Abstract
Background
The use of antibodies, such as immunoglobulin G (IgG), has faced a significant growth in the past decades for biomedical and research purposes. However, antibodies are high cost biopharmaceuticals, for which the development of alternative and cost-effective purification strategies is still in high demand.
Results
Aqueous biphasic systems (ABS) composed of poly(propylene glycol) (PPG) and cholinium-based ionic liquids (ILs) were investigated for the separation of IgG. The ABS phase diagrams were determined and characterized whenever required. Initial optimization studies with commercial IgG were carried out, followed by the extraction of IgG from rabbit serum. In all ABS, IgG preferentially partitions to the IL-rich phase, unveiling preferential interactions between IgG and ILs. Good results were obtained with commercial IgG, with extraction efficiencies ranging between 93% and 100%, and recovery yields ranging between 20% and 100%. Two of the best and two of the worst identified ABS were then evaluated in what concerns their performance to separate and recover IgG from rabbit serum. With these ABS, extraction efficiencies of 100% and recovery yields > 80% were obtained, indicating an increase in the recovery yield and extraction efficiencies when using real matrices. Under the best conditions studied, IgG with a purity level of 49% was obtained in a single-step. This purity level of IgG is higher than those previously reported using other IL-polymer ABS.
Conclusion
IgG preferentially migrates to the IL-rich phase in ABS formed by ILs and polymers, allowing the design of effective separation systems for its recovery from serum samples.
Keywords: Aqueous two-phase systems, bioseparations, downstream, liquid-liquid extraction, pharmaceuticals
Introduction
In the past years we have been facing a high demand for proteins present in the blood of mammals, specifically for immunoglobulin G (IgG) due to its potential as an alternative biopharmaceutical. Antibodies, also known as immunoglobulins, are large proteins secreted by cells of the immune system. Their function is to identify and eliminate pathogens, such as viruses, bacteria or fungi, through a recognition mechanism of high specificity. The growing interest on IgG is a result of the expanding number of biomedical applications in which this plasma-derived protein can be used.1 IgG is currently administered to prevent infections in patients with immune deficiencies,2,3 providing passive immune protection, and in the treatment of inflammatory and autoimmune diseases.4,5
Commercially available serum antibodies are typically obtained through chromatographic techniques, following a series of steps for the removal of impurities.6–8 These methods yield high purity antibodies but result in highly expensive products, limiting therefore their widespread use as recurrent biopharmaceuticals. There is thus an urge for the development of competitive cost-effective purification methods. Amongst the several possibilities, aqueous biphasic systems (ABS), also known as aqueous two-phase systems (ATPS), emerge as a possible alternative for the extraction and purification of IgG. ABS were first proposed as an alternative protein extraction technique in 1958 by P. A. Albertsson.9 They are composed of two water-soluble solutes, which above a given concentration form two immiscible aqueous-rich phases, each enriched in one of the phase-forming components. Given their high water content, ABS may be seen as a biocompatible media for the extraction and purification of a wide range of biomolecules.9,10 Furthermore, being liquid-liquid systems, ABS enable the combination of the clarification, concentration and purification steps in a single stage.11 Most works dealing with ABS and IgG have been focused on its recovery from cell culture media.12–15 However, Vargas et al.16 proposed the use of ABS for the purification of IgG from human plasma. Studies on the partitioning of pure IgG, obtained from human serum, have been also reported,17,18 as well as on the purification of IgG from transgenic plants.19
The combinations of solutes typically used for ABS formation are polymer-polymer, polymer-salt and salt-salt.20 Initial studies were chiefly focused on polymer-based ABS, consisting of two polymers or a polymer and a salting-out inducing salt in aqueous media.20 Nevertheless, this type of ABS has some downsides. Most polymer-based ABS display high viscosity and are associated to a slow phase separation.21 Moreover, when two polymers are used to create ABS, both phases display similar properties, such as hydrophobicity or polarity. Thus, polarity differences between the coexisting phases in polymer-based ABS display a limited range,21 accounting for their reduced applicability and lack of selectivity for separation purposes. To overcome this limitation, the use of salts as adjuvants and the addition of affinity ligands to polymer-based ABS have been proposed.22,23
In the past decade, a new type of ABS composed of ionic liquids (ILs) and inorganic salts in aqueous media was proposed.24 ILs are organic salts with low melting temperatures.25 IL-based ABS display a reduced viscosity and a fast phases separation rate, representing thus potential alternatives to polymer-rich systems.26,27 ILs have also been proposed as greener alternatives to volatile organic solvents commonly applied in liquid-liquid separations, mainly due to their non-flammability and negligible vapor pressure at atmospheric conditions,28 although these features may also be displayed by polymers and salts. The main advantage of IL-based ABS is the possibility to tailor the phases’ polarities and affinities, leading to improvements in selectivity, product solubility and extraction efficiency.29–36 In addition to the widely studied salt-IL ABS, more recently, ILs have been proposed as alternatives to common salts, leading to the creation of IL-polymer ABS.37–40 These systems were suggested as improved media to separate proteins, since these can bind to the metal ions of the salts commonly used and thus affect the proteins stability.41 When dealing with antibodies with potential to be applied as biopharmaceuticals, it is of significant relevance to preserve their native structure during the extraction and purification procedure, aiming at maintaining their function and activity. Shifts in the medium composition, pH and temperature can also cause modifications on the three-dimensional structure of proteins.42 Most IL-polymer ABS previously reported are formed by imidazolium-based ILs,20 which may present some degree of toxicity.43 To overcome this drawback, cholinium-based ILs were recently proposed to create IL-polymer ABS, and were shown to provide high extraction efficiencies for biologically active molecules.39,44,45
Based on the potential of IL-polymer ABS formed by cholinium-based ILs, in this work, their performance for the extraction and purification of IgG from rabbit serum was evaluated. ABS composed of cholinium-based ILs and the polymer polypropylene glycol with an average molecular weight of 400 g·mol-1 (PPG 400) were investigated. Some of the systems tested herein were previously investigated for the extraction of BSA (Bovine Serum Albumin).39 The remaining ABS are composed of a set of cholinium-based ILs with antioxidant activities,46 and for which novel ternary phase diagrams have been determined in this work. These ILs not only display higher antioxidant activity than the respective precursors, but also higher solubility in water, higher anti-inflammatory activity and thermal stability, and lower toxicity.46
Materials and Methods
Materials
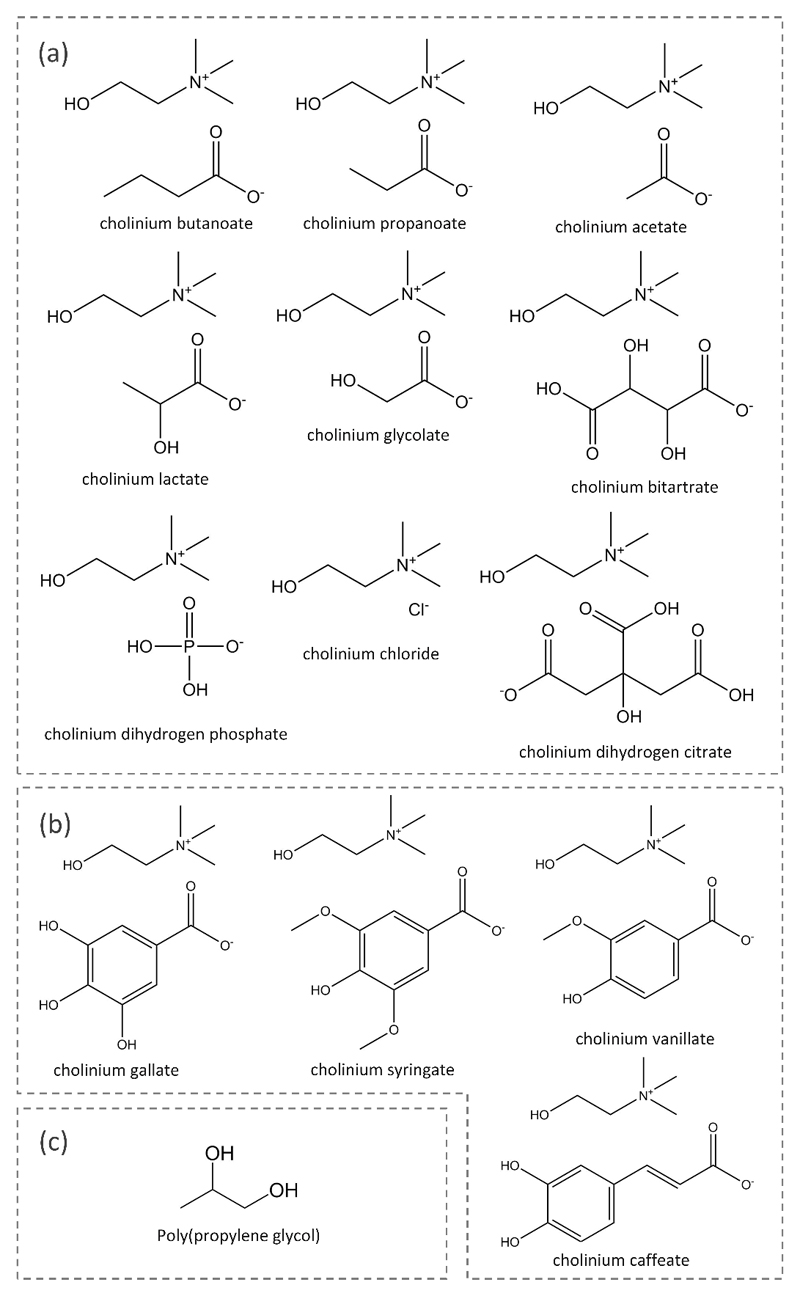
The cholinium-based ILs used for the preparation of ABS for the IgG extraction assays were cholinium chloride ([Chol]Cl, 98 wt% pure) and cholinium bitartrate ([Chol][Bit], 97 wt% pure), both from Acros Organics, cholinium dihydrogen citrate ([Chol][DHCit], 99 wt% pure) from Sigma, and cholinium dihydrogen phosphate ([Chol][DHPh], > 98 wt% pure) and cholinium acetate ([Chol][Ac], 98 wt% pure) from Iolitec. Cholinium lactate ([Chol][Lac]), cholinium glycolate ([Chol][Gly]), cholinium propanoate ([Chol][Prop]), and cholinium butanoate ([Chol][But]) were synthesized in our laboratory following previously described protocols.39,47 The antioxidant cholinium-based ILs cholinium caffeate ([Chol][Caf]), cholinium syringate ([Chol][Syr]), cholinium vanillate ([Chol][Van]), and cholinium gallate ([Chol][Gal]) were also synthesized in our laboratory, as described in a previous work.46 All the cholinium-based ILs synthetized by us showed purity values > 97 wt%, which were confirmed by 1H and 13C NMR. The polymer used was polypropylene glycol with an average molecular weight of 400 g·mol-1 (PPG 400), acquired from Aldrich. The chemical structures of the ILs investigated and PPG 400 are depicted in Figure 1.
Figure 1.
Chemical structure of the compounds used in this work. (a) cholinium-based ILs; (b) antioxidant cholinium-based ILs; (c) polymer.
For the preparation of IgG aqueous solutions, a lyophilized powder of IgG obtained from rabbit serum (reagent grade, ≥ 95%) was used, purchased from Sigma-Aldrich and kept in storage at -20 ºC. The solutions of IgG were prepared in a buffer solution composed of phosphate salts (PBS, pH = 7.4) pellets acquired from Sigma. The rabbit serum used was purchased from Sigma Aldrich, kept at -20 ºC up to use.
Determination of the ABS phase diagrams
Some ternary phase diagrams of the ABS tested for extraction purposes were determined in a previous work,39 while others were determined in this work, particularly for the systems formed by [Chol][Caf], [Chol][Syr], [Chol][Van] and [Chol][Gal] + PPG 400 + water. These were determined through the cloud point titration method at (25 ± 1) °C and atmospheric pressure. Aqueous solutions of PPG 400 at ≈ 90 wt% and aqueous solutions of the ILs with concentrations varying between 45 wt% and 70 wt% were prepared gravimetrically (± 10-4 g). The repetitive drop-wise addition of the aqueous solution of IL to the PPG-water mixture was carried out until a cloudy biphasic mixture was discerned. Subsequently, double distilled water was added drop-wise until the mixture became limpid, entering the monophasic region. This procedure was repeatedly performed under constant stirring, until no more cloud points were observed. The reverse method, consisting of the addition of the PPG 400 aqueous solution to the IL mixture was also performed to obtain more solubility points which could better describe the binodal curve. The composition of the systems after the addition of each component was determined by weight quantification (± 10-4 g). The experimental binodal curves were fitted by Eq. (1):
| (1) |
where [PPG] and [IL] correspond to the weight fraction percentages of PPG 400 and IL, respectively, while A, B and C are the fitting parameters. The tie-lines (TLs) of each phase diagram, i.e. the compositions of each phase for a common mixture composition, as well as the tie-line lengths (TLLs), were determined according to the method reported by Merchuk et al.,48 and used elsewhere to describe polymer-IL ABS.39
Extraction and separation of IgG using ABS
After gathering the knowledge on the mixture compositions required to form two-phase systems by the determination of the ABS phase diagrams, a mixture point which guarantees that all systems are within the biphasic region was selected for the commercial IgG partition assays. The overall mixture composition used was: 45 wt% of PPG 400, 25 wt% of IL and 30 wt% of a buffered PBS (pH = 7.4) aqueous solution containing IgG at 1 g·L-1. At least three individual experiments were prepared for each system. Each ABS was prepared by weighting the appropriate amount of each component, within ± 10-4 g, and mixed until all components were dissolved. All systems were centrifuged for 10 min at 1000 rpm and left to equilibrate for further 10 min at (25 ± 1) °C to promote phase separation and IgG partitioning between phases. 100 µL were collected from both the top and bottom phases of each ABS and diluted in a phosphate buffer solution (1:10 (v:v), used as the mobile phase for quantification. The IgG content in each phase was determined by size exclusion high-performance liquid chromatography (SE-HPLC). The equipment used was a Chromaster HPLC system (VWR Hitachi) equipped with a binary pump, column oven, temperature controlled auto-sampler, DAD detector and an analytical column Shodex Protein KW- 802.5 (8 mm × 300 mm). A 100 mM phosphate buffer pH 7.0 with NaCl 0.3 M was run isocratically with a flow rate of 0.5 mL.min-1 and the injection volume was of 25 µL. The wavelength was set at 280 nm. The calibration curve was established with commercial IgG from rabbit serum.
The ABS performance to extract and recover IgG to the IL-rich phase was evaluated by their extraction efficiency (EEIgG%) and yield (YIgG%), according to the following equations:
| (2) |
| (3) |
where and represent the weight of IgG in the IL-rich phase, in the polymer-rich phase, and in the initial solution, respectively.
After the identification of favorable systems for the IgG extraction to the IL-rich phase, new experiments were performed for the extraction and purification of IgG, directly from rabbit serum. The ABS chosen are composed of 45 wt% of PPG 400, 25 wt% of IL and 30 wt% of rabbit serum diluted at 1:50 (v:v) in a PBS aqueous solution, with the ILs [Chol][DHPh], [Chol][Lac], [Chol][Van] and [Chol][Gly]. Each mixture composition was weighted and mixed, centrifuged for 10 min at 1000 rpm, and left to equilibrate for 10 min at (25 ± 1) °C. Then, 100 µL of each phase were collected and diluted (1:10 (v:v)) in the mobile phase used for the analysis by SE-HPLC, as described before. In the systems containing [Chol][DHPh] and [Chol][Lac] a large amount of proteins precipitated at the interface was observed. In both cases the systems were centrifuged for 20 min at 3500 rpm, left to equilibrate under the same conditions, and the precipitate collected and diluted in 600 µL of the PBS aqueous solution for further analysis. All assays were performed at least in triplicate. The percentage purity of IgG was calculated dividing the HPLC peak area of IgG by the total area of the peaks corresponding to all proteins present at the IL-rich phase.
Results and Discussion
ABS Ternary Phase diagrams
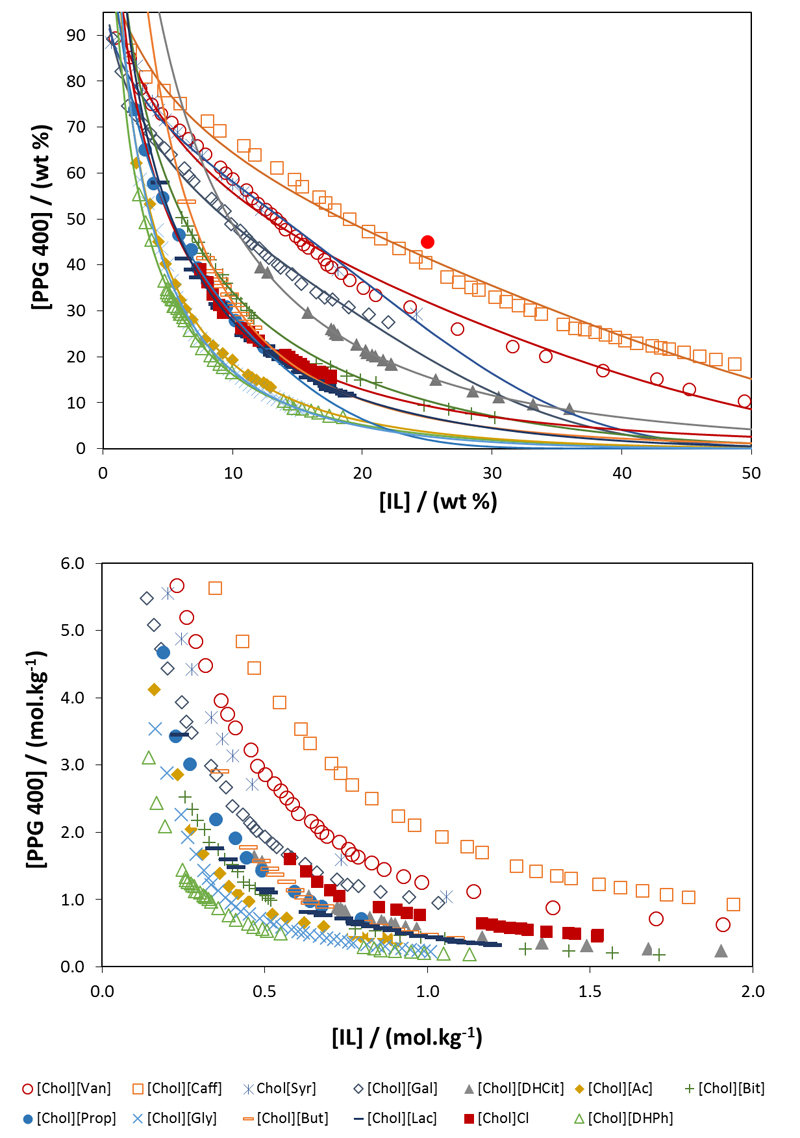
To identify the mixture compositions that can be used in the separation of IgG from rabbit serum, the respective ABS ternary phase diagrams were determined whenever required at 25 °C and atmospheric pressure. Some phase diagrams were taken from the literature,39 while those for the systems composed of the cholinium-based ILs with antioxidant properties were determined in this work, namely for [Chol][Caf], [Chol][Syr], [Chol][Van] and [Chol][Gal]. In all studied ABS the bottom phase corresponds to the IL-rich phase, while the top phase is mainly composed of PPG 400 and water. The respective phase diagrams are depicted in Figure 2, both in weight fraction and in molality units (mol·kg-1, moles of PPG or IL per kg of IL + water or PPG + water). The detailed experimental weight fraction data are given in Tables S1 and S2 in the Supporting Information. The regression parameters (A, B and C) were obtained through the least squares regression method using Eq. (1).48 Their values and the corresponding standard deviations, as well the experimental TLs and TLLs, are provided in Tables S3 and S4 in the Supporting Information.
Figure 2.
Ternary phase diagrams of ABS composed of cholinium-based ILs + PPG 400 + H2O. The ABS formed by [Chol][DHPh], [Chol][Gly], [Chol][Ac], [Chol][Lac], [Chol]Cl, [Chol][Prop], [Chol][But], [Chol][Bit] and [Chol][DHCit] have been previously reported in the literature.39 The lines correspond to the fitting of the experimental data using Eq. (1). The mixture composition used in the extraction experiments is represented by ●.
Above each binodal curve, all mixtures are biphasic. Hence, in systems with larger biphasic regions, the ability of the IL to promote liquid-liquid demixing is higher. The cholinium cation is common for all ILs; therefore, the differences observed in the phase diagrams result mainly from the IL anion nature. Contrarily to salt-based ABS, where the liquid-liquid demixing is driven by a salting-out effect of the high charge density ions over ILs,20 in IL-polymer ABS the phenomenon is more complex since the driving mechanism for the phase separation is mainly ruled by the strength of the interactions occurring between the three system components: PPG 400, IL and water.49 It was previously demonstrated that there is a competition between PPG 400 and the IL ions for water molecules, and also between themselves, where the IL anion is more prone to form hydration complexes than the corresponding IL cation.49
According to the phase diagrams depicted in Figure 2, the ability of an IL to form an ABS at a fixed molality of IL, e.g. at 0.6 mol.kg-1, decreases as follows: [Chol][DHPh] > [Chol][Gly] > [Chol][Ac] > [Chol][Bit] > [Chol][Lac] > [Chol][Prop] ≈ [Chol][DHCit] ≈ [Chol][But] > [Chol]Cl > [Chol][Gal] > [Chol][Syr] > [Chol][Van] > [Chol][Caf]. [Chol][DHPh] is the strongest IL in terms of liquid-liquid demixing, requiring thus lower amounts of IL or PPG to form ABS. In general, ILs with shorter aliphatic moieties are more prone to form ABS, as verified, for instance, with the series of ILs derived from aliphatic carboxylic acids ([Chol][Ac] > [Chol][Prop] ≈ [Chol][But]). Furthermore, the addition of hydroxyl groups to the ILs promotes phase separation ([Chol][Gly] > [Chol][Ac] and [Chol][Lac] > [Chol][Prop]). The correlation between the chemical structure of each IL and their relative tendency to promote phase demixing has been discussed in detail by Quental et al.39 These systems display larger biphasic regions when compared to the new systems composed of the antioxidant ILs herein characterized, namely [Chol][Gal], [Chol][Syr], [Chol][Van] and [Chol][Caf], which follow the trend of the octanol-water partition coefficients (Kow) of the corresponding phenolic acids.50 The higher the Kow of the phenolic acids the more difficult it is to create ABS with PPG 400. In general, the size of the biphasic regions increases with the increase in the hydrophilicity of the cholinium-based ILs.
Extraction and separation of IgG using ABS
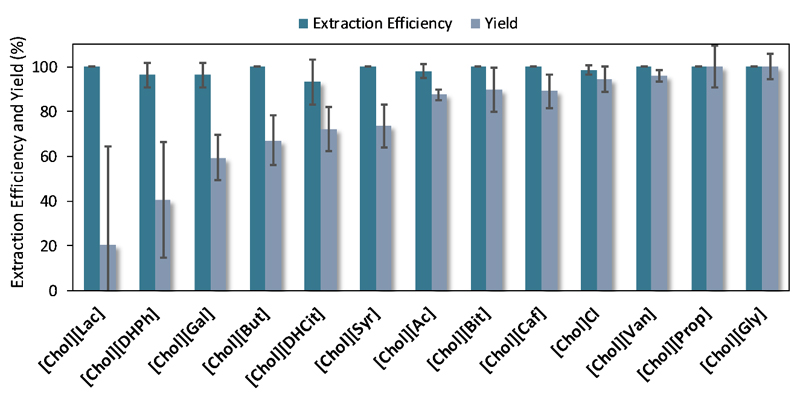
All ABS herein studied were evaluated in terms of their performance to extract and recover IgG to the IL-rich phase in a single-step. ABS constituted by the ILs [Chol][Bit], [Chol][Lac], [Chol][DHCit], [Chol][DHPh], [Chol]Cl, [Chol][Prop], [Ch][Gly], [Chol][Ac], [Chol][Caf], [Chol][Syr], [Chol][Van], [Chol][Gal], and [Chol][But] at 25 wt%, 45 wt% of PPG and 30 wt% of an aqueous solution of PBS containing IgG at 1 g·L-1 were prepared. The common mixture point used is depicted in Figure 2, along with the phase diagrams for all the ABS tested. The TLs, which correspond to the composition of each phase at the common mixture point, are provided in Table S4 in the Supporting Information. The extraction efficiencies and yields for IgG are present in Figure 3; cf. detailed data in Table S5 in the Supporting Information.
Figure 3.
Average extraction efficiencies (EEIgG %) and yields (YIgG %) of IgG to the IL-rich phase in the ABS composed of 25 wt% of IL + 45 wt% of PPG 400 + 30 wt% of a buffered aqueous solution containing pure IgG at 1 g·L-1.
Under the chromatographic conditions used, the retention time of IgG was found to be ≈ 15.6 min, within an analysis time of 45 min. It should be noted that some minor peaks are detected at lower retention times, even for the pure, commercial IgG, indicating the presence of few IgG aggregates. Figure S1 in the Supporting Information shows some examples of chromatograms corresponding to commercial IgG in the PBS aqueous solution and to the bottom phase of one of the ABS tested.
In all ABS, the antibody preferentially migrates to the more hydrophilic IL-rich phase, which corresponds to the bottom phase. In fact, in the SE-HPLC chromatograms of most systems, no IgG at the top (polymer-rich) phase was identified. The extraction efficiencies of all the investigated ABS to the IL-rich phase are higher than 93%, denoting the preference of IgG towards the IL-rich phase. Notably, all the new ABS containing the antioxidant cholinium-based ILs display high extraction efficiencies (> 96% up to complete extraction in a single-step), meaning that IgG has a high affinity for these ILs. All the antioxidant cholinium-based ILs display an aromatic group and several hydroxyl groups, which seem to be beneficial to the extraction of IgG. Other ILs allowed extraction efficiencies of 100%, namely [Chol][Lac], [Chol][But], [Chol][Bit], [Chol][Prop] and [Chol][Gly]. The ABS investigated are formed by ILs and PPG and thus no significant salting-out effects dominate the exclusion of IgG to the IL-rich phase. Therefore, it seems that the IgG partitioning is dominated by specific interactions established between the IL and the amino acids at the antibody surface.
Conventional polymer-based ABS formed by polyethylene glycol (PEG) of high molecular weight and phosphate-based salts have been used for the purification of IgG from the supernatants of Chinese Hamster Ovary (CHO) and hybridoma cell cultures.13,14 The systems investigated led to extraction efficiencies to the polymer-rich phase of 90%.13,14 Therefore, the IL-based ABS tested in the present work display higher extraction efficiencies, in addition to making use of polymers of lower molecular weight, with subsequent advantages in terms of viscosity, mass transfer, and phase demixing rate. An additional difference is the preferential migration of IgG to the polymer-rich phase observed in the literature,13,14 where salting-out effects seem to dominate the partition, in contrast to what is observed in the systems here investigated. As a way of tailoring and improving the partition of IgG for a given phase, ILs were also recently used as adjuvants in PEG-salt-based ABS.22 The use of ILs allowed the achievement of extraction efficiencies of 100% to the PEG-rich phase (the phase to which the IL preferentially partitions) in most systems investigated.22 Specific interactions between ILs and IgG were previously proposed,22 corroborating the preferential partitioning of the antibody to the IL-rich phase in the ABS studied here.
Although the ABS extraction efficiencies achieved in a single-step for IgG are > 93%, a significant effect of the IL chemical structure was observed in the recovery yields. The IgG recovery yields are depicted in Figure 3, and range from 20% to 100%. Detailed data are given in Table S5 in the Supporting Information. The ABS formed by [Chol][Gly] and [Chol][Prop] do not lead to any losses of the protein, with recovery yields of IgG at the IL-rich phase of 100%. [Chol][Van] and [Chol]Cl, although with a small loss of IgG, also lead to high recovery yields (> 94%). Since these ABS also present high extraction efficiencies (ca. 100% in a single-step), these ILs may be considered as promising phase-forming components of ABS for the purification of IgG from real matrices. ABS formed by [Chol][Ac], [Chol][Caf] and [Chol][Bit] show yields ranging from 87% to 90%. On the other hand, ABS constituted by [Chol][Lac] and [Chol][DHPh] provide the lowest recovery yields of IgG (20% and 41%, respectively). These ABS lead to the precipitation of IgG at the interface as confirmed by SE-HPLC, showing that the low recovery yield is due to the antibody precipitation.
The partitioning of proteins in ABS is driven by several interactions established between the protein surface and the phase-forming components, where steric effects may also be involved.39 These interactions comprise dispersive forces, hydrogen-bonding and electrostatic interactions.39 According to the recovery yields obtained, it seems that the IL anion has a significant influence on the IgG partitioning and on keeping its native structure. The isoelectric point of IgG is 8.6;51 therefore, the overall charge of the protein in the studied systems at pH 7.4 is positive and may justify the impact exerted by the IL anion. However, a study involving IgG using IL-salt systems reported the preferential migration of the antibody to the IL-rich phase,52 as observed in this work with polymer-IL systems, meaning that electrostatic interactions between the IL anion and the positively charged protein do not play a major role. These results support the idea that hydrogen-bonding and dispersive forces play a more significant role.
In the literature, polymer-based ABS have been described to allow recovery yields of 89% for IgG from the supernatant of CHO cell cultures.14 In a similar work, IgG from the supernatant of CHO and hybridoma cell cultures was extracted with recovery yields of 88% and 90%, respectively.13 More recently, polymer-polymer ABS were developed and tested for the purification of IgG from an hybridoma cell culture, with a recovery yield of 84%.53 Dhadge et al.54 obtained a 92% recovery yield of IgG from cell culture supernatants, using PEG-dextran ABS combined with magnetic nanoparticles. PEG-salt-based ABS using ILs as adjuvants provide recovery yields around 50%.22 Considering the results found in the literature, it can be concluded that some of the systems tested here have a great potential to extract IgG and to be exploited as separation systems of IgG from real matrices.
Two of the most promising ABS, with high extraction efficiencies and recovery yields, namely those formed by [Chol][Gly] and [Chol][Van], were then subjected to further tests regarding the extraction of IgG from rabbit serum. In addition to these, ABS formed by [Chol][Lac] and [Chol][DHPh], which correspond to the systems with the lower recovery yields and formation of large amounts of precipitate, were also investigated. The formation of an interface of precipitated proteins in these ABS could be a potential strategy to purify IgG from complex matrices within a three-phase partitioning (TPP) approach, where the target compound is enriched at the interface of two liquid phases.55 The t-butanol and ammonium sulfate aqueous solutions are often used to induce the precipitation of biomolecules at the interface,55 and recently, Alvarez et al.56,57 showed that this approach is also feasible with ILs – ionic-liquid-based three-phase partitioning (ILTPP).56,57
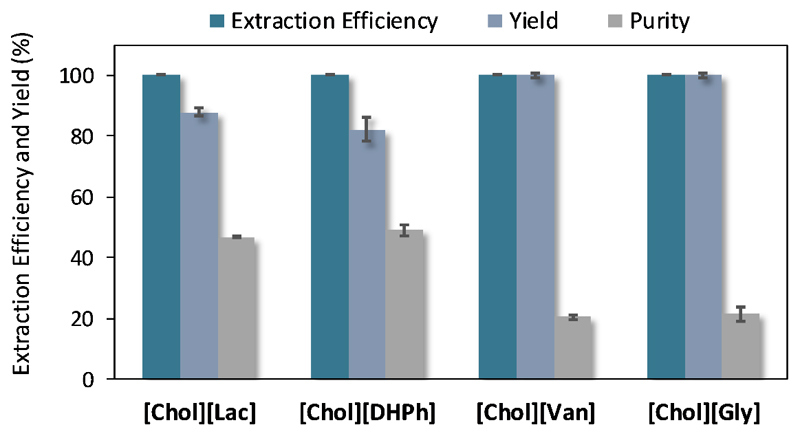
The extraction efficiencies, yields and IgG purity at the IL-rich phase, where ABS were applied to separate IgG from rabbit serum samples, are displayed in Figure 4. Detailed data are given in Table S6 in the Supporting Information. In these assays, a peak corresponding to IgG was observed at a retention time of ≈ 15.6 min, which is in agreement with the experiments using commercial IgG. As observed with pure IgG, additional peaks at lower retention times were identified in the SE-HPLC chromatograms, which correspond to protein aggregates. In the assays carried out with serum a larger peak at ≈ 16.8 min is identified, corresponding to albumin, the main serum protein. Examples of the chromatograms corresponding to rabbit serum and to the bottom phase of ABS are given in Figure S2 in the Supporting Information.
Figure 4.
Average extraction efficiencies (EEIgG %), recovery yield (YIgG %) and purity (%) of IgG at the IL-rich phase in ABS composed of 25 wt% of IL + 45 wt% of PPG 400 + 30 wt% of a buffered aqueous solution of rabbit serum (diluted at 1:50 (v:v)).
For the ABS containing [Chol][Gly] and [Chol][Van], excellent results were obtained. In both cases, the extraction efficiency and recovery yield were maintained at 100%, meaning that no losses of the target protein occurred. The IgG is completely extracted to the IL-rich phase in a single-step, even in the presence of a more complex, real matrix. This behavior is in agreement with the results obtained with pure IgG, and further indicates that the remaining proteins and other compounds present in the serum have no significant influence on the partition of IgG. These systems increase the purity of IgG from 18.1% (in the original rabbit serum) to 21-22% at the IL-rich phase. However, this is not a significant increment in the purity of IgG, and is mainly due to the large amount of albumin that migrates to the bottom phase along with IgG – cf. the chromatograms shown in Figure S2 in the Supporting Information. Nevertheless, while absolutely no IgG is detected in the top phase, small peaks corresponding to albumin and to the protein aggregates are observed in this phase, justifying the small improvement in the IgG purity.
Similarly to what occurred in the assays with pure IgG, in the assays with rabbit serum the antibody is present at the IL-rich phase and absent at the polymer-rich phase of the ABS formed by [Chol][Lac] and [Chol][DHPh], with extraction efficiencies of 100%. Surprisingly, and in contrast with the studies with pure IgG, a significant increment on the IgG recovery yield of the systems formed by [Chol][Lac] (88% vs. 20%) and [Chol][DHPh] (82% vs. 41%) when using rabbit serum was observed. Furthermore, these systems also lead to higher purity levels of IgG at the IL-rich phase (47% purity with [Chol][Lac]; 49% purity with [Chol][DHPh]) than those composed of [Chol][Gly] and [Chol][Van]. In order to investigate the presence of IgG in the precipitate as well as the possibility of using these systems as an ILTPP approach, the precipitated proteins were recovered and examined by SE-HPLC. Most of the protein found at the precipitate is albumin, leading thus to significant improvements in the IgG purity at the IL-rich phase – cf. Figure S3 in the Supporting Information. Although a significant amount of albumin is still present at the IL-rich phase, these systems allow a more significant enhancement in the purity of IgG at the IL-rich phase. Although the ILTPP approach does not induce the recovery of IgG at the interface, it however allows the precipitation of the major contaminant protein from serum (albumin), leading to an increase in the IgG purity at the IL-rich phase. Mondal et al.58 previously applied PPG-IL ABS for the separation of IgG from serum samples. Albeit high recovery yields were reported, the purification factors were considerably lower than those obtained in this work (< 30%).58 Further optimization studies aiming at both the complete partitioning of IgG to the IL-rich phase and the precipitation of albumin at the interface may be thus envisioned with IL-based ABS to confirm their potential as alternative purification systems for antibodies.
Conclusion
ABS formed by cholinium-based ILs and PPG 400 were evaluated as alternative systems for the separation of IgG. Novel ABS phase diagrams were determined at 25 °C to infer the mixture compositions required to form liquid-liquid systems. Initial assays with pure IgG showed the preferential partitioning of IgG to the IL-rich phase, with extraction efficiencies ranging between 93% and 100%, and recovery yields ranging between 20% and 100%. [Chol][Gly] and [Chol][Prop] allow recovery yields of 100%, while [Chol][Van], [Chol][Caf] and [Chol]Cl allow recovery yields higher than 89%, which are quite promising when compared to literature values using conventional polymer-salt and polymer-polymer ABS. On the other hand, ABS formed by the remaining ILs lead to lower recovery yields, with higher protein losses and precipitated protein, observed in particular in the ABS constituted by [Chol][Lac] and [Chol][DHPh].
Two of the most promising ABS, formed by [Chol][Gly] and [Chol][Van], were then tested for the purification of IgG from rabbit serum samples. The ABS comprising [Chol][Lac] and [Chol][DHPh] were tested as well. Good results were obtained with the ABS containing [Chol][Gly] and [Chol][Van], with extraction efficiencies and recovery yields kept at 100%, even for a real matrix. These systems do not allow, however, a significant increase in the purity of IgG. In the ABS formed by [Chol][Lac] and [Chol][DHPh], extraction efficiencies for IgG of 100% were obtained. However, and in contrast with the studies with pure IgG, a significant increment on the IgG recovery yield when using rabbit serum was observed. These systems lead to purity levels of IgG at the IL-rich phase of 47% for [Chol][Lac] and 49% for [Chol][DHPh] in a single-step, which is mainly due to the precipitation of the major serum protein, albumin. The purification factors here obtained are significantly higher than those previously reported using IL-polymer ABS. These systems have thus potential for the purification of IgG, and should be further investigated for the extraction and purification of other proteins.
Supplementary Material
Acknowledgements
This work was developed in the scope of the project CICECO-Aveiro Institute of Materials (Ref. FCT UID/CTM/50011/2013), financed by national funds through the FCT/MEC and co-financed by FEDER under the PT2020 Partnership Agreement. C. M. S. S. Neves and M. V. Quental acknowledge FCT for the postdoctoral (SFRH/BPD/109057/2015) and PhD (SFRH/BD/100155/2014) grants, respectively. M. G. Freire acknowledges the European Research Council (ERC) for the Starting Grant ECR-2013-StG-337753.
References
- 1.Buchacher A, Iberer G. Purification of intravenous immunoglobulin G from human plasma--aspects of yield and virus safety. Biotechnol J. 2006;1:148–163. doi: 10.1002/biot.200500037. [DOI] [PubMed] [Google Scholar]
- 2.Stiehm ER, Keller MA, Vyas GN. Preparation and use of therapeutic antibodies primarily of human origin. Biologicals. 2008;36:363–374. doi: 10.1016/j.biologicals.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Zeitlin L, Cone RA, Moench TR, Whaley KJ. Preventing infectious disease with passive immunization. Microbes Infect. 2000;2:701–708. doi: 10.1016/s1286-4579(00)00355-5. [DOI] [PubMed] [Google Scholar]
- 4.Lemieux R, Bazin R, Néron S. Therapeutic intravenous immunoglobulins. Mol Immunol. 2005;42:839–848. doi: 10.1016/j.molimm.2004.07.046. [DOI] [PubMed] [Google Scholar]
- 5.Kivity S, Katz U, Daniel N, Nussinovitch U, Papageorgiou N, Shoenfeld Y. Evidence for the Use of Intravenous Immunoglobulins—A Review of the Literature. Clin Rev Allergy Immunol. 2009;38:201–269. doi: 10.1007/s12016-009-8155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoppe HH, Mester T, Hennig W, Krebs HJ. Prevention of Rh-immunization. Modified production of IgG anti-Rh for intravenous application by ion exchange chromatography (IEC) Vox Sang. 1973;25:308–316. doi: 10.1111/j.1423-0410.1973.tb04378.x. [DOI] [PubMed] [Google Scholar]
- 7.Friesen AD, Bowman JM, Bees WC. Column ion exchange chromatographic production of human immune serum globulin for intravenous use. Vox Sang. 1985;48:201–212. doi: 10.1111/j.1423-0410.1985.tb00173.x. [DOI] [PubMed] [Google Scholar]
- 8.Teng SF, Sproule K, Husain A, Lowe CR. Affinity chromatography on immobilized ‘biomimetic’ ligands: Synthesis, immobilization and chromatographic assessment of an immunoglobulin G-binding ligand. J Chromatogr B Biomed Sci App. 2000;740:1–15. [PubMed] [Google Scholar]
- 9.Albertsson PÅ. Partition of Proteins in Liquid Polymer–Polymer Two-Phase Systems. Nature. 1958;182:709–711. doi: 10.1038/182709a0. [DOI] [PubMed] [Google Scholar]
- 10.Gündüz U. Optimization of bovine serum albumin partition coefficient in aqueous two-phase systems. Bioseparation. 2000;9:277–281. doi: 10.1023/a:1011194324047. [DOI] [PubMed] [Google Scholar]
- 11.Asenjo JA, Andrews BA. Aqueous two-phase systems for protein separation: A perspective. J Chromatogr A. 2011;1218:8826–8835. doi: 10.1016/j.chroma.2011.06.051. [DOI] [PubMed] [Google Scholar]
- 12.Andrews BA, Nielsen S, Asenjo JA. Partitioning and purification of monoclonal antibodies in aqueous two-phase systems. Bioseparation. 1996;6:303–313. [PubMed] [Google Scholar]
- 13.Azevedo AM, Rosa PAJ, Ferreira IF, Aires-Barros MR. Optimisation of aqueous two-phase extraction of human antibodies. J Biotechnol. 2007;132:209–217. doi: 10.1016/j.jbiotec.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Rosa PAJ, Azevedo AM, Sommerfeld S, Mutter M, Aires-Barros MR, Bäcker W. Application of aqueous two-phase systems to antibody purification: A multi-stage approach. J Biotechnol. 2009;139:306–313. doi: 10.1016/j.jbiotec.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Espitia-Saloma E, Vâzquez-Villegas P, Rito-Palomares M, Aguilar O. An integrated practical implementation of continuous aqueous two-phase systems for the recovery of human IgG: From the microdevice to a multistage bench-scale mixer-settler device. Biotechnol J. 2016;11:708–716. doi: 10.1002/biot.201400565. [DOI] [PubMed] [Google Scholar]
- 16.Vargas M, Segura A, Herrera M, Villalta M, Angulo Y, Gutiérrez JM, León G, Burnouf T. Purification of IgG and albumin from human plasma by aqueous two phase system fractionation. Biotechnol Prog. 2012;28:1005–1011. doi: 10.1002/btpr.1565. [DOI] [PubMed] [Google Scholar]
- 17.Wu Q, Lin DQ, Yao SJ. Evaluation of poly(ethylene glycol)/hydroxypropyl starch aqueous two-phase system for immunoglobulin G extraction. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;928:106–112. doi: 10.1016/j.jchromb.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Chow YH, Yap YJ, Show PL, Juan JC, Anuar MS, Ng EP, Ooi CW, Ling TC. Characterization of partitioning behaviors of immunoglobulin G in polymer-salt aqueous two-phase systems. J Biosci Bioeng. 2016;122:613–619. doi: 10.1016/j.jbiosc.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Lee JW, Forciniti D. Purification of human antibodies from transgenic corn using aqueous two-phase systems. Biotechnol Prog. 2010;26:159–167. doi: 10.1002/btpr.287. [DOI] [PubMed] [Google Scholar]
- 20.Freire MG, Cláudio AFM, Araújo JMM, Coutinho JAP, Marrucho IM, Canongia Lopes JN, Rebelo LPN. Aqueous biphasic systems: a boost brought about by using ionic liquids. Chem Soc Rev. 2012;41:4966–4995. doi: 10.1039/c2cs35151j. [DOI] [PubMed] [Google Scholar]
- 21.Pereira JFB, Rebelo LPN, Rogers RD, Coutinho JAP, Freire MG. Combining ionic liquids and polyethylene glycols to boost the hydrophobic–hydrophilic range of aqueous biphasic systems. Phys Chem Chem Phys. 2013;15:19580–19583. doi: 10.1039/c3cp53701c. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira AM, Faustino VFM, Mondal D, Coutinho JAP, Freire MG. Improving the extraction and purification of immunoglobulin G by the use of ionic liquids as adjuvants in aqueous biphasic systems. J Biotechnol. 2016;236:166–175. doi: 10.1016/j.jbiotec.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruiz-Ruiz F, Benavides J, Aguilar O, Rito-Palomares M. Aqueous two-phase affinity partitioning systems: current applications and trends. J Chromatogr A. 2012;1244:1–13. doi: 10.1016/j.chroma.2012.04.077. [DOI] [PubMed] [Google Scholar]
- 24.Gutowski KE, Broker GA, Willauer HD, Huddleston JG, Swatloski RP, Holbrey JD, Rogers RD. Controlling the aqueous miscibility of ionic liquids: aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations. J Am Chem Soc. 2003;125:6632–6633. doi: 10.1021/ja0351802. [DOI] [PubMed] [Google Scholar]
- 25.Wilkes JS. A short history of ionic liquids—from molten salts to neoteric solvents. Green Chem. 2002;4:73–80. [Google Scholar]
- 26.Cláudio AFM, Ferreira AM, Shahriari S, Freire MG, Coutinho JAP. Critical assessment of the formation of ionic-liquid-based aqueous two-phase systems in acidic media. J Phys Chem B. 2011;115:11145–11153. doi: 10.1021/jp204865a. [DOI] [PubMed] [Google Scholar]
- 27.Freire MG, Neves CMSS, Canongia Lopes JN, Marrucho IM, Coutinho JAP, Rebelo LPN. Impact of self-aggregation on the formation of ionic-liquid-based aqueous biphasic systems. J Phys Chem B. 2012;116:7660–7668. doi: 10.1021/jp211132z. [DOI] [PubMed] [Google Scholar]
- 28.Earle MJ, Esperança JMSS, Gilea MA, Canongia Lopes JN, Rebelo LPN, Magee JW, Seddon KR, Widegren JA. The distillation and volatility of ionic liquids. Nature. 2006;439:831–834. doi: 10.1038/nature04451. [DOI] [PubMed] [Google Scholar]
- 29.Rogers RD, Seddon KR. Ionic Liquids--Solvents of the Future? Science. 2003;302:792–793. doi: 10.1126/science.1090313. [DOI] [PubMed] [Google Scholar]
- 30.Louros CLS, Cláudio AFM, Neves CMSS, Freire MG, Marrucho IM, Pauly J, Coutinho JAP. Extraction of Biomolecules Using Phosphonium-Based Ionic Liquids + K3PO4 Aqueous Biphasic Systems. Int J Mol Sci. 2010;11:1777–1791. doi: 10.3390/ijms11041777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deive FJ, Rodríguez A, Pereiro AB, Araújo JMM, Longo MA, Coelho MAZ, Canongia Lopes JN, Esperança JMSS, Rebelo LPN, Marrucho IM. Ionic liquid-based aqueous biphasic system for lipase extraction. Green Chem. 2011;13:390–396. [Google Scholar]
- 32.Deive FJ, Rodríguez A, Rebelo LPN, Marrucho IM. Extraction of Candida antarctica lipase A from aqueous solutions using imidazolium-based ionic liquids. Sep Purif Technol. 2012;97:205–210. [Google Scholar]
- 33.Ventura SPM, De Barros RLF, Barbosa JMP, Soares CMF, Lima ÁS, Coutinho JAP. Production and purification of an extracellular lipolytic enzyme using ionic liquid-based aqueous two-phase systems. Green Chem. 2012;14:734–740. [Google Scholar]
- 34.Ding X, Wang Y, Zeng Q, Chen J, Huang Y, Xu K. Design of functional guanidinium ionic liquid aqueous two-phase systems for the efficient purification of protein. Anal Chim Acta. 2014;815:22–32. doi: 10.1016/j.aca.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 35.Taha M, E Silva FA, Quental MV, Ventura SPM, Freire MG, Coutinho JAP. Good’s buffers as a basis for developing self-buffering and biocompatible ionic liquids for biological research. Green Chem. 2014;16:3149–3159. doi: 10.1039/C4GC00328D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pereira MM, Pedro SN, Quental MV, Lima ÁS, Coutinho JAP, Freire MG. Enhanced extraction of bovine serum albumin with aqueous biphasic systems of phosphonium- and ammonium-based ionic liquids. J Biotechnol. 2015;206:17–25. doi: 10.1016/j.jbiotec.2015.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pereira JFB, Lima ÁS, Freire MG, Coutinho JAP. Ionic liquids as adjuvants for the tailored extraction of biomolecules in aqueous biphasic systems. Green Chem. 2010;12:1661–1669. [Google Scholar]
- 38.Freire MG, Pereira JFB, Francisco M, Rodríguez H, Rebelo LPN, Rogers RD, Coutinho JAP. Insight into the interactions that control the phase behaviour of new aqueous biphasic systems composed of polyethylene glycol polymers and ionic liquids. Chem Eur J. 2012;18:1831–1839. doi: 10.1002/chem.201101780. [DOI] [PubMed] [Google Scholar]
- 39.Quental MV, Caban M, Pereira MM, Stepnowski P, Coutinho JAP, Freire MG. Enhanced extraction of proteins using cholinium-based ionic liquids as phase-forming components of aqueous biphasic systems. Biotechnol J. 2015;10:1457–1466. doi: 10.1002/biot.201500003. [DOI] [PubMed] [Google Scholar]
- 40.Taha M, Almeida MR, E Silva FA, Domingues P, Ventura SPM, Coutinho JAP, Freire MG. Novel biocompatible and self-buffering ionic liquids for biopharmaceutical applications. Chem Eur J. 2015;21:4781–4788. doi: 10.1002/chem.201405693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Good NE, Winget GD, Winter W, Connolly TN, Izawa S, Singh RM. Hydrogen ion buffers for biological research. Biochemistry. 1966;5:467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- 42.Patel R, Kumari M, Khan AB. Recent advances in the applications of ionic liquids in protein stability and activity: a review. Appl Biochem Biotechnol. 2014;172:3701–3720. doi: 10.1007/s12010-014-0813-6. [DOI] [PubMed] [Google Scholar]
- 43.Petkovic M, Seddon KR, Rebelo LPN, Pereira CS. Ionic liquids: a pathway to environmental acceptability. Chem Soc Rev. 2011;40:1383–1403. doi: 10.1039/c004968a. [DOI] [PubMed] [Google Scholar]
- 44.Li Z, Liu X, Pei Y, Wang J, He M. Design of environmentally friendly ionic liquid aqueous two-phase systems for the efficient and high activity extraction of proteins. Green Chem. 2012;14:2941–2950. [Google Scholar]
- 45.Taha M, Quental MV, Correia I, Freire MG, Coutinho JAP. Extraction and stability of bovine serum albumin (BSA) using cholinium-based Good’s buffers ionic liquids. Process Biochem. 2015;50:1158–1166. doi: 10.1016/j.procbio.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sintra TE, Luís A, Rocha SN, Lobo Ferreira AIMC, Gonçalves F, Santos LMNBF, Neves BM, Freire MG, Ventura SPM, Coutinho JAP. Enhancing the Antioxidant Characteristics of Phenolic Acids by Their Conversion into Cholinium Salts. ACS Sustain Chem Eng. 2015;3:2558–2565. doi: 10.1021/acssuschemeng.5b00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muhammad N, Hossain MI, Man Z, El-Harbawi M, Bustam MA, Noaman YA, Mohamed Alitheen NB, Ng MK, Hefter G, Yin CY. Synthesis and Physical Properties of Choline Carboxylate Ionic Liquids. J Chem Eng Data. 2012;57:2191–2196. [Google Scholar]
- 48.Merchuk JC, Andrews BA, Asenjo JA. Aqueous two-phase systems for protein separation. Studies on phase inversion. J Chromatogr B Biomed Sci App. 1998;711:285–293. doi: 10.1016/s0378-4347(97)00594-x. [DOI] [PubMed] [Google Scholar]
- 49.Neves CMSS, Shariari S, Lemus J, Pereira JFB, Freire GM, Coutinho JAP. Aqueous biphasic systems composed of ionic liquids and polypropylene glycol: insights into their liquid-liquid demixing mechanisms. Phys Chem Chem Phys. 2016;18:20571–20582. doi: 10.1039/c6cp04023c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.ChemSpider. Search and share chemistry. [Accessed: 8th July 2016]; Available at: http://www.chemspider.com/
- 51.Bollen LS, Hau J. Comparison of immunospecific antibody response in young and old chickens immunized with human IgG. Lab Anim. 1999;33:71–76. doi: 10.1258/002367799780578435. [DOI] [PubMed] [Google Scholar]
- 52.Desai RK, Streefland M, Wijffels RH, Eppink MHM. Extraction and stability of selected proteins in ionic liquid based aqueous two phase systems. Green Chem. 2014;16:2670–2679. [Google Scholar]
- 53.Silva MF, Fernandes-Platzgummer A, Aires-Barros MR, Azevedo AM. Integrated purification of monoclonal antibodies directly from cell culture medium with aqueous two-phase systems. Sep Purif Technol. 2014;132:330–335. [Google Scholar]
- 54.Dhadge VL, Rosa SA, Azevedo A, Aires-Barros R, Roque AC. Magnetic aqueous two phase fishing: A hybrid process technology for antibody purification. J Chromatogr A. 2014;1339:59–64. doi: 10.1016/j.chroma.2014.02.069. [DOI] [PubMed] [Google Scholar]
- 55.Dennison C, Lovrien R. Three phase partitioning: concentration and purification of proteins. Protein Expr Purif. 1997;11:149–161. doi: 10.1006/prep.1997.0779. [DOI] [PubMed] [Google Scholar]
- 56.Alvarez-Guerra E, Irabien A. Ionic Liquid-Based Three Phase Partitioning (ILTPP) for Lactoferrin Recovery. Sep Sci Technol. 2014;49:957–965. [Google Scholar]
- 57.Alvarez-Guerra E, Irabien A. Ionic liquid-based three phase partitioning (ILTPP) systems for whey protein recovery: ionic liquid selection. J Chem Technol Biotechnol. 2015;90:939–946. [Google Scholar]
- 58.Mondal D, Sharma M, Quental MV, Tavares APM, Prasad K, Freire MG. Suitability of bio-based ionic liquids for the extraction and purification of IgG antibodies. Green Chem. 2016;18:6071–6081. doi: 10.1039/C6GC01482H. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.