Abstract
Deep eutectic solvents (DES) have been studied in a wide range of applications, and despite their potential as sustainable solvents, detailed knowledge on their solvatochromic parameters is still lacking. To overcome this problem, in this work, the Kamlet Taft (KT) solvatochromic parameters, namely the hydrogen-bond acidity, hydrogen-bond basicity and dipolarity/polarizability, of a wide range of DES composed of ammonium-based salts as hydrogen bond acceptors (HBAs), and carboxylic acids as hydrogen bond donors (HBDs), were determined aiming at better understanding the influence of the chemical structure of the DES components on their polarity. It is shown that the high acidity of the DES investigated is mainly provided by the organic acid present in the mixture, and that an increase of the alkyl side chain of both the HBA and the HBD species leads to a lower ability of the solvent to donate protons. On the other hand, the ammonium salt plays the major role on the hydrogen-bond basicity of DES. Contrarily to the hydrogen-bond acidity, an increase in the length of the aliphatic moieties of both the carboxylic acid and salt cation results in solvents with higher ability to accept protons. The dipolarity/polarizability of DES is mainly defined by the ionic species present, and tend to decrease with the increase of the aliphatic moiety of the organic acid. In general, DES composed of ammonium-based salts and carboxylic acids present a higher capacity to donate and accept protons when compared to most of the ionic liquids or organic molecular solvents.
Keywords: Deep eutectic solvents, Ammonium salts, Carboxylic acids, Solvatochromic parameters
1. Introduction
The development, identification and characterization of “greener” solvents is amongst the top research activities envisaging the use of more benign, low cost, and biomass-derived solvents to be used in several sectors [1]. Within this class of solvents, Deep Eutectic Solvents (DES) were reported in 2003 by Abbott et al. [2]. A DES is formed by the mixture of a hydrogen bond acceptor (HBA), such as a quaternary ammonium salt, and a hydrogen bond donor (HBD) species, usually an organic compound with high ability to donate protons, such as amines, carboxylic acids, alcohols, and carbohydrates [2]. By mixing these two components in proper ratios, eutectic mixtures are formed, with a significant decrease in the melting point of the mixture when compared to the melting temperatures of the starting components [2,3]. The HBD interacts by strong hydrogen-bonds with the HBA, further leading to a decrease on the cation-anion interactions of the HBA species, and resulting in a significant freezing point depression of the mixture.
DES present several interesting advantages, such as low toxicity, high biodegradability, and negligible volatility [4–6]. These solvents can be formed from natural and renewable sources, such as carbohydrates, polyols, amino acids, organic acids, among others [7]. In addition, they are usually easy to prepare and of low cost, compared for instance with other classes of solvents with similar properties such as ionic liquids [8]. The diversity of combinations of the starting materials also allows the tailoring of their physical and chemical properties, and they, therefore, present a large industrial potential. Based on these advantages, DES have been studied in a large number of applications, comprising catalysis, nanotechnology, electrochemistry, organic synthesis, and separation processes [9–13]. Amongst the most important features of DES to be used as solvents in these applications are the specific interactions occurring between the solvent and the dissolved substrate (solute-solvent interactions), usually related to the solvent polarity, and that can be characterized using semi-empirical linear free energy relationships [14].
The two most commonly used scales to characterize solvents in terms of solvatochromic parameters are the ET(30) polarity scales of Dimroth and Reichardt [15,16], and the multi-parameter scale of Kamlet and Taft (KT) [17–19]. This last approach consists on the use of a set of solvatochromic probes which allow the assessment of different parameters for the same solvent. These can be used to correlate and describe the activity and properties of the solvent by the KT equation that in its simple form of a Linear Solvation Energy Relationship (LSER) is given by the following equation:
| (1) |
where XYZ is the solute property (solubility, reaction rate, equilibrium constant, the logarithm of a gas/solvent or solvent/solvent partition coefficient, etc.) in a given solvent, (XYZ)0 is the same solute property in a reference state, π* represents the solvent's dipolarity/polarizability, α is the hydrogen-bond donating ability, β is the hydrogen-bond accepting ability and δ is the polarizability correction term. The parameters a, b, d and s represent the solvent-independent coefficients. It should be remarked that the determination of the solvatochromic parameters of a target solvent also allows to obtain insights into solvation and local density, shown to be particularly useful in the understanding of the solvent properties, as demonstrated for supercritical fluids and ionic liquids [20–24].
Although DES are seen as effective, versatile and benign alternative solvents, a widespread appraisal of their solvatochromic parameters, namely their hydrogen-bond acidity, hydrogen-bond basicity and dipolarity/polarizability, is still missing. To the best of our knowledge, there are currently no reports regarding a detailed characterization of the solvatochromic parameters of a large number of DES. There are however few and isolated studies [1,6,14,25–28] on the characterization of their polarities to better understand their behavior for a specific task or application. Furthermore, most of these works have focused on choline chloride ([N1112(OH)]Cl) as the HBA in combination with several types of HBDs [1,6,14,25–27]. For instance, Abbott and co-workers [14] determined the polarity parameters of a DES composed of [N1112(OH)]Cl and glycerol, in four mole fraction ratios (1:1; 1:1.5; 1:2; 1:3). The authors [14] shown that the ET(30), π* and α parameters increase linearly with the [N1112(OH)]Cl concentration, while the β parameter was not affected by the addition of the salt. Pandey et al. [25] studied the effect of changing temperature and the addition of water on the solvatochromic probe behavior of three DES formed by [N1112(OH)]Cl and 1,2-ethanediol, glycerol, or urea, in a 1:2 mole ratio. They [25] found that an increase in temperature results in reduced hydrogen-bond donating ability of the DES, while the dipolarity/polarizability and the hydrogen-bond accepting basicity do not change, at least in the temperature range considered (303–363 K). The addition of water resulted in an increased dipolarity/polarizability and in the hydrogen-bond basicity decrease of the DES. The same group of researchers [1] also studied the same DES and an additional one composed of [N1112(OH)]Cl and malic acid, in a 1:2 mole ratio, however using different probes. The authors [1] demonstrated that the chemical structure of the hydrogen-bond donor of the DES controls its dipolarity, whereas the hydrogen-bonding and other specific solute-solvent interactions play an important role in the solvatochromic behavior. Recently, Bharmoria et al. [28] used a mixture of two ionic liquids with a DES nature/behavior, formed by 2-hydroxyethylammonium formate ([HEA][HCOO]) and 1-butyl-3-imidazolium chloride ([C4mim]Cl), in which solvatochromic parameters were correlated with agarose dissolution data aiming to better understand the process of the solute dissolution in the ILs mixture. Kadyan et al. [26] determined the empirical solvent polarity parameter and the KT parameters (π*, α and β) of a new hybrid green non-aqueous media, namely a tetraethylene glycol (TEG)-modified DES composed of [N1112(OH)]Cl and urea, and proved that a small amount of TEG can effectively modify the chemical properties of the DES. Harifi-Mood et al. [6] studied the solvatochromic behavior of binary mixtures of dimethyl sulfoxide (DMSO) and several DES, namely [N1112(OH)]Cl-urea, [N1112(OH)]Cl-ethylene glycol, and [N1112(OH)]Cl-glycerol (molar ratio of 1:2) in order to infer on the polarity of these solvents envisaging their possible applications in chemical processes. It was shown that the polarity and hydrogen bond donor ability of the DES decreases with the DMSO content, while the opposite was observed for the hydrogen bond acceptor ability. In a different application, Kim et al. [27] determined the solvatochromic parameters of [N1112(OH)]Cl-based DES to understand the interactions between the DES and Candida rugosa lipase, when using aqueous solutions of DES in biocatalysis.
Based on the currently available data and aiming at better characterizing DES according to their polarity so that they can be properly designed for task-specific applications, in this work we determined the solvatochromic parameters, namely the hydrogen-bond acidity, hydrogen-bond basicity and dipolarity/polarizability, of a wide range of DES composed of ammonium-based salts as hydrogen bond acceptors (HBAs), and carboxylic acids as hydrogen bond donors (HBDs). The gathered data are compared and discussed in order to appraise the chemical structure influence of the DES phase-forming components on their polarity.
2. Experimental section
2.1. Materials
The investigated ammonium-based deep eutectic solvents were prepared with the following hydrogen bond acceptors: Cholinium Chloride, [N1112(OH)]Cl (Acros Organics, 98 wt%), Tetramethylammonium Chloride, [N1111]Cl (Sigma-Aldrich, 97 wt%), Tetraethylammonium Chloride, [N2222]Cl (Sigma-Aldrich, 98.0 wt%), Tetrapropylammonium Chloride, [N3333]Cl (Sigma-Aldrich, 98.0 wt%), Tetrabutylammonium Chloride, [N4444]Cl (Sigma-Aldrich, 97.0 wt%), Tetramethylammonium Bromide, [N1111]Br (Fluka, 99 wt%), Tetraethylammonium Bromide, [N2222]Br (Alfa Aesar, 98.0 wt%), Tetrapropylammonium Bromide, [N3333]Br (Aldrich, 98.0 wt%), and Tetrabutylammonium Bromide, [N4444]Br (Fluka, 98.0 wt%). The hydrogen bond donors used were: Butanoic Acid (Riedel de Haen, 99.0 wt%), Hexanoic Acid (Acros Organics, 99.0 wt%), Octanoic Acid (Sigma, 98.0 wt%), Decanoic Acid (Sigma, 98.0 wt%), and Dodecanoic Acid (Acros Organics, 99.0 wt%). To reduce the water and volatile compounds content to negligible values, all ammonium salts were dried under constant stirring at vacuum and room temperature (≈298 K) for a minimum of 48 h. A Metrohm 831 Karl Fischer coulometer using the analyte Hydranal®—Coulomat AG, from Riedel-de Haën, was used to determine the water content of the dried salts, which was found to be below 0.002 wt%. The probes used to determine the solvatochromic parameters were N,N-diethyl-4-nitroaniline (N,N) (Flourochem, 99 wt%), 4-nitroaniline (4N) (Aldrich, 99 wt%) and pyridine-N-oxide (PyO) (Aldrich, 95%).
2.2. Experimental procedure
Preparation of deep eutectic solvents
Deep eutectic solvents were prepared using ammonium-based salts as HBA and carboxylic acids as HBD species. The two components were weighted (±10−5 g) to prepare mixtures of accurately known molar ratio and then heated under constant stirring, until the complete melting of the mixture. After the complete melting of each mixture, the samples were kept in closed glass vials at 298 K, at least for 24 h, in order to infer which mixtures prepared are liquid at 298 K.
Determination of the solvatochromic parameters
The solvatochromic probes N,N-diethyl-4-nitroaniline and 4-nitroaniline were used to determine the dipolarity/polarizability, π*, and the hydrogen-bond acceptor basicity, β, of all DES found to be liquid at 298 K. To each DES (ca. 200 μL), small amounts (ca. 0.1 mg) of each probe, namely N,N-diethyl-4-nitroaniline and 4-nitroaniline, were added. After vigorous agitation for the complete dissolution of the probes, using a vortex mixer, the samples were scanned by UV-Vis spectroscopy (BioTeck Synergy HT microplate reader) at 298 K to determine the longest wavelength absorption band of each probe in each DES.
The solvatochromic parameters were determined using the following equations:
| (2) |
| (3) |
| (4) |
| (5) |
where, v is the experimental wave number and λmaxprobe is the maximum wavelength of the probe, the subscripts N,N and 4N represent the solvatochromic probes N,N-diethyl-4-nitroaniline and 4-nitroaniline, respectively, while DES, cyclohexane and DMSO correspond to the values for these solvents. At least three independent samples were prepared to determine the average solvatochromic parameters and associated standard deviation (lower than 5% in all mixtures).
The α parameter was determined by 13C nuclear magnetic resonance (NMR) spectra [29] obtained in neat conditions, using a Bruker Avance 300 equipment, operating at 75 MHz, using the probe pyridine-N-oxide (PyO), deuterium oxide (D2O) as the solvent, and trimethylsilyl propanoic acid (TSP) as the internal reference. The 13C NMR chemicals shifts, δ(Ci) (in ppm), of the carbons atoms in positions i=2 and 4 of pyridine-N-oxide were determined, and the α parameter was calculated by Eq. (6).
| (6) |
where d24 = δ4 − δ2 [29]. The analyses were carried out in triplicate and the standard deviation for the α parameter is below 0.07.
The solvatochromic parameters of the pure and liquid organic acids at 298 K were also determined using the probes and procedures previously described. The β and π* parameters were determined by a colorimetric method, while the α parameter was determined using an alternative probe based on NMR spectroscopic data. This probe was previously validated by us [30], as well as in this work by determining the hydrogen-bond acidity of the pure carboxylic acids. This probe was used because the studied DES are highly acidic and interfere with the data provided by the well-known Reichardt's dye probe.
3. Results and discussion
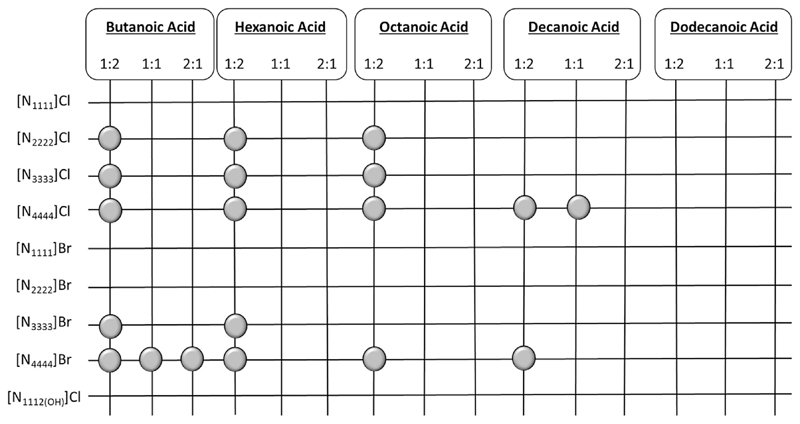
As a first attempt, a large range of DES composed of ammonium-based salts and carboxylic acids, at different mole fraction ratios, was prepared to identify the mixtures that are liquid at 298 K, and which can thus be characterized in terms of solvatochromic parameters at this temperature. Fig. 1 depicts a schematic representation of the mixtures investigated, where those identified by a circle in the respective matrix are the combinations that are liquid at 298 K. In general, carboxylic acids with shorter alkyl chains and ammonium-based salts with longer aliphatic chains are more prone to result in mixtures that are liquid at 298 K. Moreover, chloride-based salts as HBA species produce a larger number of DES liquid at 298 K than the bromide analogues. None of the mixtures investigated with cholinium chloride is liquid at 298 K. In addition, the 1:2 (HBA:HBD) mole ratio seems to lead to a higher probability of obtaining a liquid mixture at 298 K. Dodecanoic acid was the only organic acid studied that was not able to originate any liquid mixture at 298 K when combined with the various HBA investigated. This phenomenon is related with the high melting temperature of this acid (315.75 K) [31]. Amongst the studied carboxylic acids, only butanoic, hexanoic, octanoic and decanoic acids are liquid at room temperature. Therefore, their solvatochromic parameters were also determined. All HBA species are solid at 298 K, and thus we were not able to determine their solvatochromic parameters.
Fig. 1.
Combinations of ammonium-based salts and carboxylic acids to form DES, in which those able to originate liquid samples at 298 K are identified by a circle.
The KT parameters, namely the α, β and π*, were determined for all the investigated liquid mixtures at 298 K. The same solvatochromic parameters were determined for the acids that are liquid at 298 K, namely butanoic, hexanoic octanoic and decanoic acids. The values of water are also included for comparison purposes. The results obtained are reported in Table 1. The discussion of these results is provided below for each individual parameter.
Table 1.
Kamlet-Taft parameters of water, organic acids and DES composed of ammonium-based salts (HBAs) and carboxylic acids (HBDs), in different mole ratios, using the following set of probes: N,N-diethyl-4-nitroaniline, 4-nitroaniline and pyridine-N-oxide.
| HBA | HBD | Mole ratio (salt:carboxylic acid) | α | β | π* |
|---|---|---|---|---|---|
| – | Water [32] | – | 1.23 | 0.49 | 1.14 |
| – | Butanoic Acid | – | 1.06 | 0.22 | 0.47 |
| – | Hexanoic Acid | – | 1.05 | 0.21 | 0.43 |
| – | Octanoic Acid | – | 0.94 | 0.23 | 0.38 |
| [N2222]Cl | Butanoic Acid | 1:2 | 0.99 | 0.76 | 0.92 |
| Hexanoic Acid | 1:2 | 0.97 | 0.85 | 0.86 | |
| Octanoic Acid | 1:2 | 0.96 | 0.87 | 0.81 | |
| [N3333]Cl | Butanoic Acid | 1:2 | 0.94 | 0.84 | 0.93 |
| Hexanoic Acid | 1:2 | 0.91 | 0.92 | 0.85 | |
| Octanoic Acid | 1:2 | 0.90 | 0.96 | 0.80 | |
| [N4444]Cl | Butanoic Acid | 1:2 | 0.92 | 0.99 | 0.86 |
| Hexanoic Acid | 1:2 | 0.90 | 1.02 | 0.81 | |
| Octanoic Acid | 1:2 | 0.84 | 1.19 | 0.80 | |
| Decanoic Acid | 1:2 | 0.85 | 1.28 | 0.69 | |
| 1:1 | 0.91 | 1.21 | 0.86 | ||
| [N3333]Br | Butanoic Acid | 1:2 | 1.07 | 0.80 | 0.93 |
| Hexanoic Acid | 1:2 | 1.02 | 0.86 | 0.87 | |
| [N4444]Br | Butanoic Acid | 1:2 | 1.02 | 0.81 | 0.93 |
| 1:1 | 1.09 | 0.84 | 0.90 | ||
| 2:1 | 0.94 | 0.82 | 0.95 | ||
| Hexanoic Acid | 1:2 | 1.02 | 0.93 | 0.92 | |
| Octanoic Acid | 1:2 | 0.98 | 1.09 | 0.84 | |
| Decanoic Acid | 1:2 | 0.95 | 1.05 | 0.71 | |
3.1. Hydrogen-bond acidity of DES
The α parameter describes the capacity of a solvent to donate protons in solvent-to-solute hydrogen-bonding. According to the data shown in Table 1, the DES investigated present higher hydrogen-bond acidity values than alcohols [33] and ionic liquids [23,24,34], and slightly lower values than water [32] and the pure acids. In general, it is shown that the high acidity of the DES investigated is mainly provided by the organic acid present in the mixture.
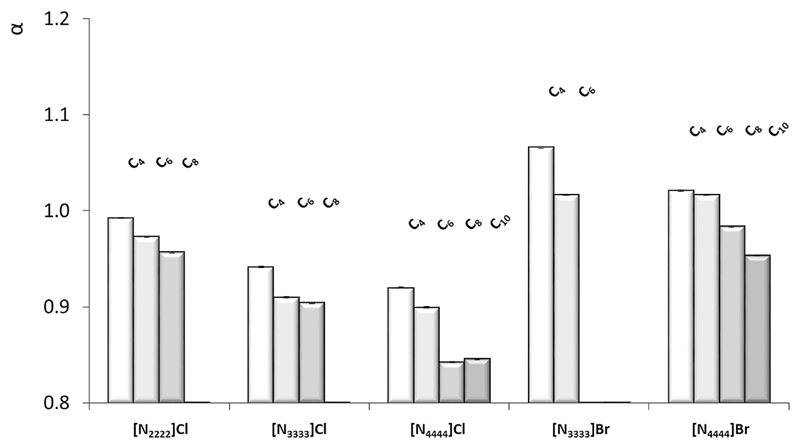
Fig. 2 depicts the α values for the studied DES, liquid at 298 K, in a mole ratio of 1:2, for a better comparison of the obtained data. The α parameter in these mixtures ranges between 0.84 ([N4444] Cl:Octanoic Acid) and 1.07 ([N3333]Br:Butanoic Acid). From the depicted data, it is shown that α values are mainly dependent on the anion of the HBA species, with larger differences observed between the chloride- and bromide-based mixtures. However, it is also observed a slight decrease of this parameter with the increase of the alkyl chains of both the HBA and the HBD. This is somehow expected since an increase in the alkyl chain length leads to a decrease on the ability of a target species to donate or accept protons, as observed for the pure organic acids. This is in agreement with previous observations concerning the marginal effect of the contribution of the alkyl chain of the salt cation in ionic liquids towards their hydrogen-bond acidity, in which the charge distribution/contribution of the cation plays the major role in their acidity [23]. The highest α values were obtained for the DES comprising bromide salts ([Nnnnn]Br, n = 3 and 4), and in particular for the [N3333]Br-based DES, evidencing thus the contribution of the salt anion to an increase of the hydrogen-bond acidity of the mixtures investigated. [N4444]Cl displays stronger cation-anion attractions [35] and higher ability to accept protons [24] than [N4444]Br, and these effects may have an impact on the hydrogen-bond interactions established between the salt anion and the carboxylic acid, and thus on the overall DES properties.
Fig. 2.
Kamlet-Taft hydrogen bond acidity (α) of DES composed of ammonium-based salts and different carboxylic acids, namely butanoic (C4), hexanoic (C6), octanoic (C8) and decanoic (C10) acids in a 1:2 mole ratio, at 298 K.
Based on the results here described combined with those reported in the literature, it is evident that DES present, in some cases, a similar or higher ability to donate protons in solvent-to-solute hydrogen bonds (high α values) when compared to some conventional organic solvents or ionic liquids. For instance, Kurnia et al. [23] reported an experimental α value of 0.583 for [N4111] [NTf2] (butyltrimethylammonium bis(trifluoromethylsulfonyl) imide), and through COSMO-RS (Conductor-like Screening Model for Real Solvents), the authors predicted α values ranging between 0.283 and 1.491 for [NTf2]-based ionic liquids combined with several tetraalkylammonium-based cations. For other molecular solvents, such as methanol, ethanol and glycerol, the α values are 0.93, 0.83, and 1.21, respectively [19,36]. For the most studied DES, for example those composed of [N1112(OH)]Cl:Glycerol, in a 1:2 mole ratio, an α = 0.937 was reported [14], being in the same range of the values reported here. For all the DES investigated in this work, α values ranging from 0.84 to 1.09 were obtained, confirming that these solvents act as efficient proton donors if a carboxylic acid is added as the HBD species, however this ability decreases with the increase on the size of the aliphatic moieties. This contribution is further supported by the α values obtained for the DES with lower amounts of the organic acid, namely in 1:1 and 2:1 mole ratios – cf. Table 1.
3.2. Hydrogen-bond basicity of DES
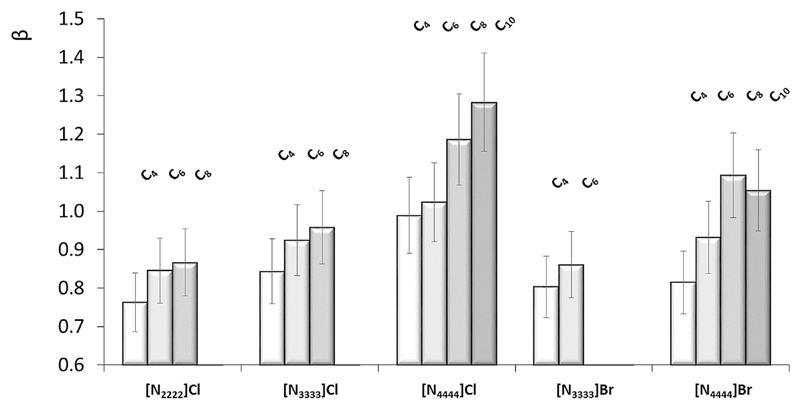
The β parameter describes the solvent capacity to accept protons or its hydrogen-bond basicity. Fig. 3 shows the β values for the studied DES, in a mole ratio of 1:2 of the salt:carboxylic acid, at 298 K. The β parameter in this DES composition ranges between 0.71 ([N1112(OH)]Cl:Butanoic Acid) and 1.28 ([N4444]Cl:Decanoic Acid). Taking into account the values shown in Table 1 for the pure organic acids, it is clear that the hydrogen-bond basicity of DES is mainly governed by the ammonium-based salt. For the DES composed of chloride-based salts ([Nnnnn]Cl, n = 2, 3, and 4) and bromide-based salts ([Nnnnn]Br, n = 3 and 4) as HBA species, the β value increases with the increase of the alkyl chains of both the organic acid and salt cation, following an opposite trend to that observed with the hydrogen-bond acidity. Moreover, a dependence of the hydrogen-bond basicity of the DES on the salt anion was found, for which the DES composed of chloride-based salts display higher values than the bromide-based counterparts, and again with an opposite behavior to that observed with the hydrogen-bond acidity. This observation is however in accordance with data previously published for pure ionic liquids [24] composed of the bromide and chloride anions, in which chloride-based ionic liquids are more prone to accept protons. However, and contrarily to data resorting to pure ionic liquids, where it was demonstrated the anion primordial effect and negligible effect of the cation in defining the hydrogen-bond basicity of the fluid [24], in this work it is shown that the hydrogen-bond basicity of DES also depends on the alkyl chain length of the salt cation and organic acid. This trend is probably related with the weaker cation-anion interaction as the alkyl chain increases [35], leaving thus the salt anion more prone to accept protons from HBD species.
Fig. 3.
Kamlet-Taft hydrogen bond basicity (β) of DES composed of ammonium-based salts and carboxylic acids, namely butanoic (C4), hexanoic (C6), octanoic (C8) and decanoic (C10) acids in a 1:2 mole ratio, at 298 K.
The β values reported in the literature for methanol, ethanol and glycerol are 0.66, 0.75 and 0.51, respectively [33,37], meaning that DES display a higher ability to accept protons than these solvents. For pure ionic liquids, Kurnia et al. [23] reported a β value of 0.273 for [N4111][NTf2], which is lower than the values reported in this work for DES. However, β values up to 1.12 have been reported for ILs composed of anions with high ability to accept protons [24]. For the most studied type of DES, for example those composed of [N1112(OH)]Cl and glycerol, in a 1:2 mole ratio, it was reported a β value of 0.544 [27]. For all the DES under study in this work, β values higher than 0.71 were obtained, confirming thus their high ability to establish hydrogen bonds. Contrarily to what is observed with the hydrogen-bond acidity, and although the chemical structure of the HBA and HBD both play a role, the β values of DES are mainly defined by the salt employed as HBA since much lower values are obtained for the pure acids (Table 1).
3.3. Dipolarity/polarizability of DES
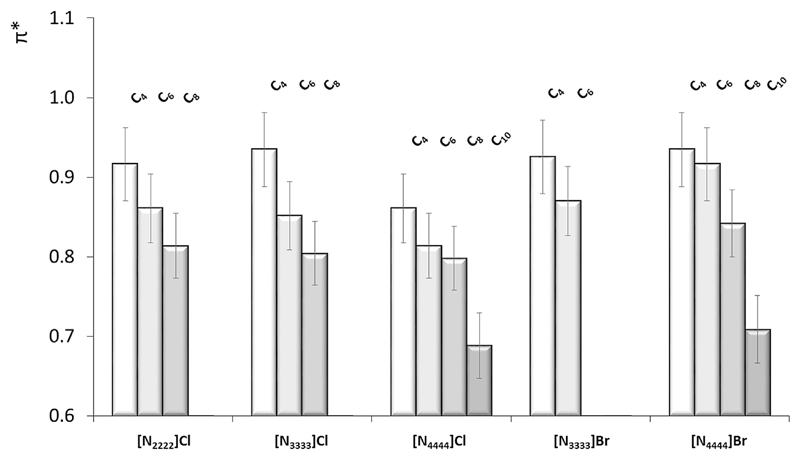
The KT π* parameter is related to non-specific interactions (polarizability, and dipole-dipole and dipole-induced dipole interactions) occurring between the solute and the solvent. Fig. 4 depicts the π* values for the studied DES, liquid at 298 K, in a mole ratio of 1:2.
Fig. 4.
Kamlet-Taft dipolarity/polarizability (π*) parameter of DES composed of ammonium-based salts and carboxylic acids, namely butanoic (C4), hexanoic (C6), octanoic (C8) and decanoic (C10) acids in a 1:2 mole ratio, at 298 K.
According to the data shown in Table 1, the π* parameter of the DES ranges between 0.69 ([N4444]Cl:Decanoic Acid, 1:2) and 0.95 ([N4444]Br:Butanoic Acid, 2:1). Considering the DES formed by both chloride ([Nnnnn]Cl, n = 2, 3, and 4) and bromide salts ([Nnnnn]Br, n = 3 and 4) as HBA species, the π* value decreases with the increase on the alkyl chain of the organic acid employed – following the same behavior of the hydrogen-bond acidity and an opposite behavior to that observed in the hydrogen-bond basicity. The π* parameter comprises the ion-dye non-specific interactions, namely the polarizability, dipole-dipole and dipole-induced dipole interactions. The gathered results suggest that less non-specific interactions occur between the DES and the dye as the aliphatic moiety of the organic acid increases. On the other hand, differences induced in the chemical structures of the salt cation and anion do not play a significant role on the DES dipolarity/polarizability. However, and taking into account the data shown in Table 1 with the solvatochromic parameters of the pure organic acids, it is clear that the dominant species ruling the values of π* is the salt/HBA.
Despite the general lack of information concerning the determination of the π* parameter for DES, some comparisons with other molecular solvents can however be drawn. DES display a higher ability to establish non-specific interactions with a solute than organic molecular solvents, as supported by the π* values of methanol, ethanol and glycerol (0.58, 0.51 and 0.62 [32] [36]). On the other hand, this parameter presents a similar magnitude to that found in pure ionic liquids (π* = 0.914 for [N4111][NTf2]) [23] as well as in other type of DES (π* = 1.161 for [N1112(OH)]Cl:Glycerol, 1:2 mole ratio [27]). For all the DES investigated in this work, π* values higher than 0.69 were obtained, confirming thus their high polarity and ability to establish dipole-dipole and dipole-induced dipole interactions. This similarity to ionic liquids results from the presence of ions in both classes of solvents. In the same line, the π* increases with the salt content in the DES composition (higher amount of ionic species).
4. Conclusions
DES have been claimed as promising solvents for a diversity of applications; to this end, their polarity is a crucial property that should be known in advance so that a rational design on new solvents based on their phase behavior and solvation ability can be undertaken. In spite of their large potential, there is still a lack of information in the literature concerning the DES solvatochromic parameters. In this work, the solvatochromic parameters of DES formed by ammonium-based salts and carboxylic acids were determined in order to better understand the chemical structure influence of the phase-forming components towards the DES hydrogen-bond acidity, hydrogen-bond basicity and dipolarity/polarizability. The DES investigated display a high hydrogen-bond acidity, which is dominated by the organic acid present in the mixture. Moreover, it is shown that the hydrogen-bond basicity is dominated by the anion of the HBA species, with differences in the hydrogen-bond basicity observed between the chloride- and bromide-based mixtures. It was also observed a slight increase of this parameter with the increase of the alkyl chain of both the HBA and the HBD – an opposite behavior to that found in the hydrogen-bond acidity. Finally, it was found that the dipolarity/polarizability of DES are controlled by the ionic species present in the mixture, which tend to decrease with the increase of the aliphatic moiety of the organic acid employed. In summary, the solvatochromic parameters of DES composed of ammonium-based salts and carboxylic acids here reported demonstrate that this type of DES displays a high capacity to donate and accept protons when compared to some organic molecular solvents or ionic liquids.
Acknowledgements
This work was developed in the scope of the project CICECO-Aveiro Institute of Materials, financed by national funds through the FCT/MEC (Ref. FCT UID/CTM/50011/2013) and co-financed by FEDER (Ref. POCI-01-0145-FEDER-007679) under the PT2020 Partnership Agreement. The research leading to reported results has also received funding from Fundação para a Ciência – Tecnologia through the DeepBiorefinery project (PTDC/AGR-TEC/1191/2014) and from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement no. 337753.
References
- [1].Pandey A, Rai R, Pal M, Pandey S. How polar are choline chloride-based deep eutectic solvents? Phys Chem Chem Phys. 2014;16:1559–1568. doi: 10.1039/c3cp53456a. [DOI] [PubMed] [Google Scholar]
- [2].Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V. Novel solvent properties of choline chloride/urea mixtures. Chem Commun. 2003:70–71. doi: 10.1039/b210714g. [DOI] [PubMed] [Google Scholar]
- [3].Abbott AP, Boothby D, Capper G, Davies DL, Rasheed RK. Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J Am Chem Soc. 2004;126:9142–9147. doi: 10.1021/ja048266j. [DOI] [PubMed] [Google Scholar]
- [4].Morrison HG, Sun CC, Neervannan S. Characterization of thermal behavior of deep eutectic solvents and their potential as drug solubilization vehicles. Int J Pharm. 2009;378:136–139. doi: 10.1016/j.ijpharm.2009.05.039. [DOI] [PubMed] [Google Scholar]
- [5].Hayyan M, Hashim MA, Hayyan A, Al-Saadi MA, AlNashef IM, Mirghani ME, Saheed OK. Are deep eutectic solvents benign or toxic? Chemosphere. 2013;90:2193–2195. doi: 10.1016/j.chemosphere.2012.11.004. [DOI] [PubMed] [Google Scholar]
- [6].Harifi-Mood AR, Ghobadi R, Matić S, Minofar B, Řeha D. Solvation analysis of some Solvatochromic probes in binary mixtures of reline, ethaline, and glyceline with DMSO. J Mol Liq. 2016;222:845–853. [Google Scholar]
- [7].Dai Y, van Spronsen J, Witkamp G-J, Verpoorte R, Choi YH. Natural deep eutectic solvents as new potential media for green technology. Anal Chim acta. 2013;766:61–68. doi: 10.1016/j.aca.2012.12.019. [DOI] [PubMed] [Google Scholar]
- [8].Li X, Row KH. Development of deep eutectic solvents applied in extraction and separation. J Sep Sci. 2016;39:3505–3520. doi: 10.1002/jssc.201600633. [DOI] [PubMed] [Google Scholar]
- [9].Hayyan A, Hashim MA, Mjalli FS, Hayyan M, AlNashef IM. A novel phosphonium-based deep eutectic catalyst for biodiesel production from industrial low grade crude palm oil. Chem Eng Sci. 2013;92:81–88. [Google Scholar]
- [10].Singh B, Lobo H, Shankarling G. Selective N-alkylation of aromatic primary amines catalyzed by bio-catalyst or deep eutectic solvent. Catal Lett. 2011;141:178–182. [Google Scholar]
- [11].Zhang Q, Vigier KDO, Royer S, Jérôme F. Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev. 2012;41:7108–7146. doi: 10.1039/c2cs35178a. [DOI] [PubMed] [Google Scholar]
- [12].Wagle DV, Zhao H, Baker GA. Deep eutectic solvents: sustainable media for nanoscale and functional materials. Accounts Chem Res. 2014;47:2299–2308. doi: 10.1021/ar5000488. [DOI] [PubMed] [Google Scholar]
- [13].Tang B, Row KH. Recent developments in deep eutectic solvents in chemical sciences. Monatsh für Chemie-Chemical Mon. 2013;144:1427–1454. [Google Scholar]
- [14].Abbott AP, Harris RC, Ryder KS, D'Agostino C, Gladden LF, Mantle MD. Glycerol eutectics as sustainable solvent systems. Green Chem. 2011;13:82–90. [Google Scholar]
- [15].Dimroth K, Reichardt C, Siepmann T, Bohlmann F. Über Pyridinium-N-phenol-betaine und ihre Verwendung zur Charakterisierung der Polarität von Lösungsmitteln. Justus Liebigs Ann Chem. 1963;661:1–37. [Google Scholar]
- [16].Reichardt C. Solvatochromic dyes as solvent polarity indicators. Chem Rev. 1994;94:2319–2358. [Google Scholar]
- [17].Kamlet MJ, Taft R. The solvatochromic comparison method. I. The. beta.-scale of solvent hydrogen-bond acceptor (HBA) basicities. J Am Chem Soc. 1976;98:377–383. [Google Scholar]
- [18].Taft R, Kamlet MJ. The solvatochromic comparison method. 2. The. alpha.- scale of solvent hydrogen-bond donor (HBD) acidities. J Am Chem Soc. 1976;98:2886–2894. [Google Scholar]
- [19].Kamlet MJ, Abboud JLM, Abraham MH, Taft R. Linear solvation energy relationships. 23. A comprehensive collection of the solvatochromic parameters., pi.*., alpha., and. beta., and some methods for simplifying the generalized solvatochromic equation. J Org Chem. 1983;48:2877–2887. [Google Scholar]
- [20].Abbott AP, Eardley CA, Scheirer JE. Analysis of dipolarity/polarisability parameter, p*, for a range of supercritical fluids. Phys Chem Chem Phys. 2001;3:3722–3726. [Google Scholar]
- [21].Abbott AP, Eardley CA. Solvent properties of liquid and supercritical hydrofluorocarbons. J Phys Chem B. 1999;103:2504–2509. [Google Scholar]
- [22].Abbott AP, Eardley CA. Solvent properties of liquid and supercritical 1, 1, 1, 2-tetrafluoroethane. J Phys Chem B. 1998;102:8574–8578. [Google Scholar]
- [23].Kurnia KA, Lima F, Cláudio AFM, Coutinho JA, Freire MG. Hydrogen-bond acidity of ionic liquids: an extended scale. Phys Chem Chem Phys. 2015;17:18980–18990. doi: 10.1039/c5cp03094c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cláudio AFM, Swift L, Hallett JP, Welton T, Coutinho JA, Freire MG. Extended scale for the hydrogen-bond basicity of ionic liquids. Phys Chem Chem Phys. 2014;16:6593–6601. doi: 10.1039/c3cp55285c. [DOI] [PubMed] [Google Scholar]
- [25].Pandey A, Pandey S. Solvatochromic probe behavior within choline chloridebased deep eutectic solvents: effect of temperature and water. J Phys Chem B. 2014;118:14652–14661. doi: 10.1021/jp510420h. [DOI] [PubMed] [Google Scholar]
- [26].Kadyan A, Behera K, Pandey S. Hybrid green nonaqueous media: tetraethylene glycol modifies the properties of a (choline chlorideþ urea) deep eutectic solvent. RSC Adv. 2016;6:29920–29930. [Google Scholar]
- [27].Kim SH, Park S, Yu H, Kim JH, Kim HJ, Yang Y-H, Kim YH, Kim KJ, Kan E, Lee SH. Effect of deep eutectic solvent mixtures on lipase activity and stability. J Mol Catal B Enzym. 2016;128:65–72. [Google Scholar]
- [28].Bharmoria P, Damarla K, Trivedi TJ, Malek NI, Kumar A. A reciprocal binary mixture of protic/aprotic ionic liquids as a deep eutectic solvent: physicochemical behaviour and application towards agarose processing. RSC Adv. 2015;5:99245–99252. [Google Scholar]
- [29].Schneider H, Badrieh Y, Migron Y, Marcus Y. Hydrogen bond donation properties of organic solvents and their aqueous mixtures from 13C NMR data of pyridine-N-oxide. Z für Phys Chem. 1992;177:143–156. [Google Scholar]
- [30].Madeira PP, Passos H, Gomes J, Coutinho JAP, Freire MG. Alternative probe for the determination of the hydrogen-bond acidity of ionic liquids and their aqueous solutions. Phys Chem Chem Phys. 2017 doi: 10.1039/C6CP08210F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Keleş S, Kaygusuz K, Sari A. Lauric and myristic acids eutectic mixture as phase change material for low-temperature heating applications. Int J energy Res. 2005;29:857–870. [Google Scholar]
- [32].Buhvestov U, Rived F, Ràfols C, Bosch E, Rosès M. Solute–solvent and solvent–solvent interactions in binary solvent mixtures. Part 7. Comparison of the enhancement of the water structure in alcohol–water mixtures measured by solvatochromic indicators. J Phys Org Chem. 1998;11:185–192. [Google Scholar]
- [33].Marcus Y. The properties of organic liquids that are relevant to their use as solvating solvents. Chem Soc Rev. 1993;22:409–416. [Google Scholar]
- [34].Ab Rani M, Brant A, Crowhurst L, Dolan A, Lui M, Hassan NH, Hallett J, Hunt P, Niedermeyer H, Perez-Arlandis J. Understanding the polarity of ionic liquids. Phys Chem Chem Phys. 2011;13:16831–16840. doi: 10.1039/c1cp21262a. [DOI] [PubMed] [Google Scholar]
- [35].Fernandes AM, Rocha MA, Freire MG, Marrucho IM, Coutinho JA, Santos LM. Evaluation of cation– anion interaction strength in ionic liquids. J Phys Chem B. 2011;115:4033–4041. doi: 10.1021/jp201084x. [DOI] [PubMed] [Google Scholar]
- [36].Marcus Y. The effectiveness of solvents as hydrogen bond donors. J Solut Chem. 1991;20:929–944. [Google Scholar]
- [37].Reichardt C, Welton T. Solvents and Solvent Effects in Organic Chemistry. John Wiley & Sons; 2011. [Google Scholar]