracts treated with non-viable cryopreserved placenta showed a significantly smaller diameter on day 40 (p = 0.043) with no infection whereas fistulas in the collagen matrix group had dehiscence and infection.38 In 2010, Kassem et al. reported use of de-epithelialized, dried and gamma-irradiated amnion that led to muscle-scleral adhesions during strabismus surgery, resulting in extraocular muscle fibrosis.25 A follow up study on nonviable cryopreserved amnion demonstrated it was ineffective in preventing conjunctival inflammation when compared to fresh human placental membranes.26
As alternatives to SOC or surgical management, the use of advanced formulations and delivery methods for living cells, tissue scaffolds, enzymatic solutions, or collagen-containing amalgams have been previously described with incomplete datasets or varying degrees of success for patient outcomes. Although a fluid or flowable matrix appears ideal for narrow tracking lesions, the bedside reconstitution and the staged use of such formulations, including dehydrated acellular micronized dermal scaffolds, prior harvest and follow up delivery of autologous PRP with fibrin glue or bone marrow aspirate in a bovine collagen, or devitalized cryopreserved amniotic fluid suspensions injected subcutaneously, have had limited clinical utility.
In a 2015 case series, Campitiello et al. evaluated the use of a flowable wound matrix composed of granulated cross-linked bovine tendon collagen for the treatment of post-surgical, post-traumatic, and neuropathic tunneling wounds. Resolution was aided by the approximation and suturing of wound edges in 61.1% of the documented closures.35 The single-arm prospective study by Werber & Martin examined subcutaneous needle injections of nonviable cryopreserved micronized amnion in amniotic fluid into foot and ankle wounds characterized by sinus tracts.34 However, the depth of sinus tracts and time to their resolution was not discussed in the study report.34
Product-related adverse events have been described in 2 studies: 8.3% for platelet-rich plasma product-related infection and 11.1% for colliquation after implantation of cross-linked bovine tendon.32, 35 Three retrospective case series have evaluated intermediate treatment progress, with Brigido et al. reporting 50% sinus depth reduction at 2 weeks in chronic lower extremity wound sinus tracts.30 Nearly ninety-percent (8/9) of published studies have included data on the proportion of patients reaching complete resolution of the tract; closure rates varied from 75 to 100% in associated time frames of 5.0–10.5 weeks. The number of treatments required to achieve reported outcomes ranged from 1 to 108 product applications.27, 28, 29, 30, 31, 32, 33, 34, 35
In this study, the patients treated with vCPM showed positive outcomes when compared to dHACM. The observed clinical course for the vCPM-treated patients included correlative reductions in sinus tract depth and exudate, contributing to decreased peri-tract tissue maceration. The progressive sinus tract depth reduction preceded surface area reduction of the surrounding wound bed. For vCPM patients, a mean 73.3% tract depth reduction was observed at 4 weeks, and complete sinus tract and surrounding wound resolution (p = 0.00216) occurred in a mean of 37.0 days and 1.7 graft applications. In comparison, sinus tract depth increased at 4 weeks and zero closures were observed in a similar timeframe and number of graft applications for dHACM. A recent surgical report by Nichols & Overly presented successful treatment of refractory low-output enterocutaneous fistulas without recurrence using a single application of vCPM.39 In a prospective multicenter clinical trial, vCPM showed clinical benefits in the management of complex diabetic foot ulcers with exposed bone and tendon.40 Results of this trial were confirmed by a head-to-toe complex wound case series.41
While research interest into nonsurgical approaches for sinus tract resolution continues to grow, optimal treatment algorithms remain undeveloped. Despite the identified limitations of this pilot study, including a small number of patients in a consecutive non-randomized versus randomized parallel cohort design, results indicate that vCPM in membrane sheet configuration can easily conform for placement within complex, treatment refractory sinus tracts with ongoing exudate while yielding positive clinical outcomes.
Acknowledgments
The authors make no acknowledgements.
Footnotes
Funding Source: None to declare. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest: GMM and YGT are employed by Osiris Therapeutics, Inc. (“Osiris”), Department of Medical Affairs. ELJ is currently a consultant for Osiris; however, he was not affiliated with Osiris during the time that the study was undertaken.
Ethics Statement/Confirmation of Patient Permission
Internal review and approval was obtained for the reported research. All patients were given a detailed explanation of the procedure and provided full written consent.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Conde-Green A., Chung T.L., Holton L.H., 3rd Incisional negative-pressure wound therapy versus conventional dressings following abdominal wall reconstruction: a comparative study. Ann Plast Surg. 2013;71(4):394–397. doi: 10.1097/SAP.0b013e31824c9073. [DOI] [PubMed] [Google Scholar]
- 2.Butcher M. Management of wound sinuses. J Wound Care. 1999;8(9):451–454. doi: 10.12968/jowc.1999.8.9.26197. [DOI] [PubMed] [Google Scholar]
- 3.Balik E., Eren T., Bulut T. Surgical approach to extensive hidradenitis suppurativa in the perineal/perianal and gluteal regions. World J Surg. 2009;33(3):481–487. doi: 10.1007/s00268-008-9845-9. [DOI] [PubMed] [Google Scholar]
- 4.Park J.J., Cintron J.R., Orsay C.P. Repair of Chronic Anorectal fistulae using commercial fibrin sealant. Arch Surg. 2000;135(2):166–169. doi: 10.1001/archsurg.135.2.166. [DOI] [PubMed] [Google Scholar]
- 5.Genua J.C., Vivas D.A. Management of nonhealing perineal wounds. Clin Colon Rectal Surg. 2007;20(4):322–328. doi: 10.1055/s-2007-991032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hazen P.G., Hazen B.P. Hidradenitis suppurativa: successful treatment using carbon dioxide laser excision and marsupialization. Dermatol Surg. 2010;36(2):208–213. doi: 10.1111/j.1524-4725.2009.01427.x. [DOI] [PubMed] [Google Scholar]
- 7.Baatenburg de Jong R.J. A new surgical technique for treatment of preauricular sinus. Surgery. 2005;137(5):567–570. doi: 10.1016/j.surg.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Mohamed H.A., Kadry I., Adly S. Comparison between three different therapeutic modalities for non-complicated pilonidal sinus disease. Surgeon. 2005;3(2):73–77. doi: 10.1016/s1479-666x(05)80065-4. [DOI] [PubMed] [Google Scholar]
- 9.Yeo S.W., Jun B.C., Park S.N. The preauricular sinus: factors contributing to recurrence after surgery. Am J Otolaryngol. 2006;27(6):396–400. doi: 10.1016/j.amjoto.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Permberton J.H. How to treat the persistent perineal sinus after rectal excision. Colorectal Dis. 2003;5(5):486–489. doi: 10.1046/j.1463-1318.2003.00520.x. [DOI] [PubMed] [Google Scholar]
- 11.Draus J.M., Jr., Huss S.A., Harty N.J. Enterocutaneous fistula: are treatments improving? Surgery. 2006;140(4):570–576. doi: 10.1016/j.surg.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Whitehouse J.D., Friedman N.D., Kirkland K.B. The impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol. 2002;23(4):183–189. doi: 10.1086/502033. [DOI] [PubMed] [Google Scholar]
- 13.Dimick J.B., Weeks W.B., Karia R.J. Who pays for poor surgical quality? Building a business case for quality improvement. J Am Coll Surg. 2006;202(6):933–937. doi: 10.1016/j.jamcollsurg.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Alexander E.S., Weinberg S., Clark R.A., Belkin R.D. Fistulas and sinus tracts: radiographic evaluation, management, and outcome. Gastrointest Radiol. 1982;7:135–140. doi: 10.1007/BF01887627. [DOI] [PubMed] [Google Scholar]
- 15.Harrison J.W., Larson W.J. The epithelialized oral sinus tract. Oral Surg Oral Med Oral Pathol. 1976;42(4):511–517. doi: 10.1016/0030-4220(76)90299-1. [DOI] [PubMed] [Google Scholar]
- 16.Davis J.W. Skin transplantation with a review of 550 cases at the John Hopkins hospital. John Hopkins Med J. 1910;15:307–396. [Google Scholar]
- 17.Stern M. The grafting of preserved amniotic membrane to burned and ulcerated skin surfaces substituting skin grafts. JAMA. 1913;60:973–974. [Google Scholar]
- 18.Sabella N. Use of fetal membranes in skin grafting. Med Rec N Y. 1913;83:478–480. [Google Scholar]
- 19.Gruss J.S., Jirsch D.W. Human amnioitic membrane: a versatile wound dressing. Can Med Assoc J. 1978;118(10):1237–1246. [PMC free article] [PubMed] [Google Scholar]
- 20.Mamede A.C., Carvalho M.J., Abrantes A.M. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012;349(2):447–458. doi: 10.1007/s00441-012-1424-6. [DOI] [PubMed] [Google Scholar]
- 21.Niknejad H., Peirovi H., Jorjani M. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88–99. doi: 10.22203/ecm.v015a07. [DOI] [PubMed] [Google Scholar]
- 22.Duan-Arnold Y., Gyurdieva A., Johnson A. Retention of endogenous viable cells enhances the anti-inflammatory activity of cryopreserved amnion. Adv Wound Care. 2015;4:523–533. doi: 10.1089/wound.2015.0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan-Arnold Y., Uveges T.E., Gyurdieva A. Angiogenic potential of cryopreserved amniotic membrane is enhanced through retention if all tissue components in their native state. Adv Wound Care. 2015;4:513–522. doi: 10.1089/wound.2015.0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan-Arnold Y., Gyurdieva A., Johnson A. Soluble factors released by endogenous viable cells enhance the antioxidant and chemoattractive activities of cryopreserved amniotic membrane. Adv Wound Care. 2015;4:329–338. doi: 10.1089/wound.2015.0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kassem R.R., Gawdat G.I., Zedan R.H. Severe fibrosis of extraocular muscles after the use of lyophilized amniotic membrane in strabismus surgery. J AAPOS. 2010;14(6):548–549. doi: 10.1016/j.jaapos.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Kassem R.R., Khodeir M.M., Salem M. Effect of cryopreserved amniotic membrane on the development of adhesions and fibrosis after extraocular muscle surgery in rabbits. Acta Opthalmol. 2013;91(2):e140–e148. doi: 10.1111/j.1755-3768.2012.02563.x. [DOI] [PubMed] [Google Scholar]
- 27.Cliffton E.E., Rees T., Spier I.R. Treatment of infected wounds and chronic sinus tracts with enzymes; plasmin (fibrinolysin) and hyaluronidase and antibiotics. Am J Surg. 1956;92(4):496–506. doi: 10.1016/s0002-9610(56)80080-9. [DOI] [PubMed] [Google Scholar]
- 28.Anstead G.M., Hart L.M., Sunahara J.F. Phenytoin in wound healing. Ann Pharmacother. 1996;30(7-8):768–775. doi: 10.1177/106002809603000712. [DOI] [PubMed] [Google Scholar]
- 29.Banta M.N., Eaglstein W.H., Kirsner R.S. Healing of refractory sinus tracts by dermal matrix injection with Cymetra. Dermatol Surg. 2003;29(8):863–866. doi: 10.1046/j.1524-4725.2003.29234.x. [DOI] [PubMed] [Google Scholar]
- 30.Brigido S.A., Schwartz E., McCarroll R. Use of an acellular flowable dermal replacement scaffold on lower extremity sinus tracts wounds: a retrospective case series. Foot Ankle Spec. 2009;2(2):67–72. doi: 10.1177/1938640009333474. [DOI] [PubMed] [Google Scholar]
- 31.de Leon J.M., Driver V.R., Fylling C.P. The clinical relevance of treating chronic wounds with an enhanced near-physiological concentration of platelet-rich plasma gel. Adv Skin Wound Care. 2011;24(8):357–368. doi: 10.1097/01.ASW.0000403249.85131.6f. [DOI] [PubMed] [Google Scholar]
- 32.Allam R.C., Partain R. Use of platelet-rich plasma in the treatment of recalcitrant sinus tracts: a case series. Wounds. 2011;23(11):322–327. [PubMed] [Google Scholar]
- 33.Williams M.L., Holewwinski J. Experience using a flowable soft tissue scaffold in conjunction with a human dermal matrix in lower extremity wounds. JDFC. 2013;5(3):55–61. [Google Scholar]
- 34.Werber B., Martin E. A prospective study of 20 foot and ankle wounds treated with cryopreserved amniotic membrane and fluid allograft. J Foot Ankle Surg. 2013;52(5):615–621. doi: 10.1053/j.jfas.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 35.Campitiello F., Della C.A., Guerniero R. Efficacy of a new flowable wound matrix in tunneled and cavity ulcers: a preliminary report. Wounds. 2015;27(6):152–157. [PubMed] [Google Scholar]
- 36.Mao Y., Hoffman T., Johnson A. Human cryopreserved viable amniotic membrane inhibits the growth of bacteria associated with chronic wounds. JDFC. 2016;8(2):23–30. [Google Scholar]
- 37.Johnson E.L., Marshall J.T., Michael G.M. A comparative outcomes analysis evaluating two different human placental membrane products for wound management. Wound Repair Regen. 2016 doi: 10.1111/wrr.12503. [DOI] [PubMed] [Google Scholar]
- 38.Kesting M.R., Loeffelbein D.J., Classen M. Repair of oronasal fistulas with human amniotic membrane in minipgs. Br J Oral Maxillofac Surg. 2010;48(2):131–135. doi: 10.1016/j.bjoms.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 39.Nichols F., Overly A. Novel approach for Enterocutaneous fistula treatment with the use of viable cryopreserved placental membrane. Case Rep Surg. 2016 doi: 10.1155/2016/8797691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frykberg R.G., Gibbons G.W., Walters J.L. A prospective, multicenter, open-label, single-arm clinical trial for the treatment of chronic complex diabetic foot wounds with exposed tendon and/or bone: positive clinical outcomes of a viable cryopreserved human placental membrane. Int Wound J. 2016 doi: 10.1111/iwj.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki K., Michael G., Tamire Y. Viable intact cryopreserved human placental membrane for a non-surgical approach to closure in complex wounds. J Wound Care. 2016;25(suppl 10):S25–S31. doi: 10.12968/jowc.2016.25.Sup10.S25. [DOI] [PubMed] [Google Scholar]