Abstract
Melatonin, a neuroendocrine hormone synthesized primarily by the pineal gland, provides various cardiovascular benefits. Regular physical activity is an effective non-pharmacological therapy for the prevention and control of hypertension. In the present study, we hypothesized that melatonin plays an important role in the aerobic exercise-induced increase of endothelium-dependent vasorelaxation in the mesenteric arteries (MAs) of spontaneously hypertensive rats (SHRs) in a melatonergic receptor-dependent manner. To test this hypothesis, we evaluated the vascular mechanical and functional properties in normotensive Wistar Kyoto (WKY), SHRs, and SHRs that were trained on a treadmill (SHR-EX) for 8 weeks. Exercise training produced a significant reduction in blood pressure and heart rate in SHR, which was significantly attenuated by the intraperitoneal administration of luzindole, a non-selective melatonin receptor (MT1/MT2) antagonist. Serum melatonin levels in the SHR group were significantly lower than those in the WKY group at 8:00–9:00 and 21:00–22:00, while exercise training reduced this difference. Endothelium-dependent vessel relaxation induced by acetylcholine was significantly blunted in SHR compared with age-matched WKY. Both exercise training and luzindole ameliorated this endothelium-dependent impairment of relaxation in hypertension. Immunohistochemistry and Western blotting showed that the protein expression of the MT2 receptor and eNOS, as well as their colocalization in the endothelial cell layer in SHRs, was significantly decreased; as exercise training suppressed this reduction. These results provide evidence that regular exercise has a beneficial effect on improving endothelium-dependent vasorelaxation in MAs, in which melatonin plays a critical role by acting on MT2 receptors to increase NO production and/or NO bioavailability.
Introduction
Melatonin (N-acetyl-5-methoxytryptamine) is synthesized primarily in the pineal gland, and its synthesis and secretion is regulated by the light/dark cycle [1]. Melatonin is reportedly involved in the regulation of the cardiovascular system by influencing smooth muscle tone and arterial pressure [2, 3]. Accumulating evidence shows that impaired melatonin production is involved in several cardiovascular pathologies [4] including hypertension [5, 6], ischemic heart disease [7–9], heart failure [10], and myocardial infarction [11].
Melatonin exhibits antihypertensive effects in humans [12] and spontaneously hypertensive rats (SHRs) [3], although the mechanism of this action has not been clearly demonstrated. Previous studies have reported that a pine-alectomy is associated with the development of hypertension in rats, which can be reversed by adding melatonin to their drinking water [13]. The intravenous injection of melatonin was observed to reduce blood pressure (BP) without altering cardiac output [14]. People with hyper-tension have lower melatonin levels than those with normal BP, and the administration of the hormone lowers BP to the normal range [15]. In patients with coronary heart disease, nocturnal serum melatonin levels are reportedly more than five times lower than in controls [7]. Pharmacological treatment with melatonin for five days in an adult SHR resulted in a gradual decrease in BP, heart rate (HR), and plasma renin activity [3]. Although numerous studies have investigated the pharmacological effects of melatonin, the mechanisms underlying the effects of melatonin on the cardiovascular system are multifactorial, complex, and still not completely understood.
In terms of vascular regulation, studies have shown that the activation of melatonin receptors on endothelial and vascular smooth muscle cells (VSMCs), in addition to the antioxidant properties of melatonin, may be responsible for the effects of melatonin on vascular contractility. Three distinct melatonin receptor subtypes have been identified and shown to mediate the physiological effects of melatonin; they are called MT1, MT2, and MT3 receptors [16]. In the vasculature, melatonin predominantly acts via two G-protein-coupled receptors: MT1 and MT2 [17]. Studies investigating the direct influence of melatonin on vascular reactivity have been performed in various laboratories. However, the data obtained from these experiments are contradictory. Melatonin was reported to have no effect on basal arterial tone [18] and to cause vasoconstriction in some studies [19–22] and vasodilatation in others [19, 23, 24]. Melatonin exhibits specific effects via its membrane receptors as well as non-specific effects through its anti-oxidant properties [25, 26].
Hypertension is associated with structural and mechanical changes of the vasculature, including vascular remodeling and increased vascular tone. Hypertension is characterized by a significant increase in contractile responses and/or decreased vasodilator responses. The mesenteric circulation of the rat receives approximately one-fifth of the cardiac output [27]. Therefore, the mesenteric arteries (MAs) contribute significantly to peripheral resistance and systemic BP. Exercise is a first-line therapeutic strategy to reduce the risk of cardiovascular diseases such as hypertension [28–30]. Accumulating evidence has shown that regular exercise, especially aerobic exercise, may increase vascular relaxation [31, 32]. In addition, acute and long-term exercise has been demonstrated to alter circulating melatonin levels [33–35]. Despite a large volume of work on melatonin, its role in exercise-induced improvement of vasoreactivity, particularly in the peripheral resistant arteries in hypertension, is poorly understood and has not been well-studied.
Therefore, in the present study, we hypothesized that melatonin plays an important role in the hypotensive effects of exercise training. To test the hypothesis, we investigated the direct role of melatonin binding to melatonergic receptors in the vasorelaxation of small MAs in SHRs with/without exercise. Furthermore, we examined the effect of melatonin/its receptors on endothelium-dependent relaxation in both normotensive rats and SHRs with/without exercise training.
Methods
Animals and exercise training
All experimental protocols were approved by the ethical committee of Beijing Sport University and were performed in accordance with the Chinese animal protection laws and institutional guidelines.
Twelve-week-old male normotensive Wistar Kyoto rats (WKY, n = 18) and SHRs (n = 48) were used. Rats were housed at a constant room temperature, humidity, and light cycle (12:12 h light–dark) with free access to tap water and fed with standard rat chow ad libitum. SHRs were randomly divided into one of the following four groups: SHR sedentary group (SHR-SED, n = 18), SHR sedentary with melatonin receptor antagonist luzindole (Luz) injection group (SHR-SED + Luz, n = 6), SHR exercise group (SHR-EX, n = 18), and SHR exercise with luzindole injection group (SHR-EX + Luz, n = 6). Luzindole injection groups were injected intraperitoneally (i.p.) with luzindole (1 mg/kg/day in sterile saline) each day at 18:00 pm [36]. After a 1-week acclimatization period, rats in the exercise groups were subjected to aerobic exercise (about 55–65% of maximal aerobic velocity, 18–20 m/min, 0% grade, 60 min, 5 days/week for 8 weeks) on a motor-driven treadmill [31]. To determine the maximal exercise capacity, rats were subjected to a progressive exercise test using an incremental speed protocol of 5 m/min every 3 min and no grade until exhaustion. Rats were considered to be exhausted when they could no longer run at the treadmill speed. The treadmill exercise test was repeated after 4 weeks in order to adjust training intensity [37].
Body weight (BW) was measured weekly. BP and HR were determined by an indirect tail-cuff method (BP-2010A, Softron Biotechnology, Beijing, China).
Serum melatonin assay
Serum melatonin levels were determined using an enzyme-linked immunosorbent assay (ELISA) kit (Melatonin Elisa, IBL Hamburg, Germany) and ELISA reader. All samples were stored at −20 °C until processing. Briefly, this assay is based on the competition principle and microtiter plate separation. First, each sample was passed through a C18 reversed phase column, extracted with methanol, evaporated to dryness, and reconstituted with bidistilled water. Then each sample was added to the corresponding well that was coated with goat-anti-rabbit antibody in a microtiter plate. An unknown amount of antigen was present in the sample, and a fixed amount of enzyme-labeled antigen competed for the binding sites of antibodies coated on the wells. After incubation and washing, an enzyme conjugate was added to each well. After further incubation and washing, p-nitrophenyl phosphate (PNPP) substrate solution was added to all wells, followed by a PNPP stop solution to halt the substrate reaction. Subsequently, optical density was measured with a photometer at 405 nm. The concentration of antigen was inversely proportional to the optical density measured. Melatonin standards were used to construct a calibration curve against which the unknown samples were calculated. The sensitivity of the melatonin assay is ~3.0 pg/mL.
Vascular reactivity
Experiments were performed on rats at the age of 21 weeks (n = 6 each group). The MAs were removed after the rats were anesthetized with sodium pentobarbitone (60 mg/kg, i.p.) and were decapitated using a guillotine. The MAs were placed in cold Krebs solution with the following composition (mM): 131.5 NaCl, 5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.5 CaCl2, 11.2 glucose, 13.5 NaHCO3, and 0.025 EDTA; gassed with 95% O2 and 5% CO2 (pH 7.4). Second-order branches (A2) of the MAs (~150–250 μm of average diameter; 2-mm long) were dissected and mounted as a ring preparation on a Multi Myograph System (620M; DMT, Aarhus, Denmark) by threading onto two stainless steel wires (40 μm diameter).
The contractile response for tension was evaluated by measuring the maximum peak height and expressed as a percentage of contraction to 120 mM K+ (Kmax). In some experiments, non-selective NOS inhibitor Nω-nitro-L-argi-nine methyl ester (L-NAME, 100 μM) was added after the Kmax measurement. To examine the vasodilation effects, tissues were first contracted with 10−5 M norepinephrine (NE). Then, responses to the cumulative addition of specific vasorelaxant agents were tested. The pIC50 (−logIC50), the negative logarithm of the half-maximal inhibitory concentration, a measure of the effectiveness of a substance in inhibiting a specific biological or biochemical function was calculated. The vessel contractile studies were separated into three sets of experiments which are described in detail in the Results section. Relaxation responses to melatonin, acetylcholine (ACh), and sodium nitroprusside (SNP) were expressed as a percentage of the NE-induced contraction immediately prior to the addition of the vasodilators.
Western immunoblotting
Segments of MAs were homogenized in ice cold Tris-EDTA buffer. Equivalent amounts (60 μg) of total protein from the MAs were added to adjacent lanes. Proteins were separated by SDS-PAGE on 12% separating gels with 5% stacking gels and then transferred to Polyvinylidene-Fluoride (PVDF) membranes. The blot membranes were blocked for 2 h at room temperature and then incubated overnight at 4 °C with rabbit polyclonal antibodies for MT1 and MT2 (1:200, Alomone Laboratories, Jerusalem, Israel) and mouse monoclonal antibodies for eNOS (1:1000, Transduction Laboratories, Lexington, UK). After another three washes with Tris-buffered saline with 0.05% Tween 20(TBST), the filters were incubated with the secondary antibodies (anti-rabbit and anti-mouse IgG-HRP, 1:8000; Proteintech Group Inc., Chicago, Illinois) for 1 h at room temperature. The immunoreactive proteins were visualized by enhanced chemiluminescence and the signals were recorded with Bio-Rad ChemiDOC XRS+ (Bio-Rad Laboratories, Hercules, CA, USA). GAPDH expression levels were used as a loading control to normalize the protein levels.
Immunofluorescence
Segments of MAs were fixed in 4% paraformaldehyde in a phosphate-buffered saline (PBS) for 12 h. The tissues went through paraffin embedding and were cut into 4-μm-thick sections. Paraffin sections (4 μm) were de-paraffined, rehydrated, antigen retrieval was performed, and then permeabilized with 0.3% Triton X-100 for 10 min. After washing with PBS, the sections were blocked for nonspecific antibody binding with 5% bovine serum albumin (BSA) for 1 h. Sections were incubated overnight at 4 °C in 1% BSA containing a primary antibody against MT2 receptors (1:300, Alomone Laboratories, Jerusalem, Israel) and eNOS (1:50, Transduction Laboratories, Lexington, UK) at the same time. The next day, Alexa Fluor 488-conjugated Goat Anti–Rabbit IgG (1:500, Molecular Probes, Grand Island, NY, USA), a secondary antibody, was used for 1 h in the dark. After washing, Texas redconjugated Goat Anti–Mouse IgG (1:300, Molecular Probes, Grand Island, NY, USA), a secondary antibody, was also used for 90 min. Sample coverslips were plated on ProLong Gold Antifade Reagent (Molecular Probes). All photographs were analyzed using Image Pro Plus (version 6.0; MediaCybernetics, Silver Spring, MD, USA).
Data analysis and statistics
Data are expressed as mean ± SEM of the number of experiments indicated in each case. Statistical analysis was performed using a Student’s t-test or analysis of variance (one-way or two-way ANOVA) in conjunction with a Bonferroni multiple comparison analysis when F values were significant. The Student’s t-test was used for comparisons between two groups. P < 0.05 was considered significant and P < 0.01 was considered highly significant.
Chemicals
All chemicals were purchased from Sigma-Aldrich (China (Mainland)) unless otherwise stated. Melatonin is first dissolved in dimethyl sulfoxide (DMSO). The final concentration of DMSO was less than 0.1% that had no effects on currents.
Results
Physical characteristics of experimental animals
Animal characteristics are shown in Table 1 for the normotensive control rats (WKY), sedentary SHRs, and the exercising SHRs. There were no significant differences in BWs among the WKY and SHR groups at the baseline (12 weeks, data not shown). However, they were significantly lower in the SHR-EX group at study end (21 weeks) when compared with their sedentary counterparts (SHR-SED). There were no significant differences in systolic BP (SBP), diastolic BP (DBP), mean arterial pressure (MAP), and HR at baseline among SHR groups including SHR-SED, SHR-SED + Luz, SHR-EX, and SHR-EX + Luz. However, these measures were significantly greater when compared with WKY (P <0.05). After 1 week of acclimatization, and 8 weeks of regular aerobic exercise, SBP, DBP, MAP, and HR were all significantly lower in SHR-EX compared to SHR-SED. Moreover, SBP, MAP, and HR in SHR-EX + Luz were significantly greater than in SHR-EX. However, in sedentary SHR groups, these parameters were not significantly different between SHR-SED + Luz and SHR-SED.
Table 1.
Physical characteristics of rats (21 week old)
| BW (g) |
SBP (mmHg) |
DBP (mmHg) |
MAP (mmHg) |
HR (beats/min) |
|
|---|---|---|---|---|---|
| WKY | 330.3 ±6.5 | 142.7 ±3.1 | 103.2 ±6.7 | 116.3 ± 5.2 | 314±7 |
| SHR-SED | 334.7 ±1.8 | 220.8 ±1.7* | 174.3 ±4.8* | 189.8 ±3.3* | 394 ± 8* |
| SHR-SED + Luz | 336.2 ±3.3 | 224.0 ± 2.2 | 172.3 ±5.9 | 189.5 ±4.4 | 393 ±4 |
| SHR-EX | 312.1 ±3.3# | 192.7 ±1.5# | 144.8 ± 2.9# | 160.8 ±1.8# | 316 ±6# |
| SHR-EX + Luz | 318.3 ±4.7 | 205.5 ± 1.0Δ& | 156.0 ±6.2 | 172.5 ±4.2Δ& | 346 ± 8Δ& |
Data are means ± SEM
SBP systolic blood pressure, DBP diastolic blood pressure, MAP mean arterial pressure, HR heart rate
P < 0.05 vs. WKY;
P < 0.05 vs. SHR-SED;
P < 0.05 vs. SHR-EX;
P < 0.05 vs. SHR-SED + Luz; n = 6 in each group
Effect of aerobic exercise on serum endogenous melatonin in SHRs
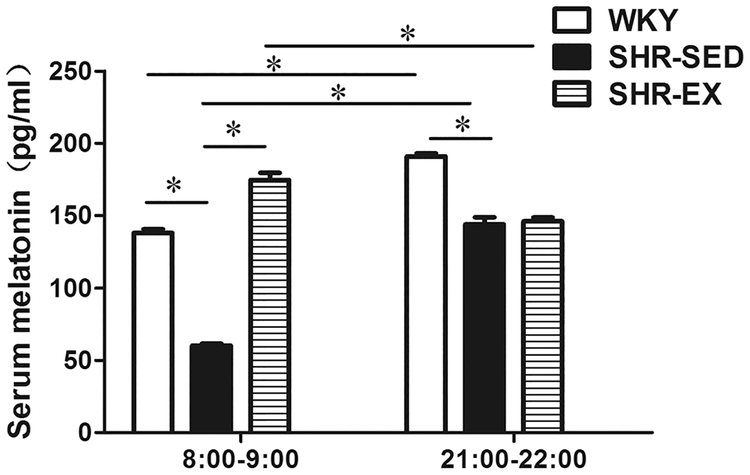
Serum melatonin concentrations were measured by ELISA at 8:00–9:00 and 21:00–22:00 in WKY, SHR-SED, and SHR-EX groups (Fig. 1). In both WKY and SHR-SED, the serum melatonin level was significantly higher at 21:00–22:00 (WKY: 190.90 ± 2.02 pg/mL; SHR-SED: 143.80 ± 5.05 pg/mL) than at 8:00–9:00 (WKY: 137.90 ± 2.53 pg/mL; SHR-SED: 60.10 ± 1.52 pg/mL); n = 6, P < 0.05. However, in SHR-EX, the melatonin level was higher at 8:00–9:00 (174.55 ± 5.15 pg/mL) compared to 21:00–22:00 (146.00 ± 2.53 pg/mL, n = 6, P < 0.05). At both testing times (day and night), the melatonin level was significantly lower in SHR-SED when compared with WKY. However, after 8 weeks of exercise (SHR-EX), the serum melatonin concentration at 8:00–9:00 was higher than that of SHR-SED (174.55 ± 5.15 pg/mL vs. 60.10 ± 1.52 pg/mL, respectively) (n = 6, P < 0.05). No significant differences in melatonin levels were observed between SHR-EX and SHR-SED at 21:00–22:00.
Fig. 1.
Serum endogenous melatonin levels in rats. *P < 0.05; n = 6 in each group
Effect of aerobic exercise on vascular tone in SHRs
Exercise training attenuated the SHR-associated decrease in melatonin-induced vasodilation of MAs
To explore the role of melatonin in exercise-induced alteration of vascular contractility in the small arteries of SHRs, three sets of experiments were conducted in arterial rings of MAs from three groups: WKY, SHR-SED, and SHR-EX. In each MA ring, KCl (120 mM) was first applied to induce maximal contraction. The maximal response induced by 120 mM KCl was similar in WKY and SHR, and exercise training did not modify the response (Table 2). However, the contractile response induced by NE was greater in arteries from SHR than WKY, and exercise training inhibited this response. In the absence of L-NAME (10−4 M), a non-selective nitric oxide synthase (NOS) inhibitor, the increase in NE-induced tension was lower than in the presence of L-NAME (Table 2) in each group (P < 0.05).
Table 2.
Peak responses and maximal inhibition on NE-induced contraction
| WKY | SHR-SED | SHR-EX | ||
|---|---|---|---|---|
| Δ Maximal tension | ||||
| Kmax (120 mM) (mN) | (−)L-NAME | 13.9±1.0 | 15.0±1.2 | 14.3 ±1.3 |
| NE (10−5M) as % Kmax | (−)L-NAME | 110.6 ±4.7 | 138.2 ±4.6* | 126.5 ±3.5# |
| (+) L-NAME | 137.8 ±3.9$ | 156.4 ± 3.1*$ | $ 143.4 ±2.8#$ | |
| Maximal inhibition on NE-induced contraction (%) | ||||
| ACh (10−5 M) | (−) Mel | 96.3 ±1.2 | 70.9 ±4.6* | 89.5 ± 2.6# |
| (+) Mel | 98.8 ±0.3 | 80.3 ±2.6* | 95.2 ±2.0# | |
| (+) Luz + Mel | 97.9 ±0.1 | 78.7 ±2.5* | 85.4 ±4.7 | |
| Melatonin (10−3 M) | (−)L-NAME | 99.1 ±1.6 | 83.7 ±2.4* | 92.6 ±3.6# |
| (+) L-NAME | 85.7 ±2.8$ | 77.3 ±1.0*$ | $ 79.7 ±2.5$ | |
| (-) Luz | 99.1 ±1.6 | 83.7 ±2.4* | 92.6 ±3.6# | |
| (+) Luz | 72.2±2.3& | 77.5 ± 1.1& | 73.3 ±4.8& | |
| SNP(10−5M) | (−) Mel | 94.0 ±2.5 | 97.8 ±0.3 | 95.6±2.6 |
| (+) Mel | 96.5±1.7 | 98.1 ±0.4 | 97.9±0.4 | |
P < 0.05 compared with WKY;
P < 0.05 compared with SHR-SED;
P < 0.05 compared with (−) L-NAME;
P < 0.05 compared with (−) Luz; n = 6 in each group
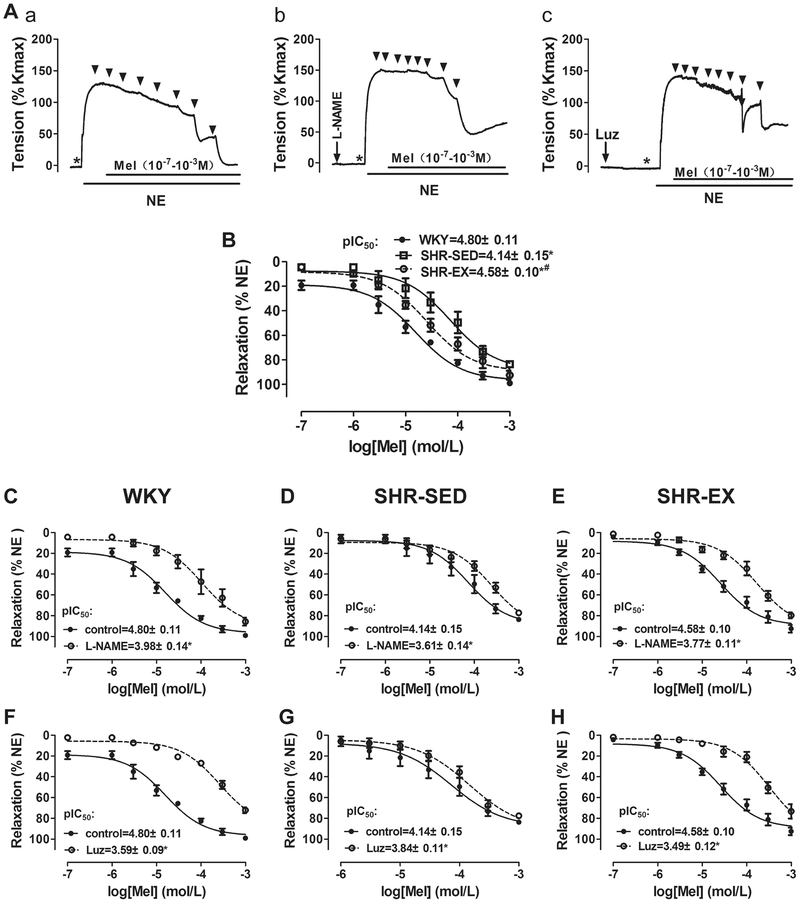
In the first set of experiments, the effects of melatonin on MA contractility were examined. As shown in Fig. 2, concentration–response curves to melatonin were generated for NE-induced vasoconstriction, with or without L-NAME (10−4 M) pretreatment. At the plateau of the 10−5 M NE-induced contraction, melatonin (10−7–10−3 M) was given in half-log increments (Fig. 2a, b). In the absence of L-NAME, the pIC50 of melatonin in SHR-SED (4.14 ± 0.15, n = 6) was significantly lower than in WKY (4.80 ± 0.11, n = 6). However, the pIC50 in SHR-EX (4.58 ± 0.10, n = 6) was greater than in SHR-SED. The maximal relaxation of MA to melatonin was also lower in SHR-SED (83.7 ± 2.4%) than in WKY (99.10 ± 1.60%), while in SHR-EX (92.6 ± 3.6%), it was greater than in SHR-SED (Table 2). In the presence of L-NAME, the melatonin dose–response curve was shifted right, with a decrease of pIC50 and maximal inhibition by melatonin, indicating that vessel sensitivity to melatonin was significantly decreased. Of note, this decrease was greatest in WKY, and least in SHR-SED. Exercise training attenuated this change in hypertensive rats (Figure 2Ab, C, D, E, and Table 2). Taken together, these results suggest that melatonin-induced vasorelaxation is mainly mediated by increasing nitric oxide (NO) production and/or NO bioavailability, which is decreased in hypertension; exercise training may prevent this.
Fig. 2.
Effect of melatonin on NE-induced vessel contraction. A Example of real-time recording of vascular contractility in mesenteric arterial rings in control (a), with L-NAME pretreatment (b), and with Luz pretreatment (c). The dose–response curves of melatonin-induced mesenteric arterial relaxation. B Comparison of dose–response relations of melatonin-induced vasorelaxation in WKY, SHR-SED, and SHR-EX in control (without L-NAME and Luz pretreatment). *P < 0.05, vs. WKY; #P < 0.05, vs. SHR-SED. C–H Cumulative concentration–relaxation (%) curves for melatonin (10−7−10−3 M) in MAs against NE in control, with L-NAME pretreatment, and with Luz pretreatment in WKY (C and F), SHR-SED (D and G), and SHR-EX (E and H). *P < 0.05, vs. control. L-NAME, 10−4 M; Luz, 2 × 10−6 M, n = 6 in each group
Furthermore, to determine whether melatonin acts on the endothelium to induce vasorelaxation through melatonergic-receptor receptors, the MT1/2 non-selective antagonist luzindole (2 × 10−6 M) was added before melatonin and in the absence of L-NAME. The luzindole itself had no effect on vascular tone. However, it inhibited the melatonin-induced vasodilation in MAs. The concentration–response curves of melatonin were shifted right, with a decrease of pIC50 and a maximal inhibition (Figure 2Ac, F, G, H, and Table 2). Similar to the results with L-NAME, this decrease was greatest in WKY, and least in SHR-SED. This indicated that melatonin-induced vasorelaxation is predominantly mediated through the MT1/2 receptors, and these receptors are significantly down-regulated in hypertension. However, regular exercise training ameliorates this effect.
Exercise training attenuated the SHR-associated decrease of ACh-induced endothelium-dependent vasodilation in MAs
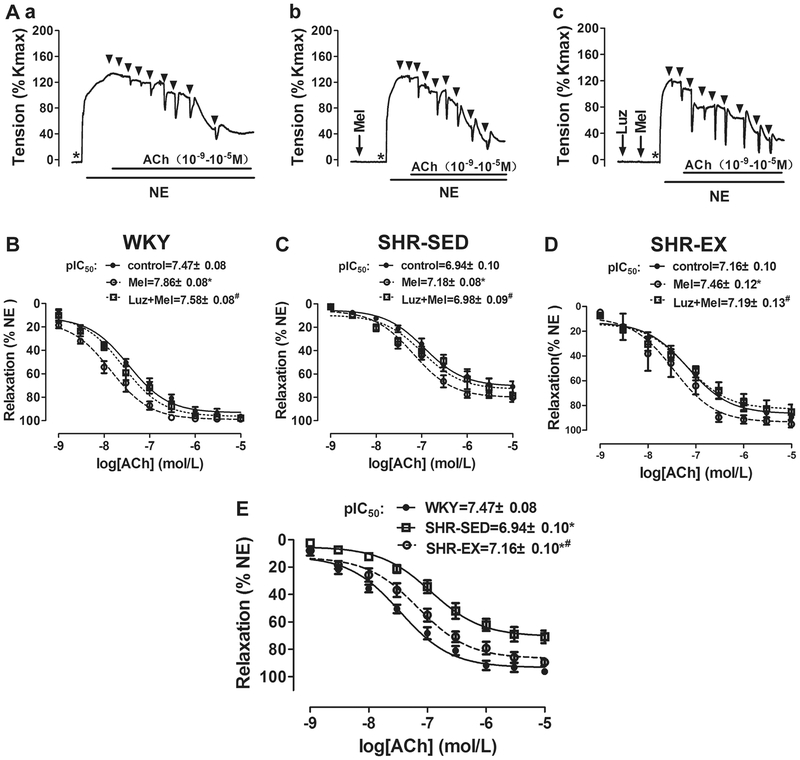
To explore the effects of exercise training on endothelium-dependent vasodilation in MAs, the concentration-dependent relaxation in arteries induced by ACh was investigated. As shown in Fig. 3Aa and B–E, at the plateau of NE-induced vasoconstriction, ACh (10−9–10−5 M) was added in half-log increments. As expected, ACh induced a concentration-dependent vasorelaxation, with a pIC50 of 6.94 ± 0.10 in SHR-SED, which was significantly lower than in WKY (7.47 ± 0.08, n = 6, P < 0.05). ACh (10−5 M) induced maximal inhibition on NE-induced contraction in SHR-SED (70.9 ± 4.6%), as this was lower than in WKY (96.3 ± 1.2%, Table 2). However, exercise training decreased this change in hypertensive rats (SHR-EX: pIC50 = 7.16 ± 0.10, maximal inhibition: 89.5 ± 2.6%, n = 6, P < 0.05). These results suggest that compared with WKY, there was a profound decrease in ACh-induced endothelium-dependent vasodilation in SHRs, and exercise training remarkably inhibited this effect.
Fig. 3.
Effects of aerobic exercise on ACh-induced endothelium-dependent vasorelaxation in MAs. A Example of real-time recording of vascular contractility in mesenteric arterial rings in control (a), with Mel pretreatment (b), and with Luz + Mel pretreatment (c). B, C, and D Cumulative concentration–relaxation (%) curves for ACh (10−9–10−5 M) in MAs against NE in control, with Mel pretreatment, and with Luz + Mel pretreatment in WKY (B), SHR-SED (C), and SHR-EX (D). *P < 0.05, vs. control; #P < 0.05, vs. Mel. E, comparison of dose–response relations of ACh-induced vasorelaxation in WKY, SHR-SED, and SHR-EX in control (without Mel and Luz pretreatment). *P < 0.05, vs. WKY; #P < 0.05, vs. SHR-SED. Luz, luzindole (2 × 10−6 M); Mel, melatonin (10−4 M); NE, norepinephrine (10−5 M). Values were expressed as mean ± SEM, n = 6 in each group
To investigate the effect of melatonin on the relaxant response to ACh, MA tissues were pre-incubated with melatonin (10−4 M) for 20 min, and the cumulative concentration–response curves for ACh (10−9–10−5 M)-induced relaxation were calculated in NE pre-constricted rings (Fig. 3Ab and B, C, D). As expected, the melatonin pretreatment produced a significant potentiation of the vasorelaxant effect of ACh on the NE pre-contracted rings in all three groups. For example, in WKY, the pIC50 of ACh-induced vasorelaxation following the melatonin pre-treatment which was 7.86 ± 0.08, this was greater than in controls (7.47 ± 0.08, n = 6, P < 0.05). This enhancement was significantly lower in SHR-SED (control: 6.94 ± 0.10; +Melatonin: 7.18 ± 0.08) than in WKY, indicating that the endothelium-dependent relaxation was significantly reduced at the same dose of melatonin in SHRs. However, exercise training inhibited the observed change in hypertension.
To analyze whether the melatonin-induced alteration on endothelium-dependent vasodilation was mediated via melatonin receptors in MA rings, luzindole (2 × 10−6 M) was added to the bath 20 min prior to and during the incubation with melatonin (Figure 3Ac and B, C, D). Cumulative concentration–response curves for ACh-induced relaxation in the NE pre-constricted rings were then calculated. Of note, luzindole significantly inhibited the melatonin-induced enhancement of the ACh-elicited vasorelaxation. Blocking of the MT1/MT2 receptors by the non-selective blocker luzindole, significantly suppressed the effects of melatonin. These results suggest that melatonin primarily exerts enhancement of endothelium-dependent vasodilation through the MT1/MT2 receptors.
Exercise training had no effect on SNP-induced endothelium-independent vasodilation in MAs
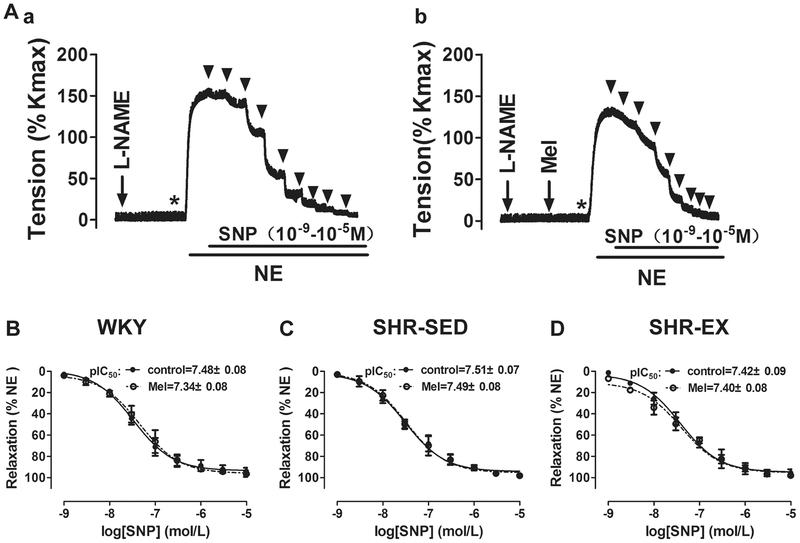
In the third set of experiments, the effects of melatonin on endothelium-independent vasodilation were examined (Fig. 4). As expected, pretreatment with melatonin (10−4 M) had no significant effect on the vasorelaxant effect of SNP (10−9–10−5 M) in all three groups. Because SNP is an exogenous NO donor, these data indicate that both exercise training and melatonin had no effect on endothelium-independent vasodilation in SHRs.
Fig. 4.
Effects of aerobic exercise on SNP-induced endothelium-independent vasorelaxation in MAs. A Example of real-time recording of vascular contractility in MAs in the presence of L-NAME in control (a) or with Mel pretreatment (b). B, C, and D Cumulative concentration–relaxation (%) curves for SNP (10−9–10−5 M) in MAs against NE in control and with Mel pretreatment in WKY (B), SHRSED (C), and SHR-EX (D). L-NAME Nω-nitro-l-arginine methyl ester (10−4 M), SNP sodium nitroprusside; n = 6 in each group
Exercise training attenuated SHR-associated downregulation of MT1/MT2 receptor expression and eNOS in MAs
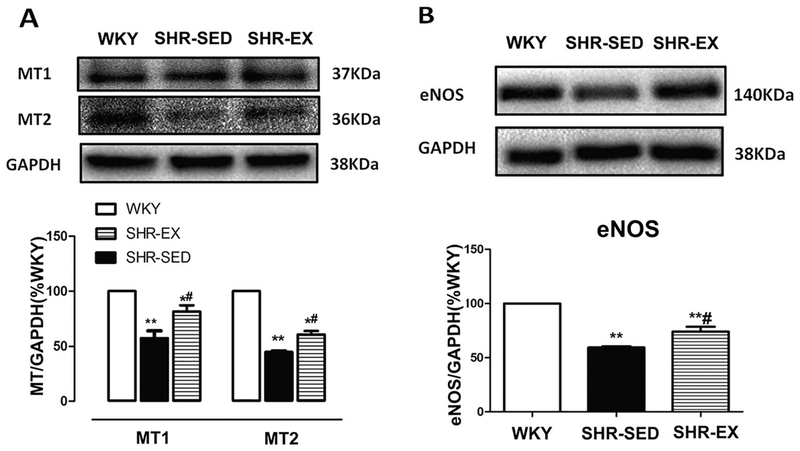
In addition, the melatonin receptor and eNOS protein expression in MAs was determined. As shown in Fig. 5, hypertension was associated with a significant decrease in protein expression of MT1, MT2, and eNOS. However, exercise diminished these alterations in SHR.
Fig. 5.
Protein expression of MT1, MT2 receptor, and eNOS in mesenteric artery homogenates. A Representative Western immunoblot and densitometric analysis of MT1 and MT2 receptor. B Representative Western blot and densitometric analysis of eNOS. *P < 0.05, **P < 0.01, relative to WKY; #P < 0.05, relative to SHR-SED; n = 6 in each group
Effects of aerobic exercise on the colocalization of MT2 receptor and eNOS in MAs
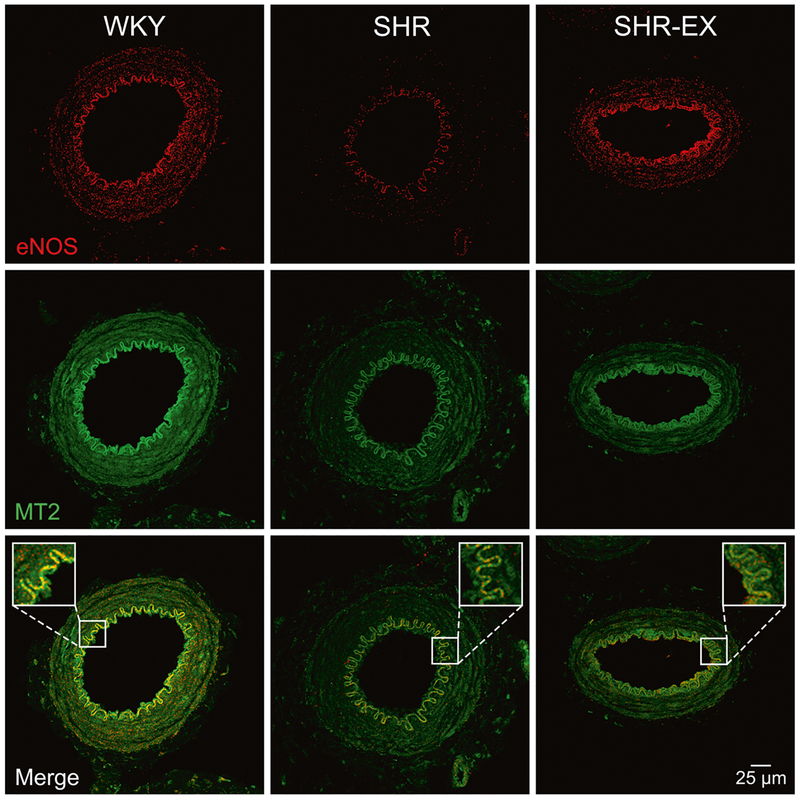
It is reported that MT1 receptor activation causes vasoconstriction, where MT2 receptor activation causes vaso-dilation [19]. Thus, we evaluated the distribution of eNOS and MT2 receptors in MAs by using immunofluorescence and confocal imaging. As shown in Fig. 6, MT2 receptors were detected in the endothelium, smooth muscle cell layer, and adventitia. The eNOS was detected predominantly in the inner layer. Further, the MT2 receptors were colocalized with eNOS in the endothelium in MAs. Colocalization was less in SHR-SED, as compared to WKY. After 8 weeks of exercise, the colocalization of MT2 receptors and eNOS was increased compared to SHR-SED.
Fig. 6.
Immunofluorescent colocalization of eNOS and MT2 receptors in MAs. Colocalization of eNOS (red) and MT2 (green) staining is confirmed by the sharp merged image (yellow). Scale bar = 25 μm
Discussion
The present study assessed the role of melatonin in aerobic exercise-induced improvement of the vasorelaxation in MAs from SHRs. This study revealed two major new findings. First, aerobic exercise training ameliorated hypertension-associated declines in serum melatonin levels and produced a significant decrease in BP and HR in hypertensive rats. In addition, blocking melatonergic receptors with luzindole attenuated the hypotensive effects of melatonin on SHR. Second, the exercise-induced increase in endothelium-dependent vasodilation in MAs from SHRs was partially mediated by melatonin acting on the MT2 receptors to increase NO production and/or NO bioavailability.
To test our hypothesis, both in vivo and in vitro studies were performed. Regular physical activity is reportedly associated with a number of health benefits, including a reduction in cardiovascular disease and mortality [38]. In our in vivo experiment, 8 weeks of treadmill training resulted in a significant decrease in HR in the SHR-EX compared to the SHR sedentary group, which was seen as an indicator of a positive training effect [39]. In agreement with previous studies, the present data confirmed that continuous low- to moderate-intensity exercise is effective in attenuating hypertension in SHRs [31, 40, 41].
To test our first hypothesis that melatonin is involved in the exercise-induced decrease of systemic BP in hyper-tension, serum melatonin levels were examined at both 8:00–9:00 and 21:00–22:00. It is well known that melatonin is formed predominantly during the night, and that light has an inhibitory effect on pineal melatonin secretion [42]. In addition, many experiments have shown that serum melatonin levels are decreased in essential hyper-tension [3]. This is supported by our results, which showed that at both test times, serum melatonin levels were lower in SHRs compared to normotensive WKY. Previous studies have shown that exercise has an impact on melatonin secretion [34]. However, the relationship between exercise and the phase of the melatonin rhythm is complex. Physical exercise causes a phase shift, and although the shifts are initially small, they can become substantial if exercise is performed regularly due to a cumulative effect. Other evidence suggests that in addition to its phase-shifting effects, exercise can also acutely alter melatonin levels [33, 34]. Interestingly, the acute increase in the circulating melatonin that occurs after exercise is attenuated by regular and vigorous training. In women subjected to a conditioning program (running), the acute peak of melatonin in response to treadmill exercise decreased by 52% as training progressed [35]. In the present study, 8 weeks of exercise training significantly blunted the decrease of serum melatonin in hypertension at both the morning and night testing times, which supports the theory that exercise has an impact on melatonin secretion. Surprisingly, serum melatonin levels at 8:00–9:00 in trained SHRs (SHR-EX) was higher than at 21:00–22:00. One explanation is that exercise changed the phase of melatonin’s rhythm. Endogenous melatonin levels in SHR are reported to be highest at midnight (11:30 pm–12:30 am) and lowest between 10:00 and11:00 am. The peak of endogenous melatonin in SHR-EX was not achieved at 21:00–22:00. Moreover, the high secretion period was prolonged, and the serum concentration decreased slowly after regular exercise training. Therefore, serum melatonin levels remained high in the morning (8:00–9:00).
In the present study, intraperitoneal administration of luzindole significantly blunted the exercise-induced decrease of BP in SHR, lending support to our hypothesis that melatonin is involved in the exercise-induced beneficial effects on the cardiovascular system in hypertension. Furthermore, these effects are partially mediated via melatonergic receptors. Of note, previous studies into the therapeutic effects of melatonin on hypertension were mainly performed using exogenously administered melatonin. In the current study, endogenous melatonin was significantly increased by regular exercise training in hypertensive rats.
Aerobic training decreases BP in hypertensive humans and animals mainly by reducing vascular resistance [43]. In our study, we further hypothesized that the increased melatonin observed in the training groups attenuated the dys-function of endothelium-dependent vasorelaxation in small MAs from SHR via binding to the melatonergic receptors and subsequent NOS pathway activation. To test this hypothesis, in vitro isometric contraction studies on MAs were performed. Results showed that melatonin induced vasorelaxation in WKY, SHR, and SHR-EX. In the presence of L-NAME, the melatonin dose–response curve was shifted right with a decrease in sensitivity and maximal relaxation to melatonin, indicating that NOS is a major target of melatonin. Of note, the observed decrease was significantly less in SHR compared to WKY, suggesting that melatonin-induced vasorelaxation is mainly mediated via an increase in NO production and/or NO bioavailability, both of which are attenuated in hypertension. In addition, luzindole significantly inhibited melatonin-induced vasorelaxation. Similarly, this inhibition by luzindole was less in SHRs compared to WKY, which indicates that the quantity or function of MT1/MT2 receptors may be downregulated in SHRs. Alternatively, exercise training reversed the effects on these changes. Taken together, these results indicate that exercise improves melatonin-induced vasodilation in MAs from SHRs, and that this is partially mediated via MT1/MT2 receptors.
It is well established that hypertensive humans and animals exhibit impaired endothelium-dependent vasorelaxation, and that eNOS dysfunction may play a role in this impairment [44]. Subsequent ACh and SNP experiments provided solid evidence to support this theory. The MAs from SHRs were significantly less responsive to the endothelium-dependent relaxing effects of ACh than those obtained from WKY, presumably due to a decrease in the synthesis and/or the release of NO [45]. However, exercise increased the maximal ACh-induced, endothelium-dependent, NO-mediated relaxation of MAs in SHRs. Endothelium-independent relaxation induced by SNP was not changed in hypertension and not affected by the exercise training. These results suggest that exercise training has an inhibitory effect on the decreased endothelium-dependent vasorelaxation observed in hypertension, which is in line with many previous studies [41]. Certainly, exercise does always induce beneficial effects in hypertensive individuals which depends on the exercise intensity and type [46, 47]. Moreover, in the present study, we found that melatonin pre-treatment potentiated vasorelaxation in response to ACh but not the NO donor SNP. This effect was significantly lower in SHR compared to WKY and was attenuated by the pharmacologic blockade of MT1/MT2 receptors. These data further suggest that MT1/2 receptors mediate the effects of melatonin, and that these receptors are downregulated in SHRs. Interestingly, exercise training reversed this alteration and increased melatonin receptor sensitivity, thus increasing endothelium-dependent relaxation in hypertension.
Luzindole is considered to be a non-selective ligand, although it has a 15- to 25-fold higher affinity for the MT2 melatonin receptor than the MT1 receptor [48, 49]. Western blotting showed that MT1, MT2, and eNOS were significantly decreased in MAs from SHRs. Previous studies have shown that MT2 receptors are mainly mediated via vasorelaxation [42], and that the activation of MT2 receptors on endothelial cells can increase cytosolic Ca2+ in endothelial cells [50]. Activated endothelial cells are then stimulated to increase the production of NO. Therefore, we examined the MT2 receptor and eNOS colocalization in MAs. In line with previous reports, the MT2 receptors appear on the endothelial as well as VSMCs [50]. Importantly, the colocalization of MT2 and eNOS in the endothelial cell layer in SHRs was significantly decreased, and the exercise training attenuated this reduction. These data provide molecular evidence that exercise enhances endothelium-dependent relaxation, partially via the MT2–eNOS–NO pathway.
Despite the high density of melatonin receptors in the vasculature, receptor-independent mechanisms of melatonin have been reported. As a powerful antioxidant and free radical scavenger, melatonin removes reactive oxygen species (ROS) [25, 51], while decreasing the conversion of NO to peroxynitrite, thereby elevating NO levels and enhancing its effects [52]. Thus, a possible mechanism of the relaxant effects of melatonin may be through the elevation of NO levels, either through stimulation of the endothelial NO production or by scavenging free oxygen and preventing its oxidation. In the present study, we cannot exclude the possibility that in addition to the MT2–eNOS pathway, exercise induces an increase in vasorelaxation in SHR that may be partially mediated by the antioxidant activity of melatonin. Moreover, the lipophilic nature of melatonin facilitates its penetration of the lipid cell membrane. Our recent study found that melatonin directly activates BKCa channels on smooth muscle cells, which can also cause vasodilation [53]. The data showed that in addition to the activation of eNOS, melatonin-induced vasorelaxation of MAs is partially attributable to its direct (passing through the cell membrane) and indirect (via MT1/MT2 receptors) activation of the BKCa channels on mesenteric arterial myocytes. In the present study, we cannot exclude such effects of melatonin in the exercise training which induced beneficial effects in MAs from SHRs.
In conclusion, our present data suggest that aerobic exercise training exerts a significant bradycardic and hypotensive effect in SHRs and increases vasorelaxation in MAs from SHRs. Enhanced serum melatonin levels, and MT2 receptor/eNOS pathway responsiveness is one of the key mechanisms through which exercise training may exert its hypotensive effects on SHRs. Our results provide novel molecular and functional evidence that melatonin mediates the exercise-induced increase in the endothelium-dependent vasorelaxation in SHRs. This suggests new insight into the mechanisms underlying the beneficial effects of exercise on hypertension.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31771312, 31371201), the Beijing Natural Science Foundation (5172023), the Chinese Universities Scientific Fund (2018GJ010), and the National Institutes of Health Grants R01HL135623 (D.X.), R01HD088039 (D.X.), and R03DA041492 (D.X.).
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Saarela S, Reiter RJ. Function of melatonin in thermoregulatory processes. Life Sci. 1994;54:295–311. [DOI] [PubMed] [Google Scholar]
- 2.Girouard H, Chulak C, Lejossec M, Lamontagne D, De Champlain J. Vasorelaxant effects of the chronic treatment with melatonin on mesenteric artery and aorta of spontaneously hypertensive rats. J Hypertens. 2001;19:1369–77. [DOI] [PubMed] [Google Scholar]
- 3.Kawashima K, Miwa Y, Fujimoto K, Oohata H, Nishino H, Koike H. Antihypertensive action of melatonin in the spontaneously hypertensive rats. Clin Exp Hypertens. 1987;9:1121–31. [DOI] [PubMed] [Google Scholar]
- 4.Tordjman S, Chokron S, Delorme R, Charrier A, Bellissant E, Jaafari N, Fougerou C. Melatonin: pharmacology, functions and therapeutic benefits. Curr Neuropharmacol. 2017;15:434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baltatu OC, Amaral FG, Campos LA, Cipollao-Neto J. Melatonin, mitochondria and hypertension. Cell Mol Life Sci. 2017. 10.1007/s00018-017-2613-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonas M, Garfinkel D, Zisapel N, Laudon M, Grossman E. Impaired nocturnal melatonin secretion in non-dipper hypertensive patients. Blood Press. 2003;12:19–24. [PubMed] [Google Scholar]
- 7.Brugger P, Marktl W, Herold M. Impaired nocturnal secretion of melatonin in coronary heart disease. Lancet. 1995;345:1408. [DOI] [PubMed] [Google Scholar]
- 8.Dwaich KH, Al-Amran FG, Al-Sheibani BI, Al-Aubaidy HA. Melatonin effects on myocardial ischemia-reperfusion injury: Impact on the outcome in patients undergoing coronary artery bypass grafting surgery. Int J Cardiol. 2016;221:977–86. [DOI] [PubMed] [Google Scholar]
- 9.Ekeløf SV, Halladin NL, Jensen SE, Zaremba T, Aarøe J, Kjærgaard B, Simonsen CW, Rosenberg H, Gögenur I. Effects of intracoronary melatonin on ischemia-reperfusion injury in ST-elevation myocardial infarction. Heart Vessels. 2016; 31:88–95. [DOI] [PubMed] [Google Scholar]
- 10.Girotti L, Lago M, Ianovsky O, Elizari MV, Dini A, Lloret SP, Albornoz LE, Cardinali DP. Low urinary 6-sulfatoxymelatonin levels in patients with severe congestive heart failure. Endocrine. 2003;22:245–8. [DOI] [PubMed] [Google Scholar]
- 11.Dominguez-Rodriguez A, Abreu-Gonzalez P. Future strategies for acute cardioprotection: ‘melatonin as promising therapy’. Cardiovasc Res. 2017;113:1418. [DOI] [PubMed] [Google Scholar]
- 12.Scheer F, Van Montfrans GA, Van Someren EJW, Mairuhu G, Buijs RM. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension. 2004; 43:192–7. [DOI] [PubMed] [Google Scholar]
- 13.Zanaboni A, Zanaboni-Muciaccia W. Experimental hypertension in pinealectomised rats. Life Sci. 1967;6:2327–31. [DOI] [PubMed] [Google Scholar]
- 14.Harlow HJ. Influence of the pineal gland and melatonin on blood flow and evaporative water loss during heat stress in rats. J Pineal Res. 1987;4:147–59. [DOI] [PubMed] [Google Scholar]
- 15.Das R, Balonan L, Ballard HJ, Ho S. Chronic hypoxia inhibits the antihypertensive effect of melatonin on pulmonary artery. Int J Cardiol. 2008;126:340–5. [DOI] [PubMed] [Google Scholar]
- 16.Dubocovich ML, Rivera-Bermudez MA, Gerdin MJ, Masana MI. Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front Biosci. 2003;8:d1093–108. [DOI] [PubMed] [Google Scholar]
- 17.Pandi-Perumal SR, Trakht I, Srinivasan V, Spence DW, Maestroni GJ, Zisapel N, Cardinali DP. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol. 2008;85:335–53. [DOI] [PubMed] [Google Scholar]
- 18.Monroe KK, Watts SW. The vascular reactivity of melatonin. Gen Pharmacol. 1998;30:31–5. [DOI] [PubMed] [Google Scholar]
- 19.Doolen S, Krause DN, Dubocovich ML, Duckles SP. Melatonin mediates two distinct responses in vascular smooth muscle. Eur J Pharmacol. 1998;345:67–9. [DOI] [PubMed] [Google Scholar]
- 20.Evans BK, Mason R, Wilson VG. Evidence for direct vasoconstrictor activity of melatonin in “pressurized” segments of isolated caudal artery from juvenile rats. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:362–5. [DOI] [PubMed] [Google Scholar]
- 21.Geary GG, Krause DN, Duckles SP. Melatonin directly constricts rat cerebral arteries through modulation of potassium channels. Am J Physiol. 1997;273:H1530–6. [DOI] [PubMed] [Google Scholar]
- 22.Viswanathan M, Scalbert E, Delagrange P, Guardiola-Lemaître B, Saavedra JM. Melatonin receptors mediate contraction of a rat cerebral artery. Neuroreport. 1997;8:3847–9. [DOI] [PubMed] [Google Scholar]
- 23.Satake N, Oe H, Shibata S. Vasorelaxing action of melatonin in rat isolated aorta; possible endothelium dependent relaxation. Gen Pharmacol. 1991;22:1127–33. [DOI] [PubMed] [Google Scholar]
- 24.Weekley LB. Melatonin-induced relaxation of rat aorta: interaction with adrenergic agonists. J Pineal Res. 1991;11:28–34. [DOI] [PubMed] [Google Scholar]
- 25.Cimen B, Uz A, Cetin I, Cimen L, Cetin A. Melatonin supplementation ameliorates energy charge and oxidative stress induced by acute exercise in rat heart tissue. Acta Cardiol Sin. 2017;33:530–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ianas O, Olinescu R, Badescu I. Melatonin involvement in oxidative processes. Endocrinologie. 1991;29:147–53. [PubMed] [Google Scholar]
- 27.Nichols AJ, Wilson AC, Hiley CR. Effects of sympathectomy with 6-hydroxydopamine on cardiac output and its distribution in the rat. Eur J Pharmacol. 1985;109:263–8. [DOI] [PubMed] [Google Scholar]
- 28.Hagberg JM, Park J-J, Brown MD. The role of exercise training in the treatment of hypertension. Sports Med. 2000;30:193–206. [DOI] [PubMed] [Google Scholar]
- 29.de Sousa EC, Abrahin O, Ferreira ALL, Rodrigues RP, Alves EAC, Vieira RP. Resistance training alone reduces systolic and diastolic blood pressure in prehypertensive and hypertensive individuals: meta-analysis. Hypertens Res. 2017;40:927–31. [DOI] [PubMed] [Google Scholar]
- 30.Inder JD, Carlson DJ, Dieberg G, McFarlane JR, Hess NC, Smart NA. Isometric exercise training for blood pressure management: a systematic review and meta-analysis to optimize benefit. Hyper-tens Res. 2016;39:88–94. [DOI] [PubMed] [Google Scholar]
- 31.Shi L, Zhang H, Chen Y, Liu Y, Lu N, Zhao T, Zhang L. Chronic exercise normalizes changes of Cav1.2 and KCa1.1 channels in mesenteric arteries from spontaneously hypertensive rats. Br J Pharmacol. 2015;172:1846–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goessler K, Polito M, Cornelissen VA. Effect of exercise training on the renin-angiotensin-aldosterone system in healthy individuals: a systematic review and meta-analysis. Hypertens Res. 2016;39:119–26. [DOI] [PubMed] [Google Scholar]
- 33.Buxton OM, L’hermite-Baleriaux M, Hirschfeld U, Cauter E. Acute and delayed effects of exercise on human melatonin secretion. J Biol Rhythms. 1997;12:568–74. [DOI] [PubMed] [Google Scholar]
- 34.Escames G, Ozturk G, Baño-Otálora B, Pozo MJ, Madrid JA, Reiter RJ, Serrano E, Concepción M, Acuña-Castroviejo D. Exercise and melatonin in humans: reciprocal benefits. J Pineal Res. 2012;52:1–11. [DOI] [PubMed] [Google Scholar]
- 35.Skrinar GS, Bullen BA, Reppert SM, Peachey SE, Turnbull BA, McArthur JW. Melatonin response to exercise training in women. J Pineal Res. 1989;7:185–94. [DOI] [PubMed] [Google Scholar]
- 36.Rezzani R, Rodella LF, Bonomini F, Tengattini S, Bianchi R, Reiter RJ. Beneficial effects of melatonin in protecting against cyclosporine A-induced cardiotoxicity are receptor mediated. J Pineal Res. 2006;41:288–95. [DOI] [PubMed] [Google Scholar]
- 37.Roque FR, Briones AM, García-Redondo AB, Galán M, Martínez-Revelles S, Avendaño MS, Cachofeiro V, Fernandes T, Vassallo DV, Oliveira EM, Salaices M. Aerobic exercise reduces oxidative stress and improves vascular changes of small mesenteric and coronary arteries in hypertension. Br J Pharmacol. 2013;168:686–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell JA, Bornstein DB, Sui X, Hooker SP, Church TS, Lee CD, Lee DC, Blair SN. The impact of combined health factors on cardiovascular disease mortality. Am Heart J. 2010;160:102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.da Costa Rebelo RM, Schreckenberg R, Schlüter KD. Adverse cardiac remodelling in spontaneously hypertensive rats: acceleration by high aerobic exercise intensity. J Physiol. 2012;590:5389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y, Zhang H, Zhang Y, Lu N, Zhang L, Shi L. Exercise intensity-dependent reverse and adverse remodeling of Cav1.2 channels in mesenteric arteries from spontaneously hypertensive rats. Hypertens Res. 2015;38:656–65. [DOI] [PubMed] [Google Scholar]
- 41.Sun MW, Qian FL, Wang J, Tao T, Guo J, Wang L, Lu AY, Chen H. Low-Intensity Voluntary running lowers blood pressure with simultaneous improvement in endothelium-dependent vasodilatation and insulin sensitivity in aged spontaneously hypertensive rats. Hypertens Res. 2008;31:543–52. [DOI] [PubMed] [Google Scholar]
- 42.Paulis L, Simko F. Blood pressure modulation and cardiovascular protection by melatonin: potential mechanisms behind. Physiol Res. 2007;56:671–84. [DOI] [PubMed] [Google Scholar]
- 43.Agarwal D, Elks CM, Reed SD, Mariappan N, Majid DS, Francis J. Chronic exercise preserves renal structure and hemodynamics in spontaneously hypertensive rats. Antioxid Redox Signal. 2012;16:139–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, Witte K, August M, Brausch I, Gödtel-Armbrust U, Habermeier A, Closs EI, Oelze M, Münzel T, Fürstermann T, Förstermann U. Reversal of endothelial nitric oxide synthase uncoupling and up-regulation of endothelial nitric oxide synthase expression lowers blood pressure in hypertensive rats. J Am Coll Cardiol. 2006;47:2536–44. [DOI] [PubMed] [Google Scholar]
- 45.Wu CC, Bohr DF. Role of endothelium in the response to endothelin in hypertension. Hypertension. 1990;16:677–81. [DOI] [PubMed] [Google Scholar]
- 46.Dekleva M, Lazic JS, Arandjelovic A, Mazic S. Beneficial and harmful effects of exercise in hypertensive patients: the role of oxidative stress. Hypertens Res. 2017;40:15–20. [DOI] [PubMed] [Google Scholar]
- 47.Battault S, Singh F, Gayrard S, Zoll J, Reboul C, Meyer G. Endothelial function does not improve with high-intensity continuous exercise training in SHR: implications of eNOS uncoupling. Hypertens Res. 2016;39:70–8. [DOI] [PubMed] [Google Scholar]
- 48.Dubocovich ML, Masana MI, Iacob S, Sauri DM. Melatonin receptor antagonists that differentiate between the human Mel1a and Mel1b recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML1 presynaptic heteroreceptor. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:365–75. [DOI] [PubMed] [Google Scholar]
- 49.Dubocovich ML, Yun K, Al-Ghoul WM, Benloucif S, Masana MI. Selective MT2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms. FASEB J. 1998;12:1211–20. [DOI] [PubMed] [Google Scholar]
- 50.Masana MI, Doolen S, Ersahin C, Al-Ghoul WM, Duckles SP, Dubocovich ML, Krause DN. MT(2) melatonin receptors are present and functional in rat caudal artery. J Pharmacol Exp Ther. 2002;302:1295–302. [DOI] [PubMed] [Google Scholar]
- 51.Reiter RJ. Antioxidant actions of melatonin. Adv Pharmacol. 1997;38:103–17. [DOI] [PubMed] [Google Scholar]
- 52.Wakatsuki A, Okatani Y. Melatonin protects against the free radicalinduced impairment of nitric oxide production in the human umbilical artery. J Pineal Res. 2000;28:172–8. [DOI] [PubMed] [Google Scholar]
- 53.Zhao T, Zhang H, Jin C, Qiu F, Wu Y, Shi L. Melatonin mediates vasodilation through both direct and indirect activation of BKCa channels. J Mol Endocrinol. 2017;59:219–33. [DOI] [PubMed] [Google Scholar]