Abstract
Purpose
Non-small cell lung cancer (NSCLC) accounts for the majority of lung cancer. Lipid–polymer hybrid nanoparticles (LPNs) combine the advantages of both polymeric nanoparticles and liposomes into a single delivery platform. In this study, we engineered LPNs as the co-delivery system of paclitaxel (PTX) and triptolide (TL) to achieve synergistic therapeutic effect and reduced drug resistance.
Materials and methods
In this study, PTX- and TL-coloaded LPNs (P/T-LPNs) were fabricated by nanoprecipitation method using lipid and polymeric materials. The P/T-LPNs combination effects on human lung cancer cells were studied. Therapeutic potentials of P/T-LPNs were further determined using lung cancer cells-bearing mice model.
Results
The average particle sizes of LPNs were around 160 nm, with narrow size distribution below 0.2. The zeta potential value of LPNs was about −30 mV. The encapsulating efficiency (EE) of PTX and TL loaded in LPNs was over 85%. The cytotoxicity of dual drug loaded LPNs was higher than single drug loaded LPNs. The combination therapy showed synergistic when PTX:TL weight ratio was 5:3, indicating the synergy effects of the LPNs. In vivo tumor growth curve of the experimental group was more gentle opposed to the control group, and tumor volumes of P/T-LPNs and control group were 392 and 1,737 mm3, respectively. The inhibition rate on day 20 was 77.4% in the P/T-LPNs group, which is higher than the free drugs solution.
Conclusion
The in vivo and in vitro results proved the synergetic effect of the two drugs coloaded in LPNs on the lung cancer xenografts, with the least systemic toxic side effect.
Keywords: non-small cell lung cancer, multidrug resistance, combination therapy, paclitaxel, triptolide
Introduction
Lung cancer is the most common cancer worldwide and nearly 23% of the total cancer-related deaths are caused by lung cancer globally.1 Non-small cell lung cancer (NSCLC) accounts for the majority (more than 80%) of lung cancer. Current therapeutic strategies for NSCLC are generally dependent on their stages at the time of diagnosis. However, in 85% of patients, NSCLC is diagnosed in their advanced stages and surgery is not suitable for them.2 Therefore, the most preferred therapy prevalent clinically is chemotherapy.3 Currently, the third-generation chemotherapy agents such as paclitaxel (PTX) in combination with a platinum compound is the recommended first-line treatment for advanced NSCLC.4,5 However, systemic toxicity of PTX or platinum and drug resistance have led to a high rate of mortality in NSCLC.6 As such, it is urgent to design novel treatment approaches for NSCLC.
Nanotechnology-based drug delivery system (DDS) potentially represents such a disruptive technology for improving therapeutic efficacy and reducing systemic toxicity by enhancing the pharmacokinetics parameters such as large volume distribution and tumor deposition of the payloads through enhanced permeability and retention (EPR) effect.7–9 Among various kinds of nanoparticles, lipid–polymer hybrid nanoparticles (LPNs) combine the advantages of both polymeric nanoparticles and liposomes into a single delivery platform.10–12 Specifically, based on the LPNs’ dual-component structure, it provides an ideal platform for combinatorial drug delivery, whereby different drugs can be loaded to either the polymer core or the lipid shell, on account of the desired release kinetics and therapeutic roles.10 In this study, we engineered LPNs as the co-delivery system of PTX and triptolide (TL) to achieve synergistic therapeutic effect and reduced drug resistance.
TL, an active compound isolated from the Chinese herb Tripterygium wilfordii, possesses a broad-spectrum therapeutic effect including anti-inflammatory, immunosuppressive, and antitumor activities.13,14 TL has also been shown to induce cell apoptosis and inhibit cell proliferation of various cancer cells including pancreatic, gastric, NSCLC, etc.15–18 Its cytotoxic effect against NSCLC has been demonstrated in vitro and in vivo via the inhibition of nuclear factor (NF)-κB; upregulation of p-p38, p-ERK, p-GSK-3β, Bax, and cleaved caspases-3 and -9; and downregulation of p-JNK, p-Akt, and Bcl-2.19 Moreover, TL exerts its anti-multidrug resistance (MDR) role on A549/Taxol cell lines by inhibiting the NF-κB signaling pathway and selective modulation of mitogen-activated protein kinase signaling.20,21 However, TL has a poor solubility in water (0.017 mg/mL).22 Significant and rapid fluctuations of TL in plasma by oral administration (Tmax from 10.0 to 19.5 minutes; t1/2 from 16.8 to 50.6 minutes) likely contribute to its toxicity, which is characterized by injury to hepatic, renal, digestive, reproductive, and hematological systems.23 Therefore, it is necessary to design LPNs for prolonging drug release and improving its safety.
In this study, PTX- and TL-coloaded LPNs (P/T-LPNs) were fabricated by nanoprecipitation method using lipid and polymeric materials (Figure 1). The P/T-LPNs combination effects on human lung cancer cells were investigated.24 The therapeutic potentials of P/T-LPNs were further determined using lung cancer cells-bearing mice model. Single drug loaded LPNs and free drugs combination were applied for contrast. As expected, such a co-delivery system would combine the desirable properties of LPNs and the synergistic effect of PTX and TL and thus provide a fascinating opportunity to treat lung cancer.
Figure 1.
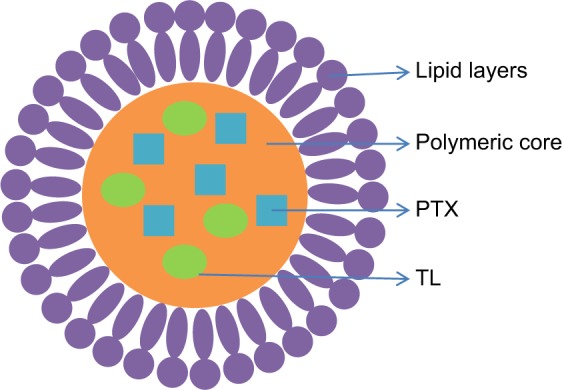
Schematic diagram PTX- and TL-coloaded LPNs (P/T-LPNs).
Abbreviations: LPN, lipid–polymer hybrid nanoparticle; P/T-LPN, PTX- and TL-coloaded LPN; PTX, paclitaxel; TL, triptolide.
Materials and methods
Chemicals and reagents
TL (isolated from T. wilfordii, ≥98% HPLC, solid) and PTX (semisynthetic drug isolated from Taxus sp., ≥97%) were purchased from Sigma-Aldrich (Shanghai, China). Poly(lactic-co-glycolic acid) (PLGA; 75/25; molecular weight, 23–37 kDa) was obtained from Dalian Meilun Biotechnology Co., Ltd. (Dalian, China). 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-5000] (DSPE-mPEG5000) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[(polyethylene glycol)-5000]-fluorescein isothiocyanate (DSPE-PEG5000-FITC) were provided by Xi’an ruixi Biological Technology Co., Ltd. (Xi’an, China). Injectable soybean lecithin (ISL) was provided by Shanghai A.V.T. Pharmaceutical Co., Ltd (Shanghai, China). DMEM, Roswell Park Memorial Institute medium (RPMI-1640), FBS, penicillin/streptomycin, and MTT were purchased from Thermo Fisher Scientific, Waltham, MA, USA. All other chemicals and reagents used were of analytical or HPLC grade.
Cells and culture
A549 human lung adenocarcinoma cells (A549 cells) were obtained from American Type Culture Collections (Manassas, VA, USA). A549/PTX (PTX resistant) cells were purchased from Shanghai MEIXUAN Biological Science and Technology Co, Ltd. (Shanghai, China). A549 cells were cultured in DMEM, and A549/PTX cells were cultured in RPMI-1640 and supplemented with 10% FBS and penicillin (100 U/mL)/streptomycin (100 U/mL) at 37°C in a humidified atmosphere with 5% CO2.25
Animals and xenografts
Balb/c-nude mice (8–10 weeks old, 20–25 g weight) were purchased from Laboratory Animal Center of Shaanxi Province (Xi’an, China) and maintained in plastic cages in an specific pathogen free-grade animal room with access to food and water ad libitum. A549/PTX cells (107) suspended in 0.9% saline (200 µL) were subcutaneously injected into the right flank of mice to produce the lung tumor xenografts. All animal experiments were complied with the guideline: National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No 8023). All the procedures were ethically and scientifically approved by the Institutional Animal Care and Treatment Committee of Tumor Hospital of Shaanxi Province (No 20180119–01, 2018-01-19).
Preparation of P/T-LPNs
P/T-LPNs were prepared by nanoprecipitation method.26 DSPE-mPEG5000 (100 mg) and ISL (100 mg) were dispersed in distilled water (aqueous phase). PTX (50 mg), TL (30 mg), and PLGA (200 mg) were dissolved in acetone (oil phase). Oil phase is added dropwise into the aqueous phase stirred at 400 rpm at room temperature for 8 hours until complete evaporation of acetone. The mixture was centrifuged to remove the unloaded PTX and/or TL at a speed of 3,000 rpm for 10 minutes and then filtered through a syringe filter (pore size, 0.45 µM). PTX-loaded LPNs (P-LPNs) were produced using the same method by dissolving PTX (100 mg) and PLGA (200 mg) in acetone to produce the oil phase. TL-loaded LPNs (T-LPNs) were produced using the same method by dissolving TL (60 mg) and PLGA (200 mg) in acetone to produce the oil phase. Blank LPNs were produced using the same method by dissolving PLGA (200 mg) in acetone to produce the oil phase. FITC-loaded LPNs were prepared using the same method, replacing DSPE-mPEG5000 with DSPE-PEG5000-FITC.
PTX solution (P solution) was prepared by dissolving 10 mg PTX in 10 mL solvent 1 (acetone:ethanol = 3:2, v:v). TL solution (T solution) was prepared by dissolving 6 mg TL in 10 mL solvent 1. PTX and TL mixed solution (P/T solution) was prepared by dissolving 5 mg PTX and 3 mg TL in 10 mL solvent 1.
Particle size and zeta potential
Particle sizes, polydispersity indexes, and zeta potentials of LPNs were determined 1 hour after preparation by placing the LPNs in a cuvette.27 A ZETA SIZER3000 laser particle size analyzer and dynamic light scattering software were used to detect their average diameter and surface potential. The tests were conducted in triplicate, and the average data were calculated.
Drug-loading (DL) content and encapsulating efficiency (EE)
DL content and EE of PTX in the LPNs was determined by UV/Vis method.28 PTX was also isolated from the carriers by centrifugation at 15,000 rpm at 4°C for 30 minutes, the supernatants were collected, and the concentration of PTX was measured by UV/Vis spectrophotometer at 233 nm. The content of TL in the nanoparticles was determined by HPLC using a reversed-phase column (C18; 250 mm × 4.6 mm, 5 µm).29 The gradient mobile phase consisted of a mixture of methanol and water, and the flow rate was 1.0 mL/min. The column effluent was detected at 218 nm, and the column temperature was 30°C. DL and EE were calculated according to the following equations:
Storage stability
LPNs dispersions were stored at 4°C, and the stability of LPNs was evaluated by measuring average vesicle size and EE during 3 months.30 Results are recorded at 0, 10, 20, 30, 60, and 90 days.
In vitro release behavior
In vitro release behavior of PTX and TL from LPNs was explored by placing 1 mL of LPNs in dialysis bags (molecular weight cutoff, 3.5 kDa).31 The dialysis bags were incubated in 30 mL phosphate buffer (pH, 7.4) containing Tween 80 (0.5%, w/w) at 37°C under gentle shaking (100 rpm). At different time intervals, incubation medium (2 mL) was taken out and replaced by the same volume of fresh preheated medium at specific time points. The release medium was vortexed and analyzed by the same method explained in the section “Drug-loading (DL) content and encapsulating efficiency (EE)”. The tests were conducted in triplicate, and the average data were calculated.
Cellular uptake
A549 and A549/PTX cells were seeded in glass bottom dishes at a density of 2 × 105 cells per dish.32 After 24 hours of incubation, cells were treated with a concentration of 20 µg/mL LPNs. The cells were washed three times with cold PBS after 2 hours of incubation at 37°C. The fluorescence intensity was measured at an excitation wavelength of 488 nm using an inversion fluorescence microscope (OLYMPUS Corporation, Tokyo, Japan), and the picture was captured. At the indicated time points, the cells were washed with PBS, harvested, and dispersed in 0.5 mL of PBS solution for flow cytometric measurements using a FACSCalibur flow cytometer (BD Bio-sciences, San Jose, CA, USA).33
Cytotoxicity assay and synergistic effects
A549 and A549/PTX cells were cultured in 96-well plate (5 × 103 cells per well) for 24 hours.34 Then, the cells were incubated with fresh culture medium containing free drugs or drugs loaded LPNs for 48 hours. The medium was replaced with the fresh medium to recapitulate the effects of the process. MTT solution (20 µL, 5 mg/mL) was then added into each well, and the cells were incubated for another 4 hours. Next, the culture medium was discarded, and 200 µL DMSO was added into the well. Finally, the absorbance of liquid in each well was measured at 490 nm by using a Microplate Reader (Bio-Rad Laboratories Inc., Hercules, CA, USA). Cell viability was calculated according to the following equation: (Absorbance of cells treated with samples/Absorbance of cells treated with fresh medium) × 100%. The mean drug concentration required for 50% growth inhibition (IC50) was calculated.
To study the synergistic effect of the combination treatment of the formulation, Combination Index (CI) analysis was undertaken.35 The results of cytotoxicity were evaluated via the CI. The CI of a combination of drugs less than 1 indicates the synergistic effect, CI equal to 1 means additive effect, and CI greater than 1 means an antagonistic effect. To evaluate the CI value, both individual drugs and drug combinations treatment in a range of concentrations were performed on the cell lines as described above. Combination index when 50% growth inhibition (CI50) was considered. Briefly, for each level of Fa (the fraction of affected cells), the CI values of PTX and TL combinations were calculated by the following equation36: CI50 = (D)A/(D50)A + (D)B/(D50)B. In this equation, (D)A and (D)B represent the IC50 value of PTX and TL alone, respectively. (D50)A and (D50)B represent the concentration of PTX and TL in the combination system at the IC50 value. The CI50 values curves were drawn according to Fa. Fa values between 0.2 and 0.8 are considered validate.
In vivo antitumor efficacy
The lung tumor xenografts were randomly assigned to eight groups (n = 10 each): 0.9% saline control, P solution (10 mg/kg), T solution (6 mg/kg), P/T solution (5 mg/kg of PTX and 3 mg/kg of TL), blank LPNs, P-LPNs (10 mg/kg), T-LPNs (6 mg/kg), and P/T-LPNs (5 mg/kg of PTX and 3 mg/kg of TL) were administered intravenously every other day for 18 days.37 Mice were sacrificed by cervical dislocation on day 20, and the tumor tissues were collected for further analysis. During the treatment, tumor size (length and width) was measured using calipers every 4 days. Tumor volumes (V) were calculated by the formula V = a×b2/2, where a is the longest axis, and b is the perpendicular shorter tumor axis. The tumor volume inhibition rate (TIR) on day 20 was calculated according to the following equation: TIR (%) = [1 − (VDay 1 treatment − VDay 20 treatment)/(VDay 1 control − VDay 20 control)] × 100, where VDay 1 treatment and VDay 20 treatment are the tumor volumes of the treatment groups at day 1 and day 20 and VDay 1 control and VDay 20 control are the tumor volumes of the control group at day 1 and day 20. Body weights of mice were measured for the evaluation of systematic toxicity.
Statistical analysis
Quantitative data were expressed as mean ± SD. Statistical analysis was performed using an unpaired t-test. P-value less than 0.05 was considered statistically significant.
Results
Characterization of P/T-LPNs
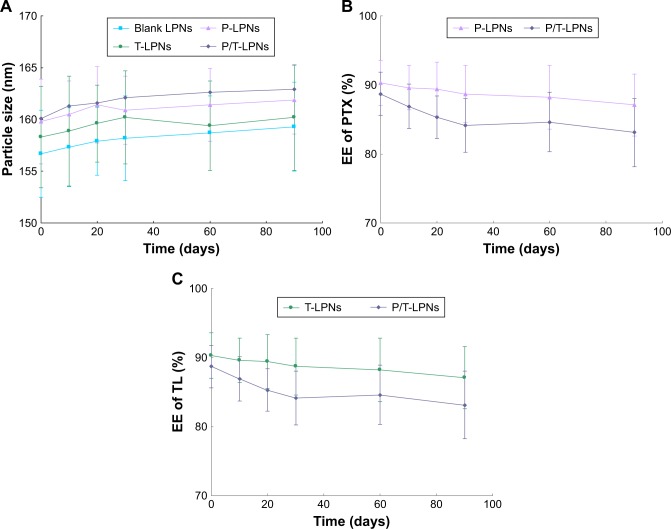
The average particle sizes of LPNs were around 160 nm, with narrow size distribution with polydispersity index values below 0.2 (Table 1). The zeta potential value of LPNs was about −30 mV. The EE of PTX and TL loaded in LPNs was over 85%. The DL of PTX and TL loaded in LPNs were approximately 10% and 6%, respectively. During the period of 90 days, LPNs showed no significant changes of average vesicle size and EE (Figure 2).
Table 1.
Characterization of LPNs (mean ± SD, n = 3)
| LPNs | Blank LPNs | P-LPNs | T-LPNs | P/T-LPNs |
|---|---|---|---|---|
| Particle size (nm) | 156.7 ± 5.1 | 159.8 ± 5.6 | 158.3 ± 5.5 | 160.1 ± 5.9 |
| Polydispersity index | 0.16 ± 0.02 | 0.15 ± 0.03 | 0.14 ± 0.02 | 0.17 ± 0.03 |
| Zeta potential (mV) | −31.2 ± 4.2 | −30.9 ± 4.6 | −29.8 ± 4.9 | −30.4 ± 4.4 |
| EE of PTX (%) | N/A | 90.3 ± 3.6 | N/A | 88.7 ± 4.1 |
| EE of TL (%) | N/A | N/A | 86.2 ± 4.5 | 85.4 ± 4.8 |
| DL content of PTX (%) | N/A | 10.4 ± 1.2 | N/A | 11.1 ± 1.5 |
| DL content of TL (%) | N/A | N/A | 6.5 ± 0.8 | 6.2 ± 0.9 |
Abbreviations: DL, drug loading; EE, encapsulating efficiency; LPN, lipid–polymer hybrid nanoparticles; P-LPN, PTX-loaded LPN; P/T-LPN, PTX- and TL-coloaded LPN; PTX, paclitaxel; TL, triptolide; T-LPN, TL-loaded LPN.
Figure 2.
Storage stability of LPNs dispersions evaluated by measuring of average vesicle size (A) and EE of PTX (B) and TL (C). LPNs showed no significant changes of average vesicle size and EE during 3 months.
Abbreviations: EE, encapsulating efficiency; LPN, lipid–polymer hybrid nanoparticle; P-LPN, PTX-loaded LPN; P/T-LPN, PTX- and TL-coloaded LPN; PTX, paclitaxel; TL, triptolide; T-LPN, TL-loaded LPN.
In vitro release behavior
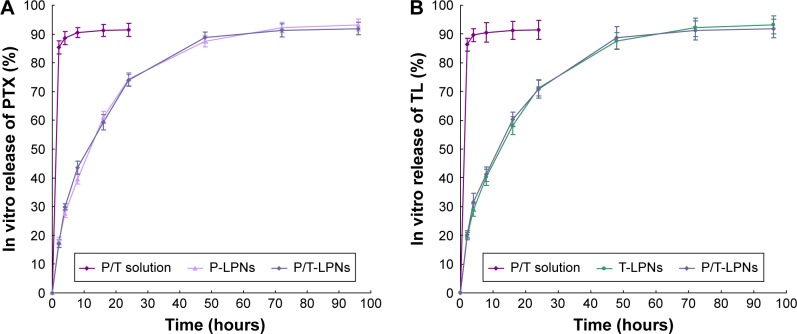
As shown in Figure 3, approximately 90% of drugs was slowly released from LPNs in a sustained behavior within 48 hours, with no burst effect. Drugs solutions showed faster release within 2 hours. Dual drugs coloaded LPNs did not exhibit differences during the release compared with single drug loaded LPNs.
Figure 3.
In vitro PTX (A) and TL (B) release behavior of drugs loaded LPNs and drugs solutions. Approximately 90% of drugs was slowly released from LPNs in a sustained behavior within 48 hours, with no burst effect.
Abbreviations: LPN, lipid–polymer hybrid nanoparticle; P-LPN, PTX-loaded LPN; P/T-LPN, PTX- and TL-coloaded LPN; PTX, paclitaxel; TL, triptolide; T-LPN, TL-loaded LPN.
Cellular uptake
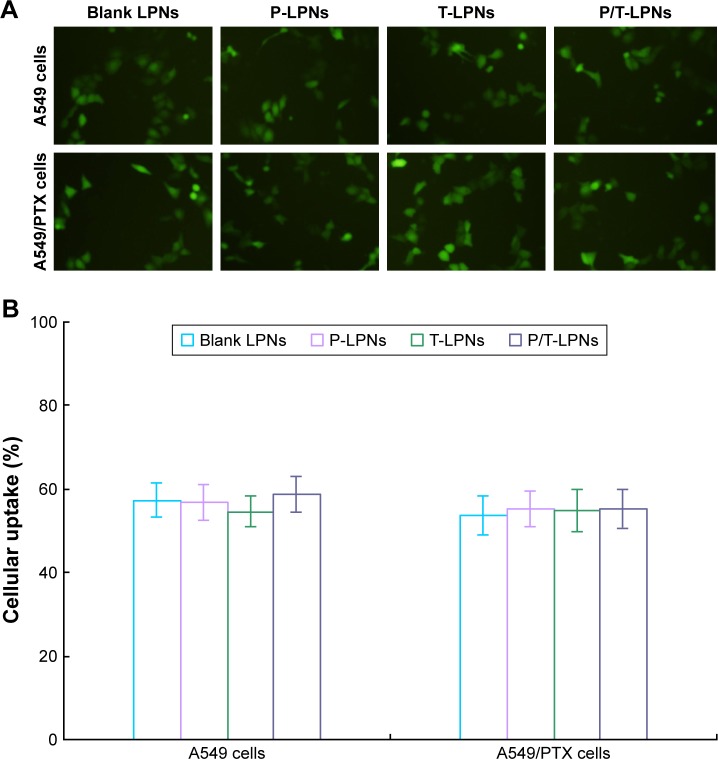
To determine the intracellular accumulation of LPNs to assess the efficacy of the formulations, qualitative and quantitative studies of cellular uptake were performed. In the qualitative study, both A549 and A549/PTX cells with LPNs exhibited obvious fluorescence at 2 hours after treatments (Figure 4A). The quantitative study of cellular uptake was further performed to confirm the percentage of cells that have been entered by LPNs (Figure 4B).
Figure 4.
Qualitative (A) and quantitative (B) studies of cellular uptake of LPNs. Both A549 and A549/PTX cells exhibited high cellular uptake by LPNs.
Abbreviations: LPN, lipid–polymer hybrid nanoparticle; P-LPN, PTX-loaded LPN; P/T-LPN, PTX- and TL-coloaded LPN; PTX, paclitaxel; T-LPN, TL-loaded LPN.
Cytotoxicity assay and synergistic effects
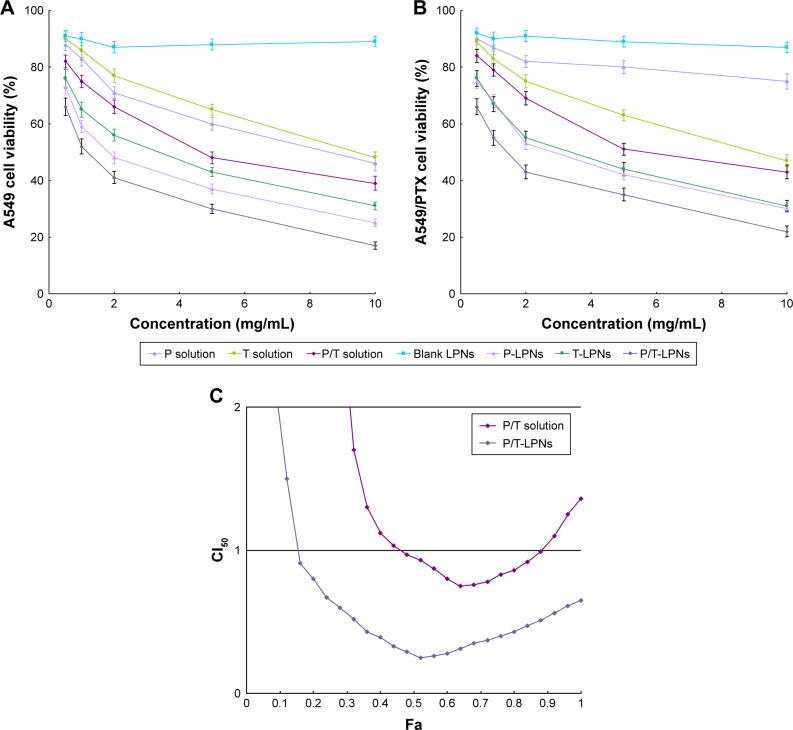
The blank LPNs exhibited no significant cytotoxicity on A549 (Figure 5A) and A549/PTX cells (Figure 5B) and when its concentration ranged from 0.5 to 10 mg/mL. Drugs loaded LPNs exhibited marked cytotoxicity on cells in a dose-dependent manner and showed higher cytotoxicity compared with their free drugs counterparts (P < 0.05). The cytotoxicity of dual drugs loaded LPNs was higher than single drug loaded LPNs (P < 0.05). Table 2 showed the effects of LPNs on A549/PTX cells: combination therapy showed synergistic when PTX:TL weight ratio was 5:3 (CI50 = 0.84), suggesting the best drugs ratios in the LPNs. This ratio was used for the in vivo antitumor study. For Fa determination on A549 cells, P/T-LPNs displayed an overall CI50 value <1 when Fa value was between 0.2 and 0.8 (Figure 5C), indicating the synergy effects of the LPNs. Table 3 summarized the lC50 values evaluated on A549 and A549/PTX cells. IC50 of PTX on A549/PTX cells was significantly higher than A549 cells, showing the drug resistance in A549/PTX cells.
Figure 5.
Cytotoxicity assay of drugs loaded LPNs and drugs solutions on A549 (A) and A549/PTX cells (B). Synergistic effects evaluated by Fa determination on A549 cells (C).
Abbreviations: LPN, lipid–polymer hybrid nanoparticle; P-LPN, PTX-loaded LPN; P/T-LPN, PTX- and TL-coloaded LPN; PTX, paclitaxel; T-LPN, TL-loaded LPN.
Table 2.
CI50 values of P/T-LPNs on A549/PTX cells with different PTX:TL ratios
| LPNs | PTX:TL (w:w) | IC50 of PTX (mg/mL) | IC50 of TL (mg/mL) | CI50 |
|---|---|---|---|---|
| P-LPNs | N/A | 2.56 | N/A | N/A |
| T-LPNs | N/A | N/A | 3.39 | N/A |
| P/T-LPNs | 5:1 | 2.49 | 0.51 | 1.12 |
| P/T-LPNs | 5:2 | 2.37 | 0.95 | 1.21 |
| P/T-LPNs | 2:1 | 2.21 | 1.10 | 1.19 |
| P/T-LPNs | 5:3 | 1.49 | 0.89 | 0.84 |
| P/T-LPNs | 5:4 | 1.68 | 1.34 | 1.05 |
| P/T-LPNs | 1:1 | 1.87 | 1.87 | 1.28 |
Abbreviations: CI50, combination index when 50% growth inhibition; IC50, 50% growth inhibition; LPN, lipid–polymer hybrid nanoparticle; P-LPN, PTX-loaded LPN; P/T-LPN, PTX- and TL-coloaded LPN; PTX, paclitaxel; TL, triptolide; T-LPN, TL-loaded LPN.
Table 3.
IC50 values of free drugs or drugs loaded LPNs on A549 and A549/PTX cells
| Samples | A549 cells
|
A549/PTX cells
|
||
|---|---|---|---|---|
| IC50 of PTX (mg/mL) | IC50 of TL (mg/mL) | IC50 of PTX (mg/mL) | IC50 of TL (mg/mL) | |
| P solution | 8.62 | N/A | 106.9 | N/A |
| T solution | N/A | 9.13 | N/A | 9.21 |
| P/T solution | 4.87 | 2.92 | 8.64 | 5.18 |
| P-LPNs | 1.92 | N/A | 2.56 | N/A |
| T-LPNs | N/A | 3.58 | N/A | 3.39 |
| P/T-LPNs | 1.27 | 0.76 | 1.49 | 0.89 |
Abbreviations: IC50, 50% growth inhibition; LPN, lipid–polymer hybrid nanoparticle; P-LPN, PTX-loaded LPN; P/T-LPN, PTX- and TL-coloaded LPN; PTX, paclitaxel; TL, triptolide; T-LPN, TL-loaded LPN.
In vivo antitumor efficacy
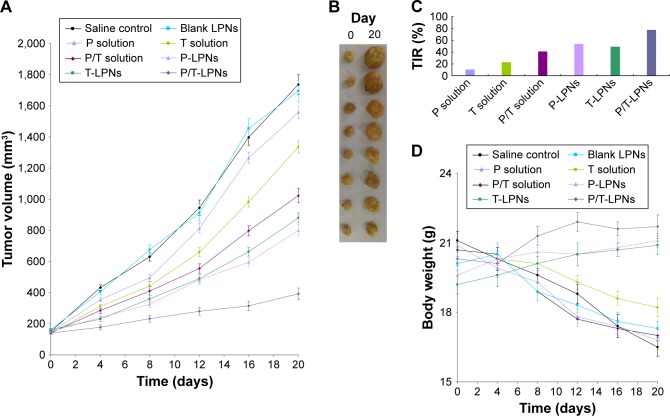
In vivo tumor growth inhibition curve of the experimental group was more gentle compared to that of the control group. After 20 days, tumor volume of the P/T-LPNs group was significantly smaller than the P-LPNs and T-LPNs groups (P < 0.05; Figure 6A). The drugs loaded LPNs exhibited smaller tumor size than the free drugs loaded counterparts (Figure 6B). The inhibition rate on day 20 was 77.4% in the P/T-LPNs group, 53.9% in the P-LPNs group, 49.3% in the T-LPNs group, and 41.1% in the P/T solution group (Figure 6C). There were no significant differences in the body weight of mice treated with drugs loaded LPNs groups, but reduction of weights were found in the drugs solution and control groups (Figure 6D).
Figure 6.
In vivo tumor growth inhibition curve of the experimental group (A), tumor images (B), tumor inhibition rate (C), and body weight of mice (D).
Abbreviations: LPN, lipid–polymer hybrid nanoparticle; P-LPN, paclitaxel-loaded LPN; P/T-LPN, paclitaxel- and TL-coloaded LPN; TIR, tumor volume inhibition rate; T-LPN, TL-loaded LPN.
Discussion
Nanoparticles are known to exploit the EPR effect for targeting the tumors, thereby increasing tumor drug concentrations while minimizing systemic toxicity.38 Many recent studies have highlighted the significance of nanoparticle sizes, which would greatly affect the fate of nanoparticle in vivo: less than 200 nm showed longer blood circulation time, greater stability, lower cytotoxicity, and favorable uptake by the EPR effect.39,40 Moreover, the EE of PTX and TL for all kinds of LPNs were over 80%, indicating the good loading ability of the LPNs.
Storage stability evaluation of the nanoparticles was essential to confirm that the structural properties were preserved over storage time, since disruption of nanocarriers in the DDSs could affect their therapeutic potential.41 For all kinds of LPNs, the particle sizes remained almost constant for 90 days. The EE of two dugs loaded in LPNs also remained stable at all the time points tested in the research, indicating that the LPNs were stable and will not disassemble or aggregate within 3 months. In this study, DSPE-mPEG10000, DSPE-mPEG5000, DSPE-mPEG2000, and DSPE-mPEG550 were tried, and only DSPE-mPEG5000 could form nanoparticles with uniform particle sizes and polydispersity index. We also tried soybean lecithin, egg lecithin, and cholesterol in the oil phase; some precipitated immediately after preparation or storage stability would not last for a week. Only ISL gain good stability. So, DSPE-mPEG5000 and ISL were chosen for making the LPNs.
In vitro drug release of the drug loaded nanoparticles may be controlled by the erosion, corrosion, and diffusion process.42 Drug depot effects could be obtained by the carriers, which could lead to the sustained release of hydrophobic drugs. More sustained-release behavior of drugs from LPNs was found in the in vitro drug release study. As the drugs are restrained within the LPNs, the cytotoxicity of drugs may be lowered until the LPNs entered the tumor cells. Once within the cells, the PTX and TL can be released from the LPNs and induce their toxic impacts.43 These results indicate that the lipid layer at the interface of the PLGA core and the PEG shell acts as a molecular fence that helps to retain the drugs inside the LPNs, thus let the drugs release slowly from the nanoparticles.
Effective cytotoxicity of nanoparticle-based therapies usually demands a high level of accumulation within the cancer cells.44 Cellular uptake of LPNs into the cells was examined to distinguish the uptake efficiency. The results of qualitative and quantitative studies of cellular uptake indicate that the functionalization of LPNs encapsulation played a key role in the uptake of drugs in lung cancer cells in vitro. High internalization of the drugs loaded nanoparticles into the cells could directly account for the anticancer efficacy, which leads to more efficacy of cell growth inhibition, induction of apoptosis, and cell cycle arrest in cancer cells.
The prepared blank LPNs nanoparticles showed no cytotoxicity on A549/PTX cells in vitro and in vivo in nude mice, demonstrating that LPNs were safe as DDS as many studies reported.45 Drugs were released in a controlled manner from the LPNs, but higher cell toxicity was observed in MTT assay than the drugs solutions. The use of multiple drugs in combination has possible favorable outcomes, such as synergism, additive, and antagonism. Evaluation of drug–drug interaction plays an important role in all areas of medicine and particularly in cancer chemotherapy where combination therapy is commonly used.46 To determine the possible effect of the drug combination, mathematical model-based method has been introduced. LPNs combing the mechanical advantages of biodegradable polymers and liposomes are core-shell nanoparticles structures comprising polymer cores and lipid shells.47 Also, dual drugs coloaded LPNs has better ability and showed obvious synergism effect than the single drug loaded systems. The novel carrier might have the enhanced ability to adhere to the cell membrane due to the similar nature of the lipids and the cell membrane. Table 3 showed the cytotoxicity data that could demonstrate the extent of drug resistance in A549/PTX cells. CI analyses are the most popular methods for evaluating drug interactions in combination cancer chemotherapy.36 In this research, CI50 was determined to validate the synergistic effect and suitable PTX to TL ratio to be loaded in the LPNs. P/T-LPNs displayed an overall CI50 value <1 when Fa value was between 0.2 and 0.8, showing the synergy effects of the LPNs. TL has been shown to induce cell apoptosis and inhibit cell proliferation of various cancer cells including NSCLC. TL may exert its anti-MDR role on A549/PTX cells by inhibiting the NF-κB signaling pathway and selective modulation of mitogen-activated protein kinase signaling.
To evaluate the effect of P/T-LPNs on lung cancer-bearing mouse xenografts, tumor volume was measured and plotted. With regard to the inhibition of tumor growth, the P/T-LPNs exhibited the strongest inhibitory effect. These results demonstrated that P/T-LPNs system was the most effective treatment in reducing the tumor volume. No noticeable loss of body weight of mice was considered as an indicator of safety.48 The in vivo antitumor results were in line with the in vitro results and proved the synergetic effect of the two drugs coloaded in LPNs in the lung cancer xenografts, with the least systemic toxic side effect.
Conclusion
The cytotoxicity of dual drugs loaded LPNs was higher than single drug loaded LPNs. Combination therapy showed synergy effects of the LPNs. In vivo tumor growth inhibition curve of the experimental group was more gentle compared to that of the control group. The drugs loaded LPNs exhibited smaller tumor size than the free drugs loaded counterparts. The in vivo and in vitro results proved the synergetic effect of the two drugs coloaded in LPNs in the lung cancer xeno-grafts, with the least systemic toxic side effect.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Provencio M, Isla D, Sánchez A, Cantos B. Inoperable stage III non-small cell lung cancer: Current treatment and role of vinorelbine. J Thorac Dis. 2011;3(3):197–204. doi: 10.3978/j.issn.2072-1439.2011.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koshkina NV, Waldrep JC, Roberts LE, Golunski E, Melton S, Knight V. Paclitaxel liposome aerosol treatment induces inhibition of pulmonary metastases in murine renal carcinoma model. Clin Cancer Res. 2001;7(10):3258–3262. [PubMed] [Google Scholar]
- 4.Chu Q, Vincent M, Logan D, Mackay JA, Evans WK, Lung Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-based Care Lung Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-based Care. Taxanes as first-line therapy for advanced non-small cell lung cancer: a systematic review and practice guideline. Lung Cancer. 2005;50(3):355–374. doi: 10.1016/j.lungcan.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Rigas JR. Taxane-platinum combinations in advanced non-small cell lung cancer: a review. Oncologist. 2004;9(Suppl 2):16–23. doi: 10.1634/theoncologist.9-suppl_2-16. [DOI] [PubMed] [Google Scholar]
- 6.Nascimento AV, Gattacceca F, Singh A, et al. Biodistribution and pharmacokinetics of Mad2 siRNA-loaded EGFR-targeted chitosan nanoparticles in cisplatin sensitive and resistant lung cancer models. Nanomedicine. 2016;11(7):767–781. doi: 10.2217/nnm.16.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aftab S, Shah A, Nadhman A, et al. Nanomedicine: An effective tool in cancer therapy. Int J Pharm. 2018;540(1–2):132–149. doi: 10.1016/j.ijpharm.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 8.He C, Lu J, Lin W. Hybrid nanoparticles for combination therapy of cancer. J Control Release. 2015;219:224–236. doi: 10.1016/j.jconrel.2015.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 10.Hadinoto K, Sundaresan A, Cheow WS. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review. Eur J Pharm Biopharm. 2013;85(3 Pt A):427–443. doi: 10.1016/j.ejpb.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Mandal B, Mittal NK, Balabathula P, Thoma LA, Wood GC. Development and in vitro evaluation of core-shell type lipid-polymer hybrid nanoparticles for the delivery of erlotinib in non-small cell lung cancer. Eur J Pharm Sci. 2016;81:162–171. doi: 10.1016/j.ejps.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Zhai J, Hinton TM, Waddington LJ, et al. Lipid-PEG conjugates sterically stabilize and reduce the toxicity of phytantriol-based lyotropic liquid crystalline nanoparticles. Langmuir. 2015;31(39):10871–10880. doi: 10.1021/acs.langmuir.5b02797. [DOI] [PubMed] [Google Scholar]
- 13.Liu Q. Triptolide and its expanding multiple pharmacological functions. Int Immunopharmacol. 2011;11(3):377–383. doi: 10.1016/j.intimp.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Wu B, Lu S-T, Zhang L-J, Zhuo R-X, Xu H-B, Huang S-W. Codelivery of doxorubicin and triptolide with reduction-sensitive lipid-polymer hybrid nanoparticles for in vitro and in vivo synergistic cancer treatment. Int J Nanomedicine. 2017;12:1853–1862. doi: 10.2147/IJN.S131235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar A, Corey C, Scott I, Shiva S, D’Cunha J. Minnelide/Triptolide Impairs Mitochondrial Function by Regulating SIRT3 in P53-Dependent Manner in Non-Small Cell Lung Cancer. PLoS One. 2016;11(8):e0160783. doi: 10.1371/journal.pone.0160783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borja-Cacho D, Yokoyama Y, Chugh RK, et al. TRAIL and triptolide: an effective combination that induces apoptosis in pancreatic cancer cells. J Gastrointest Surg. 2010;14(2):252–260. doi: 10.1007/s11605-009-1065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reno TA, Kim JY, Raz DJ. Triptolide Inhibits Lung Cancer Cell Migration, Invasion, and Metastasis. Ann Thorac Surg. 2015;100(5):1817–1824. doi: 10.1016/j.athoracsur.2015.05.074. discussion 1824–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rousalova I, Banerjee S, Sangwan V, et al. Minnelide: a novel therapeutic that promotes apoptosis in non-small cell lung carcinoma in vivo. PLoS One. 2013;8(10):e77411. doi: 10.1371/journal.pone.0077411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie CQ, Zhou P, Zuo J, Li X, Chen Y, Chen JW. Triptolide exerts pro-apoptotic and cell cycle arrest activity on drug-resistant human lung cancer A549/Taxol cells via modulation of MAPK and PI3K/Akt signaling pathways. Oncol Lett. 2016;12(5):3586–3590. doi: 10.3892/ol.2016.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang N, Dong XP, Zhang SL, You QY, Jiang XT, Zhao XG. Triptolide reverses the Taxol resistance of lung adenocarcinoma by inhibiting the NF-κB signaling pathway and the expression of NF-κB-regulated drug-resistant genes. Mol Med Rep. 2016;13(1):153–159. doi: 10.3892/mmr.2015.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng G, Wang W, Chai K, Yang S, Li F, Jiang K. Combination treatment with triptolide and hydroxycamptothecin synergistically enhances apoptosis in A549 lung adenocarcinoma cells through PP2A-regulated ERK, p38 MAPKs and Akt signaling pathways. Int J Oncol. 2015;46(3):1007–1017. doi: 10.3892/ijo.2015.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin C, Zhang X, Chen H, et al. Dual-ligand modified liposomes provide effective local targeted delivery of lung-cancer drug by antibody and tumor lineage-homing cell-penetrating peptide. Drug Deliv. 2018;25(1):256–266. doi: 10.1080/10717544.2018.1425777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C, Peng F, Liu W, et al. Nanostructured lipid carriers as a novel oral delivery system for triptolide: induced changes in pharmacokinetics profile associated with reduced toxicity in male rats. Int J Nanomedicine. 2014;9:1049–1063. doi: 10.2147/IJN.S55144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu B, Lu ST, Zhang LJ, Zhuo RX, Xu HB, Huang SW. Codelivery of doxorubicin and triptolide with reduction-sensitive lipid-polymer hybrid nanoparticles for in vitro and in vivo synergistic cancer treatment. Int J Nanomedicine. 2017;12:1853–1862. doi: 10.2147/IJN.S131235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han ML, Zhao YF, Tan CH, et al. Cathepsin L upregulation-induced EMT phenotype is associated with the acquisition of cisplatin or paclitaxel resistance in A549 cells. Acta Pharmacol Sin. 2016;37(12):1606–1622. doi: 10.1038/aps.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu J, Cai G, Liu X, Ma D. α(v)β(3) integrin receptor specific peptide modified, salvianolic acid B and panax notoginsenoside loaded nano-medicine for the combination therapy of acute myocardial ischemia. Biomed Pharmacother. 2017;96:1418–1426. doi: 10.1016/j.biopha.2017.10.086. [DOI] [PubMed] [Google Scholar]
- 27.Ge Y, Zhong Y, Ji G, et al. Preparation and characterization of Fe3O4@Au-C225 composite targeted nanoparticles for MRI of human glioma. PLoS One. 2018;13(4):e0195703. doi: 10.1371/journal.pone.0195703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu D, Li W, Zhang Y, Zhang B. Anti-tumor efficiency of paclitaxel and DNA when co-delivered by pH responsive ligand modified nanocarriers for breast cancer treatment. Biomed Pharmacother. 2016;83:1428–1435. doi: 10.1016/j.biopha.2016.08.061. [DOI] [PubMed] [Google Scholar]
- 29.Chen XY, Chen WL, Ma M, Gu C, Xiao XR, Li B. The potential of follicle-stimulating hormone peptide-modified triptolide-loaded nanoparticles to induce a mouse model of premature ovarian insufficiency. Int J Nanomedicine. 2015;10:2765–2774. doi: 10.2147/IJN.S72593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yue Y, Zhao D, Yin Q. Hyaluronic acid modified nanostructured lipid carriers for transdermal bupivacaine delivery: In vitro and in vivo anesthesia evaluation. Biomed Pharmacother. 2018;98:813–820. doi: 10.1016/j.biopha.2017.12.103. [DOI] [PubMed] [Google Scholar]
- 31.Ni XL, Chen LX, Zhang H, et al. In vitro and in vivo antitumor effect of gefitinib nanoparticles on human lung cancer. Drug Deliv. 2017;24(1):1501–1512. doi: 10.1080/10717544.2017.1384862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Zhu R, Sun X, Zhu Y, Liu H, Wang SL. Intracellular uptake of etoposide-loaded solid lipid nanoparticles induces an enhancing inhibitory effect on gastric cancer through mitochondria-mediated apoptosis pathway. Int J Nanomedicine. 2014;9:3987–3998. doi: 10.2147/IJN.S64103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang G, Wang Z, Li C, et al. RGD peptide-modified, paclitaxel prodrug-based, dual-drugs loaded, and redox-sensitive lipid-polymer nanoparticles for the enhanced lung cancer therapy. Biomed Pharma-cother. 2018;106:275–284. doi: 10.1016/j.biopha.2018.06.137. [DOI] [PubMed] [Google Scholar]
- 34.Song YF, Liu DZ, Cheng Y, et al. Charge Reversible and Mitochondria/Nucleus Dual Target Lipid Hybrid Nanoparticles To Enhance Antitumor Activity of Doxorubicin. Mol Pharm. 2018;15(3):1296–1308. doi: 10.1021/acs.molpharmaceut.7b01109. [DOI] [PubMed] [Google Scholar]
- 35.Gawde KA, Sau S, Tatiparti K, et al. Paclitaxel and di-fluorinated curcumin loaded in albumin nanoparticles for targeted synergistic combination therapy of ovarian and cervical cancers. Colloids Surf B Biointerfaces. 2018;167:8–19. doi: 10.1016/j.colsurfb.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 36.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 37.Xie F, Ding RL, He WF, et al. In vivo antitumor effect of endostatin-loaded chitosan nanoparticles combined with paclitaxel on Lewis lung carcinoma. Drug Deliv. 2017;24(1):1410–1418. doi: 10.1080/10717544.2017.1378938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan S, Wang G. Lung cancer targeted therapy: Folate and transferrin dual targeted, glutathione responsive nanocarriers for the delivery of cisplatin. Biomed Pharmacother. 2018;102:55–63. doi: 10.1016/j.biopha.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 39.Arshad A, Yang B, Bienemann AS, et al. Convection-Enhanced Delivery of Carboplatin PLGA Nanoparticles for the Treatment of Glioblastoma. PLoS One. 2015;10(7):e0132266. doi: 10.1371/journal.pone.0132266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muntimadugu E, Kumar R, Saladi S, Rafeeqi TA, Khan W. CD44 targeted chemotherapy for co-eradication of breast cancer stem cells and cancer cells using polymeric nanoparticles of salinomycin and paclitaxel. Colloids Surf B Biointerfaces. 2016;143:532–546. doi: 10.1016/j.colsurfb.2016.03.075. [DOI] [PubMed] [Google Scholar]
- 41.You P, Yuan R, Chen C. Design and evaluation of lidocaine- and prilocaine-coloaded nanoparticulate drug delivery systems for topical anesthetic analgesic therapy: a comparison between solid lipid nanoparticles and nanostructured lipid carriers. Drug Des Devel Ther. 2017;11:2743–2752. doi: 10.2147/DDDT.S141031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan S, Wang G. Redox-responsive and pH-sensitive nanoparticles enhanced stability and anticancer ability of erlotinib to treat lung cancer in vivo. Drug Des Devel Ther. 2017;11:3519–3529. doi: 10.2147/DDDT.S151422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bakhtiary Z, Barar J, Aghanejad A, et al. Microparticles containing erlotinib-loaded solid lipid nanoparticles for treatment of non-small cell lung cancer. Drug Dev Ind Pharm. 2017;43(8):1244–1253. doi: 10.1080/03639045.2017.1310223. [DOI] [PubMed] [Google Scholar]
- 44.Thapa RK, Nguyen HT, Jeong JH, et al. Progressive slowdown/prevention of cellular senescence by CD9-targeted delivery of rapamycin using lactose-wrapped calcium carbonate nanoparticles. Sci Rep. 2017;7:43299. doi: 10.1038/srep43299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo G, Fu S, Zhou L, et al. Preparation of curcumin loaded poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) nanofibers and their in vitro antitumor activity against Glioma 9L cells. Nanoscale. 2011;3(9):3825–3832. doi: 10.1039/c1nr10484e. [DOI] [PubMed] [Google Scholar]
- 46.Qu CY, Zhou M, Chen YW, Chen MM, Shen F, Xu LM. Engineering of lipid prodrug-based, hyaluronic acid-decorated nanostructured lipid carriers platform for 5-fluorouracil and cisplatin combination gastric cancer therapy. Int J Nanomedicine. 2015;10:3911–3920. doi: 10.2147/IJN.S83211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li C, Ge X, Wang L. Construction and comparison of different nanocarriers for co-delivery of cisplatin and curcumin: A synergistic combination nanotherapy for cervical cancer. Biomed Pharmacother. 2017;86:628–636. doi: 10.1016/j.biopha.2016.12.042. [DOI] [PubMed] [Google Scholar]
- 48.Yu X, Di Y, Xie C, et al. An in vitro and in vivo study of gemcitabine-loaded albumin nanoparticles in a pancreatic cancer cell line. Int J Nanomedicine. 2015;10:6825–6834. doi: 10.2147/IJN.S93835. [DOI] [PMC free article] [PubMed] [Google Scholar]