Abstract
There are approximately 6.5 million patients in the U.S. suffering from chronic wounds and approximately 140,000 patients hospitalized every year with new wounds. With a long healing process, this demands the need for a non-contact, low cost, and remote monitoring solution that can assist clinicians in diagnosing and treating a patient's wound. This will reduce the burden of countless office visits, especially for those who are elderly and incapacitated. We present a mobile platform based wound 3D imaging app. The app is the only integrated measurement solution encompassing wound area and volume through low cost yet accurate 3D imaging. Extensive experiments show the app has 1.14% and 4.41% relative errors for wound area and volume measurement respectively, far exceeding currently employed clinic methods. In addition, non-invasive volume measurement methods currently use expensive industrial 3D (>$20K) cameras, but our solution provides cheap and accurate results.
Keywords: Wound measurement, Wound assessment, Wound area and volume, 3D imaging, 2D planimetry, Ruler method
Introduction
There are approximately 6.5 million patients in the U.S. suffering from chronic wounds (e.g. diabetic foot ulcers, and pressure ulcers) and approximately 140,000 patients are hospitalized every year with new wounds.1 Currently, over 23 million people or 7.8% of the U. S. population have been diagnosed with diabetes. Among them, 5 million people suffer from chronic ulcers.1 A study from the Centers for Disease Control and Prevention (CDC) concluded that between 1995 and 2010, the prevalence of diagnosed diabetes increased by 50% or more in 42 states, and by 100% or more in 18 states.2 With soaring diabetes and obesity rates, chronic ulcers will affect more and more people's lives. Currently, an excess of $25 billion is spent annually on the treatment of wounds, and the burden is growing rapidly due to increasing health care costs, an aging population, and certainly the sharp rise in the incidence of diabetes and obesity.2, 3 Due to poor care worldwide, diabetes have caused complications in foot ulcers leading to an amputation of a lower limb every 30 s and only a 20% survival rate after 5 years—a higher mortality rate than colon, breast or prostate cancer.4 Evidence suggests that 80% of amputations are actually preventable through access of good quality and routine care,4 for example, comprehensive and accurate wound documentation, early infection diagnosis, and personalized treatment.
Reliable Wound Area/Volume Growth Tracking and Under Skin Infection Detection are Right at the Center of Good Quality Care
A wound's geometric shape and appearance contains a wealth of information about its cause, severity, length of time, change of status, and prognosis for healing. Regular wound size and appearance checkups help doctors assess progress in wound healing and validate the effectiveness of interventions. Studies have shown that documenting the reduction of wound area/volume due to the development of granulated/epithelial tissues is a vital part of wound healing and treatment assessment process.5 As an example, Flanagan discovered that percentage reduction in true wound surface area is the best way of predicting healing rates, and said that 40% reduction in wound surface area over the first two to three weeks of treatment is predictive of healing in 12–24 weeks.6
What are Current Methods on Wound Assessment and What are Their Problems?
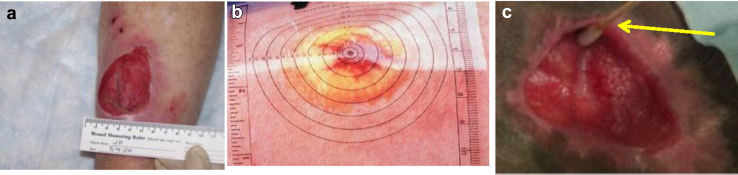
Currently, clinical assessments on wound tissues are all done through visual inspection only, and wound size measurements (width, length and depth) are commonly done through either a disposable ruler7, 8 (Fig. 1a courtesy of Reference 2), transparent tape based edge tracing (Fig. 1b courtesy of Reference 13), liquid filling, or even a dipstick swab (Fig. 1c courtesy of co-author's institute). Since it is very subjective to select reference points to measure the wound's dimension, the measured results are often non-repeatable and crude with up to 44% error in area measurement reported.7, 8 The contact based methods are invasive, painful, and difficult for patients,9 in addition, these methods are prone to contagion. On the other hand, newer non-invasive methods such as 2D digital planimetry9 and 3D stereophotogrammetry10 have been experimented or evaluated to achieve the goals of quantifying wound tissue growth through 2D and 3D imaging respectively. However, each has its own drawbacks. 2D digital planimetry was reported to have up to 10% area measurement errors,7 and produced volume measurement errors of up to 52% when relying on Q-tip for wound depth estimation.11 3D stereophotogrammetry is the most accurate measurement method reported11. However, pointed out by the co-author's institute, system's bulk size and high costs (∼tens of thousands) make it impractical for home based care or even hospital settings.10
Figure 1.
Several typical current wound measurement methods: a) ruler, b) tracing, and c) Q-tip swab (yellow arrow pointed).
We present a new measurement solution or app on mobile platforms to directly tackles the aforementioned challenges facing current chronic wound care. The app uses computer vision algorithms for quantitative measurement in 3D digital space. Since iDr utilizes a client-server framework to allow mobile app based simple wound video uploading, easy patient data access and analysis, it is designed to significantly reduce the inconvenience and high cost barriers for chronic wound care.
Research Materials and Methods
The mobile app or iDr is designed to help clinicians to objectively and conveniently monitor chronic wound healing process through 3D imaging. Through the app, clinicians can track the wound growth trend through the history data recorded in the past. The wound growth history data includes wound area and wound volume.
3D Imaging for Wound Volume and Area
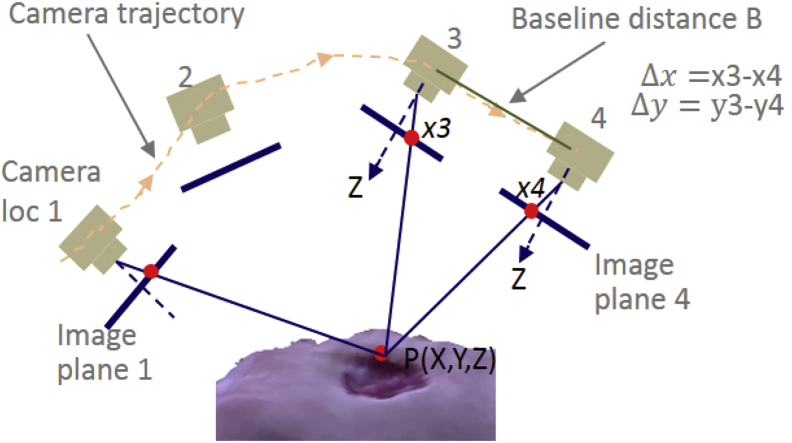
The smartphone camera is an optical imaging system. Objects in any 3D world are imaged on the sensor's image plane. While it is not possible to recover the actual 3D dimension of an imaged wound from the smartphone camera at a fixed location, it is feasible to recover a 3D digital form for a wound from two fixed camera locations. An image pair from two different camera locations forms a stereo, much like human eyes. By applying optical imaging principle on the stereo, we can recover the 3D coordinates of the imaged wound. For example, assuming two camera locations 3 and 4 in Fig. 2 where only translational motion was considered (simplest case), through the optical ray triangulation method, we can obtain the depth Z of a wound point P as with f as camera focal length and B as camera baseline distance.
Figure 2.
3D SFM when single camera is used for imaging.
For smartphone recorded videos, the wound is imaged from different viewpoints. Structure from Motion (SFM) algorithms are designed to select image pairs from different viewpoints to form stereos of different camera distances (or baselines). Using these image pairs and the classical stereo principle, the app refines and reconstructs the 3D digital wound. A black and white checkered reference marker with known size was used to recover the actual scale of the wound.
The Hypotheses for the Experiment
(1) By applying the optical imaging principle and SFM, using a smartphone video, iDr can accurately and non-invasively reconstruct 3D wound model and measure the wound's area and volume in 3D digital space. (2) Using the recorded history data on volume and area, iDr can help clinicians analyze the effectiveness of wound healing during the treatment.
Experimental Design
Two major experiments were designed: iDr 3D accuracy evaluation and live rat wound healing tracking through iDr for a period of 3 weeks.
-
1)
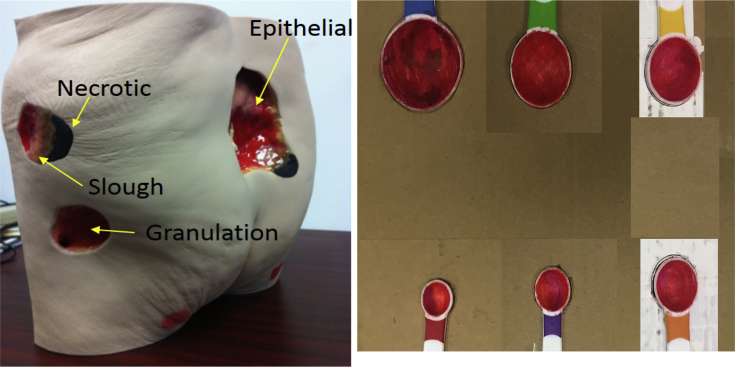
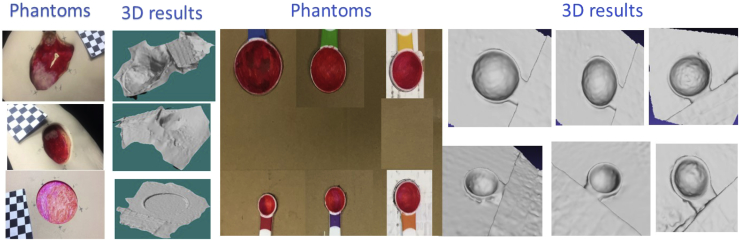
iDr 3D accuracy evaluation (dimension, area, and volume): iDr's 3D accuracy was crucial, as this directly affects the effectiveness of wound growth tracking. We used a 0910 - Seymour II wound care model as shown in Fig. 3. This staging wound model has good representation on various wounds such as diabetic ulcers and bedsores. To obtain conclusive results, we also used 6 partially spherical or ellipsoidal shaped phantom wounds with diameters varying from 15 mm to 40 mm and wound depths varying from 6.4 mm to 18.5 mm.
Figure 3.
A Seymour wound phantom model and 6 partially spherical/ellipsoidal shaped phantom used for the accuracy evaluation.
We used a commercial structured light based 3D industrial camera to capture the same wound phantom. Since the commercial camera had an accuracy level of 50 μ, the acquired 3D data was used as the baseline or ground truth.
We first assessed the accuracy level of distance measurement on iDr. This was done by randomly selecting two points on the phantoms. The distances measured from iDr and the industrial 3D camera were then compared. Multiple distances were obtained to cover the depth, width, height directions of the wound to ensure the generality of the evaluation.
We also obtained wound area and volume from iDr, and the commonly used ruler and Q-tip based methods, and benchmarked against the results from the commercial 3D industrial camera (ground truth or baseline).
The relative errors on the measurements were used for the accuracy assessment. Relative error is defined as , where Dg is the ground truth, and D is the measurement. The difference between D and Dg is taken as absolute value (abs in the formula).
-
2)
Acute rat wound healing tracking for a 3-week duration: This was done to validate the effectiveness of mobile app monitoring for assessing a skin wound. 12 rats were used for this experiment. Each rat had two wounds, one for control and one for treatment, which consisted of a non-viral DNA plasmid encoding for HIF-1alpha. Wounds were created using a 12 mm biopsy punch to achieve a full thickness. The skin was excised down all dermal layers to the panniculus carnosus, leaving the muscle intact for our excisional wounds. Wound healing was studied for a period of 3 weeks and a total of 7 data collection attempts (7 × 12 or total 84 videos) were made (Days 1, 4, 8,11,15,18 and 22 since the excision).
Fig. 4a shows the wound locations on a rat. The left side wound is for control. Fig. 4b show the videoing process for a rat's wound (Day 22).
Figure 4.
a) Rat wound locations, b) Videoing rat wounds.
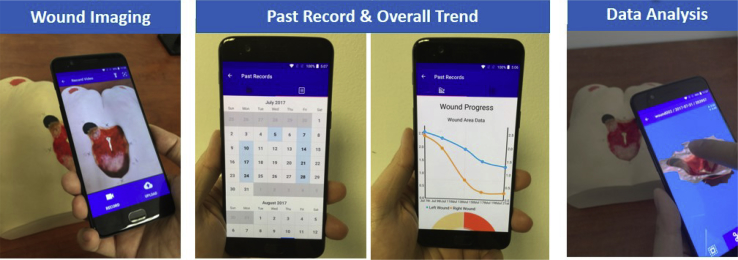
Figure 5.
Preliminary iDr app for wound imaging, wound record, trending, and 3D analysis.
Results
The iDr app contains several useful features including wound imaging, trending, and 3D analysis. As seen in Fig. 5, doctors can utilize the “Past Record” tab to view a simple graph of both wound area and volume over time. For a deeper look, doctors can swipe to the calendar screen, where all the dates with data are highlighted in light blue. Selecting a date brings them to a list of all the videos, pictures, and 3d models generated on that date, so doctors can then view each file for further analysis. iDr allows for easy access of wound history and is a great convenience for clinicians.
Figure 6.
Various 3D models from phantoms.
Through various testing, it was found that the app not only was able to accurately measure wound volume and area on wound phantoms, but it also uniquely displayed a healing trend from live rat wounds.
Accuracy Assessment
For wound distance (width, height, or depth) measurement, a total of 54 randomly selected points were picked across the phantom wounds. A total of 27 measurements were conducted. The distances measured between the iDr and 3D camera method (baseline) are shown in Table 1. Compared with the 3D camera's data, iDr yielded an overall average relative error of 1.66% among all distances (Table 1).
Table 1.
Distance Measurement Assessment on iDr app.
| 3D Camera (cm) | iDr app (cm) | Relative Error | 3D Camera (cm) | iDr app (cm) | Relative Error | 3D Camera (cm) | iDr app (cm) | Relative Error |
|---|---|---|---|---|---|---|---|---|
| 37.27 | 37.25 | 0.05366% | 59.95 | 60.3 | 0.58,382% | 13.28 | 13.235 | 0.33,886% |
| 27.26 | 26.1 | 4.25,532% | 35.81 | 36.265 | 1.27,059% | 15.07 | 14.97 | 0.66,357% |
| 38.44 | 37.745 | 1.80,801% | 39.37 | 40.285 | 2.32,410% | 12.7 | 13.555 | 6.73,228% |
| 39.17 | 38.38 | 2.01685% | 72.77 | 74.19 | 1.95,135% | 30.15 | 30.645 | 1.64,179% |
| 43.3 | 43.8 | 1.15,473% | 59.92 | 60.45 | 0.88,451% | 32.32 | 32.955 | 1.96,473% |
| 50.9 | 50.38 | 1.02161% | 45.46 | 45.775 | 0.69,292% | 30.45 | 29.36 | 3.57,964% |
| 71.3 | 71 | 0.42,076% | 78.88 | 78.95 | 0.08874% | 29.8 | 30.25 | 1.51,007% |
| 48.99 | 48.72 | 0.55,113% | 98.97 | 99.755 | 0.79,317% | 19.81 | 20.885 | 5.42,655% |
| 67.18 | 66.75 | 0.64,007% | 63.46 | 63.185 | 0.43,334% | 80.52 | 83.42 | 3.60,159% |
For the area measurement, a total of 6 different phantom wounds were used. The measurement results from iDr, ruler based method, and industrial 3D camera are obtained in Table 2. Using 3D camera's results as the baseline, the averaged relative measurement error on ruler based method is 36.21%, while iDr's averaged relative measurement error is 1.14%. The error for ruler based method is very close to reported error (up to 40%) in the literature.
Table 2.
Area Measurement Comparison Between 3D Camera, iDr, and Ruler Based Methods.
| Wound Phantoms | 3D Camera (cm2) | iDr App (cm2) | Ruler Based | Relative Error for iDr | Relative Error for Ruler |
|---|---|---|---|---|---|
| 1 | 11.90 | 11.88 | 8.82 | 0.13% | 25.88% |
| 2 | 7.56 | 7.56 | 4.65 | 0.05% | 38.49% |
| 3 | 5.82 | 5.80 | 3.78 | 0.38% | 35.05% |
| 4 | 3.79 | 3.91 | 2.2 | 3.11% | 41.95% |
| 5 | 2.48 | 2.54 | 1.53 | 2.24% | 38.31% |
| 6 | 1.57 | 1.58 | 0.98 | 0.92% | 37.58% |
For the volume measurement, we also used 6 different phantoms for the measurement comparison. Clinically the volume measurement commonly relies Q-tip for the depth estimation and ruler based method for area, we label this method as ruler/Q-tip method. Table 3 shows the volume measurement results from iDr, ruler/Q-tip, and industrial 3D camera. Using 3D camera's results as the baseline, the averaged relative error for iDr is 4.41%. However, the averaged relative error for clinically adopted ruler/Q-tip method exceeded 113%.
Table 3.
Volume Measurement Comparison Between 3D Camera, iDr, and Ruler Based Methods.
| Wound Phantoms | 3D Camera (cm3) | iDr App (cm3) | Ruler/Q-tip Based | Relative Error for iDr | Relative Error for Ruler |
|---|---|---|---|---|---|
| 1 | 14.29 | 14.51 | 33.516 | 1.59% | 134.61% |
| 2 | 7.07 | 7.22 | 14.415 | 2.02% | 103.77% |
| 3 | 4.77 | 4.48 | 10.206 | 6.00% | 114.07% |
| 4 | 2.47 | 2.36 | 4.84 | 4.74% | 95.64% |
| 5 | 1.28 | 1.20 | 2.754 | 5.68% | 115.92% |
| 6 | 0.63 | 0.59 | 1.372 | 6.43% | 116.25% |
Fig. 6 displays various 3D models on phantoms generated from iDr. Models generated from the Seymour wound model are shown on the left. These were utilized to calculate distance between the market points on the border of each wound. Models generated from other phantoms are shown on the right. On the other hand, these were used to measure area and volume.
Wound Growth Tracking
Once its accuracy was proven, iDr was taken to clinical trials on live rats. There, it was tested on a total of 12 lab rats with two wounds on each side of their backs. As mentioned previously, one wound was the control while the other was injected with a treatment.
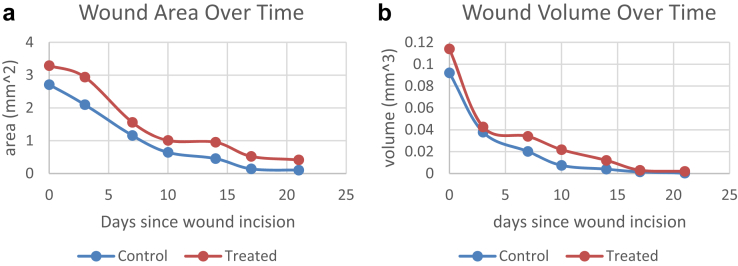
Unfortunately, the 3D commercialized camera was unable to verify these results. Even though the rats were anesthetized with isofluorane, they would still breathe during imaging. Such heavy breathing led to unstable images, so few 3D data could be recovered from commercial 3D camera. As a result, there were no ground truth measurements for calculations involving rat wounds. However, iDr's 3D calculations shows clear trends over time. While both the control and experiment wounds healed, Fig. 7 accurately reflected the decreasing area and volume trends. One of the control wounds began with an area of 2.71 square centimeters and a volume of 0.092 cubic centimeters, decreasing to an area of 0.102 square centimeters and nearly 0 volume after 21 days. Both wound area and volume exhibited similar patterns. Each wound initially decreased quickly in area and volume, then levelled off towards the later dates.
Figure 7.
Wound area (cm2) and volume (cm3) trends for one of the rats.
Discussion
The experiments conducted have answered the following key questions: How accurately does iDr measure wound area and volume? Can iDr provide meaningful or useful growth trends to help clinicians to assess wound healing status?
To assess the calculation accuracy of the iDr app, wound phantoms of different sizes and shapes were used. Wound sizes varied from 1.2 cm to 10 cm in diameter and from 0.3 cm to 3 cm in depth. Wound shapes varied from random shape (2), cylindrical shape (1), to spherical/ellipsoidal shape (6). Still, these phantoms had similar appearances to real wounds. Testing on those diverse wound phantoms ensured the accountability of the results and authenticity of the conclusion.
Measurement assessment indicated iDr has produced only 1.66% relative error on dimension measurement when compared with the ground truth or highly accurate 3D camera data. For the area and volume measurement, iDr only produced 1.14% and 4.41% relative error respectively. At the same time, the ruler and Q-tip based method produced 36.21% and 113% relative error respectively. The evaluation results have clearly showed iDr's advantage in its accuracy compared with commonly adopted ruler/Q-tip based method. It has also produced much smaller error than the 2D planimetry method for area (10%) and combined Q-tip method for volume measurement (52%) reported in the literature.7, 11
A fundamental reason for tracking certain wound parameters is that the trending helps clinicians to identity the healing status. We used iDr to track 24 acute rat wounds (12 for control, and 12 for treatment) over a period of 3 weeks (84 videos). From the experiment results plotted in previous section, it is clear that iDr presented descending trends on wound area and volume for all wounds. Fig. 8 shows that the descending trends were initially quite fast, and then started to flatten out after 10–15 days. Clinicians and residents at the co-author's institute were quite pleased and in agreement with iDr's results. Most wounds indeed started to heal after the second week.
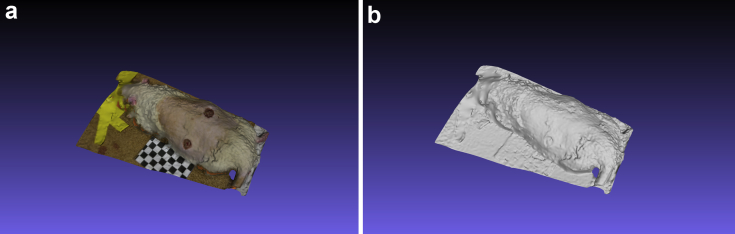
Figure 8.
Example 3D rat image acquired by iDr app: a) with color texture, b) 3D data only. Figures 8a and 8b show example iDr acquired 3D images with and without color texture respectively.
Even though iDr observed some differences between the treated and untreated (control) wound, the differences on all tracked parameters were small. This was also correct per co-author's institute conclusion. A new chitosan for the DNA plasmid on the effectiveness of the treatment was investigated through this animal study. The treatment turned out not very successful.
Conclusion
Through extensive experiments on 9 wound phantoms and 24 acute rat wounds (82 growth videos), the iDr smartphone app has successfully demonstrated the integrated capabilities of accurately measuring wound area and volume (3D). iDr has also proven to provide their trending data over time readily through internet on smartphones. While these capabilities are very important for clinicians to objectively assess wound growth and potential infection as indicated by co-author's institute, achieving them is very challenging. Ruler and 2D digital planimetry based area measurement methods, or any much needed wound volume measurement methods, are far from enough to meet the need of low cost, convenient, remote, and accurate wound assessment.
Specifically, the proposed iDr is found to be superior to the existing ruler based method as it is non-invasive, thus eliminates the chance of infection. In addition, there is no guess work needed on the wound shape. iDr outperforms digital photograph based method because it eliminates the invalid flat wound assumption and the need of orthogonal viewing by constructing a 3D model from a video (or a sequence of images) for measurement. Past researchers have calculated wound volume through invasive liquid filling or expensive 3D structured-light based industrial camera, neither of which are clinically viable solutions. Currently, no clinic technique offers remote assessment capability.
Acknowledgement
The research was partially funded through NIH grant 1R44AR072014.
Footnotes
Conflict of Interest Statement: The research was a joint effort between Xyken LLC and Johns Hopkins Medical School, and partially funded by NIH.
References
- 1.Sen C., Gordillo G., Roy S. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer A., Clark R. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 3.Crovetti G., Martinelli G., Issi M. Platelet gel for healing cutaneous chronic wounds. Transfus Apher Sci. 2004;30:145–151. doi: 10.1016/j.transci.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Office of National Statistics . 2010. Cancer Survival in England: One Year and Five Year Survival for 21 Common Cancers, by Sex and Age. [Google Scholar]
- 5.Rodheaver G., Stotts N. Methods for assessing change in pressure ulcer status. Adv Wound Care. 1995;8:28–34. [PubMed] [Google Scholar]
- 6.Flanagan M. Wound measurement: can it help us to monitor progression to healing? Wound Care Canada. 2003;2(1):46. doi: 10.12968/jowc.2003.12.5.26493. [DOI] [PubMed] [Google Scholar]
- 7.Angela Christine Chang, Bronwyn Dearman, and John Greenwood. A Comparison of Wound Area Measurement Techniques: Visitrak Versus Photography. ePlasty Web. 29 Sept. 2014, 2011. http://www.eplasty.com/index.php?view=article&catid=172%3Avolume-11-eplasty-2011&id=536&format=pdf&option=com_content. [PMC free article] [PubMed]
- 8.Louis D. Photographing pressure ulcers to enhance documentation. Decubitus. 1992;5:38–45. [PubMed] [Google Scholar]
- 9.Rogers Lee, Bevilacqua Nicholas, Andros George. Digital planimetry results in more accurate wound measurements: a comparison to standard ruler measurements. J Diabet Sci Technol. 2010;4(4) doi: 10.1177/193229681000400405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bulstrode L., Goode A., Scott P. Stereophotogrammetry for measuring rates of cutaneous healing: a comparison with conventional techniques. Clin Sci. 1986;71:437–443. doi: 10.1042/cs0710437. [DOI] [PubMed] [Google Scholar]
- 11.Shah A.J., Wollak C., Shah J.B. Wound measurement techniques: comparing the use of ruler method, 2D imaging and 3D scanner. J Am College Clin Wound Spec. 2015;5:52–57. doi: 10.1016/j.jccw.2015.02.001. 52-7. [DOI] [PMC free article] [PubMed] [Google Scholar]