Abstract
The recent evolution in immunosuppression therapy has led to significant improvement in short-term kidney allograft outcomes; however, this progress did not translate into similar improvement in long-term graft survival. The latter, at least in part, is likely to be attributed to immunosuppressant side effects. In this review, we focus on the histologic manifestations of calcineurin inhibitor and mammalian target of rapamycin inhibitor toxicity. We discuss the pathologic features attributed to such toxicity and allude to the lack of highly specific pathognomonic lesions. Finally, we highlight the importance of clinicopathologic correlation to achieve a meaningful pathologic interpretation.
Keywords: calcineurin inhibitors, drug toxicity, kidney transplantation, mammalian target of rapamycin, transplant pathology
Acute rejection is the major obstacle for allograft survival in nonimmunosuppressed animals. T-cell activation, which is the main step in initiating rejection following solid organ transplantation, needs 3 sequential signals.1 Signal 1 requires interaction of a major histocompatibility complex molecule that carries an allopeptide on an antigen-presenting cell with a T-cell receptor on the recipient T cell. Signal 2 is provided by the binding of costimulatory molecules on an antigen-presenting cell (e.g., CD80/CD86) with their T-cell counterparts (e.g., CD28). Signals 1 and 2 activate the downstream pathways of calcium-calcineurin, RAS-mitogen-activated protein kinase, and nuclear factor-kappa B pathways, which trigger the expression of several cytokines, including interleukin (IL)-2. Signal 3 follows IL-2/IL-2 receptor binding, which activates mammalian target of rapamycin (mTOR) that leads to T-cell proliferation and clonal expansion. Fully activated T cells can then initiate T-cell–mediated rejection and/or activate B cells to trigger antibody-mediated rejection.1, 2 Disrupting T-cell activation has been the target for immunosuppressive medications in solid organ transplantation. The development of potent immunosuppressive medications has dramatically decreased the incidence of early acute rejection and improved short-term allograft survival.3 Despite this significant improvement, long-term allograft survival remains suboptimal. This may be largely attributed to the limitations of the current immunosuppressive agents related to their toxic side effects and inability to control late smoldering rejection.4
Calcineurin Inhibitors
The introduction of cyclosporine and then tacrolimus has revolutionized the field of solid organ transplantation. Because cyclosporine has been used for a longer time, most data in this field pertain to cyclosporine. However, tacrolimus, which is the current first-line immunosuppressive agent in kidney transplant recipients,5, 6 causes similar functional and structural nephrotoxicity.7, 8, 9 Priming T cells via signal 1 in the presence of signal 2 leads to an increase in intracellular calcium concentration and activates calcineurin. The latter moves to the nucleus and stimulates proinflammatory cytokines that are necessary for T-cell activation.2 Calcineurin inhibitor (CNI) binds intracellular proteins called immunophilins (FK-binding proteins if tacrolimus) to form a complex that can bind and inhibit intracellular calcineurin and, thus, block further T-cell activation.2
Despite being the backbone of current immunosuppression, there is considerable concern that CNI use may lead to significant nephrotoxicity.10 CNI can cause endothelial cell injury and vasoconstriction, which is mediated by production of endothelin, activation of renin-angiotensin II system, and inhibition of vasodilators (such as cyclooxygenase-2 and nitric oxide).7 This vascular toxicity often manifests as hypertension and decreased glomerular filtration rate. CNI is also associated with acute tubular damage, which its mechanism is not completely understood. In addition to ischemia effects, tubular injury can be caused by direct toxicity probably affecting endoplasmic reticulum and mitochondria.7, 11
Acute CNI Toxicity
Acute CNI nephrotoxicity typically occurs early after kidney transplantation, correlates with the period of high CNI exposure, and is often reversible with dose reduction or discontinuation of the drug.12 There are 3 major clinico-pathologic manifestations of acute CNI toxicity: functional/acute arteriolopathy, thrombotic microangiopathy (TMA), and toxic tubulopathy.7
Acute arteriolopathy is a hemodynamically mediated phenomenon that leads to allograft dysfunction secondary to afferent arteriolar constriction and increased renal vascular resistance.13, 14 Sometimes, acute arteriolar toxicity can manifest as arteriolar wall vacuolization, which is a morphologic expression of arteriolar vasospasm (Figure 1a and b).15 That said, arteriolar wall vacuolization is neither sensitive nor specific for CNI toxicity and can be even encountered in a subgroup of implantation biopsies.16
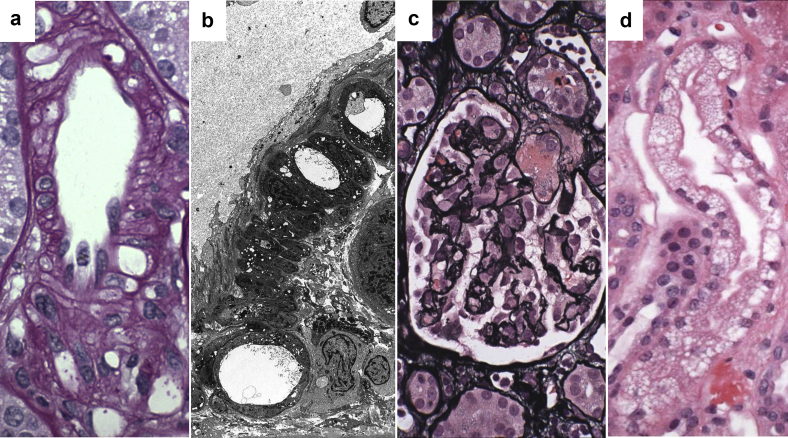
Figure 1.
Acute calcineurin inhibitor toxicity. (a) Light microscopy from a kidney allograft recipient with acute calcineurin inhibitor toxicity reveals arteriolar vacuolization (periodic acid–Schiff, original magnification ×600). (b) Ultrastructural view of coarse large vacuoles in the muscularis of an arteriole from the same patient as in (a) (electron microscopy, original magnification ×3000). (c) Afferent arteriolar thrombosis leading to glomerular wrinkling in a kidney allograft recipient with acute calcineurin inhibitor toxicity. This finding is the most severe form of acute calcineurin inhibitor toxicity (Jones methenamine silver stain, original magnification ×400). (d) Isometric tubular vacuolization in the biopsy from the same patient illustrated in (a,b). Note the small clear cytoplasmic vacuolization, which is referred to as “isometric vacuolization” (hematoxylin and eosin, original magnification ×600).
TMA is a rare manifestation of a more severe form of acute CNI-induced vascular damage.17, 18, 19 Extensive endothelial injury causes endothelial leaking, necrosis, or sloughing that consequently leads to antifibrinolysis, platelet aggregation, and activation of coagulation cascades.18, 20 Accordingly, the biopsy usually shows fibrinoid necrosis and/or thrombi affecting the afferent arterioles, and to a lesser extent, the glomeruli (Figure 1c). The differential diagnosis of CNI-induced TMA should include other forms of TMA, such as accelerated antibody-mediated rejection, anti-phospholipid syndrome, procoagulant states, and recurrent hemolytic–uremic syndrome.20 Therefore, clinical correlation is highly important to obtain a precise diagnosis.
Another feature of acute/subacute CNI toxicity is toxic tubulopathy. The most common histological finding is isometric cytoplasmic vacuolization of the proximal tubules, which is usually focal in nature. These vacuoles are composed of enlarged endoplasmic reticulum. As the name isometric implies, the vacuoles are small and evenly distributed within the cytoplasm (Figure 1d).21 In addition to the enlarged endoplasmic reticulum, the affected tubules can sometimes show giant mitochondria.22 Contrary to acute arteriolopathy, morphologic manifestations of CNI-induced acute tubular toxicity may be seen in protocol biopsies obtained from transplant patients without overt allograft dysfunction.7 Therefore, some investigators do not consider them as critical precursors for chronic allograft damage.23 Tubular vacuolization is not highly specific for acute CNI toxicity and has been documented in allograft biopsies of patients with CNI-free regimens, renal ischemia, or even in association with rejection.9 Isometric vacuolization with more pronounced swelling of the tubular cytoplasm can be seen in “osmotic nephrosis,” which can occur following treatment with i.v. Ig, radiocontrast, or dextran.24, 25 In contrast to isometric vacuolization attributed to CNI, the vacuoles in osmotic nephrosis are usually composed of lysosomes, which contain the culprit agent that enters the cytoplasm via pinocytosis.26
On the other hand, it is also important to keep in mind that CNI-induced tubular toxicity may manifest morphologically as tubular degenerative changes without overt isometric vacuolization. In an early for cause allograft biopsy without concurrent rejection or other known causes of acute tubular necrosis (ATN), the possibility of acute CNI toxicity should be raised when observing a combination of acute tubular and vascular injury, e.g., tubular degenerative changes with prominent arteriolar vacuolization or endothelial swelling.17
Chronic CNI Toxicity
Chronic CNI nephrotoxicity was first described in heart transplant recipients following prolonged exposure to cyclosporine.27 In kidney allograft recipients, chronic CNI nephrotoxicity typically occurs several months posttransplantation and the incidence increases gradually with time, probably due to cumulative and persistent vascular damage. Clinically, it is characterized by hypertension, progressive allograft insufficiency, and variable proteinuria. The histologic indicators of chronic CNI nephrotoxicity include hyaline arteriolopathy, striped tubulointerstitial scarring, and glomerulosclerosis.7, 28
Hyaline arteriolopathy is recognized by some as the “hallmark” of chronic CNI nephrotoxicity. It is likely secondary to prolonged vasoconstriction and persistent damage to endothelial and smooth muscle cells.7, 29, 30 Animal studies have shown that CNI-induced arteriolar damage affects mainly the afferent arterioles and manifests first as granular eosinophilic transformation of smooth muscle cells, which develop later to coarse vacuoles of the muscularis, and finally to amorphous hyaline material.15 In humans, hyaline arteriolopathy is characterized by hyaline nodules that replace injured smooth muscle cells and bulge into the adventitia (Figure 2a and b). With time, these growing nodules can cause significant narrowing of the arteriolar lumina.28, 31 Immunostaining reveals trapping of IgM and C3 at the sites of hyaline accumulation.17 Hyaline arteriolopathy should be distinguished from arteriolar hyalinosis that occurs secondary to persistent mechanical endothelial cell injury that is not accompanied by significant damage to the muscularis, such as seen in association with hypertension and diabetes. As such, the latter hyaline is often, but not always, subendothelial in nature (Figure 2c).17 In diabetes, glycemia can further accelerate endothelial damage through oxidative stress. The systematic toxicity of hyperglycemia can explain the characteristic presence of arteriolar hyalinosis in both afferent and efferent arterioles in diabetic nephropathy.32
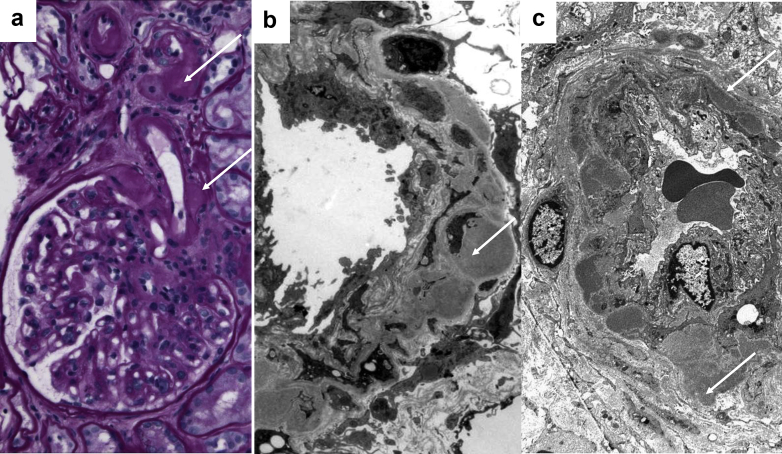
Figure 2.
(a) De novo arteriolar hyalinosis involving the muscularis of an afferent arteriole in a kidney allograft recipient with late graft dysfunction. Note the beaded nature of the hyaline accumulation (arrows, periodic acid–Schiff, original magnification ×400). (b) Electron microscopy from a kidney allograft recipient with late allograft dysfunction attributed to chronic calcineurin inhibitor toxicity reveals hyaline accumulation within the muscularis of an afferent arteriole bulging to the adventitia of the vessel (arrow, electron microscopy, original magnification ×5000). (c) Electron microscopy from a patient with diabetic glomerulosclerosis who was never exposed to calcineurin inhibitors. Note the prominent subendothelial hyaline accumulation. However, this hyalinosis focally extends to the muscularis and bulges to the adventitia to cause a vague beaded appearance (arrows). This finding is rarely observed in diabetic or hypertensive patients (electron microscopy, original magnification ×3000).
Local ischemia secondary to hyaline arteriolopathy and luminal narrowing contribute to the development of striped interstitial fibrosis/tubular atrophy. Hypoxemia from renal vasoconstriction lead to the formation of reactive oxygen species33, 34 and upregulation of transforming growth factor beta by tubular epithelial cells that promotes fibrosis.35 The above changes manifest histologically as interstitial fibrosis and tubular atrophy in a band-like or “striped” pattern.
Ischemia secondary to hyaline arteriolopathy can also lead to glomerulosclerosis and loss of functioning nephrons downstream of the significantly narrowed arterioles. With time, segmental glomerulosclerosis develops in response to compensatory glomerular hyperfiltration in these patients who often have a single functioning allograft. This focal segmental glomerulosclerosis (FSGS) is typically characterized by glomerular enlargement and focal foot process effacement. Such adaptive FSGS should be distinguished from the rare form of recurrent primary FSGS, which is characterized by extensive podocyte damage leading to near complete foot process effacement.31
In general, the histologic lesions that are described in chronic CNI nephrotoxicity are rather nonspecific.36 Clearly glomerulosclerosis is a nonspecific pattern of injury that can follow any glomerular, tubulointerstitial, or vascular damage. In addition to the subjectivity in defining striped fibrosis, the latter has been described in patients with hypertension-associated small vascular disease17, 37 and in patients with long-standing heart failure.38 Although nodular arteriolar hyalinosis of the muscularis is regarded as the typical histologic feature of chronic CNI nephrotoxicity,8, 39 Snanoudj et al.40 showed smooth muscle replacement by hyaline in 28% of kidney allograft recipients who had never received CNI. Another study reported identical degrees of arterial hyalinosis in late kidney allograft biopsies from compliant and noncompliant patients.41 Einecke et al.42 demonstrated the complexity of interpretation of arteriolar hyalinosis, which may reflect donor disease, antibody-mediated rejection, or adequate exposure to CNI rather than true toxicity.
It is becoming apparent that at least some of the chronic changes traditionally attributed to CNI nephrotoxicity in kidney transplant recipients, may be a consequence of previously unrecognized immunologic injury.43 Additionally, CNI-induced vascular sclerosis and hypoperfusion may lead to enhancement of proinflammatory milieu in the graft leading to more tubulointerstitial inflammation and fibrosis. Either way, this may question the benefit of reducing CNI dose to treat what is referred to as “chronic CNI nephrotoxicity,” which some experts even question its existence.44
Histologic Grading
Because arteriolar hyalinosis was traditionally linked to CNI toxicity, the Banff grading system for allograft biopsies had created a semiquantitative scoring system for arteriolosclerosis, which mainly emphasized hyaline accumulation: ah0–3: ah0, no periodic acid–Schiff (PAS)-positive hyaline thickening; ah1, mild to moderate PAS-positive hyaline thickening in at least 1 arteriole; ah2, moderate to severe PAS-positive hyaline thickening in more than 1 arteriole; ah3, severe PAS-positive hyaline thickening in many arterioles.45 However, the aforementioned system was criticized to be very subjective (no clear definition of hyaline location, severity, or number of affected arteries) and incomplete (e.g., no specific score for moderate to severe hyaline thickening in a single arteriole). As a result, it was not surprising that such grading system showed poor interobserver reproducibility.46
Because of these limitations, Mihatsch and colleagues proposed an alternative semiquantitative scoring system (aah0–3) based on a more objective definition of arteriolar hyaline lesions that felt to be more representative of CNI toxicity.21, 39 In this new scoring system, arteriolar hyalinosis is assessed depending on the number of arterioles affected by focal or circumferential hyaline involvement of their smooth muscle layer: aah0, no typical lesions of CNI arteriolopathy; aah1, replacement of degenerated smooth muscle cells by hyaline deposits in only 1 arteriole without circumferential involvement; aah2, replacement of degenerated smooth muscle cells by hyaline deposits in more than 1 arteriole without circumferential involvement; aah3, replacement of degenerated smooth muscle cells by hyaline deposits with circumferential involvement, independent from the number of affected arterioles (Figure 3a and b).8, 47 Compared with the original grading, the alternative scoring system showed improvement in interobserver reproducibility (kappa from 0.52 to 0.67).8 Thus, this alternative system was encouraged by the 2007 Banff meeting on Allograft Pathology to be widely tested,47 but still not formally adapted. With regard to its association with CNI toxicity, the alternative scoring system still suffers from some limitations, especially lack of sensitivity (not all cases of CNI toxicity are expected to show muscular hyalinosis that can be identified at the light microscopic level). With regard to its potential prognostic value, the alternative scoring system may dangerously ignore prominent arteriolar hyalinosis that does not extend into the media (Figure 3c). Notably, neither the original nor the alternative arteriolar hyaline scores correlated with blood levels of either cyclosporine or tacrolimus.8 This might be related to the fact that these levels were only recorded at the time of biopsy and thus may not be representative of the cumulative CNI effects.
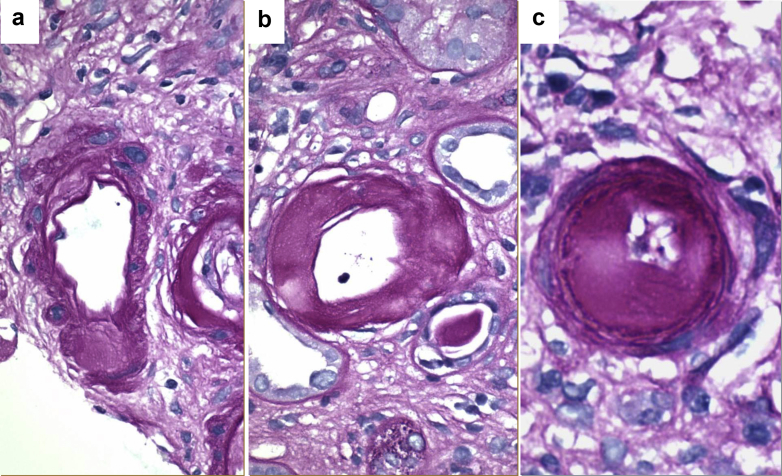
Figure 3.
Representative micro images showing different grades and some limitations of the alternative arteriolar hyalinosis scoring system. (a) Arteriole with nodular replacement of the smooth muscle by hyaline. This met criteria for aah1 or aah2 scoring depending on the number of involved arterioles. (b) Arteriole with circumferential replacement of muscularis by hyaline. These features directly meet criteria for an aah3 score regardless of the number of involved arterioles. (c) Arteriole with severe circumferential subendothelial hyalinosis not replacing the muscularis. Despite the extensive hyalinosis, this arteriole would not meet criteria for a significant aah score (aah0) (all images, periodic acid–Schiff, original magnification ×600).
To further test these scoring systems, we studied kidney transplant recipients at Columbia University who had allograft biopsies between July 2016 and January 2017 with post-reperfusion biopsies available for assessment. The institutional review board approved this unpublished research and PAS sections were used to grade arteriolar hyalinosis using both scoring systems (ah and aah). For the purpose of this study, we calculated Δ ah scores (ah at time of biopsies − ah at time of post-reperfusion biopsy) to evaluate posttransplantation evolution of ah score. The biopsies were obtained on an average of 2.2 years posttransplantation (earlier than the original study of Sis et al.,8 which was 4.4 years after transplantation). In an attempt to assess cumulative tacrolimus exposure, we used a score created by multiplying the average of tacrolimus levels with the posttransplant period (time averaged tacrolimus level = average trough tacrolimus levels [C1min + C2min + C3min … + C×min/×] × posttransplant time [mo]). Our study revealed a few findings that deserve comment. First, we found negative Δah in a minority of patients suggesting sampling error and/or probably some posttransplantation regression of donor-derived arteriolar hyalinosis. Second, our analysis revealed that ah score is significantly higher than aah scores (and also than Δah scores) (Figure 4a), confirming that depending only on the alternative scoring system may hazardously ignore some arteriolar hyalinosis, which may potentially impact prognosis. Third, although all 3 scoring systems correlated significantly with cumulative tacrolimus levels, the best correlation was observed using the Δah scoring system (ah [r = 0.41, P < 0.001], aah [r = 0.38, P < 0.001], and Δah [r = 0.57, P < 0.001]) (Figure 4b). In conclusion, we found no overt advantage of relying only on the alternative scoring system and we recommend comparing arteriolar hyalinosis scores with the ones obtained earlier on to develop an idea about cumulative tacrolimus effect.
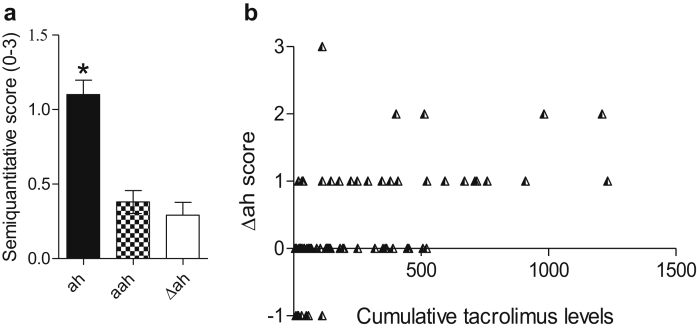
Figure 4.
(a) Average scores for arteriolar hyalinosis (ah; 1.1 ± 0.9), alternative arteriolar hyalinosis (aah; 0.4 ± 0.7), and Δah (0.3 ± 0.8) in our cohort. The ah scores are significantly higher than other scores (1-way analysis of variance: P < 0.001; Tukey multiple comparison test: P < 0.05 ah vs. aah or Δah). (b) Correlation of Δah scores with cumulative tacrolimus levels (Spearman test; r = 0.57, P < 0.001).
mTOR Inhibitors
The use of mTOR inhibitors (e.g., sirolimus and everolimus) aimed to prevent rejection while avoiding undesirable toxic effects attributed to CNI.48 Although these new medications brought high expectations initially because of their additional antifibrosis and anticancer effects,49, 50, 51 mTOR inhibitors could not replace CNI as the main immunosuppression maintenance. In fact, none of the clinical trials have demonstrated improvement in patient or allograft survival in mTOR inhibitor regimens when compared with CNI.52, 53
Similar to tacrolimus, mTOR inhibitors form a complex with the FK506-binding protein 12. However, unlike the tacrolimus–FK506-binding protein 12 complex that inhibits calcineurin, this newly formed complex effectively inhibits mTOR to prevent subsequent protein synthesis and lymphocyte division mainly by blocking IL-2.54, 55 Importantly, mTOR inhibitors can also inhibit vascular endothelial growth factor, which has the same downstream cascade of IL-2.56
Although the main reason for using mTOR inhibitors was to prevent CNI toxic side effects, mTOR inhibitors did not emerge as completely safe medications. Evidence that mTOR inhibitors cause de novo or increased proteinuria is unequivocal.57 Other histological features that have been described with mTOR inhibitors involve FSGS58 (including collapsing FSGS59), TMA,60, 61 ATN,62 and atypical casts.63
Proteinuria and FSGS
In 2003, the development of proteinuria was noted in a subpopulation of renal transplant recipients after conversion from CNI to sirolimus.64 Since then, several studies have documented nephrotic range proteinuria in some patients after such conversion.65, 66 Moreover, a clinical trial in patients with autosomal dominant polycystic kidney disease has revealed higher proteinuria levels in patients who were randomized to receive sirolimus compared with those who received placebo.67
Cinà et al.68 demonstrated that mTOR knockout mice develop proteinuria at 3 weeks of age and end-stage renal failure by 5 weeks. When further assessed, podocytes from these mice showed accumulation of phagolysosomes and damaged mitochondria, suggesting that the proteinuria is likely of glomerular origin.68 This notion is further supported by the high rate of patients with nephrotic range proteinuria and the efficiency of angiotensin conversion enzyme inhibitors in reducing mTOR-induced proteinuria.66, 69 Podocyte injury secondary to downregulation of vascular endothelial growth factor expression is a potential contributing factor.60, 68, 69
The association between mTOR inhibitors and FSGS is more complex. Several case series in kidney transplant patients treated with mTOR inhibitors have described the development of de novo FSGS.58, 59, 64, 70, 71 The few cases that were further characterized by immunohistochemistry showed decreased podocyte expression of vascular endothelial growth factor-A, PAX2, synaptopodin, podocin, and nephrin.58, 72 Only a few small studies showed de novo FSGS development in renal transplant recipients who were maintained on sirolimus and never received CNI.71, 73
Although it is plausible to believe that proteinuria and podocyte damage may accelerate the development of FSGS in the allograft, this phenomenon remains unproven. First FSGS is not infrequently encountered in late allograft biopsies (these patients often have 1 kidney and are prone to several immune and nonimmune injuries that can lead to secondary FSGS). Second, the patients who are usually switched from CNI to mTOR inhibitors most often have significant tubulointerstitial scarring, a well-known risk factor for adaptive FSGS. Third, although it is expected that up to 10% of patients in CNI regimens may develop FSGS lesions,74 there have been no randomized clinical trials to compare FSGS incidence between patients on CNI and a CNI-free mTOR regimen.
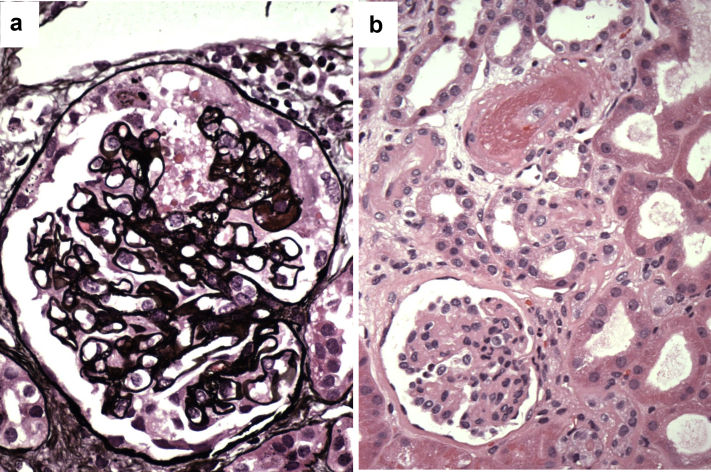
The association of mTOR inhibitors with collapsing FSGS, a poor prognosis variant of FSGS that is characterized by proliferation of glomerular epithelial cells and glomerular tuft collapse, is even more controversial. De novo collapsing FSGS has been reported in 2 patients who were maintained on mTOR inhibitors.59, 70 At Columbia University, we observed 5 cases of collapsing FSGS in patients who were on sirolimus at the time of biopsy (Figure 5a). This is striking given that <5% of our kidney transplant recipients are maintained on such therapy. However, when further assessed, we found that 2 of these 5 patients were simultaneously maintained on tacrolimus and showed TMA on their allograft biopsies. Another patient had an infection with HIV and recent history of TMA, whereas the 2 remaining patients had features of T-cell–mediated rejection on their biopsies (grade 1B and borderline changes). This may suggest that a second hit is needed in patients on mTOR inhibitors to develop collapsing FSGS.
Figure 5.
Mammalian target of rapamycin–induced toxicity. (a) Collapsing focal segmental glomerulosclerosis in a kidney allograft recipient maintained on a combination of rapamycin and tacrolimus who developed proteinuria and acute renal failure. Note the segmental proliferation of glomerular epithelial cells that is associated with glomerular tuft collapse (Jones methenamine silver stain, original magnification ×400). (b) The same biopsy reveals fibrinoid necrosis affecting an afferent arteriole leading to severe glomerular retraction due to secondary ischemia (hematoxylin and eosin, original magnification ×400).
In contrast to the allograft setting, sirolimus has been used with limited success as a treatment for idiopathic/primary FSGS in the native kidney. A clinical trial reported complete or partial remission of proteinuria in 12 of 21 (57%) patients with steroid-resistant primary FSGS who were treated with sirolimus,75 whereas another study reported successful treatment of 3 patients with primary FSGS using sirolimus combined with low-dose steroids.76 It has been proposed that prolonged mTOR activation can lead to podocyte dedifferentiation, thus offering a rationale to use mTOR inhibitors in treating primary FSGS.77
Thrombotic Microangiopathy
Data from several clinical trials suggest an association between sirolimus and TMA.60, 61, 78 This is likely mediated by blocking vascular endothelial growth factor effects and interrupting the cell cycle, and thus, leading to decreased endothelial survival.60, 69
mTOR-associated TMA appears to occur preferentially in patients with concomitant renal injury, such as acute rejection (immunologically mediated)60 or combining CNI and mTOR inhibitor treatment (additional drug-induced injury) (Figure 5b).18, 79, 80 From this point of view, sirolimus may act as a subsequent aggressor to intensify endothelial cell damage and enhance the risk of overt TMA.18, 60, 78
ATN and Atypical Casts
A study published in 2002 evaluated the impact of immunosuppression in kidney transplant recipients with delayed graft function.63 The investigators found that delayed graft function was more common in patients treated with sirolimus and that 12 patients treated with both sirolimus and tacrolimus developed intratubular cast formation that morphologically resembles myeloma cast nephropathy. Although data on kappa and lambda light chain staining were not provided, these casts were composed, at least in part, of degenerating renal tubular cells suggesting a form of tubular injury rather than a dysproteinemia-related renal disease.63
In 2013, ATN was described in 4 patients who were treated with mTOR inhibitors.62 These nontransplant patients received mTOR inhibitors for cancer, in higher doses than that usually received by transplant patients. The authors suggest that induction of autophagy by mTOR inhibitors aggravates tubular dysfunction and leads to ATN.62
In conclusion, both CNI and mTOR inhibitors can cause functional and structural injury to the kidney allografts, which may affect long-term allograft function. At least some of the drug-induced toxicities appear to be dose-related and multifactorial in nature. The limitations of studying CNI and mTOR toxicity include the lack of highly specific morphologic changes or precise immunologic markers that are pathognomonic for such toxicity. The low interobserver reproducibility for CNI toxicity and the lack of randomized trials for mTOR inhibitors further add to these limitations. Therefore, it is of major importance to integrate clinical data to interpret histological changes and guide therapeutic interventions in the right direction.
Disclosure
All the authors declared no competing interests.
References
- 1.Lim M., Kohli J., Bloom R. Immunosuppression for kidney transplantation: Where are we now and where are we going? Transplant Rev (Orlando) 2017;31:10–17. doi: 10.1016/j.trre.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Halloran P. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 3.Sá H., Leal R., Santos Rosa M. Renal transplant immunology in the last 20 years: a revolution towards graft and patient survival improvement. Int Rev Immunol. 2016;28:1–29. doi: 10.1080/08830185.2016.1225300. [DOI] [PubMed] [Google Scholar]
- 4.Modena B., Kurian S., Gaber L. Gene expression in biopsies of acute rejection and interstitial fibrosis/tubular atrophy reveals highly shared mechanisms that correlate with worse long-term outcomes. Am J Transplant. 2016;16:1982–1998. doi: 10.1111/ajt.13728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.KDIGO KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9:S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 6.Webster A., Woodroffe R., Taylor R. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: Meta-analysis and meta-regression of randomised trial data. BMJ. 2005;331:810. doi: 10.1136/bmj.38569.471007.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naesens M., Kuypers D., Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4:481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 8.Sis B., Dadras F., Khoshjou F. Reproducibility studies on arteriolar hyaline thickening scoring in calcineurin inhibitor-treated renal allograft recipients. Am J Transplant. 2006;6:1444–1450. doi: 10.1111/j.1600-6143.2006.01302.x. [DOI] [PubMed] [Google Scholar]
- 9.Randhawa P., Shapiro R., Jordan M. The histopathological changes associated with allograft rejection and drug toxicity in renal transplant recipients maintained on FK506. Am J Surg Pathol. 1993;17:60–68. doi: 10.1097/00000478-199301000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cattaneo D., Perico N., Gaspari F., Remuzzi G. Nephrotoxic aspects of cyclosporine. Transplant Proc. 2004;36:234–239. doi: 10.1016/j.transproceed.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Pallet N., Rabant M., Xu-Dubois Y. Response of human renal tubular cells to cyclosporine and sirolimus: a toxicogenomic study. Toxicol Appl Pharmacol. 2008;229:184–196. doi: 10.1016/j.taap.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Thölking G., Gerth H., Schuette-Nuetgen K., Reuter S. Tacrolimus metabolism rate on renal function after solid organ transplantation. World J Transplant. 2017;7:26–33. doi: 10.5500/wjt.v7.i1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray B., Paller M., Ferris T. Effect of cyclosporine administration on renal hemodynamics in conscious rats. Kidney Int. 1985;28:767–774. doi: 10.1038/ki.1985.196. [DOI] [PubMed] [Google Scholar]
- 14.Bobadilla N., Gamba G. New insights into the pathophysiology of cyclosporine nephrotoxicity: a role of aldosterone. Am J Physiol Renal Physiol. 2007;293:F2–F9. doi: 10.1152/ajprenal.00072.2007. [DOI] [PubMed] [Google Scholar]
- 15.Young B., Burdmann E., Johnson R. Cyclosporine A-induced arteriolopathy in a rat model of chronic cyclosporine nephropathy. Kidney Int. 1995;48:431–438. doi: 10.1038/ki.1995.311. [DOI] [PubMed] [Google Scholar]
- 16.Horike K., Takeda A., Yamaguchi Y. Is arteriolar vacuolization a predictor of calcineurin inhibitor nephrotoxicity? Clin Transplant. 2011;25:23–27. doi: 10.1111/j.1399-0012.2011.01474.x. [DOI] [PubMed] [Google Scholar]
- 17.Liptak P., Ivanyi B. Primer: histopathology of calcineurin-inhibitor toxicity in renal allografts. Nat Clin Pract Nephrol. 2006;2:398–404. doi: 10.1038/ncpneph0225. [DOI] [PubMed] [Google Scholar]
- 18.Ponticelli C. De novo thrombotic microangiopathy. An underrated complication of renal transplantation. Clin Nephrol. 2007;67:335–340. doi: 10.5414/cnp67335. [DOI] [PubMed] [Google Scholar]
- 19.Bren A., Pajek J., Grego K. Follow-up of kidney graft recipients with cyclosporine-associated hemolytic-uremic syndrome and thrombotic microangiopathy. Transplant Proc. 2005;37:1889–1891. doi: 10.1016/j.transproceed.2005.02.112. [DOI] [PubMed] [Google Scholar]
- 20.Ponticelli C., Banfi G. Thrombotic microangiopathy after kidney transplantation. Transpl Int. 2006;19:789–794. doi: 10.1111/j.1432-2277.2006.00354.x. [DOI] [PubMed] [Google Scholar]
- 21.Mihatsch M., Ryffel B., Hermle M. Morphology of cyclosporine nephrotoxicity in the rat. Clin Nephrol. 1986;25:S2–S8. [PubMed] [Google Scholar]
- 22.Chapman J. Chronic calcineurin inhibitor nephrotoxicity—lest we forget. Am J Transplant. 2011;11:693–697. doi: 10.1111/j.1600-6143.2011.03504.x. [DOI] [PubMed] [Google Scholar]
- 23.Naesens M., Kambham N., Concepcion W. The evolution of non-immune histological injury and its clinical relevance in adult-sized kidney grafts in pediatric recipients. Am J Transplant. 2007;7:2504–2514. doi: 10.1111/j.1600-6143.2007.01949.x. [DOI] [PubMed] [Google Scholar]
- 24.Bollée G., Anglicheau D., Loupy A. High-dosage intravenous immunoglobulin-associated macrovacuoles are associated with chronic tubulointerstitial lesion worsening in renal transplant recipients. Clin J Am Soc Nephrol. 2008;3:1461–1468. doi: 10.2215/CJN.00500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haas M., Sonnenday C., Cicone J. Isometric tubular epithelial vacuolization in renal allograft biopsy specimens of patients receiving low-dose intravenous immunoglobulin for a positive crossmatch. Transplantation. 2004;24:549–556. doi: 10.1097/01.tp.0000137199.32333.03. [DOI] [PubMed] [Google Scholar]
- 26.Dickenmann M., Oettl T., Mihatsch M. Osmotic nephrosis: acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am J Kidney Dis. 2008;51:491–503. doi: 10.1053/j.ajkd.2007.10.044. [DOI] [PubMed] [Google Scholar]
- 27.Myers B., Ross J., Newton L. Cyclosporine-associated chronic nephropathy. N Engl J Med. 1984;311:699–705. doi: 10.1056/NEJM198409133111103. [DOI] [PubMed] [Google Scholar]
- 28.Nankivell B., P’Ng C., O’Connell P., Chapman J. Calcineurin inhibitor nephrotoxicity through the lens of longitudinal histology: comparison of cyclosporine and tacrolimus eras. Transplantation. 2016;100:1723–1731. doi: 10.1097/TP.0000000000001243. [DOI] [PubMed] [Google Scholar]
- 29.Amberg G., Rossow C., Navedo M. NFATc3 regulates Kv2.1 expression in arterial smooth muscle. J Biol Chem. 2004;279:47326–47334. doi: 10.1074/jbc.M408789200. [DOI] [PubMed] [Google Scholar]
- 30.Nieves-Cintron M., Amberg G., Nichols C. Selective downregulation of KV2.1 Function contributes to enhanced arterial tone during diabetes. J Biol Chem. 2007;282:3231–3240. [Google Scholar]
- 31.Morozumi K., Takeda A., Uchida K., Mihatsch M. Cyclosporine nephrotoxicity: how does it affect renal allograft function and transplant morphology? Transpl Proc. 2004;36:S251–S256. doi: 10.1016/j.transproceed.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 32.Alsaad K., Herzenberg A. Distinguishing diabetic nephropathy from other causes of glomerulosclerosis: an update. J Clin Pathol. 2007;60:18–26. doi: 10.1136/jcp.2005.035592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Djamali A. Oxidative stress as a common pathway to chronic tubulointerstitial injury in kidney allografts. Am J Physiol Renal Physiol. 2007;293:F445–F455. doi: 10.1152/ajprenal.00037.2007. [DOI] [PubMed] [Google Scholar]
- 34.Longoni B., Boschi E., Demontis G. Apoptosis and adaptive responses to oxidative stress in human endothelial cells exposed to cyclosporin A correlate with BCL-2 expression levels. FASEB J. 2001;15:731–740. doi: 10.1096/fj.00-0163com. [DOI] [PubMed] [Google Scholar]
- 35.Vieira J.J., Noronha I., Malheiros D., Burdmann E. Cyclosporine-induced interstitial fibrosis and arteriolar TGF-beta expression with preserved renal blood flow. Transplantation. 1999;68:1746–1753. doi: 10.1097/00007890-199912150-00019. [DOI] [PubMed] [Google Scholar]
- 36.Mengel M., Mihatsch M., Halloran P. Histological characteristics of calcineurin inhibitor toxicity—there is no such thing as specificity! Am J Transplant. 2011;11:2549–2550. doi: 10.1111/j.1600-6143.2011.03719.x. [DOI] [PubMed] [Google Scholar]
- 37.Farris A., Alpers C. What is the best way to measure renal fibrosis? A pathologist's perspective. Kidney Int Suppl. 2011;4:9–15. doi: 10.1038/kisup.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis R., Verani R., Vo C. Evaluation of chronic renal disease in heart transplant recipients: importance of pretransplantation native kidney histologic evaluation. J Heart Lung Transplant. 1994;13:376–380. [PubMed] [Google Scholar]
- 39.Mihatsch M., Kyo M., Morozumi K. The side-effects of ciclosporine-A and tacrolimus. Clin Nephrol. 1998;49:356–363. [PubMed] [Google Scholar]
- 40.Snanoudj R., Royal V., Elie C. Specificity of histological markers of long-term CNI nephrotoxicity in kidney-transplant recipients under low-dose cyclosporine therapy. Am J Transplant. 2011;11:2635–2646. doi: 10.1111/j.1600-6143.2011.03718.x. [DOI] [PubMed] [Google Scholar]
- 41.Lerut E., Kuypers D., Verbeken E. Acute rejection in noncompliant renal allograft recipients: a distinct morphology. Clin Transplant. 2007;21:344–351. doi: 10.1111/j.1399-0012.2007.00647.x. [DOI] [PubMed] [Google Scholar]
- 42.Einecke G., Reeve J., Halloran P. Hyalinosis lesions in renal transplant biopsies: time-dependent complexity of interpretation. Am J Transplant. 2017;17:1346–1357. doi: 10.1111/ajt.14136. [DOI] [PubMed] [Google Scholar]
- 43.Gaston R. Chronic calcineurin inhibitor nephrotoxicity: reflections on an evolving paradigm. Clin J Am Soc Nephrol. 2009;4:2029–2034. doi: 10.2215/CJN.03820609. [DOI] [PubMed] [Google Scholar]
- 44.Matas A. Chronic progressive calcineurin nephrotoxicity: an overstated concept. Am J Transplant. 2011;11:687–692. doi: 10.1111/j.1600-6143.2011.03505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Racusen L., Solez K., Colvin R. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1990;55:713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 46.Furness P., Taub N., Assmann K. International variation in histologic grading is large, and persistent feedback does not improve reproducibility. Am J Surg Pathol. 2003;27:805–810. doi: 10.1097/00000478-200306000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Solez K., Colvin R., Racusen L. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 48.Kahan B. Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. The Rapamune US Study Group. Lancet. 2000;356:194–202. doi: 10.1016/s0140-6736(00)02480-6. [DOI] [PubMed] [Google Scholar]
- 49.Weichhart T., Costantino G., Poglitsch M. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Pontrelli P., Rossini M., Infante B. Rapamycin inhibits PAI-1 expression and reduces interstitial fibrosis and glomerulosclerosis in chronic allograft nephropathy. Transplantation. 2008;85:125–134. doi: 10.1097/01.tp.0000296831.91303.9a. [DOI] [PubMed] [Google Scholar]
- 51.Chiarini F., Evangelisti C., McCubrey J.A., Martelli A.M. Current treatment strategies for inhibiting mTOR in cancer. Trends Pharmacol Sci. 2015;36:124–135. doi: 10.1016/j.tips.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Webster A., Lee V., Chapman J., Craig J. Target of rapamycin inhibitors (TOR-I; sirolimus and everolimus) for primary immunosuppression in kidney transplant recipients. Cochrane Database Syst Rev. 2006;(2):CD004290. doi: 10.1002/14651858.CD004290.pub2. [DOI] [PubMed] [Google Scholar]
- 53.Lim W., Eris J., Kanellis J. A systematic review of conversion from calcineurin inhibitor to mammalian target of rapamycin inhibitors for maintenance immunosuppression in kidney transplant recipients. Am J Transplant. 2014;14:2106–2119. doi: 10.1111/ajt.12795. [DOI] [PubMed] [Google Scholar]
- 54.Wiederrecht G., Sabers C., Brunn G. Mechanism of action of rapamycin: new insights into the regulation of G1-phase progression in eukaryotic cells. Prog Cell Cycle Res. 1995;1:53–71. doi: 10.1007/978-1-4615-1809-9_5. [DOI] [PubMed] [Google Scholar]
- 55.Weichhart T., Hengstschläger M., Linke M. Regulation of innate immune cell function by mTOR. Nat Rev Immunol. 2015;15:599–614. doi: 10.1038/nri3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ko H., Yin J., Wyburn K. Sirolimus reduces vasculopathy but exacerbates proteinuria in association with inhibition of VEGF and VEGFR in a rat kidney model of chronic allograft dysfunction. Nephrol Dial Transplant. 2013;28:327–336. doi: 10.1093/ndt/gfs453. [DOI] [PubMed] [Google Scholar]
- 57.Diekmann F., Andrés A., Oppenheimer F. mTOR inhibitor–associated proteinuria in kidney transplant recipients. Transplant Rev (Orlando) 2012;26:27–29. doi: 10.1016/j.trre.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 58.Letavernier E., Bruneval P., Mandet C. High sirolimus levels may induce focal segmental glomerulosclerosis de novo. Clin J Am Soc Nephrol. 2007;2:326–333. doi: 10.2215/CJN.03751106. [DOI] [PubMed] [Google Scholar]
- 59.Dogan E., Ghanta M., Tanriover B. Collapsing glomerulopathy in a renal transplant recipient: potential molecular mechanisms. Ann Transplant. 2011;16:113–116. doi: 10.12659/aot.881874. [DOI] [PubMed] [Google Scholar]
- 60.Sartelet H., Toupance O., Lorenzato M. Sirolimus-induced thrombotic microangiopathy is associated with decreased expression of vascular endothelial growth factor in kidneys. Am J Transplant. 2005;5:2441–2447. doi: 10.1111/j.1600-6143.2005.01047.x. [DOI] [PubMed] [Google Scholar]
- 61.Crew R., Radhakrishnan J., Cohen D. De novo thrombotic microangiopathy following treatment with sirolimus: report of two cases. Nephrol Dial Transplant. 2005;20:203–209. doi: 10.1093/ndt/gfh334. [DOI] [PubMed] [Google Scholar]
- 62.Izzedine H., Escudier B., Rouvier P. Acute tubular necrosis associated with mTOR inhibitor therapy: a real entity biopsy-proven. Ann Oncol. 2013;24:2421–2425. doi: 10.1093/annonc/mdt233. [DOI] [PubMed] [Google Scholar]
- 63.Smith K., Wrenshall L., Nicosia R. Delayed graft function and cast nephropathy associated with tacrolimus plus rapamycin use. J Am Soc Nephrol. 2003;14:1037–1045. doi: 10.1097/01.asn.0000057542.86377.5a. [DOI] [PubMed] [Google Scholar]
- 64.Morelon E., Kreis H. Sirolimus therapy without calcineurin inhibitors: Necker Hospital 8-year experience. Transplant Proc. 2003;35:52S–57S. doi: 10.1016/s0041-1345(03)00244-6. [DOI] [PubMed] [Google Scholar]
- 65.Sennesael J., Bosmans J., Bogers J. Conversion from cyclosporine to sirolimus in stable renal transplant recipients. Transplantation. 2005;80:1578–1585. doi: 10.1097/01.tp.0000184623.35773.6a. [DOI] [PubMed] [Google Scholar]
- 66.Letavernier E., Peraldi M., Pariente A. Proteinuria following a switch from calcineurin inhibitors to sirolimus. Transplantation. 2005;80:1198–1203. doi: 10.1097/01.tp.0000185200.17589.74. [DOI] [PubMed] [Google Scholar]
- 67.Serra A., Poster D., Kistler A. Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med. 2010;363:820–829. doi: 10.1056/NEJMoa0907419. [DOI] [PubMed] [Google Scholar]
- 68.Cinà D., Onay T., Paltoo A. Inhibition of MTOR disrupts autophagic flux in podocytes. J Am Soc Nephrol. 2012;23:412–420. doi: 10.1681/ASN.2011070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Letavernier E., Legendre C. mTOR inhibitors-induced proteinuria: mechanisms, significance, and management. Transplant Rev (Orlando) 2008;22:125–130. doi: 10.1016/j.trre.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 70.Izzedine H., Brocheriou I., Frances C. Post-transplantation proteinuria and sirolimus. N Engl J Med. 2005;353:2088–2089. doi: 10.1056/NEJM200511103531922. [DOI] [PubMed] [Google Scholar]
- 71.Sabry A., Elagroudy A., El-dahshan K. Is sirolimus a nephrotoxic drug? A report of five cases. Transplant Proc. 2007;39:1406–1409. doi: 10.1016/j.transproceed.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 72.Stallone G., Infante B., Pontrelli P. Sirolimus and proteinuria in renal transplant patients: evidence for a dose-dependent effect on slit diaphragm-associated proteins. Transplantation. 2011;91:997–1004. doi: 10.1097/TP.0b013e318211d342. [DOI] [PubMed] [Google Scholar]
- 73.Markowitz G., Bomback A., Perazella M. Drug-induced glomerular disease: direct cellular injury. Clin J Am Soc Nephrol. 2015;10:1291–1299. doi: 10.2215/CJN.00860115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Takeda A., Uchida K., Haba T. Chronic cyclosporin nephropathy: long-term effects of cyclosporin on renal allografts. Clin Transplant. 2001;15(Suppl 5):22–29. doi: 10.1034/j.1399-0012.2001.0150s5022.x. [DOI] [PubMed] [Google Scholar]
- 75.Tumlin J., Miller D., Near M. A prospective, open-label trial of sirolimus in the treatment of focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2006;1:109–116. doi: 10.2215/CJN.00120605. [DOI] [PubMed] [Google Scholar]
- 76.Tsagalis G., Psimenou E., Iliadis A. Rapamycin for focal segmental glomerulosclerosis: a report of 3 cases. Am J Kidney Dis. 2009;54:340–344. doi: 10.1053/j.ajkd.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 77.Zschiedrich S., Bork T., Liang W. Targeting mTOR signaling can prevent the progression of FSGS. J Am Soc Nephrol. Mar 2017;28:2144–2157. doi: 10.1681/ASN.2016050519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saikali J., Truong L., Suki W. Sirolimus may promote thrombotic microangiopathy. Am J Transplant. 2003;3:229–230. doi: 10.1034/j.1600-6143.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 79.Robson M., Côte I., Abbs I. Thrombotic micro-angiopathy with sirolimus-based immunosuppression: potentiation of calcineurin-inhibitor-induced endothelial damage? Am J Transplant. 2003;3:324–327. doi: 10.1034/j.1600-6143.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 80.Fortin M., Raymond M., Madore F. Increased risk of thrombotic microangiopathy in patients receiving a cyclosporin-sirolimus combination. Am J Transplant. 2004;4:946–952. doi: 10.1111/j.1600-6143.2004.00428.x. [DOI] [PubMed] [Google Scholar]