Abstract
Aqueous biphasic systems (ABS) composed of polypropylene glycol and carbohydrates, two benign substances, are proposed to separate two food colorants (E122 and E133). ABS are promising extractive platforms, particularly for biomolecules, due to their aqueous and mild nature (pH, temperature), reduced environmental impact and processing costs. Another major aspect considered, particularly useful in downstream processing, is the “tuning” ability for the extraction and purification of these systems by a proper choice of the ABS components. In this work, our intention is to show the concept of ABS as an alternative and organic-free tool to separate two different biomolecules in a simple way, so simple than teachers can actually adopt it in their classes to explain the concept of bioseparation processes. Thus, the students should use different carbohydrates to study the partition of two food color dyes (synthetic origin), and evaluate the ability of these ABS to separate both food colorants. Through these experiments the students get acquainted with ABS, learn how to determine solubility curves and perform extraction procedures using colorant food additives, that can also be applied in the extraction of various (bio)molecules.
Keywords: biotechnology education, bioseparation, aqueous biphasic systems, phase diagrams, partition, food colorants
Introduction
The study of the separation of target compounds from complex mixtures using liquid-liquid extraction (LLE) techniques is a formative experiment in undergraduate Chemistry, Chemical Engineering and Biotechnology laboratories. Presently, liquid-liquid extractions are widely used in the industry for the concentration, extraction or purification of a variety of compounds. By definition, extraction represents the recovery of a target molecule from one (organic or aqueous) phase to another (aqueous or organic) phase; concentration is defined as the maintenance of the target compound in one phase, if possible in a low-volume phase; and purification is defined as the migration of the target compound for the opposite phase of the contaminants in which the contaminant molecules are concentrated. In this technique and independently of being a concentration, extraction or purification, two liquid phases, partially or totally immiscible, are in contact, and the transference of the solute from one phase to the other is related with the respective affinity of the target molecule and the phase components but also the main contaminants with the phase split solvents and the interaction of the two phase promoters as well. The technique is hence a useful tool to apply the knowledge of relevant topics such as molecular polarity, thermodynamic equilibrium, intermolecular forces, acid-base reactions, and several laboratory works regarding LLE were recently proposed in Chemical Education Journals [1–4]. In those, as in typical conventional industrial and laboratorial systems, at least one liquid phase is formed by an organic liquid. However, organic solvents may have deleterious effects in the environment, and the education of students in the XXI Century should focus on the crucial relevance of environmental issues and societal concerns. In one of the published LLE experiments, environmental concerns are addressed by reducing the amounts of solvents employed [3]. Although useful, this is a limited improvement in reducing environmental impacts. Besides, another relevant feature that should be considered when targeting biomolecules is the (in)compatibility of organic solvents with biological media. This is a most important issue at times where biotechnological processes assume paramount importance. To overcome these difficulties, Albertsson proposed in 1986 the use of two partially immiscible aqueous phases for the partition of cells and cell particles [5]. These systems are formed by a pair of solutes that are able to form two macroscopic aqueous liquid phases when dissolved in water above certain concentrations. Aqueous two-phase systems (ATPS) or aqueous biphasic systems (ABS) are promising extractive platforms for water-soluble molecules and particularly for biomolecules, due to their aqueous mild nature, principally related with the high water contents [6].
To make students aware of environmental concerns and to emphasize the biocompatibility concept in technological separation procedures, they must be safer alternatives to the conventional methodologies that use organic solvents. Henceforth, a set of experiments using ABS formed by a synthetic but innocuous polymer, polypropylene glycol 400 g·mol-1 (PPG 400), and a carbohydrate is proposed for undergraduate students majoring Biotechnology, Biochemistry, Chemical Engineering or Chemistry. To evaluate the influence of the carbohydrate structure in the phase separation ability, various carbohydrates were investigated, namely monosaccharides (mannose, fructose and xylose) and disaccharides (sucrose and mannose). The students will firstly determine the solubility curve (also designated as binodal curve) to obtain the ternary phase diagrams at room temperature, each group being assigned a different carbohydrate. The design of the respective ABS is proposed and will be further analyzed taking into account their application in separation processes (definition of binodal curve, mixture point, monophasic and biphasic region). Next, an appraisal of the extractive potential of the studied ABS will follow by using a suitable composition (biphasic region) to separate (or purify) two synthetic food additives (E122 and E133).
Aqueous biphasic systems
Aqueous biphasic systems (ABS) form because some solutes cause an aqueous solution to fully separate into two phases. This phenomenon was firstly observed by Beijerink in 1986, when agar was mixed with soluble starch or gelatin [7]. Originally, these systems were based on aqueous mixtures of two incompatible polymers, such as polyethylene glycol (PEG), dextran and/or maltodextrin [5–6]. Dextran and polyethylene glycol (PEG) form one of the most studied systems; dextran, more dense and hydrophilic in the lower phase and PEG, more hydrophobic and less dense, in the upper phase. Since then, many immiscible aqueous systems were found using mainly hydrophilic polymers. In the recent years, however, with the purpose of increasing the mass transfer rates and selectivity of fractionation of biomolecules, other types of ABS have been studied, in which different phase components are used. Ionic liquids (ILs), inorganic salts and carbohydrates are three examples of solutes used in ABS [8–11] that were applied in the separation or purification of a wide range of compounds, from proteins and enzymes to antibiotics and organic acids and many other bio- or synthetic molecules [8–9].
Polymer-carbohydrate based ABS extraction
For this laboratory experiment, ABS formed by the synthetic polymer poly(propylene glycol) 400 g·mol-1 (PPG 400) and a carbohydrate were chosen since these are novel and completely benign phase compounds. PPG is a biocompatible polymer that has many properties in common with PEG: both are liquid at room temperature and their solubility in water decreases rapidly while increasing their molar mass. PPG is similar to PEG, but the replacement of a methyl group in the ethylene glycol repeating unit renders PPG more hydrophobic than PEG. Moreover, secondary hydroxyl groups in PPG are less reactive than primary hydroxyl groups in polyethylene glycol (PEG). Both polymers are considered safe and have been approved for human injections and oral application but PPG is considered less toxic than PEG, so biotechnological matrices are now being produced in PPG and are widely used by the chemical, food, and pharmaceutical industries [12].
Carbohydrates or saccharides (or sugars) are a large and diverse group of organic compounds that include sugars, cellulose and starch. Carbohydrates are polyhydroxy aldehydes or ketones with high affinity for water since they exhibit several -OH groups, with a dual donor/acceptor character. For that reason, they can be involved in hydrogen bonding, and thus, present a strong ability to bind water. As a result, carbohydrates are potential substitutes to conventional compounds used in the formation of ABS, with the advantage of being more biocompatible routes for the extraction of biomolecules [11,13].
Food color additives
Food color additives are any dye, pigment or substance that imparts color when it is added to food or drink. Food additives play an important role in today's complex food supply since their use has become more prominent in recent years due to the increased production of prepared, processed, and convenience foods. Brilliant Blue FCF (E133) is a blue color synthetic dye produced using aromatic hydrocarbons from petroleum. This food additive is found in diverse range of foods such as ice cream, canned peas, packet soups, bottled food colorants, icings, ice pops, blue raspberry flavored products, dairy products and sweets. It is also used in drinks and tooth elixir. Brilliant Blue FCF is an approved food colorant and pharmacologically inactive substance for drug formulations in the EU and the United States. Azorubine, also known as carmoisine (E122), is a synthetic red color dye allowed as a food additive in the EU (e.g. cheeses, dried fruit, and some alcoholic beverages) and is permitted for use as an excipient in pharmaceutics but it was never used in food in the US.
Experimental procedures
Experimental overview
The laboratory experiment proposed intend to give tools to design an extraction procedure at reduced cost. This is a two-class work where they will firstly determine and interpret binodal (or solubility) curves, in order to identify monophasic and biphasic regions and to understand the formation and the meaning of an ABS biphasic region. Instead of a triangular phase diagram with 3 components, the orthogonal representation using concentrations is to be preferred for its simplicity. Then, they ascertain the potential of the ABS as an extraction platform by performing the partition of food colorants as model substances. The color of the target molecules facilitates the visualization and understanding of their partition between the two phases [14] and ABS are shown to be efficient extractive platforms for food additives [15–17]. Moreover, the quantification of the dyes is easy to perform and interpret (UV-Vis spectroscopy); the calibration curves may be determined by the students or by the instructor according to the duration of the lab class.
In the first class, the students should determine the solubility curves of PPG 400 + carbohydrates (Experiment 1 in Supporting information). An initial 30-minute lecture is recommended to explain the fundamentals and also the experimental details of ABS formation. The students should be then organized into 5 pair-groups and a different carbohydrate is assigned to each group: d-(-)-fructose; d-(+)-mannose; d-(+)-xylose; d-(+)-maltose and d-(+)-sucrose. Then, the experimental determination of the solubility curve (taking 90-100 minutes) will follow. Mass data should be registered and treated using a spread sheet.
The students will use information from the solubility curve to establish the composition to adopt in the partition of the dyes. A fixed composition is recommended for each of the ABS defined in the first part of the class by the students, so that the results for the different carbohydrates can be compared (40 wt. % of PPG, 20 wt. % of carbohydrate and 40 wt. % of food additive solution). In the next class, the students should study the partition of the two dyes (during approximately 2 hours) - Experiment 2 in Supporting Information. Briefly, the binodal curve determined by each group during the first class with the carbohydrate pre-selected will be the basis to perform the food dyes partition. The calibration curves needed to compute the concentration of the dyes in each phase may be experimentally determined by the students or given by the instructor, according to the duration of the class and/or desired pedagogic goals. The complete set of final results should be made available to all students so that they can analyze and compare them.
Prelab
Students must learn how to use the spectrophotometer UV-Vis, the analytical balance and micropipets. Knowledge on how to prepare a calibration curve is needed.
Materials
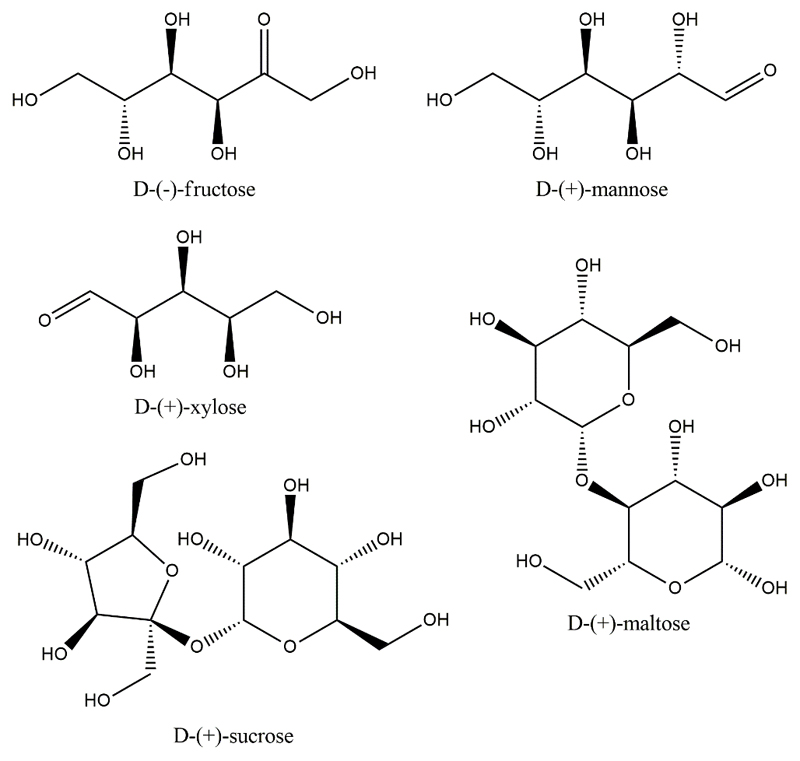
The carbohydrates used were: d-(-)-fructose (98.0 wt. % pure from Panreac), d-(+)-mannose (99.5 wt. % pure from Sigma-Aldrich), d-(+)-xylose (≥ 99.0 wt. % pure from Sigma-Aldrich), d-(+)-maltose (≥ 98.0 wt. % pure from Fisher Bioreagents) and d-(+)-sucrose (99.5 wt. % pure from HiMedia). The polypropylene glycol with a molecular weight of 400 g·mol-1 (PPG 400) was bought highly pure from Sigma-Aldrich. The chemical structures of the carbohydrates used are shown in Fig. 1. The food additives: azorubine (E122) and brilliant blue FCF (E133) were purchased from Vahiné with a purity of > 98.0 wt. %. Distilled and deionized water was used in all experiments. For more details on the equipment and materials used please check Supporting information file.
Fig 1.
Chemical structures of the carbohydrates studied in this work.
Design of binodal curves of PPG 400 + carbohydrates + H2O
The ternary phase diagrams were determined at 298 (±1) K and at atmospheric pressure by the cloud point titration method. Stock solutions 40–60 wt. % of the carbohydrates, depending on the carbohydrate solubility saturation in water (see ESI), are previously prepared to use in the determination of the phase diagrams. Repetitive drop-wise addition of the carbohydrate solution to pure PPG 400 was carried out until the detection of a cloudy solution, followed by the drop-wise addition of deionized water until the detection of a monophasic region (clear and limpid solution). In case of any doubts the teacher should do one assay to show how the turbidity of the system looks like. All these additions are carried out under continuous stirring, in a 20 mL glass vial or test tube. This procedure should be explained in detail and strictly followed by the class as defined in Supporting Information – Experiment 1.
Partition of food additives through polymer-carbohydrate based ABS
A calibration curve should be prepared for each food additive with concentration ranges of 0.2 - 1.5 mg.mL-1 (E122) and 0.5 - 4.0 mg·mL-1 (E133); the absorbance of the aqueous solutions being measured at 524 nm (E122) and 633 nm (E133).
The partition systems were prepared in Eppendorf tubes by weighing the appropriate amounts of carbohydrate, PPG 400 and the aqueous solution containing food additives (E122 and E133), in order to obtain a mixture point of fixed composition for all systems (40 wt. % of PPG 400, 20 wt. % of carbohydrate and 40 wt. % of food colorant aqueous solution). The teacher should guarantee that the students understand why is this mixture point used, and why it should be located in the biphasic region. The detailed procedure is described with all the details as Experiment 2 in Supporting Information.
After the thermodynamic equilibrium is attained and the two phase separation is visually detected, the separation of top and bottom-phases is accomplished. Using a Pasteur pipette, each phase is transferred to microvials and the respective mass is measured. Next, the concentration of food color additive in each phase is quantified through UV-spectroscopy, using the calibration curves previously established for each food colorant. This protocol should be strictly followed by the class.
Hazards
PPG 400 is not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008 nor classified as dangerous according to Directive 67/548/EEC. Prolonged contact with PPG is essentially non-irritating to the skin. Undiluted PPG is minimally irritating to the eye, and can produce slight transient conjunctivitis (the eye recovers after the exposure is removed). Carbohydrates and food additives are benign, since they can enter the food supply. Students should wear laboratory coats, gloves and safety goggles during this experiment in order to minimize possible safety risks.
Discussion
Development and analysis of the phase diagrams
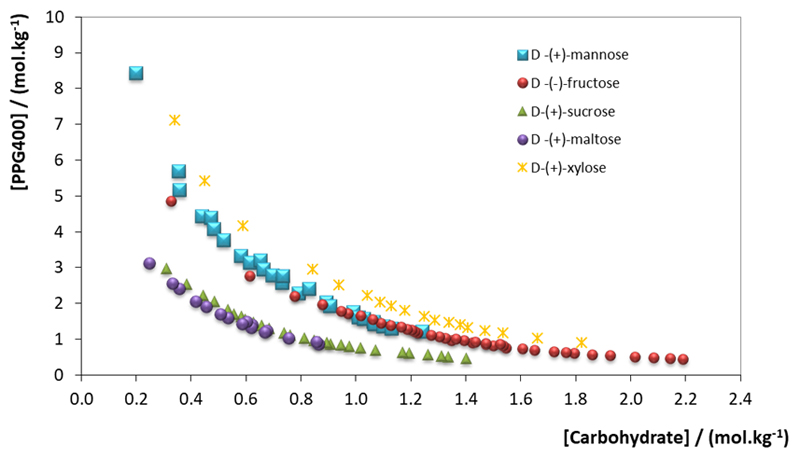
The binodal curves corresponding to the systems investigated in this work, combining PPG 400 and each of the five carbohydrates, are presented in Fig. 2.
Fig 2.
Binodal curves for the ternary systems composed of PPG 400 + carbohydrate + H2O at 298 K: (●) d-(-)-fructose; (■) d-(+)-mannose; (●) d-(+)-maltose; (▲) d-(+)-sucrose and (✳) d-(+)-xylose.
It should be mentioned to the students that the closer the curve is to the axes, the least amount of solute is required to promote the separation of the two aqueous phases. Moreover, all binodal curves are represented in molality units to avoid disparities in the evaluation of the carbohydrate potential in inducing the liquid–liquid demixing that could result from their distinct molecular weights.
The experimental ternary phase diagrams should be calculated by the students in mass units, similarly to those provided in Supporting Information (Fig. S4 and Tables S1-S2). These curves indicate that the carbohydrates capability to induce ABS follows the decreasing order: d-(+)-maltose ≈ d-(+)-sucrose >> d-(-)-fructose > d-(+)-mannose > d-(+)-xylose. Maltose and sucrose are both disaccharides and have higher molar mass and more -OH groups than fructose or mannose, therefore they interact more extensively with water and exhibit higher capacity to form ABS. Moreover, due to their disaccharide composition, they exhibit similar biphasic regions. In terms of monosaccharides, the gathered data suggests that hexoses [d-(-)-fructose and d-(+)-mannose] are more effective in promoting the formation of two-phase systems than pentoses [d-(+)-xylose]. The number of hydroxyl groups results in a higher number of potential hydrogen bonds with water turning them into stronger salting-out agents. This tendency is actually in agreement with previous studies, which used ionic liquid-based ABS formed with carbohydrates [11]. Students should be advised to search into literature and to find other examples of phase diagrams were these tendencies are found.
Development and analysis of the food colorants partition
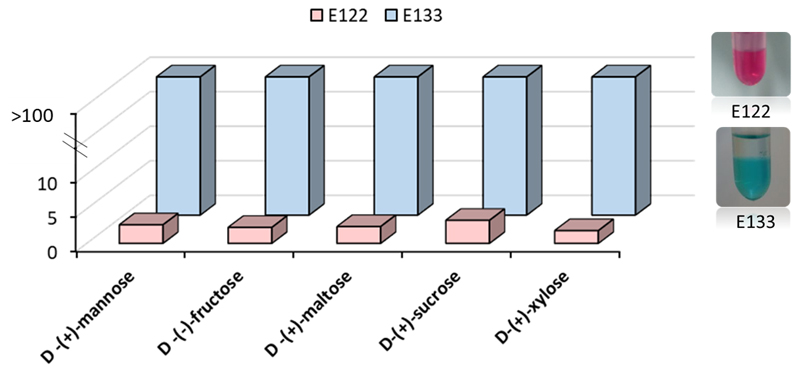
The partition of the food colorants was performed using a fixed composition for each studied ABS (40 wt. % of PPG 400 + 20 wt. % of carbohydrate + 40 wt. % of food colorants aqueous solution). The results of partition coefficient (K – Eq. 1 in Supporting Information) and extraction efficiency (EE% - Eq. 2 in Supporting Information) for the partitioning of each dye are presented in Table 1 and equally illustrated in Fig. 3. Students should be guided to prepare a similar table and figure after their experiments.
Table 1. Extraction parameters (EE%, K and S) regarding the partition of both E122 and E133 in different polymer-carbohydrate-based ABS.
| E122 | E133 | S | |||
|---|---|---|---|---|---|
| Carbohydrate | EE% | K(E122) | EE% | K(E133) | |
| D -(+)-mannose | 76.98 ± 4.18 | 2.68 ± 0.94 | 104.72 ± 4.43 | > 100 | 37.29 |
| D -(-)-fructose | 72.96 ± 2.20 | 2.31 ± 0.17 | 112.58 ± 3.65 | > 100 | 43.35 |
| D -(+)-maltose | 74.23 ± 0.60 | 2.43 ± 0.66 | 104.61 ± 1.75 | > 100 | 41.16 |
| D-(+)-sucrose | 82.72 ± 0.67 | 3.34 ± 0.29 | 100.73 ± 3.74 | > 100 | 29.98 |
| D-(+)-xylose | 70.02 ± 4.81 | 1.84 ± 0.48 | 103.35 ± 1.25 | > 100 | 54.31 |
Fig 3.
Partition coefficients (K) for both food additives - E122 and E133 in the ABS composed of 40 wt. % of PPG 400 + 20 wt. % of carbohydrate + 40 wt. % of food colorant aqueous solution.
About the analysis of the partition phenomena, for Brilliant Blue FCF (E133), a complete partition towards the bottom carbohydrate-rich phase was observed (the concentration of this dye in the PPG-rich top phase is below the detection limit), therefore, no equilibrium constants are provided. Because the partition of this dye is complete for one of the phases (Table 1, Fig. 3), by convention the K(E133) is presented as > 100. This phenomena should be explained in detail to the students and they should define the results for this specific food colorant following the same convention here described. Regarding the E122, this also partitions predominantly to the carbohydrate-rich phase but a significant amount is still present in the polymer-rich phase. The partition constants of this dye do not vary much with the carbohydrate; from K(E122) = 1.8 when xylose was used and K(E122) = 3.3 for sucrose, the correspondent extraction efficiencies being 70 % for xylose and 83 % for sucrose. The ability to promote phase separation seems to be related to the partition results. The extended results are depicted in Table S3, in which the concentration of top and bottom phases and the mass balance values (Eq. 4 in Supporting Information) are available. These parameters should be also calculated and presented by the students for further discussion helping to point out the conclusions previously described.
This experiment will allow students to calculate the selectivity value of the different ABS. Selectivity can be estimated by the ratio between the partition constants of the two dyes, S = K(E133)/K(E122) (Eq. 3 in Supporting Information), presented in Table 1. In this particular case, the K values of E133 > 100, and for that reason it was considered that K(E133) ≈ 100 to the selectivity value calculation. For every system, the students should calculate the experimental S values. From these S results, it should be concluded that the E133 manifested a stronger preference for the carbohydrate rich-phase, while the highest selectivity was provided by the xylose-based ABS (S = 54.31).
Conclusion
ABS are excellent alternative techniques to promote the partition/separation and purification of different (bio)molecules, from the simplest like amino-acids or phenolic compounds to the most complex namely proteins and enzymes. Actually, ABS are claimed as promising extractive platforms, particularly for biomolecules, due to their aqueous and mild nature (pH, temperature), reduced environmental impact and processing costs. In this work, our intention was to show the concept of preparing and using ABS as an alternative and organic-free tool to separate two different biomolecules in a simple way, so simple than teachers can actually adopt it in their classes to explain the concept of benign bioseparation processes. This work is actually described in the form of a two-class laboratory experiment, which could be proposed as an alternative to conventional liquid-liquid extraction using organic solvents in separation processes. Because the ABS concept may not be easy to grasp by lecturing alone, thereupon by doing the experiments and building the solubility curves, the students will become more involved and at ease with both the fundamental and the practical concepts of phase diagrams. In particular, they will understand how to choose a point in the biphasic region to implement a totally benign LLE. Another major aspect to be considered, particularly useful in downstream processing, is the “tuning” ability of these systems by the proper choice of the ABS components. In these experiments, a good selectivity was achieved for the food colorant molecules with one of the systems, the ABS based in maltose. Also, since PPG and carbohydrates are two substances present in food additive formulations, no further polishing steps to remove the phase compounds are required. So, students will be able to infer about the influence of using different components, in the case, carbohydrates, as phase compounds in two main areas: the ability to form ABS and the extraction yield and selectivity. This experiment will familiarize undergraduate students with new concepts through interdisciplinary roots in food industry, downstream processing and Green Chemistry.
Supplementary Material
All the instructor notes, laboratory protocols and compiled experimental values are given in Supporting Information allowing the application of this work in class.
Acknowledgments
This work was developed within the scope of the project CICECO-Aveiro Institute of Materials, POCI-01-0145-FEDER-007679 (FCT Ref. UID/CTM/50011/2013), financed by national funds through the FCT/MEC and when appropriate co-financed by FEDER under the PT2020 Partnership Agreement. E.V. Capela, J.H.P.M. Santos and S.P.M. Ventura acknowledges FCT for the PhD grants SFRH/BD/126202/2016, SFRH/BD/102915/2014 and the IF contract IF/00402/2015, respectively. M. G. Freire acknowledges the European Research Council (ERC) for the Starting Grant ERC-2013-StG-337753.
Footnotes
Conflict of Interest:
There are no conflict of interests associated to this work.
References
- [1].Michalowski T. Effect of Mutual Solubility of Solvents in Multiple Liquid-Liquid Extraction Systems. J Chem Educ. 2002;79:1267. [Google Scholar]
- [2].Raydo ML, Church MS, Taylor ZW, Taylor CE, Danowitz AM. A guided inquiry liquid/liquid extractions laboratory for introductory organic chemistry. J Chem Educ. 2015;92:139–142. [Google Scholar]
- [3].Radford SA, Hunter RE, Jr, Barr DB, Ryan PB. Liquid–Liquid Extraction of Insecticides from Juice: An Analytical Chemistry Laboratory Experiment. J Chem Educ. 2013;90:483–486. [Google Scholar]
- [4].Harris MF, Logan JL. Determination of log K ow Values for Four Drugs. J Chem Educ. 2014;91:915–918. [Google Scholar]
- [5].Albertsson P-Å. Partition of cell particles and macromolecules: separation and purification of biomolecules, cell organelles, membranes, and cells in aqueous polymer two-phase systems and their use in biochemical analysis and biotechnology. Vol. 5. Wiley; New York, NY: 1986. pp. 233–234. [Google Scholar]
- [6].Zaslavsky BY. Aqueous two-phase partitioning: physical chemistry and bioanalytical applications. Vol. 99. M. Dekker; New York, NY: 1995. p. 694. [Google Scholar]
- [7].Beijerinck MW. Kulturversuche mit Amoeben auf festem Substrate. Cent f Bakteriol Orig. 1896;19:257–267. [Google Scholar]
- [8].Freire MG, Cláudio AFM, Araújo JMM, Coutinho JAP, Marrucho IM, Lopes JNC, Rebelo LPN. Aqueous biphasic systems: a boost brought about by using ionic liquids. Chem Soc Rev. 2012;41:4966–4995. doi: 10.1039/c2cs35151j. [DOI] [PubMed] [Google Scholar]
- [9].Hatti-Kaul R. Aqueous two-phase systems. Mol Biotechnol. 2001;19:269–277. doi: 10.1385/MB:19:3:269. [DOI] [PubMed] [Google Scholar]
- [10].Chen Y, Wang Y, Cheng Q, Liu X, Zhang S. Carbohydrates-tailored phase tunable systems composed of ionic liquids and water. J Chem Thermodyn. 2009;41:1056–1059. [Google Scholar]
- [11].Freire MG, Louros CLS, Rebelo LPN, Coutinho JAP. Aqueous biphasic systems composed of a water-stable ionic liquid + carbohydrates and their applications. Green Chemistry. 2011;13:1536–1545. [Google Scholar]
- [12].Moon HJ, Ko DY, Park MH, Joo MK, Jeong B. Temperature-responsive compounds as in situ gelling biomedical materials. Chem Soc Rev. 2012;41:4860–4883. doi: 10.1039/c2cs35078e. [DOI] [PubMed] [Google Scholar]
- [13].Antoniou E, Buitrago CF, Tsianou M, Alexandridis P. Solvent effects on polysaccharide conformation. Carbohydr Polym. 2010;79:380–390. [Google Scholar]
- [14].Shugrue CR, Mentzen HH, Linton BR. A Colorful Solubility Exercise for Organic Chemistry. J Chem Educ. 2015;92:135–138. [Google Scholar]
- [15].Huddleston JG, Willauer HD, Boaz KR, Rogers RD. Separation and recovery of food coloring dyes using aqueous biphasic extraction chromatographic resins. J Chromatogr B Biomed Sci Appl. 1998;711:237–244. doi: 10.1016/s0378-4347(97)00662-2. [DOI] [PubMed] [Google Scholar]
- [16].Ventura SPM, Santos-Ebinuma VC, Pereira JFB, Teixeira MFS, Pessoa A, Coutinho JAP. Isolation of natural red colorants from fermented broth using ionic liquid-based aqueous two-phase systems. J Ind Microbiol Biotechnol. 2013;40:507–516. doi: 10.1007/s10295-013-1237-y. [DOI] [PubMed] [Google Scholar]
- [17].Santos JHPM, e Silva FA, Coutinho JAP, Ventura SPM, Pessoa A. Ionic liquids as a novel class of electrolytes in polymeric aqueous biphasic systems. Process Biochem. 2015;50:661–668. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.