Abstract
Biologically engineered products are medical devices offer support and structure for wound healing by providing a scaffold for cell growth and proliferation. In the field of plastic surgery, these devices are being used to improve the outcomes of surgical closure in selected patients. The purpose of this article is to provide an overview of the source, indications, mechanisms, and outcomes of commonly used biologic products in wound healing. It will also provide an understanding of how biologics can be of value to patients with significant tissue defects requiring plastic surgery.
Keywords: Skin substitutes, Bioprosthetic mesh, Allografts, Xenografts, Plastic surgery
Introduction
There has been an explosion of biologic tissue devices recently. The promise of regenerative medicine with improved healing and faster return to normal function has led to a demand for these products. The demand is primarily driven by health care providers seeking improved outcomes for patients with challenging clinical problems, along with extensive commercial marketing efforts. Most of these products have significant basic science research touting potential clinical benefits. However, high quality randomized clinical trials are lacking for these products. In addition, the cost of these products can significantly impact the financial viability of wound care programs. Physicians and health systems are becoming responsible for the cost of care provided. The purpose of this article is to describe the use of selected biologic products the senior author (R.M.J.) has used as an adjunct to plastic surgery procedures. An introduction to value analysis of biologic products will be also covered.
Methods
Representative plastic surgery case studies utilizing commercially available biologic regenerative medicine products are presented. The authors have no financial relationship to the manufacturers of these products. A background of the mechanism of biologic activity, and approved FDA indications are reported. The senior author's experience with key clinical tips are provided. A primer on value analysis of biologic products is also given.
Case Study 1
A 44 year old female involved in a motorcycle crash suffering a 4th degree road rash injury to the right foot presents with two areas of exposed bone approximately 2 by 2 cm, and the entire wound approximately 15 by 10 cm. Following thorough debridement, the areas of exposed bone were covered with porcine UBM powder and a wound matrix sheet. A wound Vac® was placed over the sheet and two weeks later, a small local flap and skin graft were performed. Complete healing occurred in approximately 6 weeks without the need for free flap reconstruction Fig. 1.
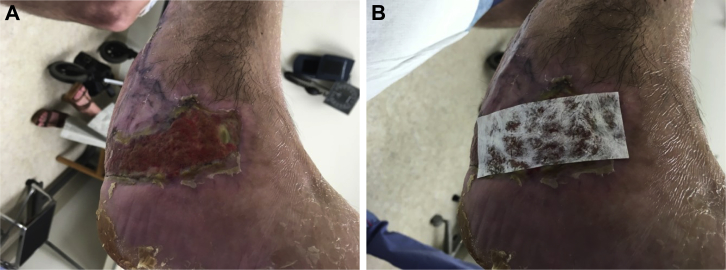
Figure 1.
Case 1 figure A- Motor vehicle crash with exposed bone two areas, B reconstruction of radial collateral ligament of 1st metatarsal with UBM sheet and coverage with UBM micromatrix. And sheet, C wound closure after autograft and local flap, D- final closure 6 months post injury scar still improving.
Acellular Porcine Urinary Bladder
The acellular UBM is a xenograft, derived from porcine urinary bladder and acts as a basement membrane scaffold.1 This product matrix is commercially available as ACell® in both a powder and sheet format. The powder formulation permits distribution throughout the wound due to the powder formulation, and in its low production cost. FDA approved indications include partial and full thickness wounds, second degree burns, pressure ulcers, venous ulcers, diabetic ulcers, tunneling wounds, surgical wounds such as graft donor sites and post Mohs surgical sites, post laser, wound dehiscence, abrasion, laceration, and draining wounds.2 Anecdotally experience has shown improvement in ulcers related to radiation burns.
Key Clinical Tips
Acellular UBM is useful to convert small areas of non-graftable exposed bone into an appropriate wound bed for skin grafting. This can prevent the need for more extensive and costly microsurgical distant tissue transfers.
Case Study 2
A 53 year old male involved in a motor vehicle crash suffered a grade 3 B open fracture to the right leg with extensive de-gloving injury. The patient underwent broad debridement, and a free latissimus dorsi muscle flap with autograft on post trauma day 5. The flap and graft did well but there was further demarcation of the skin on the opposite side of the foot. Local debridement helped to promote granulation tissue development leaving approximately 80 cm2 of remaining open wound. The patient did not desire an additional skin graft, therefore, the option of a skin substitute was given. Oasis® was used as a keratinocyte scaffold to promote re-epithelialization. Successful healing occurred after 5 applications without a return trip to the operating room Fig. 2.
Figure 2.
Case study 2 figure A - large soft tissue defect treated with early Latissimus dorsi free flap and skin graft but further demarcation of lateral soft tissue led to eschar and a small area of tendon exposure. The patient did not want further surgery, B- Oasis® applied to wound 4 times closed without further surgery.
Porcine Small Intestine Submucosa
Commercially available as OASIS® wound matrix, the product is derived from the submucosa layer of the porcine small intestine. The submucosa layer provides much of the strength and flexibility to the small intestine and these properties provide unique aspects to this form of graft.1 OASIS® is available in sheets and creates a scaffold for tissue regrowth. The advantages include ease of application and the ability to avoid autologous skin grafts and donor site morbidity. FDA approved indications include diabetic, pressure, venous, and chronic vascular ulcers, abrasion, laceration, second degree burns, partial and full thickness wounds, draining wounds, post Mohs procedure, post laser, podiatric, wound dehiscence, and skin graft donor site. OASIS® wound matrix is not indicated for patients with porcine allergy or in the setting of third degree burns.3
Key Clinical Tips
Early coverage of extensive soft tissue loss in the lower extremity is best covered with free tissue transfer, and this is best performed in the early post-injury period to reduce the risks of osteomyelitis. Occasionally, touch up grafting or treatment of delayed skin loss is required. These areas may be managed without a return to surgery with skin substitutes such as OASIS®.
Case Study 3
A 67 year old female with morbid obesity and diabetes suffered a short distance fall resulting in an open fracture that was treated with ORIF by the orthopedic service. The patient also had dermal atrophy, where the combination of thin skin and postoperative edema prevented soft tissue closure over the hardware. There was approximately a 4 by 1.5 cm area of exposed hardware evident on examination. Due to the patient's body habitus and location of the wound, free flap reconstruction was not a desirable option. The patient was treated with placement of thick 2 by 4 cm ADM over the exposed hardware. There was some available viable soft tissue that was then tacked over the ADM leaving an exposed area of approximately 3 by 1 cm. The ADM and subcutaneous fat was covered with Integra® and a wound vac. The silastic layer of the Integra was later removed and the entire area was autografted approximately 3 weeks later. Complete healing occurred without the need for flap reconstruction and the graft remained stable at the 2 year follow up appointment Fig. 3.
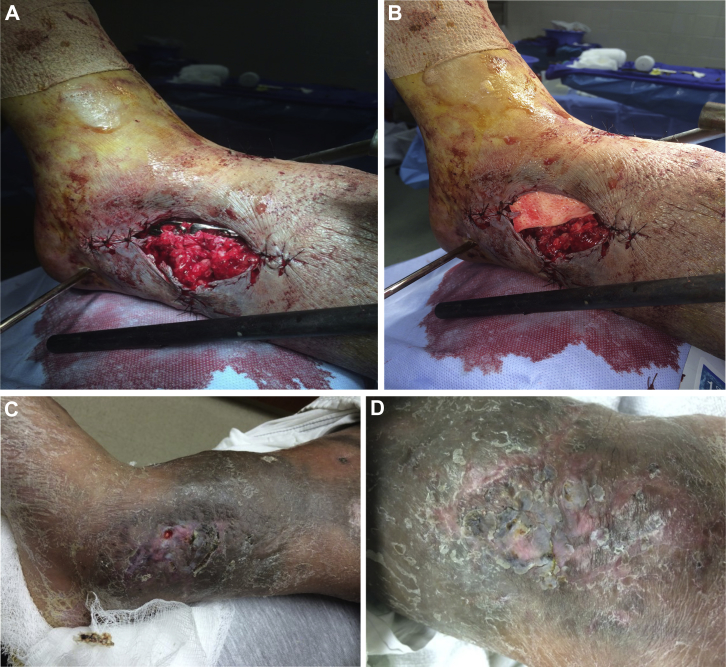
Figure 3.
Case study 3 figure A exposed hardware with small soft tissue deficit morbidly obese unhealthy patient, poor candidate for flap reconstruction, B alloderm™ coverage of the hardware. (partial coverage of the alloderm™ with adjacent fat and integra™ with 2 weeks of negative pressure therapy not shown), C approx. 4 weeks post skin graft, D 3 month post op complete closure with only biologics and skin graft under local anesthesia.
Bilaminate Bovine Dermal Regeneration Template
The acellular bovine dermal regeneration template is commercially available as Integra®. The product is a bioengineered device that is bi-layered with a deep layer consisting of collagen and chondroitin which is used for fibro-vascular ingrowth, and a superficial layer consisting of silicone that functions as a barrier for prevention of moisture loss or microbe invasion.1 The advantages include use for breast and abdominal wall reconstruction, coverage of tendons, reduction in pain to the patient, reduced scarring, and provide excellent wound seal. FDA approval for use of this product includes the repair, reinforcement, or replacement of damaged or inadequate skin or other homologous uses of human integument. Contraindications include any patients exhibiting autoimmune connective tissue disease or gross infection at site of planned application.4
Acellular Dermal Matrix
Commercially, available as Alloderm®, these acellular dermal allograft are composed of extracellular components consisting of the collagen structure of the dermal matrix which has had the epidermis and subcutaneous tissue removed by either freeze drying or chemical detergents.1 These are used in a variety of clinical applications in plastic surgery beyond wound healing and include replacement of fascia for body wall reconstruction, support of marginal tissue coverage, and for use in breast reconstruction. These products are offered in either thick or thin sheets that are terminally sterilized. The advantages in clinical use include less extrusion than prosthetic meshes, and that it can be used in tendon coverage. FDA approval is granted for the use of the AlloDerm® matrix for repair or replacement of damaged or inadequate integument or other homologous uses of human integument. Allergies to any of the antibiotics listed on its package or Polysorbate 20 are contraindications to the use of AlloDerm® matrix.5
Key Clinical Tips
Biologic products are not first line treatment for exposed hardware in lower extremity reconstruction. However, combination therapy (ADM for hardware coverage, Integra® dermal regeneration template and negative pressure therapy) or coverage of small defects can be effective if major flap reconstruction is not ideal. The concept of coverage of avascular surfaces with avascular biologic products is counterintuitive to reconstructive principles. However, these products can be useful as regenerative scaffolds to bridge small gaps of avascular surfaces as long as there is viable adjacent soft tissue. While it is possible these areas may granulate and be grafted over time, the areas tend to be less stable and increase the risk of bone infection.
Case Study 4
A 49 year old male underwent robotic extended abdominoperineal resection for low rectal cancer. He had previously received external beam radiation to the surgical site. At the time of surgery, the radiated levator muscles were not amenable to approximation. A 6 by 6 cm porcine crosslinked dermis commercially available as STRATTICE® was used for repair of the pelvic inlet defect, and a left gracilis muscle was also tunneled into the perineal defect and secured onto the STRATTICE®. The purpose of the muscle was to provide vascularized tissue. The skin and donor sites were closed primarily over drains, and complete healing occurred in 2 weeks.
Acellular Dermal Matrix
STRATTICE® is an acellular dermal xenograft that is FDA approved for use as a soft tissue patch reinforcing where soft tissue weakness exists for damaged or ruptured soft tissue membranes. Known sensitivity to porcine derived materials or Polysorbate 20 are contraindications.6
Key Clinical Tips
Reinforcement of perineal defects are important to prevent difficult-to-treat perineal hernias in a radiated field. The senior author has found the Strattice® product to be ideal in this location since it does not attenuate over time and it is not a synthetic mesh that can adhere and irritate radiated bowel causing extraction or enterocutaneous fistula.
Discussion
Cutaneous wound healing is multifaceted and involves simultaneous activities that intertwine in the process of transforming from a traumatic injury into a stable scar. Classically, the process is broken down into an inflammatory phase involving intrinsic and extrinsic clotting system and acute and chronic inflammatory responses. This is followed by the fibroproliferative stage involving matrix formation, neovascularization by way of angiogenesis and vasculogenesis, and re-epithelialization. Finally, the maturation phase which involves remodeling of the wound structure. The fibroproliferative phase, though typically said to occur 4–21 days following an injury, actually begins almost immediately following tissue damage by the process of re-epithelialization. Cellular adaptations to adjacent keratinocytes allows for migration to the site of the wound. This temporary formed matrix is then replaced over time by granulation tissue consisting of mainly fibroblasts, endothelial cells, and macrophages creating a scaffold for the neovascularization of the wound site. Over time, this temporary matrix will be replaced by collagen formation in the maturation phase.7
The purpose of biologically engineered products for use in plastic surgery is in its application as a scaffold for the acceleration of tissue regeneration during the fibroproliferative phase of wound healing. The products are designed to adhere to the wound bed and help in wound repair by acting as an attachment site for cellular re-epithelialization and proliferation.1, 8 There are many different types of products currently in use with a variety of sources, indications and mechanisms in an attempt to replicate the physiologic and mechanical properties of normal skin. The ideal biomaterial properties would be effective in promoting tissue formation, provide support as a scaffold for cellular growth and proliferation, be chemically inert, non-allergenic, non-carcinogenic, sterilized, be simple to use, and be cost effective.8
The indications for the use of such biologically engineered grafts include exposed tendon, bone, or neovascular structure, radiation wounds, and wounds that have not healed in four months within proper standards of wound care.9 Biologic products come both as allografts, which are derived from cadaveric or neonatal donors, as well as xenografts which are derived from porcine or bovine donors. Additionally, these products can be acellular grafts, which contain no living cells and are immunologically inert, or cellular grafts, which retain living cells. Those grafts that contain living cells may cause an immunogenic host response, and should be considered during discussion of outcomes with the patient. All xenografts are of the acellular type to avoid a host immunogenic response. Acellular allografts are derived from decellularized cadavers and cellular allografts are derived from neonatal foreskin.1 We have highlighted specific products currently available on the market and FDA approved for use, and elaborated on indicated uses as well as off-label applications and outcomes identified within our departments clinical experiences.
The balance in determining the value of different biologic engineered products for use in wound healing is a difficult challenge for many physicians. Outcomes that matter to the patient must be determined and weighed against the costs of the different products to the medical system. Standardization of comorbidities and outcome measures are challenging in the treatment of open wounds. Frequently clinical decision-making is based on opinion and experience alone. Additionally, patient factors vary wildly in the many cofactors that affect the graft outcome and success of the procedure. Furthermore, there are inadequate resources and infrastructure for the collection of such data needed for a true value analysis in most health systems. There is a wide range of cost difference between the types of grafts and price negotiations may play a large role in the actual final cost. This actual cost information is frequently not made available to physicians and thus are not made part of the clinical decision making process.
Conclusion
The senior author's clinical experiences show that biologically engineered products are useful adjuncts to surgical closure in selected patients. These products serve as an effective scaffold that aids in the general goal of wound healing in plastic surgery. In addition, these products can provide structural support and tissue regeneration. The potential to achieve rapid wound closure, and improved functional performance is likely increased with the newer biologic products. However, the majority of current decision-making on which products to use is largely based on experience and clinical judgment alone. Value analysis of these products is complicated and requires more data collection from patients for outcomes and hospital systems for actual product cost and reimbursement information.
References
- 1.Novitsky Y.W., Rosen M.J. The biology of biologics: basic science and clinical concepts. Plast Reconstr Surg. 2012;130(5 suppl 2):S9–S17. doi: 10.1097/PRS.0b013e31825f395b. [DOI] [PubMed] [Google Scholar]
- 2.ACell, ACell Inc., 2015. http://www.acell.com.
- 3.OASIS Wound Matrix, Smith & Nephew Inc., 2015. http://www.oasiswoundmatrix.com.
- 4.Integra. Integra LifeSciences Corporation, 2017. http://www.Integralife.com.
- 5.ALLODERM SELECT Regenerative Tissue Matrix, Allergan, 2017. http://www.lifecell.com/products/allodermtm/selecttm-regenerative-tissue-matrix/.
- 6.STRATTICE Reconstructive Tissue Matrix, Allergan, 2017. http://www.lifecell.com/products/stratticetm/reconstructive-tissue-matrix/.
- 7.Janis J.E., Harrison B. Wound healing: part I. Basic science. Plast Reconstr Surg. 2014;133(2):199e–207e. doi: 10.1097/01.prs.0000437224.02985.f9. [DOI] [PubMed] [Google Scholar]
- 8.Shridharani S.M., Tufaro A.P. A systematic review of acellular dermal matrices in head and neck recon-struction. Plast Reconstr Surg. 2012;130(5 suppl 2):S35–S43. doi: 10.1097/PRS.0b013e31825eff7a. [DOI] [PubMed] [Google Scholar]
- 9.Iorio M.L., Shuck J., Attinger C.E. Wound healing in the upper and lower extremities: a systematic review on the use of acellular dermal matrices. Plast Reconstr Surg. 2012;130(5 suppl 2):S232–S241. doi: 10.1097/PRS.0b013e3182615703. [DOI] [PubMed] [Google Scholar]