Abstract
Phenolic acids are ubiquitous biomolecules exhibiting a wide range of physiological properties, with application in the pharmaceutical and nutraceutical fields. In this work, aqueous biphasic systems (ABS) formed by polyethylene glycol and sodium polyacrylate, and inorganic salts or ionic liquids as electrolytes, were applied to the purification of caffeic, ferulic and protocatechuic acids, followed by the use of centrifugal partition chromatography (CPC) to reinforce the fractionation process scale-up. In single-step experiments in ABS, high selectivities (SFA/CA = 12.09; SCA/PA = 6.32; SFA/PA = 1.91) and adequate partition coefficients (KCA = 2.78 ± 0.20; KPA = 0.44 ± 0.04; KFA = 0.23 ± 0.01) were achieved using ABS formed by sodium chloride as electrolyte. This system was further applied in CPC, allowing an efficient separation of the three phenolic acids after the optimization of the equipment operational conditions, while demonstrating the potential of polymer-based ABS to be used in liquid-liquid chromatography. Finally, the recovery of the phenolic acids (≥ 65%) with high purity from the ABS phases was demonstrated, followed by the reuse of the phase-forming components.
Introduction
Phenolic compounds are relevant biomass building blocks. They are considered as one of the most versatile and important industrial organic chemicals,1 widely used in the food2,3 (e.g. as dyes and food additives), pharmaceutical4,5 (e.g. natural antioxidants or raw materials for producing medical drugs like aspirin), chemical6–9 (e.g. resins, plastics and polycarbonates) and cosmetic3,10 (e.g. natural additives) industries. Due to their wide range of applications, these products are economically attractive11 in particular when compared with petrochemical phenolic compounds. In in 2015, the price of synthetic phenols achieved values around 1000$/tonne,11 while the price of phenolic compounds derived from the lignocellulosic biorefinery ranged from 1000-12000$/tonne12 depending on the phenolic compound (e.g. 12000$/tonne for vanillin; 4500$/tonne for eugenol; and 2000$/tonne for syringols/conyferols/guaiacol). Their high price is a result of significant drawbacks regarding the lignocellulosic biorefinery processing, especially considering the need of more effective purification methods and downstream processes. The alkaline oxidative process13,14 and the hydrothermal processing15 of lignin are the conventional depolymerisation platforms used to produce monomeric aromatic compounds, mainly due to their greener and cheaper characteristics. Nevertheless, one of the main concerns is the difficulty to fractionate the heterogeneity of phenolic compounds resulting from the lignin depolymerisation process.16 Therefore, there is an effective and crucial need to develop more efficient, yet scalable, fractionation processes, operating at mild conditions with minimal waste formation, in order to selectively separate and purify each phenolic compound produced by lignin depolymerisation, in which aqueous biphasic systems (ABS) can be envisioned as a promising alternative. Previous successful studies used ABS to purify structurally similar biomolecules,17–19 including phenolic compounds.20 However, none of these works reported the scale-up of the technique.
Two polymers, a polymer and a salt or two salts dissolved in water, are the most common combinations to form ABS. Polymer-based ABS are commonly composed of polyethylene glycol (PEG) and dextran.21–23 However, due to the high cost of dextran and the high viscosity that PEG-dextran-based systems present,24 other polymer combinations have been proposed.25–27 Amongst these, sodium polyacrylate (NaPA)-PEG-based ABS appeared as a promising combination of phase-forming components that have been successfully applied in the purification of a wide variety of biomolecules.18,28,29 These novel polymer–based aqueous systems have some important advantages when compared to other polymer combinations, namely a lower viscosity and faster separation rate, beneficial to reduce energetic inputs and improve mass transfer.27 Moreover, PEG-NaPA-based ABS exhibit a remarkable high water content, with phase separation occurring at very low concentrations of polymer (3–5 wt% of each polymer).27 These two polymers are biocompatible, relatively inexpensive, and easy to recycle and reuse.20,27 PEG is an uncharged polymer whereas NaPA is a polyelectrolyte. To form an ABS at reasonable polymer concentrations, a minimum amount of an electrolyte is however required.27 Various authors have evaluated the effect of different electrolytes, principally inorganic salts,26,27 and more recently, ionic liquids30 and surfactants20 to induce the phase separation. Some of these authors suggested that the entropy penalty upon compartmentalization of the counter ions present27 and/or the competition of the charged species for the water molecules are the driving forces behind the phase separation.26 Meanwhile, when ionic liquids are used as electrolytes30 the interactions are far more complex, being the two phase formation not only driven by NaPA compartmentalization effect. Even though there are still different visions on the molecular-level phenomenon which rules the phase separation, these systems have shown some interesting results regarding the purification of biomolecules.27 In general, in PEG-NaPA systems, the manipulation of the electrolyte nature affects the biomolecules partition and their selectivity to one of the phases, which are a result of specific interactions occurring between the phase-forming components, the electrolyte, and the target biomolecules or their contaminants.20,25,29 Biomolecules of higher complexity and molecular weight, like cytochrome c,30 hemoglobin,31 lysozyme31 and protease,28 and simpler biomolecules, such as clavulanic29 and chloranilic acids30, were studied using PEG-NaPA-based ABS with different electrolytes. In these works, authors draw particular attention to the maintenance of the biomolecules biological activity and stability during the purification process. However, one of the major drawbacks associated with the use of polymer-based ABS is the difficulty to transpose the high yields of extraction and purity levels obtained in the lab, to continuous processes at a larger scale, culminating in an industrial process. To this end, centrifugal partition chromatography (CPC) can enhance the resolution of the separations and convert liquid-liquid extractions based on ABS into processes scalable to large flow rates.32 This chromatographic downstream technology operates with liquid stationary and mobile phases, which in this work will correspond to the two phases of the polymer-based ABS. The stationary phase is immobilized by a strong centrifugal force while the mobile phase is pumped through the stationary phase,33 allowing a multistage separation process by the continuous partition of the biomolecules between the two phases. CPC does not need an expensive solid stationary phase, and both the quantity and quality requirements regarding the solvents/phases to be used could be much more similar in terms of their properties (e.g. viscosity and density), than those applied in the more standard liquid chromatographic techniques, such as CCC (counter current chromatography).33 Compared to traditional chromatographic techniques which employ stationary phases, CPC reduces the ample losses which could occur by irreversible adsorption, imposing no restrictions regarding the flow related to solids or the adsorbent porosity, and no changes of the analytes structure.34 Due to the different affinity of the target compounds and contaminants for each phase, all components of a sample mixture injected at the beginning of the multistage cascade will elute at different times. CPC has been applied to fractionate various biomolecules derived from biomass, showing separations with high selectivities and extraction efficiencies. Phenolic compounds from plant extracts were previously successfully purified by CPC using other immiscible phases, e.g. heptane/ethyl acetate/methanol/water (1:5:1:5; v/v) and hexane/ethyl acetate/ethanol/water (4:5:3:3; v/v).35–37
In this work, PEG 8000 + NaPA 8000-based ABS, using ionic liquids or inorganic salts as electrolytes, were studied in the separation of three model phenolic compounds (caffeic, ferulic and protocatechuic acids), derived from lignocellulosic depolymerisation. The selection of the best ABS and its optimization was performed, followed by its application in CPC to reinforce the technique scale-up.
Experimental
Materials
The polymer-based ABS studied are formed by two polymers, namely polyethylene glycol (PEG 8000 g mol-1; purum) and sodium polyacrylate (NaPA 8000 g mol-1; 45 wt% in water), both from Sigma-Aldrich.
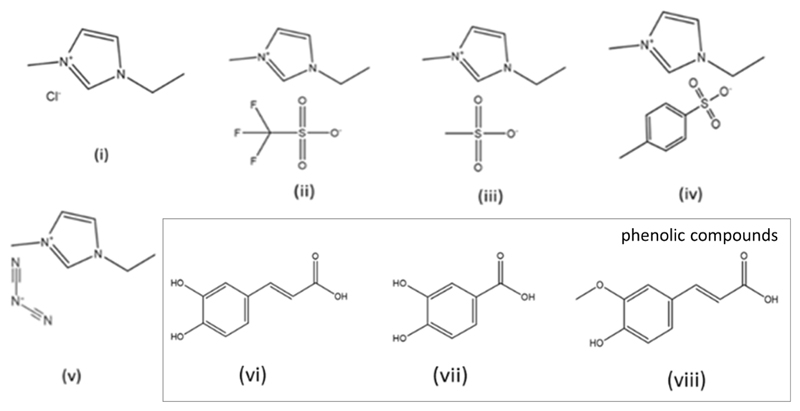
Inorganic salts used as electrolytes were sodium chloride (NaCl) and sodium sulphate (Na2SO4), both purchased from Sigma-Aldrich, with a purity ≥ 99 wt%. Ionic liquids used as electrolytes were 1-ethyl-3-methylimidazolium chloride ([C2mim]Cl), 1-ethyl-3-methylimidazolium triflate [C2mim][CF3SO3], 1-ethyl-3-methylimidazolium methanesulfonate [C2mim][CH3SO3], 1-ethyl-3-methylimidazolium tosylate [C2mim][TOS], and 1-ethyl-3-methylimidazolium dicyanamide [C2mim][N(CN)2]. The ionic liquids were purchased from Iolitec, with a purity > 97 wt% (Fig. 1A).
Fig. 1.
Chemical structure of ionic liquids and phenolic compounds studied in this work: (i) [C2mim]Cl, (ii) [C2mim][CF3SO3], (iii) [C2mim][CH3SO3], (iv) [C2mim][TOS], (v) [C2mim][N(CN)2], (vi) caffeic acid, (vii) protocatechuic acid, and (viii) ferulic acid.
The investigated phenolic acids (Fig. 1B), namely caffeic, ferulic and protocatechuic acids, were acquired at Sigma-Aldrich (purity > 98 wt%).
Preparation of polymer-based ABS using inorganic salts or ionic liquids as electrolytes to separate phenolic acids
The ABS used on the partition studies of phenolic acids were prepared using graduated centrifuge tubes by weighting the appropriate amount of each phase component, and the proper amount of each phenolic aqueous solution. The extraction point adopted to study the partition of phenolic compounds was 15 wt% of PEG 8000 + 4.5 wt% of NaPA 8000 + 5 wt% of each electrolyte + 75.5 wt% of an aqueous solution containing each phenolic compound, namely caffeic acid (28 µg.mL-1), ferulic acid (20 µg.mL-1), and protocatechuic acid (20 µg.mL-1). This mixture point falls within the biphasic region and was chosen based on phase diagrams reported elsewhere.30 The different phenolic concentrations were selected to ensure sufficient accuracy within the analytical technique employed for their quantification.
After the complete dissolution of all components by their stirring, all mixtures were left to equilibrate for 12 hours in an air oven, at (298 ± 1) K, to achieve the complete partitioning of each phenolic compound between the two aqueous phases. The phases were then carefully separated and the phenolic acids were quantified at both top and bottom phases by UV-spectroscopy, using a Synergy HT spectrometer microplate reader, at the wavelengths of 287, 277, and 256 nm, for caffeic, ferulic, and protocatechuic acids, respectively. Calibration curves for each phenolic compound were established at the respective maximum absorption wavelengths. Three independent assays were prepared for each mixture, and the quantification of each phenolic compound was performed in triplicate, where the final absorbance results are reported as the average of with the respective standard deviation. Possible interferences of the phase-forming electrolytes were taken into account using blank controls (represented by the same mixture points, but without the presence of the phenolic compound under study). Different parameters were determined in order to evaluate the partition performance of each phenolic compound (PC), namely their partition coefficients (KPC) those representing KCA, KFA and KPA, their recovery in the top phase (RecTopPC%), which corresponds to the PEG-rich phase, and the selectivity data (S), as represented by Eqs. 1 to 3, respectively.
| (Eq. 1) |
| (Eq. 2) |
| (Eq. 3) |
where [PC]Top and [PC]Bot represent the phenolic compound concentration at the top and bottom phases, respectively. Specific abbreviations representative of the partition coefficients for each phenolic compound, namely KCA, KFA and KPA, are used for caffeic, ferulic and protocatechuic acids, respectively. Rv represents the volume ratio between the top and bottom phase volumes, and KPC1 and KPC2 represent the partition coefficients of two different phenolic compounds, in which KPC1 ≥ KPC2 represent S ≥ 1. The detailed recovery data are presented in Table S1 in the ESI†.
Isolation of phenolic acids from aqueous polymeric phases
Phenolic acids were isolated from the PEG-8000- and NaPA-8000-rich phases through dialysis. A Spectra/Por membrane (cut-off: 1 KDa; diameter 24 mm) was used, against a volume of 10 mL of ultra-pure water, at room temperature. The dialysate was evaluated by UV-Vis spectroscopy in order to quantify the amount of each phenolic acid present. When the equilibrium was reached, a new volume of water (10 mL) was added to recover the remaining phenols, being the PEG 8000 retained in the dialysis membrane (this process was applied just for caffeic acid since the remaining phenols were recovered in a single dialysis step). The purity of the recovered protocatechuic and ferulic acids was evaluated by 1H NMR. The 1H NMR measurements were performed on a Bruker Avance 300 spectrometer operating at 300.13 MHz.
Centrifugal partition chromatography to separate phenolic acids
A Fast Centrifugal Partition Chromatography (FCPC)® system, model FCPC-C, from Kromaton Rousselet-Robatel (Annonay, France), was used for the phenolic compounds separation. The equipment design comprises a pattern of cells interconnected by duts and dug in a stainless steel disk. The cell design, also called as twin cells, contains a restriction in the middle ducts of the canal creating two superimposed chambers. The rotor consists of 13 associated disks, each one containing 64 twin cells, making a total of 832 twin cells. The total cell volume is of 50 mL, with 10 mL or 20 % of the column volume corresponding to the connecting ducts. The maximum theoretical liquid stationary phase retention factor (Sf = VS/ VC) is of 80 %, since 20 % of the connecting duct volume can only contain the mobile phase. The maximum rotor rotation is 3000 rpm generating a maximum centrifugal field of ~ 1500 G. Two rotating seals are displayed at the rotor entrance and exit (also called, respectively, “head” and “tail”), which allow a maximum pressure of 7 MPa. The CPC system is connected to an ECOM ECB2004 Gradient box with degasser, an ECOM ECP2010 Analytical HPLC pump, an ECOM Flash 14 DAD detector (four wavelengths are simultaneously analysed), and to a continuous scan (ECOM spol. S.r.o., Czech Republic). Several fractions are collected with an ADVANTEC® Super Fraction Collector CHF122SC (Advantec Toyo Kaisha, Ltd., Tokyo, Japan). Each sample was injected manually using a Rheodyne valve model 3055-023 through a 10 mL sample loop. Analogical detector signals were processed using the ECOMAC software (ECOM spol. S.r.o., Czech Republic).
The CPC separations were carried out using the system composed of 15 wt% of PEG 8000 + 4.5 wt% of NaPA 8000 + 75.5 wt% of water + 5 wt% of NaCl. This system was set to work in the ascending mode. The rotor was entirely filled with the NaPA-(bottom)-rich phase at 600 rpm to achieve the homogeneous solvent re-equilibration on the rotor. Then, the rotation was set up at the highest speed (2000 rpm), needed for the appropriate stationary phase retention. After the working rotational speed was set up, the PEG-8000-rich-(top) phase was pumped through the stationary phase to reach the equilibrium, i.e. when only the mobile phase came out of the column and the signal baseline is stabilized. The mobile phase flow rate was studied to increase the stationary phase retention ratio and, simultaneously, decrease the purification time, and the best flow rate found was of 1.5 mL.min-1. The stationary phase retention, Sf, was calculated by the ratio of the stationary phase volume (VS) and the column volume (VC): Sf= VS/VC. In this case, values of 37 % and 20 % of Sf for 1.5 and 1.0 mL.min-1 of flow rate, respectively, were achieved.
The sample loop was filled with 5 mL of the ABS composed of 15 wt% of PEG 8000 + 4.5 wt% of NaPA 8000 + 75.5 wt% of water + 5 wt% of NaCl containing the caffeic, ferulic and protocatechuic acids at higher concentrations (0.4 mg/mL) than those used in the lab-scale ABS experiments. After 55 min of elution with the top PEG-rich phase to extract the caffeic acid, the mobile phase was changed to the NaPA-rich (bottom) phase to elute separately the protocatechuic and ferulic acids, by applying an elution-extrusion process.38
Results and discussion
Evaluation of PEG-NaPA-based ABS to separate phenolic acids
The phase diagrams of the systems investigated in this work, as well as the electrolytes preferential partition, were reported in a previous work.30 Aiming at better understanding the separation of the three phenolic compounds, the pH of the phases was also determined, as presented in Table S2 in the SI†. Table S2 also presents the partition coefficients data for the three phenolic compounds between the two phases. Despite the absence of a buffering agent to maintain the pH, the pH of the studied systems is ca. 7 (6.7 < pH top phase < 7.5 and 6.6 < pH bottom phase < 7.4). From these results, it seems that this condition (pH) is independent of the electrolyte nature and type, probably because these are present in low concentrations. At this pH values, all phenolic acids are negatively charged in all the partition experiments (cf. their dissociation curves in Figs. S1-S3 in the ESI†).
In this work, two types of electrolytes were investigatedd30, five imidazolium-based ionic liquids and two inorganic salts. The partition tests were performed using systems composed of 15 wt% of PEG 8000 + 4.5 wt% of NaPA 8000 + 75.5 wt% of water + 5 wt% of electrolyte. The electrolyte concentration of 5 wt% since it corresponds to the biphasic regions according to binodal curves previously reported, and since these conditions lead to high selectivity and good performance on the separation of molecules.30
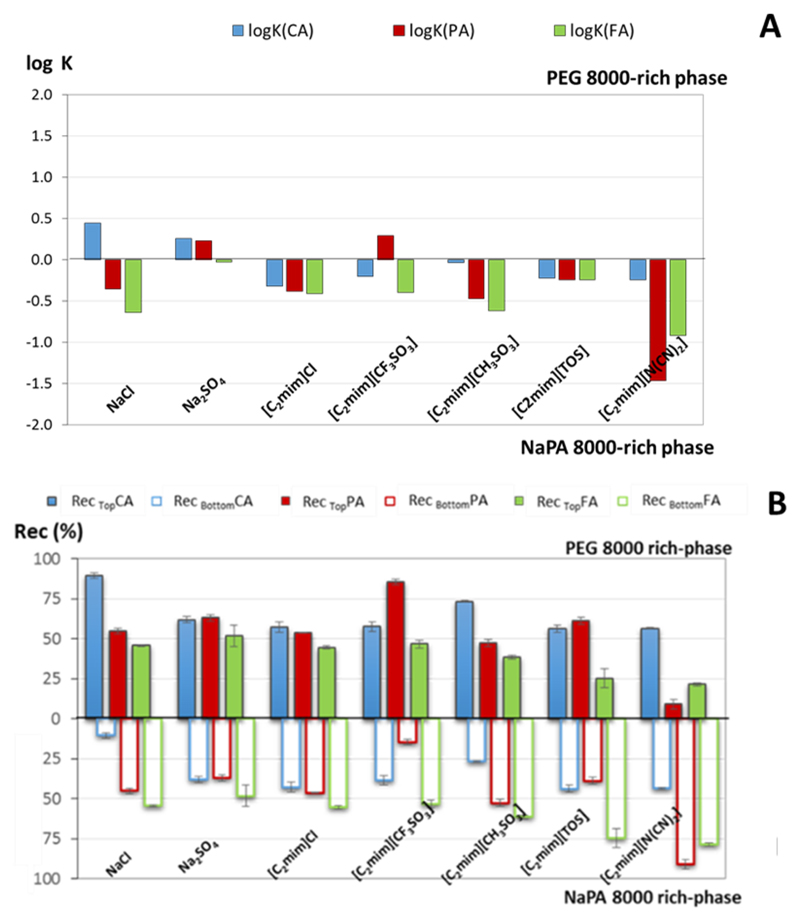
Fig. 2 depicts the partition coefficients and recovery results achieved, in which two profiles can be distinguished for the phenolic acids partition: (i) non-electrolyte dependent (ferulic acid); and (ii) electrolyte-dependent (protocatechuic and caffeic acid). The preferential partition of ferulic acid (log Kow = 1.6739) for the NaPA-8000-rich phase (log KFA < 0; Fig. 2A) observed in this work follows the results previously reported20, similarly to the protocatechuic partition profile, which in general displays a higher affinity to the more hydrophobic phase (being the systems based in Na2SO4 and [C2mim][CF3SO3] the exceptions). The partition of both protocatechuic and caffeic acids seems to be however dependent on the phenolic-acid-electrolyte interactions, which and -1.47 < log KPA < 0.29; Fig. 2A) and recovery experimental is patent in their partition coefficients (-0.32 < log KCA < 0.44 data (Fig. 2B).
Figure 2.
(A) Partition coefficient in log10, (logK), and (B) Recovery, Rec (%), of phenolic acids between the PEG-8000-(top)- and NaPA-8000-(bottom)-rich phases in different polymer-based ABS.
In general, phenolic acids preferentially partition towards the NaPA-8000-rich phase (log K < 0), the same partition tendency described for some of the electrolytes used in the ABS formation.30 Exceptions to this behaviour are observed with the systems formed by [C2mim]Cl and [C2mim][N(CN)2]. In fact, and despite the preferential partition of the phenolic compounds towards the NaPA-8000-rich phase, the electrolytes, specifically the ILs, are equally distributed between both phases (K ~ 1).30 On the other hand, for ABS using the inorganic salts (NaCl and Na2SO4) and the ionic liquid [C2mim][CF3SO3] with a lower partition coefficient (K <<1), it is observed that at least one of the phenolic acids partition to the PEG-8000-rich phase.
The protocatechuic acid is partitioned to the PEG-8000-rich phase in the ABS using Na2SO4 (log KPA= 0.23 and RecTopPA = 64.31 ± 0.10%) and [C2mim][CF3SO3] (log KPA= 0.29 and RecTopPA = 85.5 ± 1.7%) as electrolytes. The caffeic acid also partitions to the PEG-8000-rich phase when NaCl (log KCA = 0.44 and RecTopCA = 89.6 ± 1.8%) and Na2SO4 (log KCA = 0.26 and RecTopCA = 62.0 ± 1.9%) are used as electrolytes. The selectivity was also determined (Table 1) to assess the capacity of the investigated ABS to separate a mixture of the phenolic compounds. The capacity of each ABS to separate each phenolic compound is different and depends on the electrolyte used. The best results of selectivity were achieved for the ABS formed by NaCl (1.91 < S < 12.09), [C2mim][CF3SO3] (1.58 < S < 4.86), [C2mim][CH3SO3] (1.41 < S < 3.85) and [C2mim][N(CN)2] (3.54 < S < 16.8) as electrolytes. Taking into account the results achieved in the optimization step, the ABS considered as the most promising to be used in the following CPC studied was the one using NaCl as electrolyte. This ABS was selected due to its low cost, high selectivity and good separation performance, i.e. low partition coefficients, yet sufficiently different for the various phenolic compounds.
Table 1.
Selectivity values (S) obtained for the ferulic (FA), protocatechuic (PA) and caffeic (CA) acids using inorganic salts and ionic liquids as electrolytes in PEG/NaPA-based ABS.
| Electrolyte used in the PEG/NaPA-based ABS | SFA/CA | SCA/PA | SFA/PA |
|---|---|---|---|
| NaCl | 12.09 | 6.31 | 1.91 |
| Na2SO4 | 1.93 | 1.07 | 1.80 |
| [C2mim]Cl | 1.23 | 1.17 | 1.05 |
| [C2mim][CF3SO3] | 1.58 | 3.10 | 4.86 |
| [C2mim][CH3SO3] | 3.85 | 2.73 | 1.41 |
| [C2mim][TOS] | 1.05 | 1.05 | 1.00 |
| [C2mim][N(CN)2] | 4.75 | 16.81 | 3.54 |
Separation of phenolic acids using centrifugal partition chromatography (CPC)
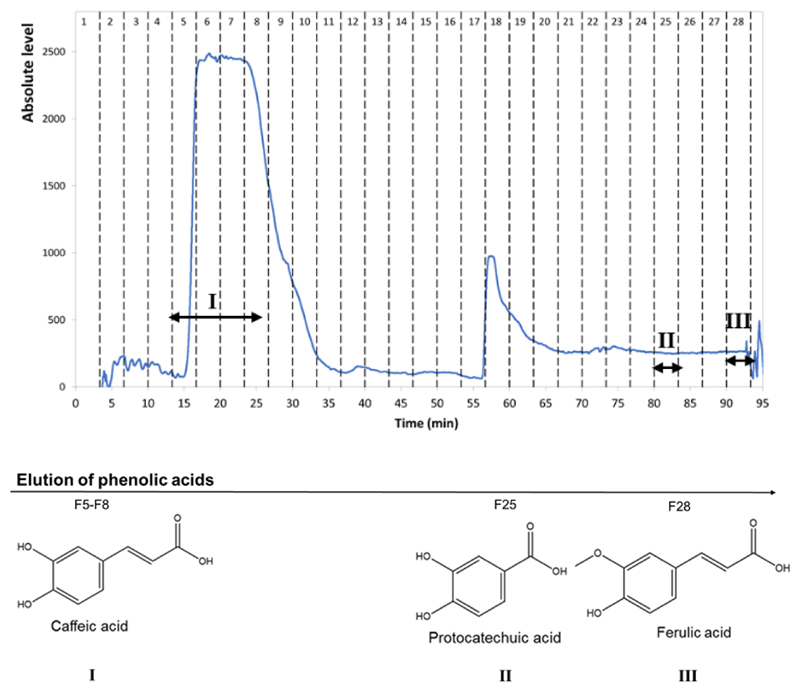
The best ABS identified before, composed of 15 wt% of PEG 8000 + 4.5 wt% of NaPA 8000 + 5 wt% of NaCl + 74.5 wt% of water, was used for the separation of the three phenolic compounds using CPC. As previously mentioned, this ABS presents partition coefficients (K) for the phenolic acids in an adequate range for use in CPC (KCA = 2.78 ± 0.2, KPA = 0.44 ± 0.04 and KFA =0.23 ± 0.01). In order to select the optimum conditions to separate the three phenolic acids, preliminary studies were performed considering the combination of two different mobile phases, respectively the PEG- and NaPA-8000-rich phases; flow rates, respectively 1.0 and 1.5 mL.min−1; rotation speeds, respectively 1500 and 2000 rpm; and ascending or descending modes. After some preliminary essays, the flow rate of 1.5 mL.min−1, rotation speed of 2000 rpm, and the ascending mode, were selected to perform further studies. These conditions provide a stationary phase retention (i.e. the volume ratio between the stationary phase and the column volume) of 37% allowing to perform a fast separation of the target acids under constant pressure (4.5 MPa). Fig. 3 presents the chromatogram resulting from the batch injection of a mixture (5 mL) of caffeic (2 mg), protocatechuic (2 mg), and ferulic (2 mg) acids, performed in the ascending mode and with a mobile phase flow rate of 1.5 mL.min−1. The top PEG-8000-rich phase was used as mobile phase during the initial stage of elution (from 0 to 55 min). Afterwards, and for the elution of caffeic acid, the mobile phase was changed to the NaPA-8000-rich phase, to separately elute the protocatechuic and ferulic acids, respectively. This change on the mobile phase was required to decrease the elution time of the two last phenolic acids, since both acids have a higher affinity to the stationary phase used during the initial stage of elution, the NaPA-8000-rich phase. No losses of stationary phase were observed during the separation run, and the complete separation of the three compounds was achieved, being the caffeic acid eluted in fractions 5-8, the ferulic acid in fraction 25, and the protocatechuic acid in fraction 28. Since NaPA 8000 absorbs strongly in the UV region it was not possible to observe the phenolic characteristic peaks of ferulic and protocatechuic acids since these are masked by the polymer. The identification of these two phenolic acids in the respective fractions was achieved after removal of NaPA 8000 from the fractions collected using sulphuric acid. The peak observed in the fractions 18-19 corresponds to the change of the mobile phase from PEG to NaPA aqueous solutions. In agreement with the partition coefficients determined for the PEG 8000 + NaPA 8000 + water + NaCl system (presented in Table 1), it is here confirmed that caffeic acid has the highest affinity to the PEG-8000-rich phase, being the first compound to be eluted. Both protocatechuic and ferulic acids have a low affinity to the PEG-8000-rich phase (K < 1), which justifies the application of an elution-extrusion process.38 Changing the mobile phase from a PEG-8000- to a NaPA-8000-rich phase, the ferulic acid was first eluted (due to its smaller K value), followed by the protocatechuic acid. A higher separation resolution was achieved at a mobile phase flow rate of 1.5 mL.min−1 (Fig. 3) than with the rate of 1.0 mL.min−1 (Fig. S4 in the ESI†). This was attributed to the lower retention time of the phenolic acids elution and also to the higher stationary phase retention (two times greater Sf), being the compounds eluted at lower elution times. In addition, by increasing the flow rate from 1.0 mL.min−1 to 1.5 mL.min−1, a better resolution of protocatechuic (PA) and ferulic (FA) acids was obtained. With a flow rate of 1.0 mL.min-1, the protocatechuic (PA) and ferulic (FA) acids were co-eluted.
Figure 3.
Separation of phenolic acids by CPC using the system composed of 15 wt% of PEG 8000 + 4.5 wt% of NaPA 8000 + 75.5 wt% of water + 5 wt% of NaCl. Experimental conditions: rotation speed of 2000 rpm.min-1; flow-rate of 1.5 mL.min-1; Sf = 37 %; P ≈ 38 bar.
Summing up, the experiments and results discussed before demonstrate the potential of polymer-based ABS as alternative and versatile platforms for the separation of phenolic acids using CPC. It was shown that any of the studied polymeric phases could be used as the stationary or the mobile phase in CPC. In spite of the viscosity of the NaPA-8000-rich phase, the design of the twin-cells of the CPC column enabled the use of high flow rates, while attaining a high stationary phase retention.40
Integrated purification process: isolation of phenolic acids and ABS phase-forming components recycling
The isolation of the studied phenolic acids, namely caffeic acid from the PEG-8000-rich phase and the other two phenolic compounds from the NaPA-8000-rich phase, after their separation by CPC, was experimentally addressed through dialysis. The dialysis of both the PEG-8000-rich and NaPA-8000-rich phases was executed using a membrane with a MWCO = 1 kDa, thus allowing the polymer retention inside the membrane, and the release of the phenolic acids in the dialysate. Around 87% of the initial mass of caffeic acid added to the system was recovered after two washing steps with water (68% and 19% in the first and second steps of dialysis, respectively). It should be highlighted that after the dialysis step, not only the caffeic acid was removed, but also the PEG 8000-rich phase was cleaned, allowing thus the polymer recycling and consequent reuse in a new purification step. For the isolation of ferulic and protocatechuic acids from the NaPA-8000-rich phase, a dialysis step was also carried out, followed by the phenolic acids lyophilisation. In this polishing step, 84% of ferulic acid and 65% of protocatechuic acid were recovered by a single washing dialysis step. After lyophilisation, a 1H NMR (depicted in Figs. S5 and S6 in the ESI†) analysis was carried out confirming both the removal of the polymers and the high purity of the lyophilized samples of each phenolic acid (Fig. S7 in the ESI†). After the phenolic compounds removal, NaPA 8000 aqueous solutions could be directly reused in new ABS formation.
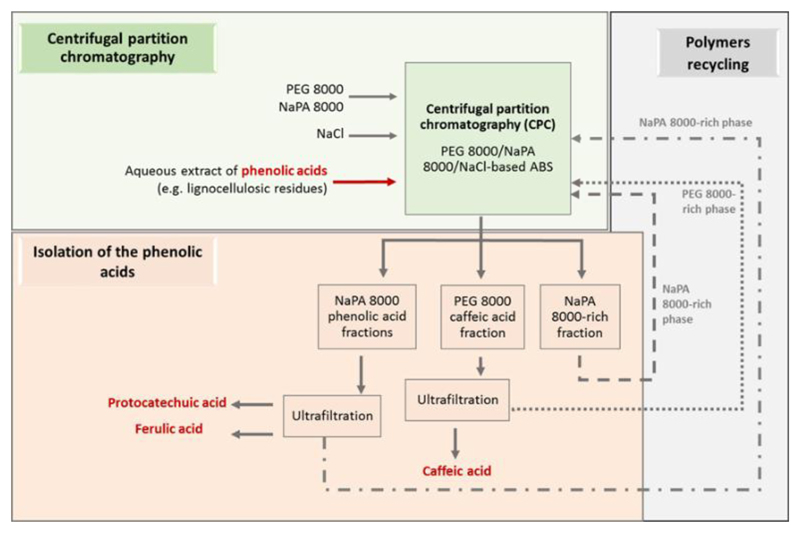
Taking the previous results into account and envisaging the industrial application of the technique here developed, an integrated process for the purification of phenolic compounds was designed, presented in Fig. 4.
Figure 4.
Schematic representation the envisioned scaled-up process of purification of three phenolic acids by applying PEG 8000/NaPA 8000-based ABS using NaCl as electrolyte and CPC. The isolation of the phenolic acids from each aqueous phase and the respective phase-forming components’ reuse is also represented.
The process here proposed uses PEG 8000/NaPa 8000/NaCl-based ABS combined with CPC to efficiently separate ferulic, protocatechuic, and caffeic acids. Moreover, the polishing (isolation) of phenolic acids from the phase-forming components was also proposed by a membrane-based purification step, for which ultrafiltration can be used when envisaging the technology scale-up. Overall, this work describes an integrated process for the separation of three structurally similar phenolic acids, by applying low-cost components (low polymer contents and low cost inorganic salt) and eco-friendly (mild conditions) polymeric-based ABS.
Conclusions
PEG/NaPA-based ABS using inorganic salts or ionic liquids as electrolytes were investigated to separate phenolic acids, for which a purification process using these polymeric-based ABS integrated with CPC is here shown for the first time. The separation of three structurally similar natural compounds (phenolic acids) was firstly optimized, allowing to prove the importance of the electrolyte nature, not only in terms of the phase diagrams design (as discussed elsewhere30), but mainly in the different partition tendencies observed for each phenolic compound. The PEG 8000/NaPA 8000/NaCl-based ABS was identified as the most efficient system for the separation of the three phenolic acids, and was further used in CPC to support its scale-up. After the optimization of some operational conditions, successful results, i.e. the complete separation of the three phenolic acids, were obtained with CPC. Further, novel approaches for the recovery of the phenolic acids and reuse of the phase-forming components have been developed. Based on the success of this work, its applicability for the processing of real matrices, e.g. agro-industrial lignocellulosic residues, biomass wastes and forestry residues, is certainly an open topic for future investigations.
Supplementary Material
†Electronic Supplementary Information (ESI) available: Detailed data for phase diagrams, extraction efficiencies, 1H NMR data and See DOI: 10.1039/x0xx00000x
Acknowledgments
This work was developed within the scope of the project CICECO-Aveiro Institute of Materials, POCI-01-0145-FEDER-007679 (FCT Ref. UID/CTM/50011/2013), financed by national funds through the FCT/MEC and when appropriate co-financed by FEDER under the PT2020 Partnership Agreement. The authors are grateful for the financial support of the Portuguese Foundation for Science and Technology (FCT) for the doctoral grant of SFRH/BD/102915/2014 of J.H.P.M. Santos. S.P.M. Ventura acknowledges the FCT/MCTES for a contract under Investigador FCT 2015 contract number IF/00402/2015. M.G.F. acknowledges the European Research Council (ERC) for the grant ERC-2013-StG-337753.
References
- 1.Kleinert M, Barth T. Chem Eng Technol. 2008;31:736–745. [Google Scholar]
- 2.Škerget M, Kotnik P, Hadolin M, Hraš AR, Simonič M, Knez Ž. Food Chem. 2005;89:191–198. [Google Scholar]
- 3.Miguel MG, Nunes S, Dandlen SA, Cavaco AM, Antunes MD. Food Chem Toxicol. 2010;48:3418–3423. doi: 10.1016/j.fct.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Khalatbary AR. Nutr Neurosci. 2013;16:243–249. doi: 10.1179/1476830513Y.0000000052. [DOI] [PubMed] [Google Scholar]
- 5.Bolling BW, McKay DL, Blumberg JB. Asia Pac J Clin Nutr. 2010;19:117–123. [PMC free article] [PubMed] [Google Scholar]
- 6.Ramires EC, Megiatto JD, Gardrat C, Castellan A, Frollini E. Bioresour Technol. 2010;101:1998–2006. doi: 10.1016/j.biortech.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Ibeh CC, Sidney HG. Handbook of Thermoset Plastics. Second Edition. 1999. pp. 23–71. [Google Scholar]
- 8.Charrouf Z, Guillaume D. Am J Food Technol. 2007;2:679–683. [Google Scholar]
- 9.Bolton DH, Woolsey KL. Macromolecules. 1997;30:1890–1896. [Google Scholar]
- 10.Kunicka-Styczyńska A, Sikora M, Kalemba D. J Appl Microbiol. 2009;107:1903–1911. doi: 10.1111/j.1365-2672.2009.04372.x. [DOI] [PubMed] [Google Scholar]
- 11.S. G. Platts, .
- 12.Varanasi P, Singh P, Auer M, Adams PD, Simmons BA, Singh S. Biotechnol Biofuels. 2013;6:14. doi: 10.1186/1754-6834-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos SG, Marques AP, Lima DLD, Evtuguin DV, Esteves VI. Ind Eng Chem Res. 2011;50:291–298. [Google Scholar]
- 14.Rodrigues Pinto PC, Borges Da Silva EA, Rodrigues AE. Industrial and Engineering Chemistry Research. 2011;50:741–748. [Google Scholar]
- 15.Ruiz HA, Rodríguez-Jasso RM, Fernandes BD, Vicente AA, Teixeira JA. Renew Sustain Energy Rev. 2013;21:35–51. [Google Scholar]
- 16.Xu C, Arancon RAD, Labidi J, Luque R. Chem Soc Rev. 2014;43:7485–7500. doi: 10.1039/c4cs00235k. [DOI] [PubMed] [Google Scholar]
- 17.Dembczyński R, Białas W, Regulski K, Jankowski T. Process Biochem. 2010;45:369–374. [Google Scholar]
- 18.Alcântara LAP, Amaral IV, Bonomo RCF, da Silva LHM, do C. H. da Silva M, Minim VPR, Minim LA. Food Bioprod Process. 2014;92:409–415. [Google Scholar]
- 19.Chen Y, Meng Y, Yang J, Li H, Liu X. J Chem Eng Data. 2012;57:1910–1914. [Google Scholar]
- 20.Santos JHPM, Martins M, Silvestre AJD, Coutinho JAP, Ventura SPM. Green Chem. 2016;18:5569–5579. [Google Scholar]
- 21.Diamond AD, Hsu JT. AIChE J. 1990;36:1017–1024. [Google Scholar]
- 22.Karakatsanis A, Liakopoulou-Kyriakides M. J Food Eng. 2007;80:1213–1217. [Google Scholar]
- 23.Rito-Palomares M, Negrete a, Miranda L, Flores C, Galindo E, Serrano-Carreón L. Enzyme Microb Technol. 2001;28:625–631. doi: 10.1016/s0141-0229(01)00298-8. [DOI] [PubMed] [Google Scholar]
- 24.Kim BK, Ban YB, Kim JD. Korean J Chem Eng. 1992;9:219–224. [Google Scholar]
- 25.Pereira Alcântara LA, Do Nascimento KS, Mourão CA, Minim VPR, Minim LA. Sep Purif Technol. 2013;118:888–894. [Google Scholar]
- 26.Gupta V, Nath S, Chand S. Polymer (Guildf) 2002;43:3387–3390. [Google Scholar]
- 27.Johansson H-O, Feitosa E, Junior AP. Polymers (Basel) 2011;3:587–601. [Google Scholar]
- 28.Barros KVG, Souza PM, Freitas MM, Filho EXF, Junior AP, Magalhães PO. Process Biochem. 2014;49:2305–2312. [Google Scholar]
- 29.Pereira JFB, Santos VC, Johansson H-O, Teixeira JAC, Pessoa A. Sep Purif Technol. 2012;98:441–450. [Google Scholar]
- 30.Santos JHPM, e Silva FA, Coutinho JAP, Ventura SPM, Pessoa A. Process Biochem. 2015;50:661–668. [Google Scholar]
- 31.Johansson H-O, Magaldi FM, Feitosa E, Pessoa A, Jr, Pessoa A. J Chromatogr A. 2008;1178:145–53. doi: 10.1016/j.chroma.2007.11.071. [DOI] [PubMed] [Google Scholar]
- 32.Schwienheer C, Merz J, Schembecker G. J Liq Chromatogr Relat Technol. 2015;38:929–941. [Google Scholar]
- 33.Berthod A, Maryutina T, Spivakov B, Shpigun O, Sutherland IA. Pure Appl Chem. 2009;81:355–387. [Google Scholar]
- 34.Friesen JB, McAlpine JB, Chen SN, Pauli GF. J Nat Prod. 2015;78:1765–1796. doi: 10.1021/np501065h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Destandau E, Boukhris MA, Zubrzycki S, Akssira M, El Rhaffari L, Elfakir C. J Chromatogr B Anal Technol Biomed Life Sci. 2015;985:29–37. doi: 10.1016/j.jchromb.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 36.Trabelsi N, Oueslati S, Henry-Vitrac C, Waffo-Téguo P, Medini F, Mérillon JM, Abdelly C, Ksouri R. Ind Crops Prod. 2013;49:740–746. [Google Scholar]
- 37.Delaunay JC, Castagnino C, Chèze C, Vercauteren J. J Chromatogr A. 2002;964:123–128. doi: 10.1016/s0021-9673(02)00355-2. [DOI] [PubMed] [Google Scholar]
- 38.Berthod A, Ruiz-Angel MJ, Carda-Broch S. Anal Chem. 2003;75:5886–5894. doi: 10.1021/ac030208d. [DOI] [PubMed] [Google Scholar]
- 39.Chemspider, – The free chemical database. at http://www.chemspider.com.
- 40.Roehrer S, Bezold F, García EM, Minceva M. J Chromatogr A. 2015;1434:102–110. doi: 10.1016/j.chroma.2016.01.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.