ABSTRACT
Objective: The present study was designed to investigate the molecular mechanism and biological roles of lncRNA brain-derived neurotrophic factor antisense (lncRNA BDNF-AS) in acute spinal cord injury (ASCI). Methods: The rat model of ASCI and hypoxic cellular model were established to detect the expression of BDNF-AS, miR-130b-5p, PR (PRDI-BF1 and RIZ) domain protein 5 (PRDM5) and cleaved caspase 3 (c-caspase 3) using qRT-PCR and western blot. Basso, Beattie and Bresnahan (BBB) score was carried out to assess neurological function. Flow cytometry was used to determine the apoptosis of neuronal cells. The association among BDNF-AS, miR-130b-5p and PRDM5 were disclosed by RNA immunoprecipitation (RIP) assay, RNA pull-down assay and dual-luciferase reporter assay. Results: BDNF-AS, PRDM5 and c-caspase 3 expression were significantly upregulated, while miR-130b-5p was suppressed in the ASCI group and neuronal cells following hypoxia treatment. BDNF-AS knockdown inhibited neuronal cell apoptosis. Further studies indicated that BDNF-AS functioned as a competing endogenous RNA (ceRNA) by sponging miR-130b-5p in neuronal cells. Further investigations demonstrated that PRDM5 was a target of miR-130b-5p and BDNF-AS knockdown exerted anti-apoptotic effects via miR-130b-5p/PRDM5 axis. Conclusion: The lncRNA BDNF-AS/miR-130b-5p/PRDM5 axis might be a promising therapeutic target for ASCI.
KEYWORDS: Acute spinal cord injury, lncRNA BDNF-AS, miR-130b-5p, PRDM5, apoptosis
Introduction
It was well documented that spinal cord injury (SCI) was a serious and disabling condition with high mortality and disability that caused a series of clinical symptoms, including severe movement dysfunction, muscle atrophy and changes in sensation, resulting in severe neurological damage and dysfunction [1]. Despite great efforts have been made to improve the functional outcome of patients with SCI, secondary injury induced by acute SCI (ASCI) still remains the main cause of neurological dysfunction which is characterized by neuronal apoptosis in the central nervous system [2]. Therefore, exploration of molecular mechanisms mediating neuronal cell apoptosis in ASCI was useful in clinical diagnosis and treatment of ASCI.
PR (PRDI-BF1 and RIZ) domain proteins (PRDM) were a subfamily of the kruppel-like zinc finger gene products that modulated cellular processes such as cell differentiation, growth and apoptosis. PRDM5 was a recently identified family member that was functioned as a novel transcriptional depressor and a broad tumor suppressor in various types of human cancers, such as gastric, colorectal, liver and lung cancers [3]. Recently, accumulating evidence has strongly implied that the expression of PRDM5 was significantly increased in neurons in a rat model of ASCI [3]. Moreover, the depletion of PRDM5 in vitro by short interfering RNA (siRNA) significantly inhibited neuronal cell apoptosis [4]. Ling et al reported that the inhibition of PRDM5 decreased the apoptosis of spinal cord neurons (SCN) and improved the neurological function of rats [5], indicating that PRDM5 played a vital role in ASCI. However, the regulation of the PRDM5 inhibition-mediated protective effect on neuronal cell apoptosis remained elusive.
Long noncoding RNAs (lncRNAs) were defined as a novel class of RNA transcripts longer than 200 nucleotides with narrow protein coding functions. Emerging data have revealed that lncRNAs were involved in regulating various cell biological processes, such as cell growth, differentiation and apoptosis [6]. The brain-derived neurotrophic factor antisense RNA (BDNF-AS) was a naturally conserved noncoding antisense RNA transcript that negatively regulated the transcription of BDNF in various human and animal tissues [7]. A recent study has declared that BDNF-AS knockdown was a novel method to prevent neurotoxicity in mouse embryonic neural stem cell (ESC)-derived neurons [8]. However, the function of BDNF-AS on the apoptosis of neurons in ASCI has not been reported yet.
MiRNAs were another crucial class of non-coding with endogenous 21–23 nucleotides, which effectively regulated post-transcriptional eukaryotic gene expression [9]. MiRNAs have become critical regulators in the pathophysiology of ASCI. Recently, Jonsson et al. [10] found miR-130b-5p to be specifically associated with neural progenitors, implying the functional role of miR-130b-5p in neuronal development. At the post-transcriptional level, lncRNAs could serve as miRNA sponges and modulate the occurrence and development of ASCI [11]. Therefore, we focused on the interaction between BDNF-AS and miR-130b-5p in neuronal cell apoptosis.
In the present study, BDNF-AS was up-regulated while miR-130b-5p was decreased in the spinal cord tissues in ASCI rat model. Besides, knockdown of BDNF-AS in vitro reduced the neuron apoptosis. According to the bioinformatics analysis, we found that miR-130b-5p might be bound to BDNF-AS, and miR-130b-5p were predicted to have the bind site on PRDM5. Hence, we speculated that BDNF-AS served as a competing endogenous RNA (ceRNA) to up-regulate PRDM5 via sponging miR-130b-5p in the progression of ASCI. Our study aimed to explore the underlying mechanisms of BDNF-AS inhibition in the attenuation of neuronal apoptosis via miR-130b-5p/PRDM5 axis in ASCI rats.
Materials and methods
Animals
A total of 14 male Sprague-Dawley (SD) rats (weighing 230–270 g) were obtained from Center for Animal Experiment of Henan province (Zhengzhou, Henan, China). All animals were housed in standard conditions of controlled temperature (23–25°C) with 12 h light/dark cycle and freely fed and watered. These rats were randomized to two groups: Sham group and ASCI group (n = 7 per group). Animal experiments performed in our study were approved by the Animal Ethics Committee of The First Affiliated Hospital of Zhengzhou University.
ASCI model
The rat ASCI model was induced by extradural compression using a modification of Allen’s method as previously described [12]. Briefly, SD rats were anaesthetized by intraperitoneal injection of 10% chloral hydrate (3 mL/kg). Under aseptic conditions, rats’ backs were shaved to expose the T9-11 spinous process and vertebral segments. Afterwards, the T10 spinous process was subjected to impact trauma by compression at an interval of 12.5 mm for 20 s to produce severe injury. The successful ASCI model was defined as the paralysis of the lower limbs. Besides, rats in the Sham group underwent accordant surgery except inflicted crush injury. On the 1st day after operation, hind limb paralysis and motor deficits appeared in rats. Seven days after operation, locomotor behavior was monitored and scored using the Basso, Beattie and Bresnahan (BBB) open field test. Subsequently, rats were euthanized with an overdose of 10% chloral hydrate (10 mg/kg) and spinal cord tissues at the injury epicenter were isolated for quantitative real-time PCR (qRT-PCR) and western blot.
Cell line and hypoxia treatment
Neuronal cell lines AGE1.HN (American Type Culture Collection, ATCC, Rockville, MD, USA) and PC12 (BioVector NTCC, Beijing, China) were maintained in dulbecco’s modified eagle medium (DMEM; Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, US) and 100 μg/mL penicillin/streptomycin (Life Technologies) in an incubator at 37°C with 5% CO2. AGE1.HN and PC12 cells were cultured in 96-well plates and subjected to hypoxic treatment in a hypoxic incubator containing 3% O2, 5% CO2 and 92% N2 at 37°C for 24 h or incubated under normoxic conditions as controls. qRT-qPCR and western blot analysis were performed to detect the expression of BDNF-AS, miR-130b-5p, PRDM5 and cleaved caspase 3 (c-caspase 3).
RNA interference
To explore the effect of BDNF-AS knockdown on neuronal cell apoptosis, AGE1.HN and PC12 cells were respectively divided into four groups: control, hypoxia, hypoxia+ si-control and hypoxia+ siRNA-BDNF-AS. The siRNA transfection was performed on cells seeded at 1 × 104 cells/well in 96-well plates using X-tremeGENE siRNA Transfection Reagent (Roche Applied Science, Mannheim, Germany) following the manufacturer’s instructions. The concentration of siRNA was 1nM (Supplemental Figure1(a)), and the treatment time was 24 h. Cells in each group were harvested to determine cell apoptosis using flow cytometry and c-caspase 3 protein levels using western blot, respectively.
Figure 1.
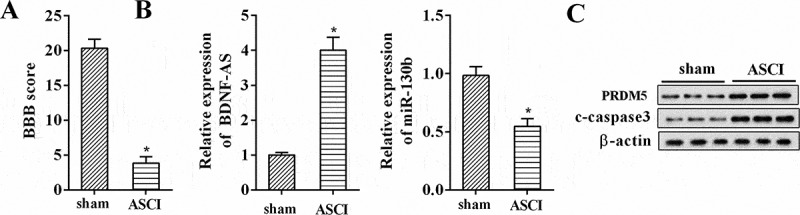
Changes of gene expression in rat model of ASCI. (a) Basso, Beattie, and Bresnahan (BBB) scores for hindlimb locomotion in the rat SACI model group and the sham group (n = 7 per group). (b) The relative expression levels of BDNF-AS and miR-130b-5p in the rat SACI model group and the sham group (n = 7 per group) using qRT-PCR. (c) The protein expression levels of PRDM5 and c-caspase 3 in the rat SACI model group and the sham group (n = 7 per group) using western blot. Data were expressed as mean ± SD. *P < 0.05 vs. the sham group.
RNA immunoprecipitation and RNA pull down
DIANA tools (http://carolina.imis.athena-innovation.gr/) were used to predict the target sites of BDNF-AS and miR-130b-5p. RIP assay was conducted using Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, Billerica, MA, USA) according to the manufacturer’s instructions. In brief, PC12 cells were rinsed with cold PBS and lysed in radioimmunoprecipitation buffer at 4°C for 30min. Cell extracts were incubated with protein A/G sepharose beads conjugated to antibodies against Ago2 (Millipore) or normal mouse immunoglobulin G (IgG; Millipore). Immunoprecipitated RNA and total RNA from the whole cell lysates (input controls) were extracted for western blot and qRT-PCR analysis.
The interaction between BDNF-AS and miR-130b-5p was further examined by RNA pull-down using a Pierce Magnetic RNA-Protein Pull-Down Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Protein extracts from PC12 cells were mixed with 50 pmol of biotinylated BDNF-AS and incubated with 50 µL of streptavidin agarose magnetic beads (Life Technologies) at 4°C for 1 h. The associated RNA-protein complex was eluted with Biotin Elution Buffer and then boiled in SDS buffer for another 10 min. The retrieved Ago2 protein levels were detected using western blot, while miR-130b-5p mRNA expression levels were measured by qRT-PCR using IgG as the negative control (NC).
Luciferase reporter assay
Targetscan online bioinformatics software (http://www.targetscan.org) was used to identify the underlying binding sites of miR-130b-5p and PRDM5. To verify whether PRDM5 was a direct target of miR-130b-5p, we carried out luciferase experiments in PC12 cells. The recombinant plasmids (PRDM5-WT and PRDM5-Mut) were respectively co-transfected with miR-130b-5p mimic or inhibitor or their corresponding negative controls into PC12 cells. Twenty-four hours later, cells were harvested and incubated with passive lysis buffer at room temperature for 10 min. Luciferase activity was determined using the Dual Luciferase Assay kit (Promega, Madison, WI, USA) following the manufacturer’s instructions.
Cell transfection
The BDNF-AS sequence was synthesized and subcloned into the pcDNA3.1 vector (pcDNA-BDNF-AS; Invitrogen), while BDNF-AS-specific siRNA (siRNA-BDNF-AS), miR-130b-5p mimics and miR-130b-5p inhibitors were designed and synthesized by GenePharma Corporation (Shanghai, China). The empty pCDNA3.1 vector (pcDNA) and scrambled siRNA (si-control) as well as the corresponding scrambled miRNAs (pre-NC and NC) were used as the negative controls.
We then investigated the molecular mechanism of BDNF-AS in neuronal cell lines. AGE1.HN and PC12 cells were randomized to 4 groups as follows: pcDNA, pcDNA-BDNF-AS, pcDNA-BDNF-AS+ pre-NC and pcDNA-BDNF-AS+ miR-130b-5p mimic or si-control, siRNA-BDNF-AS, siRNA-BDNF-AS+ NC and siRNA-BDNF-AS+ miR-130b-5p inhibitor. Before transfection, cells were seeded in 6-well plates at a density of 4 × 105 cells/ml for 24 h. When cell confluence reached more than 70%, transfection of plasmids was performed using Lipofectamine 2000 reagent (Invitrogen). After 24 h transfection, cells in each group were subjected to qRT-PCR analysis to determine the expression levels of miR-130b-5p and western blot to measure the protein levels of PRDM5.
To further explore the biological function of BDNF-AS in neuronal cell lines, AGE1.HN and PC12 cells were transfected with si-control, siRNA-BDNF-AS, siRNA-BDNF-AS+ NC and siRNA-BDNF-AS+ miR-130b-5p inhibitor followed by hypoxia treatment. Cell apoptosis and c-caspase 3 protein levels were detected 24h post-transfection.
Lentivirus production and infection
The siRNA-BDNF-AS or scrambled oligonucleotides were ligated into the LV-3 vector (GenePharma). The viruses were packaged in HEK293T cells according to standard protocols. A total volume of 5 µL packaged lentivirus (LV-si-control and LV-siRNA-BDNF-AS) were intrathecally injected into ASCI rats using a glass micro-pipette (n = 7 per group). Seven days after injection, locomotor behavior was scored using BBB open field test. Then, rats were euthanized and spinal cord tissues at the injury epicenter were isolated for qRT-PCR analysis.
BBB score
Spinal cord function was measured by BBB score at one-week post-injury. Three same independent and well-trained investigators blinded to the treatment group observed the movement of rats and scored the locomotor function according to the BBB scales. The final score of each animal was obtained by averaging the values from every examiner.
RNA extraction and quantitative real-time PCR
Total RNA was extracted from spinal cord tissues or neuronal cells using TRIzol (Life Technologies) according to the manufacturer’s protocols. To quantify BDNF-AS and miR-130b-5p expression, 1 μg RNA was reverse-transcribed to cDNA using a MiRcute miRNA First-strand cDNA synthesis kit (Tiangen Biotech, Beijing, China) according to the manufacturer’s protocol. Relative quantification of lncRNA and miRNA expression levels were performed on an ABI 7500 Real-Time PCR system (Applied Biosystems, Carlsbad, CA, USA) and calculated by the 2−ΔΔCt method using MiRcute miRNA qPCR detection kit (Tiangen Biotech). The relative expression of lncRNAs and miRNAs are normalized to GAPDH and U6 snRNA expression, respectively.
Western blot
Total protein was obtained using Radio-Immunoprecipitation Assay (RIPA) buffer (Beyotime, Shanghai, China) followed by separated with 6% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred to polyvinylidene fluoride (PVDF) membranes with 350mA for 4h. Afterwards, PVDF membranes were blocked with tris buffered saline tween (TBST) containing 5% skim milk at room temperature for 1 h, followed by incubation with primary antibodies against PRDM5 (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and c-caspase 3 (1:500; Cell Signaling Technology, Boston, MA, USA) at 4°C overnight. Blots were incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibodies (1:200; Abcam, Cambridge, UK) at room temperature for 1–2 h and visualized using enhanced chemiluminescence (Thermo Scientific) by a Molecular Imager ChemiDoc XRS System (Bio-Rad Laboratories, Hercules, CA, USA). Band intensities were standardized to β-action (Sigma-Aldrich, St. Louis, MO, USA) as a loading control.
Cell apoptosis assay
Cell apoptosis was analyzed by flow cytometry using Annexin V-FITC apoptosis detection kit (BD Biosciences; San Jose, CA, USA) according to the manufacturer’s protocols. AGE1.HN and PC12 cells following transfection and hypoxia treatment were collected and washed with cold PBS. Then, cells (1 × 105 cells/mL) were stained with binding buffer containing Annexin V-FITC and propidine iodide (PI) at 4°C under darkness for 15 min. Afterwards, cells were recorded using flow cytometry (Beckman Coulter, Fullerton, CA, USA).
Terminal-deoxynucleotidyl transferase mediated nick end labeling (TUNEL)
Spinal cord tissues were paraffin-embedded and cut into 5-μm-thick slides for TUNEL assay. Briefly, slides were de-waxed in xylene for 2 times, and rehydrated in graded alcohols (100%, 95%, 80%, 75%) for 5 min each. Then, slides were placed in 3% H2O2 at room temperature for 10 min. After that, slides were incubated with 20 μg/mL Proteinase K at room temperature. Fifteen minutes later, slides were washed with PBS for 3 times and incubated in TUNEL reaction mixture at 37°C for 60 min (protected form light). Next, slides were washed with PBS for 3 times and incubated with horse reddish peroxidase-streptavidin reagent (1:200) for at room temperature. Thirty minutes later, slides were washed with PBS 3 times, and then counterstained with hematoxylin. After washed with distilled water for 2, slides were cover-slipped with mounting medium and observed under a light microscope. The number of TUNEL positive cells was counted.
Statistical analysis
The data were expressed as mean ± standard deviation (SD). Statistical analyses were performed using SPSS version 22.0 (SPSS, Chicago, IL, USA). Significant differences between groups were analyzed using two-sided Student’s t test, and P < 0.05 was considered to be statistically significant.
Results
Expression patterns of BDNF-AS, miR-130b-5p, PRDM5 and c-caspase 3 following ASCI in rats and hypoxia treatment in neuronal cells
In this study, we firstly established the animal ASCI model and the BBB open field test was used to evaluate locomotor behaviour. As shown in Figure 1(a), the BBB score of rats in ASCI model group was significantly lower than those of rats in the sham group (P < 0.05), indicating that the rat model of ASCI had been successfully established. We then examined the differential expressed lncRNA BDNF-AS, miR-130b-5p, PRDM5 and c-caspase 3 in ASCI using qRT-PCR and western blot, respectively. The results demonstrated that BDNF-AS was increased in the ASCI group compared with the sham group, whereas miR-130b-5p was decreased (Figure 1(b), P < 0.05). Meanwhile, PRDM5 and c-caspase 3 protein levels were up-regulated as expected (Figure 1(c)). The same expression changes were observed in AGE1.HN and PC12 cell lines after hypoxia treatment (Figure 2(a) and 2(b), P < 0.05).
Figure 2.
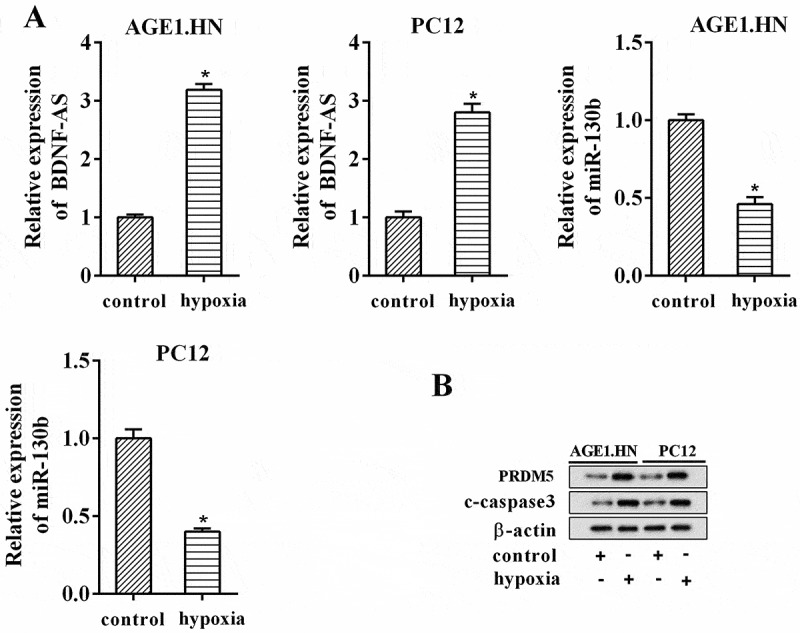
Changes of gene expression in hypoxia-induced cellular ASCI model. (a) The relative expression levels of BDNF-AS and miR-130b-5p in neuronal cell lines AGE1.HN and PC12 following hypoxia and normoxia treatment. (b) The protein expression levels of PRDM5 and c-caspase 3 in neuronal cell lines AGE1.HN and PC12 following hypoxia and normoxia treatment. Data were expressed as mean ± SD. *P < 0.05 vs. the control group.
Knockdown of BDNF-AS reduced neuronal cell apoptosis
To confirm the effect of BDNF-AS on neuronal cell apoptosis, neuronal cells were treated with hypoxia and siRNA-BDNF-AS. As shown in Figure 3(a), hypoxia treatment induced the apoptosis of neuronal cells (P < 0.05), while knockdown of BDNF-AS attenuated the apoptosis after hypoxia treatment (P < 0.05). In addition, we also measured the changes in c-caspase-3 expression after hypoxia treatment and inhibition of BDNF-AS using western blot. Compared with the neuronal cells in the control group, hypoxia treatment led to an increase in c-caspase-3 expression, however, knockdown of BDNF-AS alleviated the expression of c-caspase-3 (Figure 3(b)). These data manifested that BDNF-AS knockdown had a protective effect on ASCI.
Figure 3.
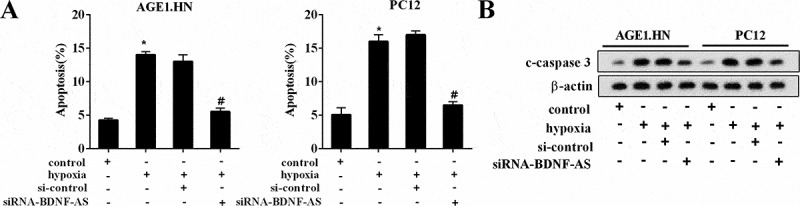
Effects of BDNF-AS on neuronal cell apoptosis. The percentage of apoptotic neuronal cells (AGE1.HN and PC12 cells) in the group of control, hypoxia, hypoxia+si-control and hypoxia+siRNA-BDNF-AS detected by flow cytometry. The protein expression levels of c-caspase 3 in neuronal cells (AGE1.HN and PC12 cells) in the group of control, hypoxia, si-control and siRNA-BDNF-AS. Data were expressed as mean ± SD. *P < 0.05 vs. the control group. #P < 0.05 vs. the hypoxia+ si-control group.
BDNF-AS directly targeted mir-130b-5p
As described above, lncRNA could serve as miRNA sponge, thereby mediating the development of ASCI [11]. We thus hypothesized that BDNF-AS knockdown-induced neuroprotective effects might operate through regulating miRNAs expression. Firstly, according to bioinformatics tools that were used to predict the potential miRNAs regulated by BDNF-AS, we found that BDNF-AS had binding sites with miR-130b-5p, miR-489-3p, miR-136-5p, miR-129-5p, miR-30a-5p and miR-185 (all of the miRNAs were associated with SCI [13–17], data not shown). To investigate which miRNA expression could be significant affected by BDNF-AS, PC12 cells were transfected with pcDNA-BDNF-AS or si-BDNF-AS. Results showed that overexpression or interference of BDNF-AS had the most significant effect on miR-130b-5p expression (Supplemental Figure 1(b,c)). Therefore, we chose miR-130b-5p for follow-up experiments.
The binding sites between BDNF-AS and miR-130b-5p were illustrated in Figure 4(a). RNA immunoprecipitation was performed using an antibody against AGO2 from nuclear extracts of PC12 cells. We found the significant enrichment of miR-130b-5p (Figure 4(b), P < 0.05) and BDNF-AS (Figure 4(b), P < 0.05) using AGO2 antibody compared with IgG antibody. We further confirmed the association between BDNF-AS and miR-130b-5p using RNA pull down. Figure 4(c) showed a higher expression level of biotin-labeled miR-130b-5p in BDNF-AS pulled down pellet was than that of beads and negative control (P < 0.05), suggesting an association between BDNF-AS and miR-130b-5p.
Figure 4.
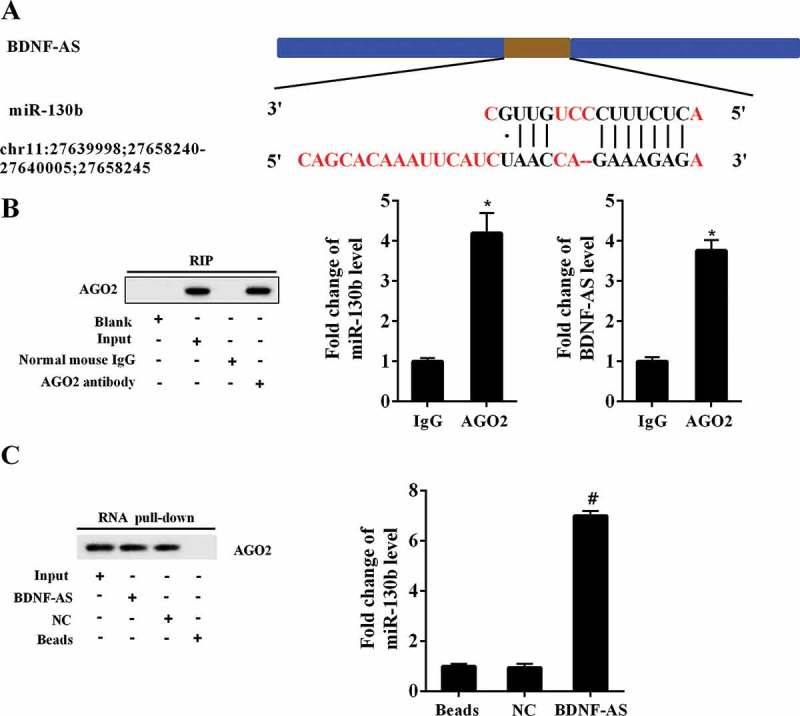
The interaction between BDNF-AS and miR-130b-5p. (a) The binding sites on the BDNF-AS and miR-130b-5p predicted by DIANA tools. (b) RIP experiment with anti-Ago2, IgG as negative control or input as a positive control from PC12 cells extracts using western blot and qRT-PCR. Data were expressed as mean ± SD. *P < 0.05 compared with IgG group. (c) Ago2 expression levels and fold enrichment of miR-130b-5p expression after RNA pull-down experiment with PC12 cells extracts in different groups. Data were expressed as mean ± SD. #P < 0.05 compared with NC group.
PRDM5 was a downstream target of mir-130b-5p
Bioinformatics analysis (TargetScan) found that PRDM5 3ʹUTR had binding sites with miR-130b-5p, miR-129-5p, miR-494, miR-30b and miR-34a (all of the miRNAs were associated with SCI [15,18–20], data not shown). To investigate which miRNA could significant affect PRDM5 expression, PC12 cells were transfected with miRNAs mimic or inhibitor. Results showed that overexpression or interference of miR-130b-5p had the most significant effect on PRDM5 expression (Supplemental Figure 1(d,e)). Therefore, we chose miR-130b-5p for follow-up experiments. The binding sites between miR-130b-5p and PRDM5 3ʹUTR were shown in Figure 5(a). Then, we performed luciferase reporter assay to verify whether miR-130b-5p targeted PRDM5. The results showed that miR-130b-5p overexpression significantly decreased the luciferase activity of PRDM5-WT (Figure 5(b), P < 0.05), whereas knockdown of miR-130b-5p increased luciferase activity (Figure 5(c), P < 0.05). However, PC12 cells co-transfected with PRDM5-Mut and miR-130b-5p mimic or inhibitor showed no obvious changes in their luciferase activity. In addition, we found that PRDM5 mRNA and protein expressions were decreased after overexpressing miR-130b-5p, whereas they were increased after transfection with miR-130b-5p inhibitor. Collectively, miR-130b-5p negatively regulated PRDM5 expression.
Figure 5.
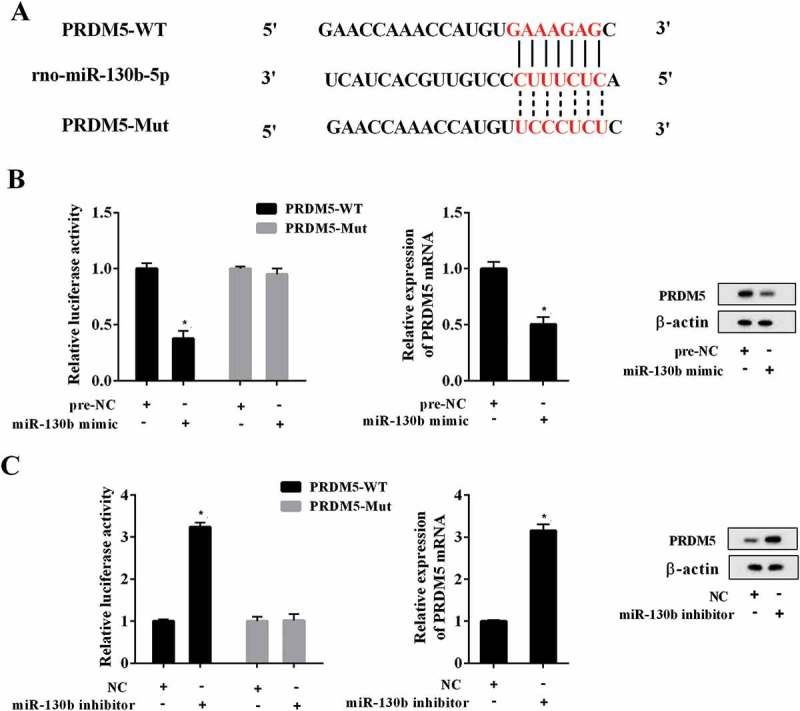
Effects of miR-130b-5p on PRDM5 expression. (a) The potential gene target of miR-130b-5p predicted by mircoRNA.org online database. (b) Relative luciferase activity of PC12 cells co-transfected with miR-130b-5p mimic/pre-NC and PRDM5-3ʹUTR-WT or PRDM5-3ʹUTR-MUT plasmid. The mRNA and protein expression levels of PRDM5 in PC12 cells transfected with miR-130b-5p mimic or pre-NC. (C) Relative luciferase activity of PC12 cells co-transfected with miR-130b-5p inhibitor/NC and PRDM5-3ʹUTR-WT or PRDM5-3ʹUTR-MUT plasmid. The mRNA and protein expression levels of PRDM5 in PC12 cells transfected with miR-130b-5p inhibitor or NC. Data were expressed as mean ± SD. *P < 0.05 compared with the pre-NC or NC group.
BDNF-AS positively regulated PRDM5 via targeting mir-130b-5p in neuronal cells
We then elucidated the underlying molecular mechanism about how BDNF-AS exerted its effects on neuronal cells. Notably, BDNF-AS overexpression reduced the expression of miR-130b-5p (Figure 6(a), P < 0.05) and increased PRDM5 protein expression (Figure 6(b)), while miR-130b-5p overexpression in BDNF-AS-overexpressing AGE1.HN and PC12 cells led to an opposite effect. Conformably, the inhibition of BDNF-AS upregulated miR-130b-5p (Figure 6(c), P < 0.05) and decreased PRDM5 expression (Figure 6(d)). However, the knockdown of BDNF-AS and miR-130b-5p reversed above-mentioned trends. Jointly, BDNF-AS directly targeted miR-130b-5p, thereby regulating PRDM5 expression in neurocytes.
Figure 6.
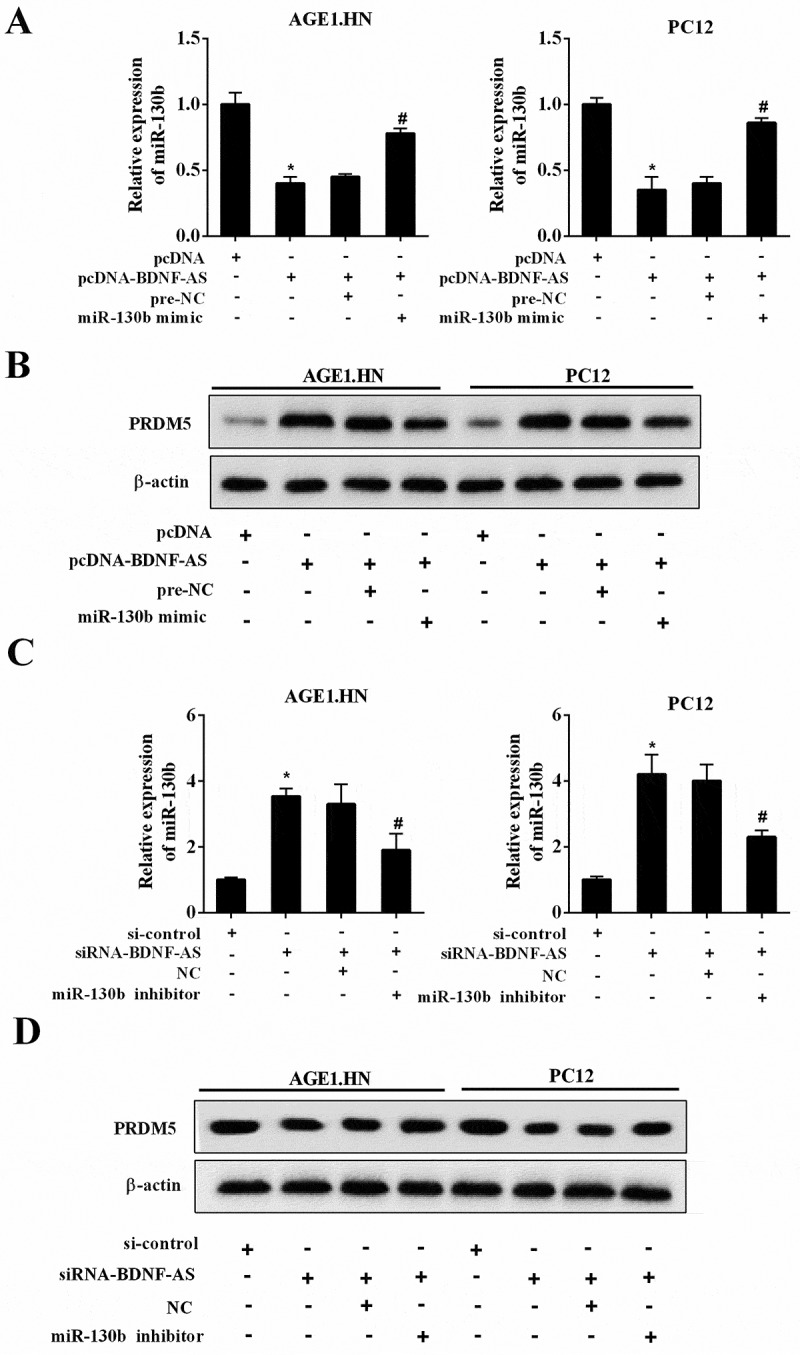
The molecular mechanism of BDNF-AS in neuronal cells. (a) The mRNA expression of miR-130b-5p in neuronal cells (AGE1.HN and PC12 cells) in the group of pcDNA, pcDNA-BDNF-AS, pcDNA-BDNF-AS+pre-NC and pcDNA-BDNF-AS+miR-130b-5p mimic. (b) The protein expression of PRDM5 in neuronal cells (AGE1.HN and PC12 cells) in the group of pcDNA, pcDNA-BDNF-AS, pcDNA-BDNF-AS+pre-NC and pcDNA-BDNF-AS+miR-130b-5p mimic. Data were expressed as mean ± SD. *P < 0.05 vs. the pcDNA group. #P < 0.05 vs. the pcDNA-BDNF-AS+pre-NC group. (c) The mRNA expression of miR-130b-5p in neuronal cells (AGE1.HN and PC12 cells) in the group of si-control, siRNA-BDNF-AS, siRNA-BDNF-AS+NC and siRNA-BDNF-AS+miR-130b-5p inhibitor. (d) The protein expression of PRDM5 in neuronal cells (AGE1.HN and PC12 cells) in the group of si-control, siRNA-BDNF-AS, siRNA-BDNF-AS+NC and siRNA-BDNF-AS+miR-130b-5p inhibitor. Data were expressed as mean ± SD. *P < 0.05 vs. the si-control group. #P < 0.05 vs. the siRNA-BDNF-AS+NC group.
BDNF-AS attenuated neuronal cell apoptosis by regulating mir-130b-5p
Next, we investigated whether BDNF-AS could exert its inhibitory effects on the apoptosis of neuronal cells via targeting miR-130b-5p. Dramatically, BDNF-AS knockdown reduced the neuronal cell apoptosis (Figure 7(a), P < 0.05) and downregulated c-caspase 3 expression (Figure 7(b)) following hypoxia treatment. Cell apoptosis and c-caspase 3 expression were significantly reversed in cells co-transfected with siRNA-BDNF-AS and miR-130b-5p inhibitor. Thus, we demonstrated that BDNF-AS could exert its inhibitory effects of neuronal cell apoptosis via targeting miR-130b-5p.
Figure 7.
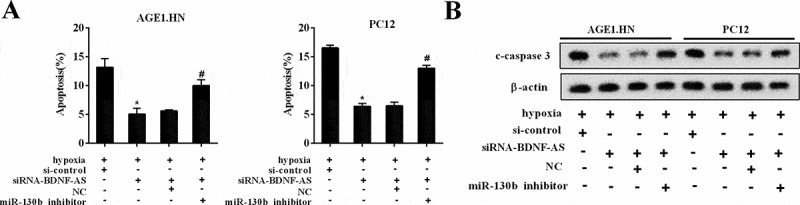
The biological functions of BDNF-AS in neuronal cells. (a) The percentage of apoptotic neuronal cells (AGE1.HN and PC12 cells) in the group of si-control, siRNA-BDNF-AS, siRNA-BDNF-AS+NC and siRNA-BDNF-AS+miR-130b-5p inhibitor. (b) The protein expression of c-caspase-3 in neuronal cells (AGE1.HN and PC12 cells) in the group of si-control, siRNA-BDNF-AS, siRNA-BDNF-AS+NC and siRNA-BDNF-AS+miR-130b-5p inhibitor. Data were expressed as mean ± SD. *P < 0.05 vs. the si-control group. #P < 0.05 vs. the siRNA-BDNF-AS+NC group.
BDNF-AS knockdown relieved apoptosis through mir-130b-5p/prdm5 axis in the rat ASCI model
To further validate whether the miR-130b-5p/PRDM5 axis was involved in the anti-apoptotic effects of BDNF-AS knockdown in rats of ASCI model, LV-si-control and LV-siRNA-BDNF-AS were respectively injected into the ASCI model rats. The BBB score was reduced in the LV-siRNA-BDNF-AS group compared with the LV-si-control group (Figure 8(a), P < 0.05), revealing that BDNF-AS knockdown promoted neural functional recovery. Furthermore, qRT-PCR analysis showed that LV-siRNA-BDNF-AS injection in ASCI model decreased BDNF-AS expression and upregulated miR-130b-5p expression (Figure 8(b), P < 0.05). Western blot analysis showed that BDNF-AS knockdown inhibited the expression of PRDM5 and c-caspase3 (Figure 8(c)). TUNEL assay revealed that LV-siRNA-BDNF-AS inhibited neuronal apoptosis in spinal cord tissue of ASCI rats (Figure 8(d), P < 0.01). Taken together, these results suggested that BDNF-AS knockdown inhibited the expression of PRDM5 through competitively binding with miR-130b-5p, resulting in alleviating apoptosis after ASCI.
Figure 8.
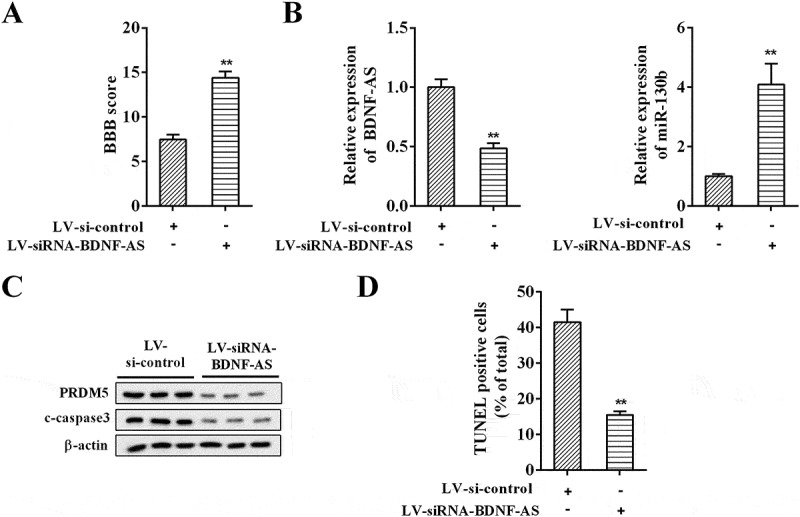
The anti-apoptotic effects of BDNF-AS in rat ASCI model. (a) The BBB scores for hindlimb locomotion in rat model of ASCI injected of LV-si-control or LV-siRNA-BDNF-AS (n = 7 per group). (b) The expression of BDNF-AS and miR-130b-5p in rat model of ASCI injected of LV-si-control or LV-siRNA-BDNF-AS (n = 7 per group). (c) The expression of PRDM5 and c-caspase3 in rat model of ASCI injected of LV-si-control or LV-siRNA-BDNF-AS (n = 7 per group). (d) The neuronal apoptosis in spinal cord tissue of ASCI rats. Data were expressed as mean ± SD. **P < 0.05 or **P < 0.01 vs. the LV-si-control group.
Discussion
Our results suggested that the expressions of BDNF-AS and PRDM5 were significantly up-regulated, whereas, miR-130b-5p expression was decreased in the spinal cord tissues of ASCI model rats. In addition, knockdown of BDNF-AS improved hindlimb locomotor activity in rats following ASCI and inhibited neuronal cell apoptosis. Our findings also demonstrated that BDNF-AS inhibition exerted an anti-apoptotic effect by targeting miR-130b-5p, thereby negatively regulating an important pro-apoptotic gene, PRDM5 in ASCI rats.
In the past years, studies showed that lncRNAs played important roles in the occurrence and progression of several neurological disorders. For instances, it has been shown that a novel lncRNA, microtubule-associated protein tau antisense (MAPT-AS) has an inhibitory effect on the expression of a major neurodegenerative gene [21]. BDNF-AS, a conservative antisense transcriptional product of BDNF, has been showed a regulation effect on neuron cells. Zhang et al. [7] reported that knockdown of BDNF-AS could reduce the apoptosis of dorsal root ganglion neurons. Zheng et al. [8] revealed that siRNA-mediated BDNF-AS downregulation ameliorated the apoptosis of mouse embryonic neural stem cell-derived neurons and induced neurite outgrowth. These implied that BDNF-AS could regulate apoptosis of neuron related cells. However, to data, little was known about the underlying role of BDNF-AS on neurons apoptosis after ASCI. In the present study, BDNF-AS was observed to be up-regulated in ASCI model rats and neurocytes following hypoxia treatment. Additionally, we also found a significant therapeutic effect of BDNF-AS knockdown on ASCI. These findings indicated that BDNF-AS might play a significant role in the progression of ASCI.
It was generally known that lncRNAs might act as ceRNAs, thereby modulating the expression of different miRNAs in a cell-type dependent manner [22]. The binding of miRNAs to lncRNAs decreased miRNA levels and led to the increase in the expression of miRNA target genes [23,24]. Therefore, in this study, we investigated whether BDNF-AS functioned as a ceRNA by interacting with miRNAs. Using bioinformatics software, we found that BDNF-AS contained several target binding sites for miR-130b-5p.
MiR-130b-5p has been considered as a tumor suppressor in several cancers, such as prostate cancer, esophageal squamous cell carcinoma (ESCC) and diabetic nephropathy [25–27]. In addition, it has been showed that miR-130b-5p is specifically related to neural progenitors, and overexpression of miR-130b-5p was found to promote its proliferation [10]. However, the functional role of miR-130b-5p on the apoptosis of neurons after ASCI was still unclear. Our study showed a direct binding existed between BDNF-AS and miR-130b-5p, and knockout of BDNF-AS could attenuate neuronal cell apoptosis by up-regulating miR-130b-5p. Additionally, luciferase activity assay showed that PRDM5 was a downstream target of miR-130b-5p, and miR-130b-5p could negatively regulate PRDM5 expression. PRDM5 is a member of PRDM family, which played a vital role in neuronal cell apoptosis. Several studies demonstrated that knockout of PRDM5 could inhibit neuronal cell apoptosis [3–5]. In the current study, we had the similar results. We found that knockdown of BDNF-AS could suppress neuronal cell apoptosis by down-regulating PRDM5 through up-regulating miR-130b-5p. Furthermore, the effects of BDNF-AS knockdown on promoting neurological function recovery were confirmed in vivo.
However, there are still some deficiency in this study. The spinal cord contains neurons and non-neuronal cells. In the current study, we focused on the role of BDNF-AS and miR-130b-5p in neuronal cells. Whether they play a role in microglia or other non-neuronal cells, we will further study in the following experiment.
In summary, our study for the first time revealed the important role of the BDNF-AS/miR-130b-5p/PRDM5 signaling pathway in the development of ASCI. These results might provide a novel insight into the prevention and treatment of ASCI.
Funding Statement
This work was supported by the Henan Provincial Department of Science and Technology [142300410379 and 152102410062].
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here
References
- 1.Bowes AL, Yip PK.. Modulating inflammatory cell responses to spinal cord injury: all in good time. J Neurotrauma. 2014;31(21):1753–1766. [DOI] [PubMed] [Google Scholar]
- 2.Liu XJ, Zheng XP, Zhang R, et al. Combinatorial effects of miR-20a and miR-29b on neuronal apoptosis induced by spinal cord injury. Int J Clin Exp Pathol. 2015;8(4):3811–3818. [PMC free article] [PubMed] [Google Scholar]
- 3.Shu XS. Emerging role of PR domain containing 5 (PRDM5) as a broad tumor suppressor in human cancers. Tumour Biol. 2015;36(1):1–3. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Wu W, Hao J, et al. PRDM5 expression and essential role after acute spinal cord injury in adult rat. Neurochem Res. 2016;41(12):3333–3343. [DOI] [PubMed] [Google Scholar]
- 5.Ling W, Xu X, Liu J A causal relationship between the neurotherapeutic effects of miR182/7a and decreased expression of PRDM5. Biochem Biophys Res Commun. 2017;490(1):1–7. [DOI] [PubMed] [Google Scholar]
- 6.Chen LL, Zhao JC. Functional analysis of long noncoding RNAs in development and disease. Adv Exp Med Biol. 2014;825:129–158. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Yan L, Cao Y, et al. Long noncoding RNA BDNF-AS protects local anesthetic induced neurotoxicity in dorsal root ganglion neurons. Biomed Pharmacother. 2016;80:207–212. [DOI] [PubMed] [Google Scholar]
- 8.Zheng X, Lin C, Li Y, et al. Long noncoding RNA BDNF-AS regulates ketamine-induced neurotoxicity in neural stem cell derived neurons. Biomed Pharmacother. 2016;82:722–728. [DOI] [PubMed] [Google Scholar]
- 9.Yang JC, Wu SC, Rau CS, et al. TLR4/NF-kappaB-responsive microRNAs and their potential target genes: a mouse model of skeletal muscle ischemia-reperfusion injury. Biomed Res Int. 2015;2015:410721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonsson ME, Nelander Wahlestedt J, Akerblom M, et al. Comprehensive analysis of microRNA expression in regionalized human neural progenitor cells reveals microRNA-10 as a caudalizing factor. Development. 2015;142(18):3166–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang WT, Ye H, Wei PP, et al. LncRNAs H19 and HULC, activated by oxidative stress, promote cell migration and invasion in cholangiocarcinoma through a ceRNA manner. J Hematol Oncol. 2016;9(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu JZ, Huang JH, Zeng L, et al. Anti-apoptotic effect of microRNA-21 after contusion spinal cord injury in rats. J Neurotrauma. 2013;30(15):1349–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang R, Zhang C, Gu R, et al. MicroRNA-489-3p inhibits neurite growth by regulating PI3K/AKT pathway in spinal cord injury. Die Pharmazie. 2017;72(5):272–278. [DOI] [PubMed] [Google Scholar]
- 14.He J, Zhao J, Peng X, et al. Molecular mechanism of MiR-136-5p targeting NF-kappaB/A20 in the IL-17-mediated inflammatory response after spinal cord injury. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2017;44(3):1224–1241. [DOI] [PubMed] [Google Scholar]
- 15.Li XQ, Chen FS, Tan WF, et al. Elevated microRNA-129-5p level ameliorates neuroinflammation and blood-spinal cord barrier damage after ischemia-reperfusion by inhibiting HMGB1 and the TLR3-cytokine pathway. J Neuroinflammation. 2017;14(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu X, Shen Y, Wang W, et al. MiR-30a-5p ameliorates spinal cord injury-induced inflammatory responses and oxidative stress by targeting Neurod 1 through MAPK/ERK signalling. Clin Exp Pharmacol Physiol. 2018;45(1):68–74. [DOI] [PubMed] [Google Scholar]
- 17.Zhao P, Wang S, Zhou Y, et al. MicroRNA-185 regulates spinal cord injuries induced by thoracolumbar spine compression fractures by targeting transforming growth factor-beta1. Exp Ther Med. 2017;13(3):1127–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu S, Xie R, Liu X, et al. Long coding RNA XIST contributes to neuronal apoptosis through the downregulation of AKT phosphorylation and is negatively regulated by miR-494 in rat spinal cord injury. Int J Mol Sci. 2017;18(4). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Shao J, Cao J, Wang J, et al. MicroRNA-30b regulates expression of the sodium channel Nav1.7 in nerve injury-induced neuropathic pain in the rat. Mol Pain. 2016;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Cao S, Xu P, et al. Changes in the expression of miR-34a and its target genes following spinal cord injury in rats, medical science monitor: international medical. J Exp Clin Res. 2016;22:3981–3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coupland KG, Kim WS, Halliday GM, et al. Role of the long non-coding RNA MAPT-AS1 in regulation of microtubule associated protein tau (MAPT) expression in Parkinson’s disease. PLoS One. 2016;11(6):e0157924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang WC, Fu WM, Wong CW, et al. The lncRNA H19 promotes epithelial to mesenchymal transition by functioning as miRNA sponges in colorectal cancer. Oncotarget. 2015;6(26):22513–22525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon JH, Abdelmohsen K, Srikantan S, et al. LincRNA-p21 suppresses target mRNA translation. Mol Cell. 2012;47(4):648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kallen AN, Zhou XB, Xu J, Qiao C, Ma J, Yan L, Lu L, Liu C, Yi JS, Zhang H, Min W, Bennett AM, Gregory RI, Ding Y, Huang Y The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol Cell. 2013;52(1):101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Q, Zhao X, Zhang H, et al. MiR-130b suppresses prostate cancer metastasis through down-regulation of MMP2. Mol Carcinog. 2015;54(11):1292–1300. [DOI] [PubMed] [Google Scholar]
- 26.Yu T, Cao R, Li S, et al. MiR-130b plays an oncogenic role by repressing PTEN expression in esophageal squamous cell carcinoma cells. BMC Cancer. 2015;15:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lv C, Zhou YH, Wu C, et al. The changes in miR-130b levels in human serum and the correlation with the severity of diabetic nephropathy. Diabetes Metab Res Rev. 2015;31(7):717–724. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


