Abstract
Generating a hematopoietic stem cell (HSC) in vitro from nonhematopoietic tissue has been a goal of experimental hematologists for decades. Until recently, no in vitro–derived cell has closely demonstrated the full lineage potential and self-renewal capacity of a true HSC. Studies revealing stem cell ontogeny from embryonic mesoderm to hemogenic endothelium to HSC provided the key to inducing HSC-like cells in vitro from a variety of cell types. Here we review the path to this discovery and discuss the future of autologous transplantation with in vitro–derived HSCs as a therapeutic modality.
Visual Abstract
Introduction
Hematopoietic stem cells (HSCs) are rare, long-lived cells that can produce all mature blood cell lineages, self-renew for the lifetime of the organism, and fully reconstitute hematopoiesis in appropriately conditioned recipients after transplantation. Studies identifying the pathways that regulate HSC induction from embryonic mesodermal tissue, permit HSC self-renewal, and guide hematopoietic lineage commitment have brought us closer to generating HSCs in vitro from nonhematopoietic tissue. Two landmark discoveries have built on this knowledge and used defined culture conditions and ectopic expression of transcription factors to make HSC-like cells in vitro.1,2 This review will attempt to show how understanding the mechanisms of HSC ontogeny made this achievement possible and to identify the next questions to be answered if using autologous in vitro–derived HSCs as an alternative to allogeneic hematopoietic stem cell transplantation (HSCT) is to become a reality.
Directed differentiation of pluripotent stem cells (PSCs)
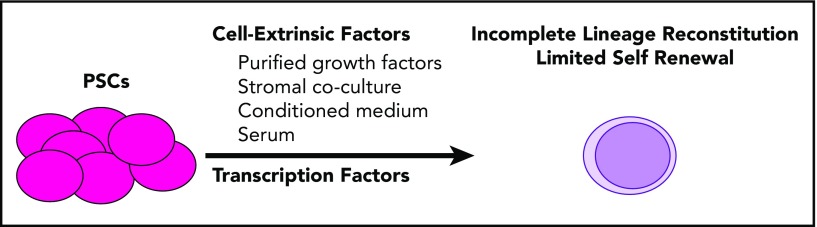
Embryonic stem (ES) cells are derived from the blastocyst stage of murine or human embryos and under appropriate conditions can differentiate into three-dimensional aggregates of endoderm, ectoderm, and mesoderm called embryoid bodies (EBs).3-6 EBs plated in semisolid medium with hematopoietic cytokines (eg, Epo, interleukin-1, interleukin-3, granulocyte-macrophage colony-stimulating factor) were differentiated further as nucleated, hemoglobinized erythrocytes and macrophage-like cells, resembling the primitive wave of hematopoiesis observed in the mammalian yolk sac.7-11 Extended culture of EBs in hematopoietic cytokines produced colonies with multilineage potential,9 yet these cells did not have long-term repopulating ability in irradiated mice and could not be considered definitive HSCs (Figure 1).8,11 Many protocols for induction and expansion of HSC-like cells from ES cells using defined growth factors, with or without serum, conditioned media, or coculture with stromal cell lines were subsequently reported.12-18 Similar findings have been made using EBs derived from induced pluripotent stem cell lines (iPSCs).1,19 Thus, the cell-extrinsic factors used in these studies were unable by themselves to differentiate PSCs directly to definitive HSCs.
Figure 1.
Directed differentiation of PSCs. PSCs differentiated by extended culture in hematopoietic cytokines or by ectopic expression of HoxB4 or Cdx4 generate cells capable of producing granulocyte, monocyte, B-cell, erythrocyte, and megakaryocyte lineages. T lymphopoiesis is limited to absent, and self-renewal is poor.
To overcome this deficiency in self-renewal, transgenic murine ES cells were generated that conditionally express HoxB4, 1 of several homeotic selector genes involved in hematopoiesis (Figure 1).20-24 HoxB4-induced cells supported long-term multilineage hematopoiesis in primary and secondary transplant recipients. Yet, in this work, overall chimerism was modest, and lymphoid engraftment, particularly T-cell engraftment, was low.20 A parallel study using somatic cell nuclear transfer with constitutive overexpression of HoxB4 also demonstrated robust engraftment (94%) of myeloid cells but poor lymphoid engraftment.25 Subsequently, the caudal-related homeobox gene Cdx4 was also shown to induce HSC-like cells from ES cells, likely via modulation of Hox gene expression, although T lymphopoiesis was similarly low.26,27 Although promising in mice, overexpression of HOXB4 was not sufficient to convert human ES cells into definitive HSCs capable of engrafting murine recipients.27,28 Nevertheless, these studies showed that specific culture conditions with enforced expression of HSC-specific transcription factors such as HoxB4 or Cdx4 could direct PSC differentiation toward an HSC-like phenotype.
Reprogramming committed and pluripotent cells
Reprogramming is the process of converting one differentiated cell type to another either directly or via a less differentiated intermediate.29 Alteration of lineage-specific transcription factors has proved to be an effective means of reprogramming committed hematopoietic cells into other cell lineages. Pax5−/− pro-B cells acquire an undifferentiated state in culture and can be reprogrammed into monocytes, granulocytes, natural killer cells, or T cells under appropriate conditions via derepression of lineage-specific transcripts such as Csf1r, Mpo, Gata1, Prf1, and Ptcra.30-32 Subsequently, it was shown that enforced expression of Cebpa and Cebpb in B cells could reprogram them into macrophage-like cells via downregulation of Pax5.33 Sequential enforced expression of Cebpa and Gata2 over 24 hours in common lymphoid progenitor cells can reprogram these cells to either basophils or eosinophils depending on the order in which the transcription factors are introduced.34 Together, these studies uncovered tremendous dormant plasticity of otherwise committed hematopoietic cells. This plasticity allows reprogramming to other hematopoietic lineages, provided the appropriate transcription factors are expressed at the correct levels and within the correct epigenetic landscape.
To generate HSC-like cells via reprogramming, human PSCs were differentiated into CD34+CD45+ committed hematopoietic progenitor cells, and a small library of HSC-specific transcription factors were screened to identify those that could confer self-renewal.35 Expression of HOXA9, ERG, RORA, SOX4, and MYB in hematopoietic progenitor cells generated a population of CD34+CD38low/−CD90+CD49f+ cells, phenotypically similar to umbilical cord blood HSCs but unable to support long-term engraftment in mice.36,37 The pooled factor approach was subsequently used in an in vivo screen of 36 murine transcription factors, revealing 8 (Hlf, Runx1t1, Pbx1, Lmo2, Zfp37, Prdm5, Meis1, and Mycn) that could support multilineage reconstitution of primary and secondary murine recipients when introduced into B lymphocytes, common myeloid progenitor cells, or Mac1+ myeloid cells.38 Engraftment and lineage potential of the induced HSCs was variable and changed over time, suggesting further reprogramming by the niche was required in vivo after transplantation. Additionally, because much of the study was carried out in vivo, it was not possible to assess any intermediate stages of reprogramming. Studies aiming to recreate HSC ontogeny in vitro would provide answers to these questions.
HSC-like cells produced via hemogenic endothelium
In the developing mouse, HSCs arise beginning embryonic day 10.5 from a small population of endothelial cells with hemogenic potential (hemogenic endothelium) located within the aorta-gonad-mesonephros region.39-42 A similar observation has been made in the zebrafish from 30 to 60 hours postfertilization.43-45 In a process known as endothelial to hematopoietic transition (EHT), endothelial cells in the floor of the aorta round up and bud into the extravascular space followed by reentry into the circulation via the underlying vein.44 These are the first definitive HSCs produced by the organism, and they go on to seed various hematopoietic niches throughout development.46 Expression of specific transcription factors including Runx1, Scl, Gfi1/Gfi1b, and Sox17 have a stem cell-autonomous role in EHT.40,44,47-50 Likewise, extrinsic signals from the microenvironment such as Hedgehog, Notch, BMP4, WNT, prostaglandin E2, CXCL12, adenosine, CXCL8, epoxyeicosatrienoic acid, and others are critical for the development of hemogenic endothelium and subsequent EHT.51-60
Culture conditions that support specification of hemogenic endothelium from PSCs have been identified, and EHT has been directly observed in vitro.47,61-64 A series of studies subsequently showed that acquisition of the hemogenic endothelial phenotype permits generation of hematopoietic progenitor-like cells with multilineage potential. Human PSCs cultured in BMP-4, bFGF, and VEGF in the absence of exogenous transcription factors acquired markers indicative of hemogenic endothelium and after culture in hematopoietic cytokines on OP9 stroma could be differentiated into myeloid or T cells.64 Human PSCs transduced with ETV2 were found to acquire an endothelial cell morphology, endothelial gene expression profile, and the ability to take up lipids while retaining expression of pluripotency genes.65 The addition of GATA2 to ETV2 generated a pan-myeloid developmental program with activation of almost all genes considered essential for definitive hematopoiesis and production of granulocytes, monocytes, macrophages, mast cells, megakaryocytes, and erythrocytes in culture.65 Mouse embryonic fibroblasts transduced with either Gata2, Gfi1b, cFos, and Etv6 or Erg, Gata2, Lmo2, Runx1c, and Scl developed into hematopoietic progenitor-like cells after passing through an intermediate cell type expressing endothelial cell genes.66,67 Yet, in all of these cases, the HSC-like cells derived from PSCs lacked sufficient self-renewal to engraft recipient mice.
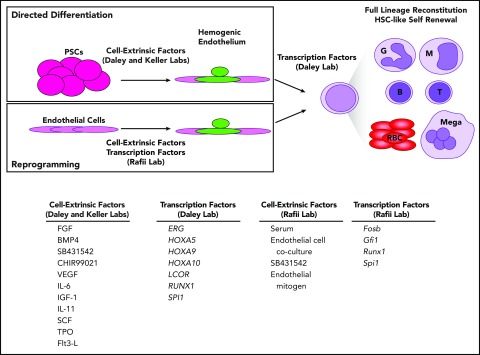
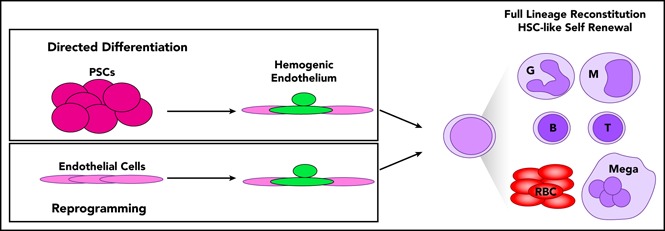
The most robust generation of in vitro–derived HSC-like cells to date was reported in the mouse2 and human1 in 2017 by the Rafii and Daley laboratories, respectively (Figure 2). The Rafii laboratory directly reprogrammed murine endothelial cells from lung, brain, and other tissues into hemogenic endothelium by transduction of Fosb, Gfi1, Runx1, and Spi1 and coculture with an immortalized endothelial cell line.2 The hematopoietic cells emerging from these cultures could fully reconstitute myeloid and lymphoid compartments and generate adaptive immune responses, in contrast to previous work in the human.68 The cell-intrinsic and cell-extrinsic factors used in this study conferred sufficient self-renewal properties to the hemogenic cultures to permit serial transplantation of HSC-like cells. A chemical screen identified CXCL12/CXCR4 and BMP signaling to be positive regulators of induction of hemogenic endothelium, whereas transforming growth factor β and Nodal signaling were negative regulators of EHT.
Figure 2.
Generation of hemogenic endothelium enables HSC-like self-renewal and lineage potential. By first making hemogenic endothelium, either by directed differentiation of PSCs or reprogramming endothelial cells from various tissues and expressing pools of transcription factors, full lineage potential and HSC self-renewal could be achieved. (SB431542 is an inhibitor of Activin/Nodal signaling, and CHIR99021 is a GSK-3 inhibitor.)
Using human PSCs and culture conditions previously described by the Keller laboratory,62,64 the Daley laboratory differentiated PSCs to hemogenic endothelium and transduced these cells with a pool of 12 transcription factors enriched in fetal liver HSCs relative to hemogenic endothelium plus 14 additional transcription factors identified in previous studies.35,38,68 Transduced cells were enriched by fluorescence-activated cell sorting and injected intrafemorally into immunodeficient mice, transcription factor expression was induced in vivo by doxycycline administration, and 12 weeks later, 5/40 mice were found to have multilineage (myeloid, erythroid, B, T) human cell engraftment. Transcription factors enriched in engrafted human cells included ERG, HOXA5, HOXA9, HOXA10, LCOR, RUNX1, and SPI1. These 7 factors conferred sufficient self-renewal capacity to allow transduced cells to engraft secondary transplant recipients. Induction of these factors in PSCs prior to generation of hemogenic endothelium was not sufficient to permit multilineage engraftment, highlighting the importance of developmental context and recreating HSC ontogeny in vitro. Analysis of engrafted cells showed expression of fetal and adult hemoglobin in similar ratios found in human umbilical cord blood together with erythrocyte enucleation and expression of GLY-A, induction of myeloperoxidase expression by neutrophils, and antigen-specific B- and T-cell responses with class switching and T-cell receptor gene rearrangement.
Summary and conclusions
These latest reports show that it is possible to create a self-renewing HSC-like cell from nonhematopoietic tissue, a long-sought goal in experimental hematology research. The approaches used by the Daley and Rafii laboratories highlight the importance of cell-intrinsic and cell-extrinsic signals acting in the correct developmental context (ie, hemogenic endothelium) to confer HSC-like self-renewal and lineage potential. It is noteworthy that most of the transcription factors uncovered in both of these studies had previously been tested in prior screens. Consequently, the hemogenic endothelial state may have a favorable epigenetic landscape to allow these transcription factors access to genetic loci necessary for self-renewal and hematopoietic differentiation. The transcription factors uncovered in these studies include specifiers of endothelial and hematopoietic identity (ERG, HOX), critical regulators of hematopoiesis (Runx1, Gfi1), and lineage specifiers (LCOR, Spi1). We do not yet know how or where these transcription factors bind during the transition to HSC-like cells. Further, in vitro–derived HSC-like cells still show significant gene expression differences compared with umbilical cord blood CD34+ HSCs.1 This suggests that these cells still lack a critical reprogramming signal or that reprogramming is highly dependent on an epigenetic state that was not completely achieved in the hemogenic endothelium created in these experiments. A screen in the zebrafish identified 29 factors associated with chromatin remodeling, histone modification, and the Polycomb repressive complex that are required for generation of definitive HSCs.69 Activity of 1 or more of these may need to be modulated in the hemogenic endothelium to make heterochromatin accessible and improve reprogramming transcription factor binding. Alternatively, identification of pioneer transcription factors that can directly bind heterochromatin and allow access to all factors necessary to make an HSC could reduce this dependence on epigenetic state and narrow the gap between in vitro–derived cells and true HSCs. Genome-wide screens may help identify such factors which may not have been included in the few dozen studied heretofore in targeted screens.
Efforts to generate in vitro-derived HSCs have repeatedly shown the importance of cell-extrinsic factors in HSC specification. Notch, BMP, WNT, and CXCR4/CXCL12 play a role, but there are likely many other factors produced in vitro by the stromal and nonhemogenic endothelial cells used in these culture systems that remain to be identified. The final steps of HSC reprogramming in these systems occur after transplantation and thus are dependent on in vivo niche factors. The identity of these in vivo reprogramming factors remains largely unknown, although CXCR4/CXCL12 signaling is likely of central importance.70 Experimental systems that permit live imaging cellular interactions may play an important role in further defining the cell-extrinsic factors involved in HSC specification and engraftment into the niche.71
The ability to derive HSCs from somatic tissue raises the possibility of using autologous in vitro–derived HSCs as an alternative to allogeneic HSCT. Yet many barriers remain before this becomes a reality. In clinical HSCT, the minimum cell dose required for acceptable engraftment is based on body weight (2 × 106 CD34+ cells per kg). Using in vitro–derived HSCs would require a ∼1000-fold increase in scale of production compared with the murine transplants described here. Significant levels of engraftment were only detected many weeks after transplantation in these studies, likely complicating clinical HSCT. Further, intrabone injection is most commonly used to transplant in vitro–derived HSC-like cells, likely improving engraftment efficiency of these cells, which may lack the appropriate chemokine receptors, integrins, and selectin ligands to exit the bloodstream and engraft the marrow.36 Although intrabone injection has not shown any advantage in human umbilical cord blood transplants,72,73 it may be necessary to successfully transplant in vitro–derived HSCs into humans. For these reasons, protocols that do not require in vivo reprogramming steps may need to be developed. The health of the marrow microenvironment must also be taken into account. Myeloid malignancies alter CXCL12/CXCR4 signaling in the marrow; induce angiogenic, fibrotic, and inflammatory gene expression programs; and promote fatty acid metabolism, all of which may affect the engraftment and survival of transplanted in vitro–derived HSCs or the completion of their in vivo reprogramming.74 Likewise, a senescent, DNA-damaged microenvironment in a patient with Fanconi anemia or dyskeratosis congenita or one who has been repeatedly exposed to environmental toxins, radiation, or chemotherapy may be unable to support in vitro–derived HSCs.75 In these cases, agents that condition the marrow microenvironment without cytotoxic effects may be helpful to aid engraftment.76-78
Two important goals for HSCT are elimination of minimal residual disease and correction of defective hematopoiesis. Autologous transplantation with in vitro–derived HSCs for myelodysplastic syndromes or acute myeloid leukemia, currently the most common disease indications for allogeneic HSCT, may lead to an unacceptably high rate of relapse because of the absence of graft-versus-leukemia effects, although this would be balanced by the absence of graft-versus-host disease.79,80 Combining autologous in vitro–derived HSCs with chimeric antigen receptor–modified T-cell or natural killer–cell therapy may be an option for patients with acute myeloid leukemia, acute lymphoblastic leukemia, or myeloma.81 When paired with gene correction or gene replacement techniques, autologous HSCT with in vitro–derived HSCs may be most useful for treating congenital marrow failure syndromes.82 In all such scenarios, but particularly in patients with genomic instability, the potential risk of malignant transformation cannot be ignored. It may be preferable to use direct reprogramming techniques that require transduction with fewer potential oncogenes and avoid the transition through a pluripotent state.2 Reprogramming approaches that avoid integration may be attractive alternatives to current strategies.83-89
Once an optimal reprogramming strategy has been devised, preclinical studies in nonhuman primates should be performed to demonstrate the feasibility of generating in vitro–derived HSCs on the scale required for large animals. Expansion of the in vitro–derived cells with agents such as prostaglandin E2 or aryl hydrocarbon receptor antagonists may be necessary to obtain adequate rates and tempo of engraftment.53,90,91 First in human studies should be performed in younger patients with congenital marrow failure syndromes where successful transplantation with autologous, gene-corrected, in vitro–derived HSCs could represent a cure without the attendant risks of allogeneic HSCT.82 For example, following gene correction, dermal fibroblasts from patients with Fanconi anemia have been reprogrammed successfully into iPSCs.92,93 These could be differentiated into HSCs and returned to the patient (Figure 3). Although patients with these disorders do have an elevated risk of myeloid malignancies, restoration of normal, gene-corrected hematopoiesis may be adequate to suppress outgrowth of any premalignant clones in the marrow.
Figure 3.
A potential first in human study of in vitro–derived HSCs. Dermal fibroblasts are collected from a patient with a congenital marrow failure syndrome such as Fanconi anemia. Gene therapy corrects the primary genetic defect. Gene-corrected fibroblasts are used to generate iPSCs by expression of OCT4, SOX2, KLF4, and c-MYC. Hemogenic endothelium is generated in culture, and HSCs are induced by transcription factor transduction. It may be necessary to complete HSC induction and expand these cells in vitro prior to infusion into the patient. Appropriate conditioning regimens must be identified to permit efficient engraftment of these cells while causing minimal toxicity to the patient.
Efforts toward creating HSCs in vitro have taught us that understanding and recreating HSC ontogeny appropriately tunes the conditions for exogenous transcription factors to successfully confer HSC-like self-renewal and lineage potential. Future studies will further define the molecular mechanisms of HSC production from hemogenic endothelium and perhaps will more closely approximate all the properties of a true HSC. Although many biological and clinical questions remain to be answered, in vitro–derived HSCs are closer to changing the way we transplant patients with benign and malignant hematologic disorders.
Acknowledgments
This work was supported by grants from the National Heart, Lung, and Blood Institute, National Institutes of Health (R01HL04880, P01HL032262, and U01HL10001) and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (P30DK049216, R01DK53298, R24DK092760, and K08DK111920). In addition, L.I.Z. is a Howard Hughes Medical Institute Investigator, and B.W.B. is supported by Pelotonia.
Authorship
Contribution: B.W.B. and L.I.Z. conceived and wrote the manuscript.
Conflict-of-interest disclosure: L.I.Z. is a founder and stockholder of Fate Therapeutics, Marauder Therapeutics, and Scholar Rock. B.W.B. declares no competing financial interests.
Correspondence: Leonard I. Zon, Boston Children’s Hospital-HHMI, 1 Blackfan Circle, Karp 5211, Boston, MA 02115; e-mail: zon@enders.tch.harvard.edu.
REFERENCES
- 1.Sugimura R, Jha DK, Han A, et al. . Haematopoietic stem and progenitor cells from human pluripotent stem cells. Nature. 2017;545(7655):432-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lis R, Karrasch CC, Poulos MG, et al. . Conversion of adult endothelium to immunocompetent haematopoietic stem cells. Nature. 2017;545(7655):439-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78(12):7634-7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154-156. [DOI] [PubMed] [Google Scholar]
- 5.Itskovitz-Eldor J, Schuldiner M, Karsenti D, et al. . Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6(2):88-95. [PMC free article] [PubMed] [Google Scholar]
- 6.Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27-45. [PubMed] [Google Scholar]
- 7.Wiles MV, Keller G. Multiple hematopoietic lineages develop from embryonic stem (ES) cells in culture. Development. 1991;111(2):259-267. [DOI] [PubMed] [Google Scholar]
- 8.Müller AM, Dzierzak EA. ES cells have only a limited lymphopoietic potential after adoptive transfer into mouse recipients. Development. 1993;118(4):1343-1351. [DOI] [PubMed] [Google Scholar]
- 9.Keller G, Kennedy M, Papayannopoulou T, Wiles MV. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993;13(1):473-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore MA, Metcalf D. Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol. 1970;18(3):279-296. [DOI] [PubMed] [Google Scholar]
- 11.Perah G, Feldman M. In vitro activation of the in vivo colony-forming units of the mouse yolk sac. J Cell Physiol. 1977;91(2):193-199. [DOI] [PubMed] [Google Scholar]
- 12.Chadwick K, Wang L, Li L, et al. . Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102(3):906-915. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy M, D’Souza SL, Lynch-Kattman M, Schwantz S, Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109(7):2679-2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2001;98(19):10716-10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pick M, Azzola L, Mossman A, Stanley EG, Elefanty AG. Differentiation of human embryonic stem cells in serum-free medium reveals distinct roles for bone morphogenetic protein 4, vascular endothelial growth factor, stem cell factor, and fibroblast growth factor 2 in hematopoiesis. Stem Cells. 2007;25(9):2206-2214. [DOI] [PubMed] [Google Scholar]
- 16.Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265(5175):1098-1101. [DOI] [PubMed] [Google Scholar]
- 17.Cho SK, Webber TD, Carlyle JR, Nakano T, Lewis SM, Zúñiga-Pflücker JC. Functional characterization of B lymphocytes generated in vitro from embryonic stem cells. Proc Natl Acad Sci USA. 1999;96(17):9797-9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vodyanik MA, Bork JA, Thomson JA, Slukvin II. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105(2):617-626. [DOI] [PubMed] [Google Scholar]
- 19.Lengerke C, Grauer M, Niebuhr NI, et al. . Hematopoietic development from human induced pluripotent stem cells. Ann N Y Acad Sci. 2009;1176(1):219-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyba M, Perlingeiro RCR, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109(1):29-37. [DOI] [PubMed] [Google Scholar]
- 21.Sauvageau G, Lansdorp PM, Eaves CJ, et al. . Differential expression of homeobox genes in functionally distinct CD34+ subpopulations of human bone marrow cells. Proc Natl Acad Sci USA. 1994;91(25):12223-12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGrath KE, Palis J. Expression of homeobox genes, including an insulin promoting factor, in the murine yolk sac at the time of hematopoietic initiation. Mol Reprod Dev. 1997;48(2):145-153. [DOI] [PubMed] [Google Scholar]
- 23.Sauvageau G, Thorsteinsdottir U, Eaves CJ, et al. . Overexpression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes Dev. 1995;9(14):1753-1765. [DOI] [PubMed] [Google Scholar]
- 24.Helgason CD, Sauvageau G, Lawrence HJ, Largman C, Humphries RK. Overexpression of HOXB4 enhances the hematopoietic potential of embryonic stem cells differentiated in vitro. Blood. 1996;87(7):2740-2749. [PubMed] [Google Scholar]
- 25.Rideout WM III, Hochedlinger K, Kyba M, Daley GQ, Jaenisch R. Correction of a genetic defect by nuclear transplantation and combined cell and gene therapy. Cell. 2002;109(1):17-27. [DOI] [PubMed] [Google Scholar]
- 26.Davidson AJ, Ernst P, Wang Y, et al. . cdx4 mutants fail to specify blood progenitors and can be rescued by multiple hox genes. Nature. 2003;425(6955):300-306. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Menendez P, Shojaei F, et al. . Generation of hematopoietic repopulating cells from human embryonic stem cells independent of ectopic HOXB4 expression. J Exp Med. 2005;201(10):1603-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee GS, Kim BS, Sheih J-H, Moore M. Forced expression of HoxB4 enhances hematopoietic differentiation by human embryonic stem cells. Mol Cells. 2008;25(4):487-493. [PubMed] [Google Scholar]
- 29.Graf T. Historical origins of transdifferentiation and reprogramming. Cell Stem Cell. 2011;9(6):504-516. [DOI] [PubMed] [Google Scholar]
- 30.Nutt SL, Heavey B, Rolink AG, Busslinger M. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature. 1999;401(6753):556-562. [DOI] [PubMed] [Google Scholar]
- 31.Cobaleda C, Jochum W, Busslinger M. Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. Nature. 2007;449(7161):473-477. [DOI] [PubMed] [Google Scholar]
- 32.Rolink AG, Nutt SL, Melchers F, Busslinger M. Long-term in vivo reconstitution of T-cell development by Pax5-deficient B-cell progenitors. Nature. 1999;401(6753):603-606. [DOI] [PubMed] [Google Scholar]
- 33.Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. Cell. 2004;117(5):663-676. [DOI] [PubMed] [Google Scholar]
- 34.Iwasaki H, Mizuno S, Arinobu Y, et al. . The order of expression of transcription factors directs hierarchical specification of hematopoietic lineages. Genes Dev. 2006;20(21):3010-3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doulatov S, Vo LT, Chou SS, et al. . Induction of multipotential hematopoietic progenitors from human pluripotent stem cells via respecification of lineage-restricted precursors. Cell Stem Cell. 2013;13(4):459-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazurier F, Doedens M, Gan OI, Dick JE. Rapid myeloerythroid repopulation after intrafemoral transplantation of NOD-SCID mice reveals a new class of human stem cells. Nat Med. 2003;9(7):959-963. [DOI] [PubMed] [Google Scholar]
- 37.Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333(6039):218-221. [DOI] [PubMed] [Google Scholar]
- 38.Riddell J, Gazit R, Garrison BS, et al. . Reprogramming committed murine blood cells to induced hematopoietic stem cells with defined factors[published correction appears in Cell 2014;158(1):226]. Cell. 2014;157(3):549-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86(6):897-906. [DOI] [PubMed] [Google Scholar]
- 40.North T, Gu TL, Stacy T, et al. . Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126(11):2563-2575. [DOI] [PubMed] [Google Scholar]
- 41.Zovein AC, Hofmann JJ, Lynch M, et al. . Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3(6):625-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boisset J-C, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464(7285):116-120. [DOI] [PubMed] [Google Scholar]
- 43.Vogeli KM, Jin SW, Martin GR, Stainier DY. A common progenitor for haematopoietic and endothelial lineages in the zebrafish gastrula. Nature. 2006;443(7109):337-339. [DOI] [PubMed] [Google Scholar]
- 44.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464(7285):112-115. [DOI] [PubMed] [Google Scholar]
- 45.Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DYR, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464(7285):108-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen AT, Zon LI. Zebrafish blood stem cells. J Cell Biochem. 2009;108(1):35-42. [DOI] [PubMed] [Google Scholar]
- 47.Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457(7231):892-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lancrin C, Mazan M, Stefanska M, et al. . GFI1 and GFI1B control the loss of endothelial identity of hemogenic endothelium during hematopoietic commitment. Blood. 2012;120(2):314-322. [DOI] [PubMed] [Google Scholar]
- 49.Choi E, Kraus MR, Lemaire LA, et al. . Dual lineage-specific expression of Sox17 during mouse embryogenesis. Stem Cells. 2012;30(10):2297-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakajima-Takagi Y, Osawa M, Oshima M, et al. . Role of SOX17 in hematopoietic development from human embryonic stem cells. Blood. 2013;121(3):447-458. [DOI] [PubMed] [Google Scholar]
- 51.Kim PG, Albacker CE, Lu YF, et al. . Signaling axis involving Hedgehog, Notch, and Scl promotes the embryonic endothelial-to-hematopoietic transition. Proc Natl Acad Sci USA. 2013;110(2):E141-E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jing L, Tamplin OJ, Chen MJ, et al. . Adenosine signaling promotes hematopoietic stem and progenitor cell emergence. J Exp Med. 2015;212(5):649-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.North TE, Goessling W, Walkley CR, et al. . Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447(7147):1007-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nguyen PD, Hollway GE, Sonntag C, et al. . Haematopoietic stem cell induction by somite-derived endothelial cells controlled by meox1. Nature. 2014;512(7514):314-318. [DOI] [PubMed] [Google Scholar]
- 55.Li P, Lahvic JL, Binder V, et al. . Epoxyeicosatrienoic acids enhance embryonic haematopoiesis and adult marrow engraftment. Nature. 2015;523(7561):468-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hadland BK, Varnum-Finney B, Poulos MG, et al. . Endothelium and NOTCH specify and amplify aorta-gonad-mesonephros-derived hematopoietic stem cells. J Clin Invest. 2015;125(5):2032-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gori JL, Butler JM, Chan Y-Y, et al. . Vascular niche promotes hematopoietic multipotent progenitor formation from pluripotent stem cells. J Clin Invest. 2015;125(3):1243-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rafii S, Kloss CC, Butler JM, et al. . Human ESC-derived hemogenic endothelial cells undergo distinct waves of endothelial to hematopoietic transition. Blood. 2013;121(5):770-780. [DOI] [PubMed] [Google Scholar]
- 59.Uenishi GI, Jung HS, Kumar A, et al. . NOTCH signaling specifies arterial-type definitive hemogenic endothelium from human pluripotent stem cells. Nat Commun. 2018;9:1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clements WK, Kim AD, Ong KG, Moore JC, Lawson ND, Traver D. A somitic Wnt16/Notch pathway specifies haematopoietic stem cells. Nature. 2011;474(7350):220-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125(4):725-732. [DOI] [PubMed] [Google Scholar]
- 62.Ditadi A, Sturgeon CM, Tober J, et al. . Human definitive haemogenic endothelium and arterial vascular endothelium represent distinct lineages. Nat Cell Biol. 2015;17(5):580-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eilken HM, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457(7231):896-900. [DOI] [PubMed] [Google Scholar]
- 64.Kennedy M, Awong G, Sturgeon CM, et al. . T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Reports. 2012;2(6):1722-1735. [DOI] [PubMed] [Google Scholar]
- 65.Elcheva I, Brok-Volchanskaya V, Kumar A, et al. . Direct induction of haematoendothelial programs in human pluripotent stem cells by transcriptional regulators. Nat Commun. 2014;5:4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Batta K, Florkowska M, Kouskoff V, Lacaud G. Direct reprogramming of murine fibroblasts to hematopoietic progenitor cells. Cell Reports. 2014;9(5):1871-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pereira C-F, Chang B, Qiu J, et al. . Induction of a hemogenic program in mouse fibroblasts. Cell Stem Cell. 2013;13(2):205-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sandler VM, Lis R, Liu Y, et al. . Reprogramming human endothelial cells to haematopoietic cells requires vascular induction. Nature. 2014;511(7509):312-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang HT, Kathrein KL, Barton A, et al. . A network of epigenetic regulators guides developmental haematopoiesis in vivo. Nat Cell Biol. 2013;15(12):1516-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reid JC, Tanasijevic B, Golubeva D, et al. . CXCL12/CXCR4 signaling enhances human PSC-derived hematopoietic progenitor function and overcomes early in vivo transplantation failure. Stem Cell Reports. 2018;10(5):1625-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perlin JR, Robertson AL, Zon LI. Efforts to enhance blood stem cell engraftment: Recent insights from zebrafish hematopoiesis. J Exp Med. 2017;214(10):2817-2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brunstein CG, Barker JN, Weisdorf DJ, et al. . Intra-BM injection to enhance engraftment after myeloablative umbilical cord blood transplantation with two partially HLA-matched units. Bone Marrow Transplant. 2009;43(12):935-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frassoni F, Gualandi F, Podestà M, et al. . Direct intrabone transplant of unrelated cord-blood cells in acute leukaemia: a phase I/II study. Lancet Oncol. 2008;9(9):831-839. [DOI] [PubMed] [Google Scholar]
- 74.Medyouf H. The microenvironment in human myeloid malignancies: emerging concepts and therapeutic implications. Blood. 2017;129(12):1617-1626. [DOI] [PubMed] [Google Scholar]
- 75.Ramalingam P, Poulos MG, Butler JM. Regulation of the hematopoietic stem cell lifecycle by the endothelial niche. Curr Opin Hematol. 2017;24(4):289-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318(5854):1296-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Palchaudhuri R, Saez B, Hoggatt J, et al. . Non-genotoxic conditioning for hematopoietic stem cell transplantation using a hematopoietic-cell-specific internalizing immunotoxin. Nat Biotechnol. 2016;34(7):738-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blaser BW, Moore JL, Hagedorn EJ, et al. . CXCR1 remodels the vascular niche to promote hematopoietic stem and progenitor cell engraftment. J Exp Med. 2017;214(4):1011-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.D’Souza A, Fretham C Current uses and outcomes of hematopoietic cell transplantation (HCT): CIBMTR summary slides, 2017. http://www.cibmtr.org. Accessed 17 August 2018.
- 80.Horowitz MM, Gale RP, Sondel PM, et al. . Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555-562. [PubMed] [Google Scholar]
- 81.Smith M, Zakrzewski J, James S, Sadelain M. Posttransplant chimeric antigen receptor therapy. Blood. 2018;131(10):1045-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alter BP. Inherited bone marrow failure syndromes: considerations pre- and posttransplant. Blood. 2017;130(21):2257-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322(5903):945-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nishimura K, Sano M, Ohtaka M, et al. . Development of defective and persistent Sendai virus vector: a unique gene delivery/expression system ideal for cell reprogramming. J Biol Chem. 2011;286(6):4760-4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu J, Hu K, Smuga-Otto K, et al. . Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322(5903):949-953. [DOI] [PubMed] [Google Scholar]
- 87.Warren L, Manos PD, Ahfeldt T, et al. . Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7(5):618-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim D, Kim CH, Moon JI, et al. . Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4(6):472-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hou P, Li Y, Zhang X, et al. . Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341(6146):651-654. [DOI] [PubMed] [Google Scholar]
- 90.Goessling W, Allen RS, Guan X, et al. . Prostaglandin E2 enhances human cord blood stem cell xenotransplants and shows long-term safety in preclinical nonhuman primate transplant models. Cell Stem Cell. 2011;8(4):445-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boitano AE, Wang J, Romeo R, et al. . Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329(5997):1345-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Müller LU, Milsom MD, Harris CE, et al. . Overcoming reprogramming resistance of Fanconi anemia cells. Blood. 2012;119(23):5449-5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raya A, Rodríguez-Pizà I, Guenechea G, et al. . Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature. 2009;460(7251):53-59. [DOI] [PMC free article] [PubMed] [Google Scholar]