Abstract
A solid tumor is like an aberrant organ – comprised of cancer cells and a variety of host cells embedded in an extracellular matrix – nourished by blood vessels and drained by lymphatic vessels. In its journey from the blood stream to cancer cells, a therapeutic agent must cross the vessel wall and the extracellular matrix that cancer cells are ensconced in. Growth of tumors in a confined space along with deposition of matrix components, including collagen (yellow) and hyaluronan (pink), increases ‘solid stress’, which compresses blood and lymphatic vessels and impairs their function. The leakiness of tumor vessels also impairs tumor blood flow and increases ‘intratumor fluid pressure’. The abnormal blood flow not only impedes drug delivery, but the resulting hypoxia also aids tumor invasion, metastasis, immunosuppression, in flammation, fibrosis, and treatment resistance. Engineers and physical scientists have dissected the molecular, cellular, and physical mechanisms underlying these abnormalities and developed a number of strategies to reengineer the tumor microenvironment to overcome these barriers and thus improve delivery and efficacy of treatments. Finally, these strategies have been translated from bench to bedside for treatment of cancer and have the potential to improve the treatment outcome for many diseases characterized by an abnormal microenvironment.
Graphical Abstract
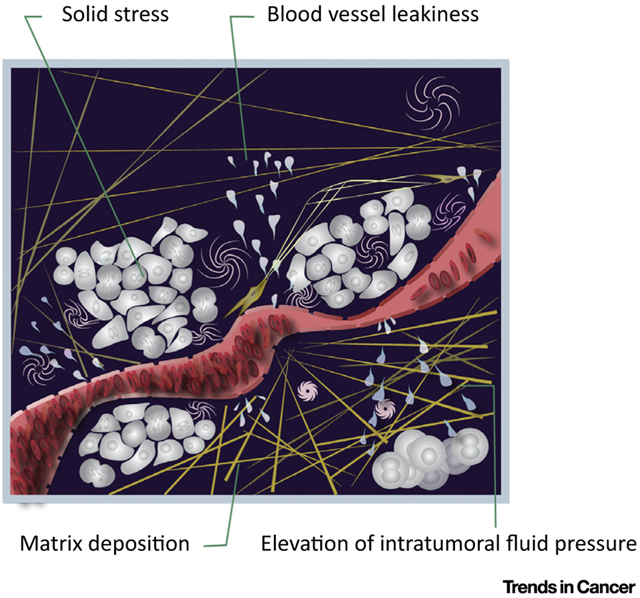
KEY FACTS:
Mathematical modeling, intravital microscopy, and animal models of primary and metastatic tumors have provided unprecedented insights into the tumor microenvironment.
All components of the tumor microenvironment – blood and lymphatic vessels, immune and other stromal cells, and associated extracellular matrix – are abnormal.
Obesity can further increase these abnormalities in the tumor microenvironment.
Abnormal vessels and matrix create a hostile metabolic and physical tumor microenvironment, characterized by hypoxia, low pH, and high solid stress and interstitial fluid pressure
Forces exerted by cancer and stromal cells, as well as the extracellular matrix, can directly affect cells and compress blood and lymphatics vessels, resulting in impaired blood flow and lymphatic function.
Fluid flow within the tumor interstitial space can affect blood vessel formation and metastasis.
Poor blood flow impairs the delivery of drugs and immune cells in tumors, compromises their efficacy after they accrue in tumors, and, independent of these, fuels tumor progression and metastasis and induces immunosuppression.
Reengineering the physical microenvironment of tumors by normalizing the vessels and matrix can improve delivery and efficacy of standard and emerging treatments and reduce their toxicity.
Normalizing blood vessels using direct or indirect antiangiogenic agents can make tumor vessels less leaky and better organized, resulting in improved vascular function, reduced interstitial fluid pressure, restoration of a pressure gradient across the vessel wall, and a more uniform drug delivery into the tumor tissue.
Normalizing the extracellular matrix by targeting stromal cells can lower solid stress, reopen collapsed vessels restoring tumor blood flow, and improve the delivery and penetration of drugs into the tumor.
Over the past three decades, preclinical and clinical studies have provided evidence in support of these concepts and offered novel strategies to improve cancer treatment.
BY THE NUMBERS:
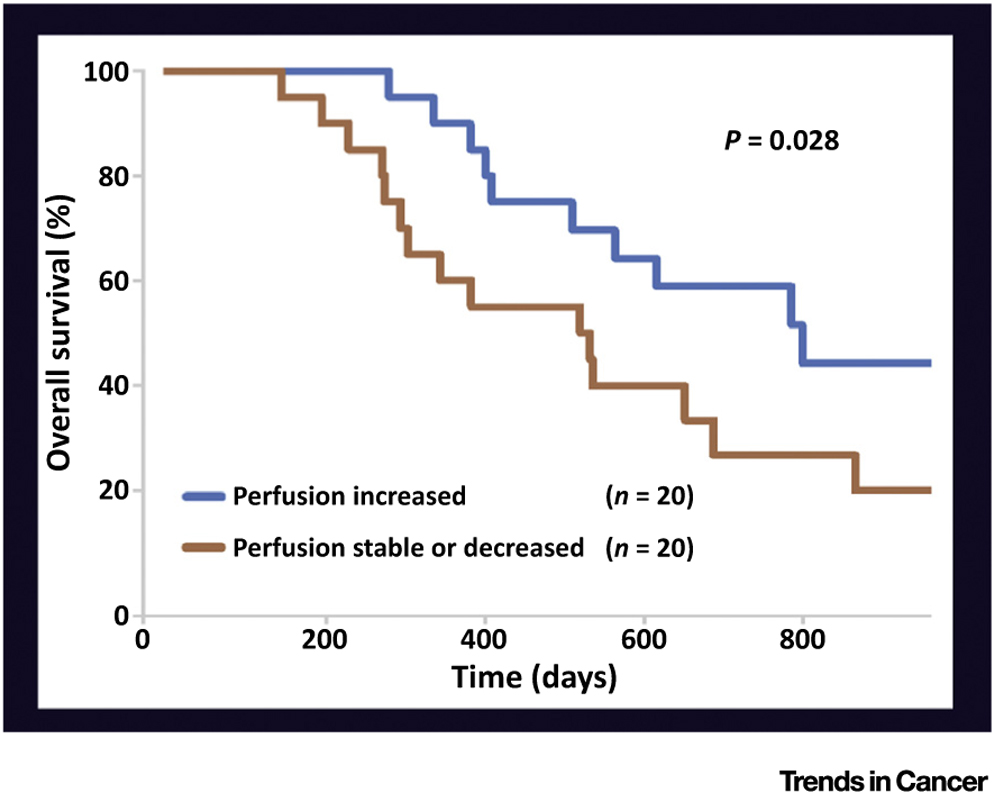
Improving blood flow in tumors can increase survival of cancer patients. Newly diagnosed glioblastoma patients received antiangiogenic treatment and chemoradiation. The patients whose tumor perfusion increased during the therapy (blue line) survived ~9 months longer than those whose tumor was stable or decreased (brown line). (Reproduced with permission from T. Batchelor et al. (2013) Proc. Natl. Acad. Sci. U. S. A. 10, 19059–19064.)
Figure.
Literature
- 1.Jain RK (2005) Normalization of the tumor vasculature: an emerging concept in anti-angiogenic therapy of cancer. Science 307, 58–62 [DOI] [PubMed] [Google Scholar]
- 2.Tse JM et al. (2011) Mechanical compression drives cancer cells toward invasive phenotype. Proc. Natl. Acad. Sci. U. S. A 109, 911–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stylianopoulos T et al. (2012) Causes, consequences and remedies for growth-induced solid stress in murine and human tumors. Proc. Natl. Acad. Sci. U. S. A 109, 15101–15108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chauhan VP et al. (2013) Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumor blood vessels. Nat. Commun 4, 2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batchelor TT et al. (2013) Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc. Natl. Acad. Sci. U. S. A 110, 19059–19064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain RK (2013) Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J. Clin. Oncol 31, 2205–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain RK (2014) Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell 26, 605–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jain RK et al. (2014) The role of physical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng 16, 321–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Incio J et al. (2016) Obesity-induced in flammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov 6, 852–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nia HT et al. (2016) Solid stress and elastic energy as measures of tumour mechanopathology. Nat. Biomed. Eng 1, 0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinter M and Jain RK (2017) Targeting the renin-angiotensin system to improve cancer treatment: implications for immunotherapy. Sci. Transl. Med 9, eaan5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell MJ et al. (2017) Engineering and physical sciences in oncology: challenges and opportunities. Nat. Rev. Cancer 17, 659–675 [DOI] [PMC free article] [PubMed] [Google Scholar]


