Abstract
Background.
While thyroid cancer risks from exposure to ionizing radiation early in life are well-characterized quantitatively, the association of radiation with non-malignant, functional thyroid disorders has been less studied. The present study analyzes risk of hypothyroidism with radiation dose to the thyroid gland and the hypothalamic-pituitary axis among survivors of childhood cancer.
Methods.
Utilizing data from the Childhood Cancer Survivor Study, a cohort of 14,364 five-year survivors of childhood cancer diagnosed at 26 hospitals in the United States and Canada between 1970 and 1986 and followed through 2009, the occurrence of hypothyroidism was ascertained among 12,015 survivors through serial questionnaires. Radiation doses to the thyroid gland and pituitary gland were estimated from radiotherapy records. Binary outcome regression was used to estimate prevalence odds ratios for hypothyroidism at five years from diagnosis of childhood cancer and Poisson regression to model incidence rate ratios (RR) after the first five years.
Results.
A total of 1,193 cases of hypothyroidism were observed, 777 (65%) of which occurred five or more years after cancer diagnosis. The cumulative proportion affected with hypothyroidism (prevalence at five years post-cancer diagnosis plus incidence through 30 years after cancer diagnosis) was highest among five-year survivors of Hodgkin lymphoma (32.3%; 95% CI: 29.5–34.9) and cancers of the central nervous system (17.7%; 95% CI: 15.2–20.4). The incidence rate was significantly associated with radiation dose to the thyroid and pituitary. The joint association of hypothyroidism with thyroid and pituitary dose was sub-additive for pituitary doses greater than 16 Gy. In particular, a very strong thyroid radiation dose-dependence at low-to-moderate pituitary/hypothalamic doses was diminished at high pituitary doses. Radiation-related risks were higher in males than females and inversely associated with age at exposure and time since exposure but remained elevated more than 25 years after exposure. Hypothyroidism was significantly associated with treatment with bleomycin (RR=3.4; 95% CI: 1.6–7.3) and the alkylating agents cyclohexyl-chloroethyl-nitrosourea (CCNU) (RR=3.0; 95% CI: 1.5–5.3) and cyclophosphamide (RR=1.3; 95% CI: 1.0–1.8), with a significant dose-response for CCNU (P< 0.01).
Conclusions.
The risk of hypothyroidism among childhood cancer survivors treated with radiation depends both on direct, dose-dependent radiation-induced damage to the thyroid gland and on dose-dependent indirect effects secondary to irradiation of the hypothalamic-pituitary axis. The dose-response relation for each site depends on dose to the other. Radiation-related risk persists for more than 25 years after treatment. Treatment with certain chemotherapy agents may increase the risk of hypothyroidism.
Keywords: Hypothyroidism, ionizing radiation, radiotherapy, childhood cancer, epidemiology, thyroid disorders
INTRODUCTION
The thyroid gland is a well-established radiosensitive site, particularly following radiation exposures occurring early in life (1, 2). Exposure to therapeutic doses of radiation during childhood has been linked to increased risks of subsequent thyroid cancer as well as benign disorders of the thyroid gland, including hypothyroidism, thyroid nodules and, less consistently, hyperthyroidism (3–10). Relative to thyroid cancer, the effects of irradiation during childhood on the occurrence of benign thyroid disorders have been much less studied. While there is evidence that irradiation at moderate-to-high doses is associated with increased risk of benign thyroid conditions, the dose-response relationships for specific conditions and the factors that modify the dose-response relationships remain poorly understood (7). The most common of these benign disorders is hypothyroidism.
A complicating factor in quantifying radiation-related risks of hypothyroidism is that radiation effects can, potentially, be mediated through effects on the hypothalamus and anterior pituitary gland as well as the thyroid gland. Thyroid-releasing hormone (TRH) secreted by the hypothalamus promotes secretion of thyroid-stimulating hormone (TSH) by the pituitary gland, which, in turn, stimulates secretion of the hormones triiodothyrine) (T3) and thyroxine (T4) by the thyroid; serum T3 and T4 levels, in turn, exert negative feedback on the hypothalamus (11, 12). Disruption of any of the links in this loop can lead to thyroid dysfunction (11, 12). For example, radiation-related hypothyroidism can develop as a result of direct damage to the thyroid gland (primary hypothyroidism) as well as by damage to the hypothalamic-pituitary axis (central or secondary hypothyroidism) (11, 12). While previous studies have described the occurrence of hypothyroidism (5, 6, 13) and TSH deficiency (14, 15) following radiotherapy among childhood cancer survivors, none, to our knowledge, have addressed the joint radiation dose-dependence of irradiation of the thyroid gland and hypothalamic-axis in a detailed way
In the present study, we evaluated the risk of self-reported hypothyroidism in relation to radiation dose to the thyroid gland and hypothalamic-pituitary axis and the possible importance of other factors, including age at radiation exposure, time since exposure, sex, chemotherapy and type of childhood cancer, in a large cohort of childhood cancer survivors. The inclusion of different types of childhood cancer increases the range of thyroid and hypothalamic/pituitary radiation doses, which enables analysis of the specific contributions to risk from dose to the thyroid gland and to the hypothalamic-pituitary axis.
METHODS
Study population
The Childhood Cancer Survivor Study (CCSS) is a cohort study of survivors of childhood cancer treated at 26 institutions in the United States and Canada. Eligibility criteria included a diagnosis of leukemia, central nervous system (CNS) cancer, Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), neuroblastoma, kidney cancer (Wilms tumor), soft tissue sarcoma or bone sarcoma, diagnosis and treatment of cancer between 1970 and 1986, age at cancer diagnosis less than 21 years and minimum survival of five years after diagnosis (16,17). The study was approved by institutional review boards at each participating hospital. Informed consent was obtained directly from study participants age 18 years or older at time of contact and from parents for those younger than 18 years and those who had died or were too ill to participate directly. Of 20,276 potentially eligible survivors, 14,364 were located and agreed to participate in the study. Details about the CCSS study design and cohort characteristics have been described previously (16, 17) and also are available online at https://ccss.stjude.org.
We excluded patients who had not signed medical release forms allowing for review and abstraction of cancer treatment information (n=1608), had not completed a baseline or 2007 follow-up questionnaire (n=3), had reported thyroid gland removal before the cancer treatment (n=17), or had missing or incomplete radiation treatment information (n=487). We excluded an additional 234 survivors who reported hypothyroidism before the cancer diagnosis (n=73) or who did not report age at diagnosis of hypothyroidism (n=161). These exclusions resulted in 12,015 eligible persons. For incidence rate analyses only, we excluded an additional 416 persons who reported hypothyroidism within the first five years of cancer treatment and 96 who reported thyroid removal or hyperthyroidism within the first five years, leaving 11,503 persons and 777 hypothyroidism cases for incidence rate analysis.
Collection of treatment, outcome and covariate information
Medical records at hospitals where participants were treated for their initial cancer were reviewed by trained abstractors according to a standardized protocol. Data concerning start and stop dates of drug administration were abstracted for all chemotherapy drugs, and information about dose (mg/m2) and route of administration was abstracted for 22 agents. Radiotherapy records were photocopied and sent to collaborating medical physicists for use in radiation dosimetry, as described below. Abstract forms are available at https://ccss.stjude.org.
Study participants were asked to complete a baseline questionnaire and follow-up resurveys (every 2–3 years), including a 2007 questionnaire that included questions similar to those administered at baseline (time at entry to the cohort). Data used in the current analysis were taken from the baseline and 2007 questionnaires, which were completed between 1992–2003 and 2007–2009, respectively. The two questionnaires obtained information on a variety of health-related outcomes and practices, including physician-diagnosed thyroid disorders, prescription thyroid medication use, thyroid surgery and new primary cancers. With regards to hypothyroidism, questions included “Have you ever been told by a doctor or other health care professional that you have or have had an underactive thyroid gland (hypothyroid)?” and, “If yes, age at first occurrence.” The questionnaires asked about hyperthyroidism in a similar manner. The questionnaires also asked about thyroid medications “taken consistently for more than one month or for a total of 30 days in one year” during the two-year window prior to mailing of the questionnaire. The baseline and 2007 questionnaires are available at: https://ccss.stjude.org. Of 12,015 survivors who completed the baseline questionnaire, 7,836 (65%) also completed the 2007 questionnaire.
Radiation treatments and dosimetry
Nearly all radiation treatments involved exclusively external beam high-energy photons administered in a standardized fractionation regimen.
Methods for radiation dose reconstruction were described in detail previously (18) and are summarized here. Radiation therapy records were reviewed at the CCSS Radiation Physics Center at The University of Texas MD Anderson Cancer Center. Each patient’s record was abstracted for date of radiotherapy, radiation field location, delivered dose, energy, configuration, field size, blocking and anatomic borders. For this study, doses from radiation treatments given within ten years after the cancer were included. Doses to the thyroid gland and pituitary gland were estimated for each individual using a mathematical phantom and adjusting for the ages of patients at the time of first cancer treatment (18). Dose estimations accounted for typical beam blocking. Thyroid dose was estimated for each lobe of the gland, and these doses were averaged to provide a single dose for analysis. Given the close anatomic proximity of the pituitary gland and the hypothalamus, we assume that the estimated dose to the pituitary gland represents dose to the hypothalamic-pituitary axis and use “pituitary dose” as shorthand for dose to both the pituitary and hypothalamus, with the caveat that doses to the pituitary and hypothalamus could differ for some patients with partial brain irradiation, such as CNS cancer patients.
Statistical analysis
Prevalence.
All CCSS cohort participants, by definition, had survived at least five years after childhood cancer diagnosis. Information about health outcomes, including hypothyroidism, was not collected for cancer patients who did not survive at least five years. Because hypothyroidism occurred frequently within the first few years after therapy, we computed the prevalence of hypothyroidism at five years after cancer diagnosis to compare with the more detailed incidence rate analysis. We computed prevalence odds ratios (ORs) using unconditional binary regression models with an additive adjustment for radiation dose and chemotherapy (equation 1, below) and baseline adjusted for sex, attained age and type of cancer. All parameter estimates, likelihood ratio tests (LRTs), and likelihood-based 95% confidence intervals (CIs) were computed using the GMBO module of the EPICURE statistical program (Risk Sciences International, Ottawa, Canada).
Follow-up.
Follow-up for incidence rate analyses began five years after the date of childhood cancer diagnosis and was truncated at the earliest of the following events: a) diagnosis of hypothyroidism or hyperthyroidism, b) thyroid removal, c) diagnosis of second cancer (exclusive of non-melanoma skin cancer), d) death; or e) last contact.
Incidence rate.
Case counts and person-years (PYR) of follow-up were summarized in a multidimensional cross-tabulation based on categorized demographic, diagnostic and treatment-related variables. These included categories of sex, age at childhood cancer diagnosis (<1, 1-<5, 5-<10, 10-< 15, 15-<21 years), attained age (<20, 20-<25, 25-<30, …, 50-<55, ≥ 55 years), type of childhood cancer (leukemia, CNS cancer, HL, NHL, kidney cancer, neuroblastoma, soft tissue sarcoma, bone sarcoma), calendar year of follow-up (<1980, 1980-<1985, 1985-<1990, …, 2000-<2005, ≥2005), time since cancer diagnosis (5-<6, 6-<7, 7-<8, 8-<9, 9-<10, 10-<15, 15-<20, 20-<25, 25-<30, 30-<35 , ≥35 years), thyroid and pituitary gland radiation dose (0, >0-<0.2, 0.2-<0.3, 0.3-<0.4, 0.4-<0.5, 0.5-<1.0, 1.0-<2.0, 2.0-<3.0, 3.0-<4.0, 4.0-<10.0, 10.0-<15.0, 15.0-< 20.0, 20.0-<25.0, … , ≥60 Gy), chemotherapy (yes/no/unknown) and variables (yes/no/unknown ) for treatment with various classes of chemotherapeutic agents (alkylating agents, anthracyclines, bleomycin, epipodophyllotoxins, platinum-based compounds), as well as specific alkylating agents: cyclohexyl-chloroethyl-nitrosourea (CCNU); bis-chloroethyl-nitrosourea (BCNU), cyclophosphamide, mechlorethamine, procarbazine, melphalan, and triethylenethiophosphoramide (Thiotepa), plus categories based on tertiles of dose for selected alkylating agents. The DATAB module of EPICURE was used to construct the events/person-years tables.
Risk models.
We modeled the incidence rate for hypothyroidism, r(x,dt,dp,c), as a function of a vector of explanatory variables (x), which included sex, attained age, calendar year of follow-up and type of cancer, thyroid radiation dose (dt), pituitary radiation dose (dp) and chemotherapy exposure (c, 1 if yes and 0 if no). The model form was r(x,dt,dp,c) = r0(x) × RP(dt,dp ,c), where r0(x) = exp(αx) was the rate of hypothyroidism among non-radiation, non-chemotherapy exposed individuals with α a vector of parameters, and RR(.) was the relative risk for the joint association of radiation exposures and chemotherapy.
Preliminary analysis based on the Akaike Information Criterion (AIC) revealed that an additive RR model was the preferred form for radiation doses and chemotherapy. For I categories of thyroid radiation dose, denoted dt,1, ...,dt,I, where dt,i is zero/one indicator variable for thyroid dose in the ith category, and J categories of pituitary radiation dose, denoted dp,1, ...,dp,J, where dp,j is zero/one indicator variable for pituitary dose in the jth category, we fitted the additive model:
where exp(φt,i) andexp(φp,J) were RRs for the ith and jth categories of thyroid and pituitary radiation doses, respectively, with φt,1 = φp,1=0 for identifiability, and θ was the excess relative risk (ERR) of chemotherapy.
For continuous radiation doses, we used the general form:
where K(.) and G(.) were the radiation-associated ERRs. For K(.), we considered cross-product terms of dt and dp , their exponentials and logarithms. Using the AIC, deviances (a measure of model fit) and likelihood ratio tests, we developed the following preferred model
| (1) |
where
The ERR for thyroid radiation dose,Gt (dt), had a linear-exponential(linear-quadratic) form, while the ERR for the pituitary radiation dose,Gp (dp), had a linear-exponential(linear) form. The factor, H(dp), described departures from an additive joint association for the thyroid and pituitary radiation doses. As a function of dp H(.) designated a sub-additive, additive or supra-additive relationship depending on its value being less than, equal to, or greater than one, respectively.
We extended equation 1 to evaluate a categorical effect modifier z with J levels by replacing βt with βt exp{Σjδt,jZj} and βp with βp exp{Σjδp,jZj}, where zj, j=1, …, J was an indicator variable and exp(δt,j) and exp(δp,j) represented the relative changes in the linear slope parameters for the jth category in relation to level 1, where δt,1 = 0 and δp,1 = 0 for identifiability. We used a likelihood ratio test to evaluate sex, age at radiation exposure, attained age, time since exposure, type of cancer and use of chemotherapy as potential effect modifiers of the dose effects. We initially included 2×(J-1) distinct δt,j and δp,j parameters; however, analyses revealed that, with the exception of CCNU (see below), the relative changes in the linear parameters for each level of the modifier were statistically homogeneous for thyroid dose and for pituitary dose, i.e., δt,j = δp,j for all j. For example, each category of age at exposure had the same relative effect on the linear parameter for thyroid dose βt and for pituitary dose βp
Finally, for comparison with equation 1, we also fitted a multiplicative relationship for chemotherapy:
| (2) |
AIC values revealed a slight but consistent preference for the additive form compared to the multiplicative form. Nonetheless, our evaluation of effect modifiers under a multiplicative relationship with chemotherapy resulted in similar statistical inference.
We used the AMFIT module of the EPICURE computer program for all incidence rate analyses, including incidence rate ratio (RR) estimates, LRTs and 95% likelihood-based CIs.
Cumulative proportion affected.
We also calculated a measure, “cumulative proportion affected”, combining prevalence at five years with cumulative incidence thereafter, stratified by sex, type of cancer and radiotherapy (yes/no). This measure was not further adjusted for other covariates. We calculated the cumulative incidence of hypothyroidism by time since diagnosis of cancer beginning five years after date of cancer diagnosis (19,20) using Stata (Stata, release 13.1, College Station, TX).
RESULTS
Characteristics of the study population
General characteristics of survivors are summarized in Table 1. Leukemia was the most common type of cancer, followed by HL and CNS cancer. The average age at diagnosis of the cancer was 8 years, and the average follow-up for incidence rate analyses, beginning five years after childhood cancer diagnosis, was 16.3 years. Follow-up was shortest for survivors of HL and longest for survivors of kidney cancer (Table 2). Two-thirds of the population was treated with radiation and, among irradiated five-year survivors, the average dose was 11.0 Gy to the thyroid gland and 15.2 Gy to the pituitary gland. Eighty percent of survivors received chemotherapy, which most often involved alkylating agents, anthracyclines, or both. Among persons included in the incidence rate analysis, eighty-six percent were still alive at the end of follow-up, and, among survivors, the mean age at the end of follow-up was 22 years (maximum, 57 years). There were 1,193 cases of hypothyroidism, 416 (34.9%) of which were diagnosed within five years after the diagnosis of childhood cancer (prevalence at five years=3.5%). Among these 416 cases, the median interval from diagnosis of cancer to diagnosis of hypothyroidism was 2 years. Based on the same questionnaires used to ascertain cases of hypothyroidism, there were 179 self-reported cases of hyperthyroidism, 31 (17.3%) of which were diagnosed within five years after the diagnosis of cancer.
TABLE 1.
General characteristics of study subjects in the Childhood Cancer Survivor Study cohort evaluated for hypothyroidism.
| Number of patients | 12,015 |
| Person-years of follow-upa | 187,449 |
| Average follow-up, yearsa | 16.3 |
| Number of hypothyroidism cases | 1,193 |
| Number of hypothyroidism cases <5 years of cancer (%)a | 416 (35) |
| Number of females (%) | 5,662(47) |
| Mean dose to thyroid gland, Gy (range)b | 11.0 (>0 – 60.4) |
| Mean dose to pituitary gland, Gy (range)b | 15.2 (>0–108.7) |
| Mean age at first radiation exposure, years (range)b | 8 (0–20) |
| Mean age at diagnosis of hypothyroidism, years (range) | 20 (0–54) |
| Mean age at end of follow-up, years (range)a | 22 (5–57) |
| Type of childhood cancer (%) | |
| Leukemia | 4,110 (34) |
| Central nervous system cancer | 1,516 (13) |
| Hodgkin lymphoma | 1,550 (13) |
| Non-Hodgkin lymphoma | 893 (7) |
| Kidney cancer (Wilms tumor) | 1060 (9) |
| Neuroblastoma | 819 (7) |
| Soft tissue sarcoma | 1,049 (9) |
| Bone sarcoma | 1,018 (8) |
| Radiotherapy (%) | |
| No | 3,978 (33) |
| Yes | 8,037 (67) |
| Chemotherapy (%) | |
| No | 2,256 (19) |
| Yes | 9,594 (80) |
| Missing information | 165 (1) |
| Chemotherapy drugs (%) | |
| Any alkylating agents | 6,347 (53) |
| Cyclophosphamide | 5,390 (45) |
| Procarbazine | 1,163 (10) |
| CCNU (Lomustine) | 438 (4) |
| Any anthracyclines | 4,899 (41) |
| Any bleomycin | 711 (6) |
| Any platinum-based compounds | 726 (6) |
| Any epipodophyllotoxins | 1,124 (9) |
For incidence rate analysis, follow-up began five years after diagnosis of the childhood cancer. Some patients exited the cohort before follow-up started, including patients who developed hypothyroidism within the first five years. A total of 512 patients were excluded from the incidence analysis which was based on 11,503 persons and 777 cases.
Among all patients treated with radiation
Abbreviations: CCNU=cyclohexyl-chloroethyl-nitrosourea;
TABLE 2.
Proportion (%) of five-year childhood cancer survivors given radiotherapy, mean and median thyroid and pituitary gland doses, age at first radiation treatment and follow-up information according to type of childhood cancer: Childhood Cancer Survivor Study.
| Type of childhood cancer | RT (%) | Thyroid dosea (Gy) | Pituitary dosea (Gy) | Age at first radiation treatmentb (years) | Age at diagnosis of hypothyroidism (years) | Time to diagnosis of hypothyroidismc (years) | Time to end of follow-upc(years) | ||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median (IQR) | Mean | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | ||
| Leukemia | 69 | 3.9 | 0.8 (0.6–1.5) | 20.8 | 21.2 (16.8–23.4) | 5 (3–9) | 19 (13–28) | 13.4 (7.9–20.9) | 17.7 (8.6–22.5) |
| Central nervous system | 70 | 10.9 | 1.7 (0.7–25.2) | 37.6 | 41.3 (30.2–50.4) | 7 (3–12) | 12 (8–17) | 5.5 (1.8–10.3) | 16.1 (7.0–21.0) |
| Hodgkin lymphoma | 94 | 34.8 | 38.0 (26.9–43.6) | 3.5 | 1.3 (0.9–1.7) | 15 (12–17) | 22 (17–31) | 6.4 (1.5–14.6) | 14.5 (7.3–20.8) |
| Non-Hodgkin lymphoma | 68 | 11.0 | 1.9 (0.4–20.3) | 11.0 | 1.1 (0.1–24.0) | 10 (6–14) | 18 (14–29) | 9.6 (3.2–18.5) | 18.1 (11.3–23.6) |
| Kidney cancer (Wilms) | 64 | 1.5 | 0.3 (0.1–0.7) | 0.2 | 0.1 (0.1–0.2) | 3 (2–5) | 21 (17–28) | 16.9 (14.1–21.5) | 18.9 (11.2–23.6) |
| Neuroblastoma | 49 | 5.0 | 0.6 (0.2–5.8) | 3.9 | 0.2 (0.1–0.6) | 1 (0.6–2) | 15 (9–23) | 13.4 (7.7–21.9) | 18.2 (10.6–23.4) |
| Soft tissue sarcoma | 62 | 6.4 | 0.5 (0.1–3.3) | 14.4 | 0.5 (0.1–30.5) | 7 (3–13) | 17 (10–28) | 10.6 (3.6–15.8) | 18.2 (10.2–23.7) |
| Bone sarcoma | 36 | 3.8 | 0.7 (0.1–3.8) | 3.4 | 0.1 (0.02–0.33) | 13 (10–16) | 30 (23–37) | 14.7 (7.5–24.3) | 17.8 (9.2–23.2) |
| All cancers | 67 | 11.0 | 1.1 (0.5–20.4) | 15.2 | 16.1 (0.5–23.4) | 7 (3–13) | 19 (13–28) | 8.7 (2.9–16.4) | 17.5 (8.8–22.7) |
Among patients treated with radiation.
Age at first radiation treatment is nearly equal to age at diagnosis of the cancer.
Beginning with date of cancer diagnosis. Includes prevalent and incident cases.
Abbreviations: IQR=interquartile range; RT=radiation treatment
Frequency of use of radiotherapy and radiation doses to the thyroid and pituitary glands varied widely by type of cancer (Table 2). Radiation was used for 94% of HL survivors compared to 36% of survivors of bone cancer. The median dose to the thyroid gland for HL survivors was 38 Gy, which dwarfed median thyroid doses associated with other types of cancer, although some CNS cancer and NHL survivors also received very high doses. In contrast, the median dose to the pituitary gland was highest among survivors of CNS cancer and leukemia, with some soft tissue sarcoma and NHL survivors also receiving very high doses. Leukemia and bone and soft tissue sarcoma survivors accounted for 59% of the non-irradiated group (data not shown). The median age at irradiation was youngest for survivors of neuroblastoma and kidney cancer and oldest for survivors of HL and bone cancer. The shortest median intervals from diagnosis of cancer to diagnosis of hypothyroidism were seen among survivors of CNS cancer and HL.
Incidence rate with respect to patient characteristics
Poisson regression analysis of the incidence rate of hypothyroidism beginning five years after diagnosis of childhood cancer showed the highest RRs among survivors of CNS cancer or HL (Table 3). Homogeneity of RRs by age at diagnosis of the cancer was rejected, but there was no clear trend. Incidence was significantly higher among females than males, decreased slightly for 15+ years since cancer diagnosis and increased with calendar year of follow-up. There was no trend with attained age. Incidence was 2.9-fold among persons who received radiation relative to those who did not (p<0.01) and 2.3-fold among those who received chemotherapy relative to those who did not (p=0.05).
TABLE 3.
Relative risk (rate ratio) for incidence rate of hypothyroidism according to selected demographic and clinical factors in the Childhood Cancer Survivor Study cohort.
| Characteristic | Cases | PYR/10,000 | RR (95% CI)a |
|---|---|---|---|
| Type of cancer | |||
| Other cancers | 193 | 8.1 | 1.0 |
| Leukemia | 194 | 6.5 | 1.0 (0.5–1.9) |
| Central nervous system cancer | 127 | 2.0 | 1.2 (0.8–1.8) |
| Hodgkin lymphoma | 246 | 1.8 | 1.5 (1.0–2.2) |
| Pb =0.29 | |||
| Sex | |||
| Male | 308 | 9.7 | 1.0 |
| Female | 452 | 8.7 | 1.8 (1.6–2.1) |
| Pb <0.01 | |||
| Time since cancer diagnosisc | |||
| 5-<6 | 47 | 1.1 | 1.0 |
| 6-<10 | 181 | 4.3 | 1.0 (0.7–1.4) |
| 10-<15 | 202 | 4.5 | 1.1 (0.7–1.5) |
| ≥15 | 330 | 8.6 | 0.7 (0.5–1.1) |
| Pb = 0.05 | |||
| Attained age, yearsc | |||
| <25 | 383 | 11.0 | 1.0 |
| 25-<30 | 133 | 3.2 | 0.8 (0.7–1.0) |
| 30-<35 | 118 | 2.2 | 1.0 (0.8–1.2) |
| 35-<40 | 66 | 1.3 | 0.9 (0.6–1.2) |
| 40+ | 60 | 0.8 | 1.1 (0.8–1.6) |
| Pb =0.25 | |||
| Calendar year of follow-upc | |||
| <1990 | 198 | 5.4 | 1.0 |
| 1990-<2000 | 379 | 8.3 | 1.6 (1.3–1.9) |
| 2000-<2005 | 98 | 2.9 | 1.3 (1.0–1.7) |
| ≥2005 | 85 | 1.9 | 1.9 (1.4–2.6) |
| Pb <0.01 | |||
| Age at cancer diagnosis, years | |||
| <5 | 238 | 7.1 | 1.0 |
| 5-<10 | 154 | 4.4 | 0.9 (0.7–1.1) |
| 10-<15 | 141 | 3.6 | 0.8 (0.6–1.1) |
| ≥15 | 227 | 3.3 | 1.2 (0.8–1.6) |
| Pb =0.03 | |||
| Radiation treatment | |||
| No | 117 | 6.7 | 1.0 |
| Yes | 643 | 11.8 | 2.9 (2.1–4.0) |
| Pb <0.01 | |||
| Any chemotherapy | |||
| No | 190 | 3.5 | 1.0 |
| Yes | 570 | 14.9 | 2.3 (1.1–4.7) |
| Pb =0.05 |
RRs adjusted for an additive association for categories of the cross-tabulation of radiation dose to the thyroid and pituitary, with dose categories as in Table 5, and categories of exposure to chemotherapy and type of cancer (leukemia, central nervous system cancer, Hodgkin lymphoma and other cancers). Type of first cancer included to account for possible differential surveillance and shared predisposition. Baseline adjusted for sex, attained age, type of cancer, and calendar year of follow-up. Analysis excluded patients with missing information for pituitary radiation dose and chemotherapy (177 patients, including 17 cases).
P-value for likelihood ratio test of homogeneity of risk across categories
PYR are distributed across categories as time passes and person ages; that is, PYR, rather than persons, are classified.
Abbreviations: CI=confidence interval; PYR=person-years; RR=relative risk (incidence rate ratio)
Cumulative proportion affected
Combining prevalent and incident cases through 30 years after the cancer diagnosis, the cumulative percentage of patients with hypothyroidism was 11.2% (95% CI: 10.5–11.9). The proportion was 13.8% among females (95% CI: 12.7–14.4) and 8.9% among males (95% CI: 8.1–9.7). The cumulative percentage was 32.3% for HL (95% CI: 29.5–34.9), 17.7% for CNS cancer (95% CI: 15.2–20.4), 9.2% for NHL (95% CI: 7.1–11.7), 7.3% for leukemia (95% CI: 6.2–8.3) and 5.1% for all other cancers combined (95% CI: 3.5–6.9). It was 14.5% among those who received radiotherapy (95% CI: 13.6–15.4) compared to 4.6% among those who did not (95% CI: 3.7–5.6).
Incidence rate by type of chemotherapy
The chemotherapy-associated risk was greatest among CNS cancer survivors (incidence RR=6.7; 95% CI: 3.0–14.8) (Table 4). Although homogeneity was not rejected across all types of cancer (P=0.14), homogeneity was rejected for chemotherapy-associated risk with CNS cancer compared with all other cancers (P=0.03). Significant positive associations were seen for alkylating agents as a group and bleomycin, with a borderline significant association for epipodophlyllotoxins. Among alkylating agents, we observed significant positive associations for CCNU and cyclophosphamide, with a significant dose-response for CCNU but not cyclophosphamide. The RR for CCNU was elevated among survivors of CNS cancer (RR=3.6; 95% CI: 1.2–7.1) and survivors of other cancers (RR=2.6; 95% CI: 0.8–5.6). CCNU was used to treat 16.2% of CNS cancer patients, 9.7% of HL patients, and 3.4% of NHL patients. Associations with CCNU and cyclophosphamide persisted when the analysis was restricted to patients with pituitary radiation doses < 1 Gy, whereas that for bleomycin was attenuated (Supplementary Table 1). Bleomycin was used most commonly in the treatment of bone cancer (25.4%) and HL (22.6%). Cyclophosphamide was widely used for all types of cancer other than CNS cancer and kidney cancer (data not shown).
TABLE 4.
Relative risk (rate ratio) for incidence rate of hypothyroidism according to type of treatment with chemotherapy agents in the Childhood Cancer Survivor Study cohort.
| Characteristic | Cases | PYR/10,000 | RR (95% CI)a |
|---|---|---|---|
| Any chemotherapy & type of childhood cancer | |||
| No chemotherapyb | 190 | 3.5 | 1.0 |
| Any chemotherapy & leukemia | 193 | 6.5 | 1.1 (0.2–7.1) |
| Any chemotherapy & CNS cancer | 58 | 0.4 | 6.7 (3.0–14.8) |
| Any chemotherapy & HL | 148 | 1.2 | 0.8 (0.0–20.4) |
| Any chemotherapy & other cancers | 171 | 6.7 | 1.8 (0.7–4.5) |
| Pc =0.14 | |||
| Any alkylating agentsd | |||
| No | 326 | 9.2 | 1.0 |
| Yes | 433 | 9.2 | 1.8 (1.1–3.0) |
| Pc =0.04 | |||
| Any anthracyclinesc | |||
| No | 522 | 11.3 | 1 |
| Yes | 237 | 7.1 | 1.1 (0.6–2.1) |
| Pc =0.85 | |||
| Any bleomycin | |||
| No | 690 | 17.6 | 1 |
| Yes | 69 | 0.8 | 3.4 (1.6–7.3) |
| Pc =0.03 | |||
| Any platinum-based compounds | |||
| No | 725 | 17.7 | 1 |
| Yes | 34 | 0.7 | 1.1 (0.3–4.8) |
| Pc = 0.88 | |||
| Any epipodophyllotoxins | |||
| No | 696 | 17.2 | 1 |
| Yes | 63 | 1.3 | 1.5 (1.0–2.3) |
| Pc =0.08 | |||
| Alkylating Agents | |||
| CCNU (Lomustine) | |||
| No | 704 | 18.0 | 1.0 |
| Yes | 55 | 0.4 | 3.0 (1.5–5.3) |
| Pc <0.01 | |||
| CCNU, mg/m2 | |||
| 0 | 704 | 18.0 | 1.0 |
| >0- < 411.18 | 13 | 0.1 | 1.0 (0.1–7.4) |
| 411.18- <800.0 | 23 | 0.1 | 5.0 (2.5–9.9) |
| >800.0 | 12 | 0.04 | 8.2 (3.6–18.9) |
| Pc <0.01 | |||
| CCNU & type of cancer | |||
| No CCNU | 704 | 18.0 | 1.0 |
| CCNU & CNS cancer | 35 | 0.2 | 3.6 (1.2–7.1) |
| CCNU & other cancers | 20 | 0.2 | 2.6 (0.8–5.6) |
| Pc =0.03 | |||
| BCNU (Carmustine) | |||
| No | 729 | 17.7 | 1.0 |
| Yes | 30 | 0.7 | 1.1 (0.5–2.0) |
| Pc 0.50 | |||
| Cyclophosphamide | |||
| No | 450 | 10.3 | 1.0 |
| Yes | 309 | 8.1 | 1.3 (1.0–1.8) |
| Pc =0.05 | |||
| Cyclophosphamide, mg/m2 | |||
| 0 | 450 | 10.4 | 1.0 |
| >0- < 5323.1 | 120 | 3.0 | 1.3 (0.9–1.9) |
| 5323.1- <11,448.3 | 89 | 2.4 | 1.3 (0.9–1.9) |
| >11,448.3 | 74 | 2.1 | 1.3 (0.9–2.1) |
| Pc =0.36 | |||
| Procarbazine | |||
| No | 598 | 17.1 | 1.0 |
| Yes | 161 | 1.3 | 1.0 (0.4–2.3) |
| Pc >0.50 | |||
| Mechlorethamine | |||
| No | 668 | 17.6 | 1.0 |
| Yes | 91 | 0.9 | 0.7 |
| Pc >0.50 | |||
| Melphalan | |||
| No | 745 | 18.2 | 1.0 |
| Yes | 14 | 0.2 | 2.2 (0.7– 5.2) |
| Pc =0.16 | |||
| Thiotepa | |||
| No | 747 | 18.4 | 1.0 |
| Yes | 12 | 0.05 | 5.7 (0.2–15.2) |
| Pc =0.14 |
RRs adjusted for an additive association for categories of the cross-tabulation of radiation dose to the thyroid and and pituitary, with dose categories as in Table 5, and categories of exposure to classes of chemotherapy agents or chemotherapy drugs/doses. Baseline was adjusted for sex, attained age, type of cancer, and calendar year of follow-up. Type of first cancer included to account for possible differential surveillance and shared predisposition. Patients with missing pituitary dose and missing information on the specific class of chemotherapy agent or chemotherapydrugs/doses were excluded from this analysis. Alkylating agents with fewer than 10 cases (busulfan, chlorambucil and ifosfamide) were not included in this analysis.
Number of cases who did not receive chemotherapy, by type of cancer: leukemia=1, CNS=69, HL=98 and others=22
Likelihood ratio test of homogeneity of risk across categories
Abbreviations: BCNU=bis-chloroethyl-nitrosourea, CCNU=cyclohexyl-chloroethyl-nitrosourea; CI=confidence interval;CNS=central nervous system; HL= Hodgkin lymphoma); PYR=person-years; RR=relative risk (rate ratio); Thiotepa= triethylenethiophosphoramide
Radiation dose-response
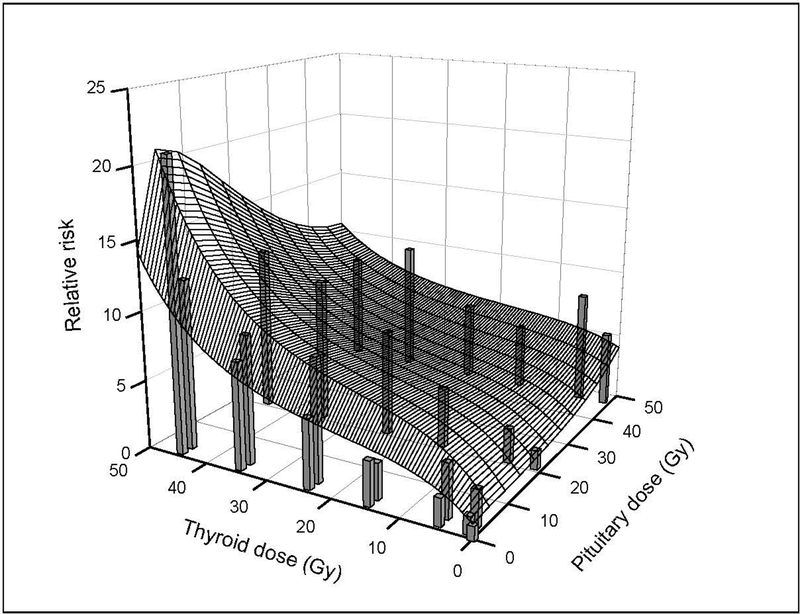
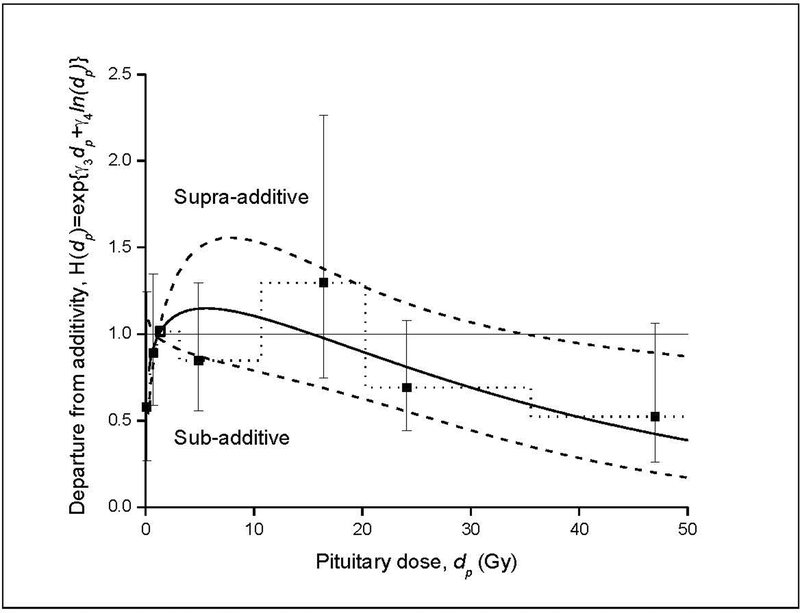
The joint thyroid and pituitary radiation dose-response for incidence of hypothyroidism is summarized in Table 5 and Figure 1. For pituitary doses < 35 Gy, there was a strongly increasing thyroid dose-response, while, at very high pituitary doses (> 40–45 Gy), RRs for thyroid dose flattened. There was a significantly positive pituitary dose-response for thyroid doses < 10 Gy, but this association was attenuated at higher thyroid doses. The joint thyroid and pituitary radiation dose-response model included a linear-exponential(linear-quadratic) term in thyroid dose (dt), linear-exponential(linear) term in pituitary dose (dp) and a multiplicative factor [H(dp)] accounting for pituitary dose-dependent departure from an additive joint association (equation 1). The analysis of the joint effects indicated a significant departure from an additive model (P=0.01), with sub-additive effects at pituitary doses above approximately 16 Gy (Figure 2). This crossover dose to sub-additivity of effects was estimated with considerable uncertainty and partially the consequence of the form of the interaction model.
Table 5.
Estimated incidence rate ratio (RRa) for hypothyroidism (95% CI) by radiation dose to the thyroid gland and pituitary gland: Childhood Cancer Survivor Study.
| RR (95% CI) | |||||
|---|---|---|---|---|---|
| Pituitary dose, Gy (mean) | |||||
| Thyroid dose, Gy (mean) | 0 | >0 - <1 (0.2) | 1 - <10 (2.6) | 10-<35 (21.0) | ≥35 (47.0) |
| 0 | 1.0b | - | - | - | - |
| >0 - <2 (0.6) | - | 1.3 (0.8–2.0) | 2.6 (1.2–5.5) | 1.4 (0.9–1.9) | 5.3 (3.3–8.7) |
| 2- <10 (4.8) | - | 2.0 (0.9–4.2) | 4.2 (1.9–9.3) | 2.4 (1.1–5.3) | 7.6 (3.9–14.7) |
| 10 - <20 (14.7) | - | 3.1 (1.5–6.3) | 3.9 (1.1–13.5) | 3.8 (2.2–6.6) | 4.7 (2.2–9.9) |
| 20 - <30 (24.1) | - | 4.9 (2.8–8.9) | 8.8 (4.5–16.9) | 6.9 (3.7–12.6) | 6.8 (3.8–12.6) |
| 30 - <40 (35.5) | - | 7.8 (3.9–15.4) | 9.0 (5.2–15.8) | 10.1 (4.2–24.4) | 9.5 (4.9–18.4) |
| ≥40 (45.3) | - | 21.0 (8.6–51.5) | 12.2 (6.9–21.6) | 12.4 (6.2–24.5) | 8.0 (3.0–21.6) |
RRs computed using Poisson regression analysis for a joint thyroid and pituitary dose-response. Fitted model includes an additive adjustment for an interaction term of chemotherapy and type of cancer (leukemia, central nervous system cancer, Hodgkin lymphoma, and other cancers). Type of first cancer included to account for possible differential surveillance and shared predisposition. Baseline was adjusted for sex, attained age, type of cancer and attained calendar year.
Reference category
Abbreviations: CI=confidence interval; RR=incidence rate ratio
FIGURE 1.
Fitted joint thyroid and pituitary dose-response for incidence rate of hypothyroidism among five-year survivors of childhood cancer (equation 1): Childhood Cancer Survivor Study. Vertical bars represent observed RRs by categories of radiation dose to the thyroid gland and pituitary gland from Table 5 and are centered at the category-specific, mean thyroid and pituitary doses. Width of bars is arbitrary.
FIGURE 2.
Departure from additivity (deviation from horizontal line) of joint effects of thyroid dose (dt) and pituitary dose (dp) on risk of hypothyroidism as a function of radiation dose to the pituitary gland (solid line) and pointwise 95% confidence interval (dash line) (equation 1). Square symbols (and dot line) with 95% confidence intervals display relative effects for categories of pituitary dose on thyroid radiation dose response. Results suggest a sub-additive effect (antagonism or impedance) for pituitary doses ≥ approximately 16 Gy.
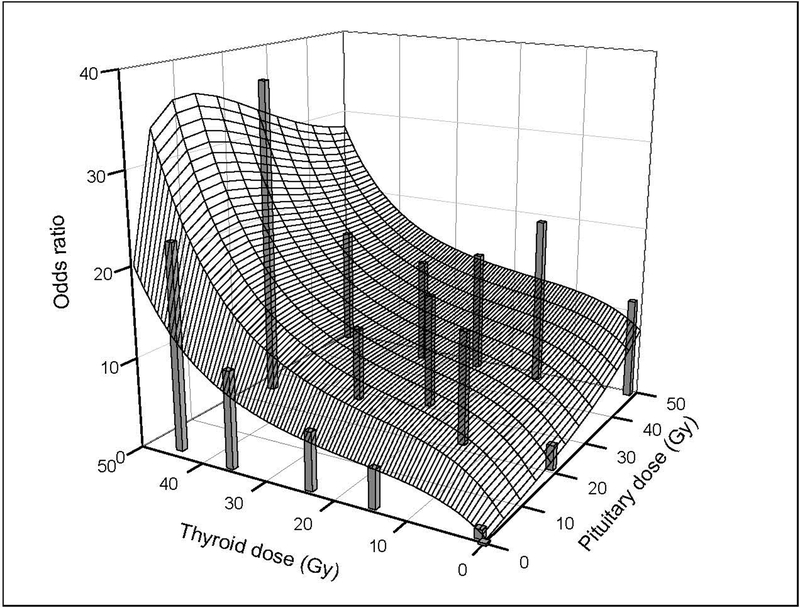
For prevalent hypothyroidism at five years after cancer diagnosis, the fitted model for the joint thyroid and pituitary dose-response was qualitatively similar to the incidence model; that is, the OR increased with thyroid dose at low-to-moderate pituitary doses and with pituitary dose at low thyroid doses, with sub-additivity at high pituitary doses (Figure 3, Supplementary Table 2). The departure from additivity [i.e., H(dp)=1 in equation 1] was statistically significant (p=0.05). Prevalence ORs generally were higher than incidence RRs and estimated with less precision. Parameter estimates for fitted incidence and prevalence dose-response models are given in Supplementary Table 3.
FIGURE 3.
Fitted joint thyroid and pituitary dose-response for prevalence of hypothyroidism five years after cancer diagnosis among five-year survivors of childhood cancer (odds ratio [OR] specified with equation 1): Childhood Cancer Survivor Study. Vertical bars represent observed prevalence ORs by categories of radiation dose to the thyroid gland and pituitary gland from Supplementary Table 2 and are centered at the category-specific, mean thyroid and pituitary doses. Width of bars is arbitrary.
Proportions of radiotherapy- and chemotherapy-associated hypothyroidism cases
Among the 760 observed hypothyroidism cases with information concerning radiotherapy and chemotherapy, the fitted dose-response model estimated that 436.8 (57.5%) were due to radiation treatment, 71.4 (9.4%) were due to chemotherapy and 251.7 (33.1%) were due to causes other than radiation or chemotherapy (background) (Table 6). For the estimated 644.5 irradiated cases (=165.7+478.8), at very low pituitary doses (< 1 Gy), the estimated attributable proportion of radiation-associated cases based on the fitted dose-response model ranged from 10% for thyroid doses < 2 Gy to 91% for thyroid doses ≥ 40 Gy (Supplementary Table 4). For high pituitary doses (> 35 Gy), the attributable proportion was approximately 70% for thyroid doses from < 2 Gy to 40 Gy. For thyroid doses < 2 Gy, the proportion increased monotonically with pituitary dose, whereas, for thyroid doses > 10 Gy, the proportion of cases attributable to radiation varied little with dose to the pituitary.
Table 6.
Observed and fitted numbers of hypothyroidism cases among five-year survivors of childhood cancer, and estimated numbers of cases due to background, radiation treatment and chemotherapy, separately by type of cancer treatment: Childhood Cancer Survivor Study.
| Treatment | Number of cases | Estimated number of cases due toa: | |||||
|---|---|---|---|---|---|---|---|
| Chemo | Radiation | Observed | Fitted | Background | Radiation | Chemo | Percentb |
| No | No | 25 | 24.6 | 24.6 | - | ||
| No | Yes | 165 | 165.7 | 28.6 | 137.1 | 82.7% | |
| Yes | No | 92 | 90.9 | 68.6 | 22.3 | 24.5% | |
| Yes | Yes | 478 | 478.8 | 130.0 | 299.7 | 49.1 | 72.8% |
| Total | 760 | 760.0 | 251.7 | 436.8 | 71.4 | ||
Based on fitted joint thyroid and pituitary dose-response model. Fitted model also includes an additive adjustment for an interaction term of chemotherapy and type of cancer (leukemia, central nervous system cancer, Hodgkin lymphoma, and other cancers). Type of first cancer included to account for possible differential surveillance and shared predisposition. Baseline was adjusted for sex, attained age, type of cancer and attained calendar year.
Percent of estimated number of cases divided by total number of fitted cases in given treatment sub-group, including background cases. For example, the estimated percentage of cases attributable to radiation among those treated by radiation only =137.1 ÷ 165.7 = 82.7%. The estimated percentage of cases due to radiation and chemotherapy among those treated by both modalities = (299.7+49.1) ÷ 478.8 = 72.8%.
Abbreviations: Chemo=chemotherapy
Modifiers of radiation dose effects
We evaluated potential modifiers of the linear coefficient of the thyroid and pituitary dose-response functions and observed homogeneity of the relative changes in linear parameters for the two organs (Table 7, rightmost column). Only for CCNU was the test of homogeneity of the relative change in the linear dose coefficient for thyroid dose and pituitary dose rejected (p<0.01). The column “exp(δj)” represents the relative change in the linear slope parameter for the jth category relative to the referent category. Main findings were as follows. (1) Dose effects (per Gy) were greater in males than females. (2) There was a significant decrease in radiation-related risk with increasing age at exposure, time since exposure and attained age. When adjusted for time since exposure, attained age was not related significantly to the association between radiation dose and hypothyroidism. Radiation-related risk remained elevated more than 25 years after exposure. (3) Radiation-related risk was not significantly associated with type of cancer or chemotherapy overall; however, it was significantly greater among persons treated with bleomycin. (4) The linear component of the radiation dose-response relation for the pituitary, but not the thyroid, was significantly increased among persons treated with CCNU. We note, however, that relatively few survivors had been treated with CCNU, and this result is based on 55 exposed hypothyroidism cases.
Table 7.
Analysis of effect modification for the effects of thyroid and pituitary radiation doses on the incidence of hypothyroidisma: Childhood Cancer Survivor Study.
| Modifier | Cases | PYR/10000 | Exp(δj) | Pb | Pc |
|---|---|---|---|---|---|
| Time since exposure, years | |||||
| <10 | 228 | 5.4 | 1.00d | <0.01 | 0.41 |
| 10–14 | 202 | 4.5 | 0.90 | ||
| 15–19 | 139 | 3.7 | 0.63 | ||
| 20–24 | 106 | 2.8 | 0.33 | ||
| ≥25 | 85 | 2.1 | 0.21 | ||
| Age at exposure, years | |||||
| <5 | 238 | 7.1 | 1.00d | 0.01 | 0.16 |
| 5–9 | 154 | 4.5 | 0.60 | ||
| ≥10 | 368 | 6.9 | 0.56 | ||
| Attained age, years | |||||
| <25 | 383 | 11.0 | 1.00d | 0.01 | 0.55 |
| 25–29 | 133 | 3.2 | 0.54 | ||
| 30–39 | 184 | 3.4 | 0.33 | ||
| ≥40 | 60 | 0.8 | 0.30 | ||
| Sex | |||||
| Male | 308 | 9.7 | 1.00d | <0.01 | 0.45 |
| Female | 452 | 8.7 | 0.41 | ||
| Type of cancere | |||||
| Other cancers | 193 | 8.1 | 1.00d | 0.08 | 0.53 |
| Leukemia | 194 | 6.5 | 0.07 | ||
| CNS cancer | 127 | 2.0 | 0.46 | ||
| HL | 246 | 1.8 | 0.33 | ||
| Chemotherapyf | |||||
| No | 190 | 3.5 | 1.00d | 0.96 | 0.18 |
| Yes | 570 | 14.9 | 0.98 | ||
| CCNU | |||||
| No | 704 | 18.0 | 1.00d | 0.33 | <0.01g |
| Yes | 55 | 0.4 | 1.39g | ||
| Cyclophosphamide | |||||
| No | 450 | 10.3 | 1.00d | 0.88 | 0.63 |
| Yes | 309 | 8.1 | 1.01 | ||
| Bleomycin | |||||
| No | 690 | 17.6 | 1.00d | <0.01 | 0.12 |
| Yes | 69 | 0.8 | 1.77 | ||
P-value for test of homogeneity across levels of modifier; δ2= =δj=0.
P-value for test of homogeneity of modifying effects for thyroid radiation dose and pituitary radiation dose, i.e., , i.e., δt,j = δp,j for all j.
Referent category.
Model includes additional terms for the interaction of type of cancer and chemotherapy, θk, k=1, …, 4.
Model includes additional terms for the interaction of CNS cancer and chemotherapy, θk, k=1, 2.
The relative effects for thyroid dose and pituitary dose differed significantly. Among persons treated with CCNU, exp(δj) was 4.5 (95% CI: 2.4–8.6) for pituitary dose and 0.82 (95% CI: 0.36–1.9) for thyroid dose relative to those not treated with CCNU. The value of 1.39 is based on a model in which relative effects for thyroid dose and pituitary dose were constrained to be the same.
Abbreviations: CCNU=cyclohexyl-chloroethyl-nitrosourea; CNS=central nervous system; HL= Hodgkin lymphoma); PYR=person-years
Although homogeneity of effects for thyroid dose and pituitary dose with respect to time since exposure as an effect modifier was not rejected (p=0.41), we further explored possible differences in latency for presumptive primary versus central hypothyroidism by assuming that most radiation-related cases among survivors with pituitary doses less than 1 Gy (mean, 0.2 Gy) were primary hypothyroidism and most radiation-related cases among survivors with thyroid doses < 2 Gy (mean, 0.6 Gy) were central hypothyroidism and then evaluating temporal trends separately for the two groups (Supplementary Table 5). We stress that this distinction between radiation-related primary and central hypothyroidism is inferential and not based on hormone measurements and that both groups also include cases due to chemotherapy and background causes. Differences are not striking, though in the direction of (presumptive) radiation-related primary hypothyroidism tending to occur relatively more often within the first 15 years after exposure.
Sensitivity analysis
Because the diagnosis of hypothyroidism was self-reported, we reanalyzed data under a more stringent definition of outcome, requiring both self-reported hypothyroidism and use of medications specific for hypothyroidism (Supplementary Tables 6, 7). When compared with Table 5, the joint thyroid and pituitary radiation dose-response pattern is the same, though with somewhat higher incidence rate ratios in the higher thyroid dose categories. Most (79%) of the 777 self-reported cases of incident hypothyroidism also reported use of a hypothyroidism medication.
DISCUSSION
In this large cohort of five-year survivors of childhood cancer followed to an average of 21 years after their cancer diagnosis, radiation dose to both the thyroid gland and hypothalamic-pituitary axis emerged as important predictors of the risk of hypothyroidism, reflecting the multilevel regulation of production of thyroid hormones. The thyroid dose-response relation was contingent on dose to the pituitary and vice-versa2, with the thyroid showing greater radiation sensitivity. This complex joint dose-response pattern reflects a combination of primary and central hypothyroidism, with the latter becoming quantitatively important only at very high doses to the pituitary and/or hypothalamus, which occurred most commonly following irradiation for cancer of the CNS (median dose, 41.3 Gy). We estimate that a transition to sub-additivity of effects occurs at a hypothalamic-pituitary dose of approximately 16 Gy, though this value was estimated with substantial uncertainty and partially the consequence of the form of the interaction model. Flattening of the thyroid dose-response only becomes readily apparent at hypothalamic-pituitary doses substantially higher than 16 Gy. Very high doses to the hypothalamic-pituitary region likely result in reduced secretion of TSH by the anterior pituitary, whether the most relevant target cells for radiation are in the pituitary or hypothalamus (21–23). Due to this radiation-induced damage to the hypothalamic-pituitary axis, the positive feedback of low levels of thyroid hormones on the pituitary is compromised. The THS deficit presumably becomes more severe with increasing hypothalamic/pituitary dose such that above some dose, perhaps 45–50 Gy, little TSH is secreted. At this point, differences in thyroid dose would be of little impact. The reverse also holds. At very high thyroid doses, secretion of T3 and T4 by the thyroid gland is minimal, regardless of level of stimulation by TSH, and differences in dose to the hypothalamic-pituitary region become less important. The present analysis provides quantitative information about the joint thyroid and pituitary/hypothalamus radiation dose-dependence of these relationships. The estimated crossover dose for sub-additivity of effects is similar to the recommended dose limit for the hypothalamus (16.1 Gy) for purposes of radiation treatment planning noted by Sklar and Wolden (24). As these authors also noted, we caution against interpreting this value as a threshold dose.
The available literature concerning effects of hypothalamic-pituitary irradiation on the occurrence of central hypothyroidism, TSH deficiency and, more generally, hypopituitarism, is broadly consistent with our findings. Radiation doses > 20 Gy to the hypothalamic-pituitary region have been reported to increase the risk of central hypothyroidism (11, 23, 25), and the cumulative incidence of hypopituitarism increased with dose to the pituitary for doses of 20->50 Gy (23, 25–28). Radiation-related damage to the hypothalamic-pituitary axis can result in deficiencies of other hormones that are normally produced by the anterior pituitary, in addition to TSH. This includes growth hormone (GH), luteinizing hormone (LH), follicle stimulating hormone (FSH) and adrenocorticotropic hormone (ACTH) (14, 15, 22, 23). In a study of 748 adult survivors of childhood cancer who were treated with cranial radiotherapy, Chemaitilly et al. (14) evaluated risk of hormonal deficiencies in relation to radiation dose (referent: ≤ 22 Gy). Doses ≥ 30 Gy were associated with deficiencies of TSH and ACTH; doses of 22-< 30 Gy were associated with a deficiency of GH; and doses > 22 Gy were associated with deficiencies of LH and FSH. Darzy and Shalet (27, 28) note that both incidence and severity of radiation-related damage to the hypothalamic-pituitary axis increase with time since exposure as well as radiation dose.
In the present study, we observed an increased occurrence (cumulative proportion affected) of hypothyroidism among survivors of HL as well as CNS cancer. Among HL survivors, the occurrence of hypothyroidism was extraordinarily high: 32% of 5-year survivors who participated in the study developed hypothyroidism by 30 years after cancer diagnosis. Among HL survivors who received radiation, thyroid doses typically were very high (median, 35 Gy), whereas pituitary doses were low (median, 1.3 Gy). It is likely that a large majority of the cases of hypothyroidism among HL survivors were primary hypothyroidism (5).
Like CNS cancer patients, ALL patients in the CCSS also were treated with cranial or craniospinal radiotherapy but with lower radiation doses. Median doses to the pituitary and thyroid glands for the leukemia survivors who received radiation in the present study were 21 Gy and 0.8 Gy, respectively, versus 41 Gy and 1.7 Gy for CNS cancer survivors. We did not observe a significantly increased incidence rate of hypothyroidism among leukemia survivors, relative to survivors of cancers other than CNS cancer or HL. We suggest that cranial or craniospinal radiotherapy for leukemia survivors in the CCSS was insufficient to cause central hypothyroidism to a marked degree. However, if, in irradiated leukemia survivors, the hypothalamus was still producing TRH and the pituitary was still producing TSH, even if at diminished levels, radiation effects on the thyroid could still be determinative of the occurrence of hypothyroidism, most of which would be expected to be primary hypothyroidism. One would expect a thyroid dose-response. We note that, unlike the earlier CCSS study by Chow et al (6), the present study also included survivors of types of leukemia other than ALL (mostly acute myelogenous leukemia) and that 83% of leukemia survivors received thyroid doses between 0 and 1.5 Gy. Our results suggest that the risk of hypothyroidism associated with thyroid doses of this magnitude is small.
The most relevant basis for comparison with our overall results comes from the British Childhood Cancer Survivor Study (BCCSS), a large cohort (N=17,981) of five-year childhood cancer survivors in the United Kingdom, 71% of whom were followed for ≥16 years (13). The overall prevalence of hypothyroidism at the end of follow-up was 7%. The highest prevalences (cumulative proportions) were seen among survivors of HL (19.9%), CNS cancer (15.3%), NHL (6.2%) and leukemia (5.2%), an ordering similar to that in the present study. The BCCSS study of hypothyroidism did not include radiation dosimetry.
Among survivors of CNS cancer and HL in the present study, hypothyroidism tended to be diagnosed relatively soon after the childhood cancer, with a median interval of 5–6 years (including prevalent and incident cases). This is not simply a matter of poor survival among patients with these cancers, insofar as appreciable numbers of persons remained under observation for more than 20 years. If radiation-related hypothyroidism tends to begin to occur very soon after radiation exposure, and the largest proportions of hypothyroidism cases due to radiation occurred among survivors of HL (mostly primary hypothyroidism) and CNS cancer (central and primary hypothyroidism), then one would expect shorter average latency intervals among survivors of these cancers. Part of the explanation also could be differential surveillance related to type of cancer and type of hypothyroidism. Close surveillance of HL survivors for thyroid abnormalities has been part of standard follow-up care for many years (3). The situation with CNS cancer survivors is less clear, as is the impact of surveillance as it relates to primary versus central hypothyroidism. Screening for thyroid disease typically has relied almost exclusively on TSH concentrations, which would detect primary hypothyroidism but not central hypothyroidism (26). For detection of the latter, serum levels of free T4 must also be addressed3, but, historically, this was not done routinely, at least in the UK (13).
Among survivors of CNS cancer in the BCCSS who received radiation treatment, the prevalence of self-reported hypothyroidism decreased with follow-up time, and the prevalence was lower among patients discharged for follow-up care to primary care physicians than among those who continued to be followed in the hospital, where more specialized care, including by endocrinologists, would be available (13). This difference increased with follow-up time. The authors hypothesized that the difference was due to under-diagnosis of central hypothyroidism among survivors of CNS cancer and that the reason for the difference between primary care and specialized care settings could be the nature of the monitoring for thyroid disease. Insofar as radiation-induced hypopituitarism may take many years to develop or become symptomatic (27, 28), under-diagnosis of central hypothyroidism might increase with time.
The incidence rate of hypothyroidism among childhood cancer survivors in the CCSS was higher for calendar years of follow-up after 1990 than in earlier years. Part of this may be due to increased awareness of the risk of treatment-related thyroid dysfunction, more frequent thyroid function testing, and/or improvements in the accuracy and sensitivity of laboratory tests. These factors could be particularly important in the context of central hypothyroidism and subclinical hypothyroidism of both types; however, the shorter average follow-up intervals for more recent calendar years may also be a factor, insofar as the age-adjusted RR for hypothyroidism decreased with time since diagnosis of childhood cancer.
While the estimated attributable risk of hypothyroidism due to chemotherapy was much lower than that for radiotherapy, we observed positive associations between risk of hypothyroidism and treatment with CCNU, bleomycin and cyclophosphamide, with the strongest chemotherapy-associated risk occurring among survivors of CNS cancer. CCNU was used most commonly to treat CNS cancer. An earlier experimental study with rats indicated that exposure to CCNU altered neurosecretory function in the hypothalamic-pituitary axis (29). In a study of fertility in the CCSS cohort, having been treated with CCNU or receiving a radiation dose >30 Gy to the hypothalamus/pituitary (relative to ≤ 10 Gy) was associated with a reduced likelihood of becoming pregnant (30). Chemaitilly et al. (14) reported that cranial radiation doses > 22 Gy were associated with deficiencies of LH and FSH. This raises the possibility that toxicity to the hypothalamus and/or anterior pituitary, whether caused by radiation or CCNU, may influence levels of LH and/or FSH as well as TSH, affecting both fertility and susceptibility to central hypothyroidism. However, few human studies have directly implicated chemotherapy in the pathogenesis of central hypothyroidism, hypopituitarism, or LH/FSH deficits (31), and effects of CCNU on the likelihood of becoming pregnant may relate to gonadal rather than pituitary dysfunction. Rose et al. (32) reported evidence of hypothalamic dysfunction among childhood cancer survivors who received chemotherapy but not radiotherapy, but most previous studies have not indicated a strong effect of chemotherapy on the risk of either primary or central hypothyroidism (31). Part of the increased risk of hypothyroidism among brain cancer survivors may relate to effects of the disease on the hypothalamic-pituitary axis other than those due to radiation or chemotherapy; these might include mechanical effects associated with the tumor (e.g., increased intracranial pressure) or tumor surgery (31). Nonetheless, we note that, in the present study, increased risk associated with CCNU was seen among survivors of other types of cancer as well as CNS cancer and among those with pituitary doses < 1 Gy, minimizing the possibility of confounding by radiation. Furthermore, treatment with CCNU appeared to modify the effect of radiation dose to the pituitary but not the thyroid. In contrast, bleomycin appeared to modify effects of radiation dose to the thyroid and pituitary non-differentially. Caution in interpretation of the association of risk with bleomycin is indicated by the fact that it was attenuated among persons who received low radiation doses to the pituitary. The association with cyclophosphamide, though modest and of borderline significance, is noteworthy in light of the frequent use of this drug. Our results concerning chemotherapy and hypothyroidism, while provocative, require confirmation.
Age at exposure, time since exposure (or attained age) and sex also appeared to modify the effect of radiation. As is the case with thyroid cancer, radiation-related risk was inversely associated with age at exposure, possibly due to greater sensitivity of the thyroid at young ages. In the present study, this modifying effect of age at exposure was equally apparent for dose to the thyroid and hypothalamic-pituitary axis. The decrease in the ERR/Gy with increasing time since exposure (or attained age),which also was seen for both thyroid dose and pituitary dose, may reflect diminution of radiation effects with time, reduced surveillance of irradiated survivors over time, or increasing relative importance of non-radiation causes of hypothyroidism. Nonetheless, a positive association with radiation persisted more than 25 years after exposure. Although the radiation-related excess relative risk of hypothyroidism was greater in males than females, the cumulative proportion affected was greater in females, due to the higher background incidence rate.
Based on Table 6, we estimate that 57.5% of hypothyroidism cases were attributable to radiotherapy, 9.4% to chemotherapy and 33.1% to background. This implies that two-thirds of cases were due to cancer treatment and that one third were unrelated to treatment, assuming that the only relevant treatment factors were radiation and chemotherapy. Multiplying one third (0.33) by the overall prevalence of hypothyroidism in our study, combining prevalent and incident cases through 30 years after the cancer diagnosis (=11.2%), yields an estimated prevalence of 3.7% in the absence of RT or CT. This value is somewhat higher than the value of 2.4% for persons ages 12–49 years in the U.S. general population for 1999–2002 based on data from the National Health and Nutrition Examination Survey (NHANES) for 1999–2002 (33). The difference could be due, in part, to cancer survivors being under closer surveillance for thyroid disorders than the general population (particularly during early years following cancer treatment), differences in underlying susceptibility to hypothyroidism, differences in how hypothyroidism was defined or measured, or effects of cancer or its treatment other than radiation and chemotherapy.
Strengths of this study include its large size, long follow-up, longitudinal ascertainment of hypothyroidism at two widely separated time points following treatment for childhood cancer, systematic and detailed ascertainment of treatments for the childhood cancer, and estimation of radiation doses to the thyroid gland and pituitary gland for individual childhood cancer survivors based on radiotherapy records.
The most important limitation is reliance on self-reported outcome information from cancer survivors in the absence of serological measurements that would allow for: (a) confirmation of the presence of reported hypothyroidism; (b) direct assessment of primary versus central hypothyroidism, and (c) detection of unreported hypothyroidism, including subclinical disease. When we used a more stringent definition of hypothyroidism that required both self-reported diagnosis of hypothyroidism by a physician and the use of prescription medications for hypothyroidism, the joint thyroid and pituitary radiation dose-response pattern was little changed. This suggests that under-reporting or under-diagnosis of hypothyroidism may be a more important source of outcome misclassification than over-reporting of hypothyroidism. Our overall estimates of absolute risks of hypothyroidism also could be biased downward because we excluded persons with missing age at diagnosis of the thyroid disorder.
Because the CCSS was limited to 5-year cancer survivors, we could not address latency for hypothyroidism occurring within the first five years. Radiation-related risk is high during this early time window, as reflected in the generally higher dose-specific prevalence odds ratios (Supplementary Table 2) than incidence rate ratios (Table 5). Furthermore, reliance on self-reported date (age) of diagnosis of hypothyroidism means that we might have erroneously placed the date as being inside or outside of the five-year window following date of diagnosis of cancer.
We cannot distinguish between radiation-induced damage to the hypothalamus (and attendant reduction in TRH secretion) from damage to the pituitary gland; either can ultimately result in reduced serum levels of TSH (21–23). It has been suggested that the hypothalamus is somewhat more radiosensitive than the pituitary (22) and that effects on the hypothalamus appear earlier (23). In the context of partial brain irradiation, doses to the pituitary and hypothalamus may differ. We estimated only the former and assumed it to be applicable to both sites.
Misclassification of treatment, including radiation dose, also is possible. Radiotherapy and chemotherapy given at another institution or facility for a recurrence of the cancer may have been under-ascertained. While persons with hyperthyroidism may go on to develop hypothyroidism, sometimes as a result of radiation treatment for hyperthyroidism, this should not be an important issue here, because we censored persons from follow-up at the time of diagnosis of the first functional thyroid disorder. A further limitation is our inability to evaluate aspects of radiation treatment apart from organ dose. Nearly all irradiated patients in the present study were treated with high energy photons, and fractionation schedules were highly standardized. With respect to chemotherapy, we evaluated specific chemotherapeutic agents rather than chemotherapy regimens.
Two-thirds of persons eligible for membership in the cohort were located and agreed to participate. Their experience with respect to the occurrence of hypothyroidism might not be representative of the experience of all eligible persons (34, 35).
In summary, the present study provides new information concerning the radiation dose-dependence for hypothyroidism, in particular, concerning the joint thyroid and pituitary dose-response and factors that modify the dose-response. The combined effects of thyroid and hypothalamic-pituitary doses appear to be less than additive when pituitary or thyroid doses are very high. Although we could not address primary versus central hypothyroidism explicitly, it is very likely that radiation-related cases of hypothyroidism occurring among survivors of CNS cancer were a mixture of central and primary hypothyroidism (primarily the former), and that a large majority of radiation-related cases among survivors of all other types of childhood cancer were primary hypothyroidism. For low pituitary doses (e.g., < 10 Gy), the thyroid dose-response may approximate the dose-response for primary hypothyroidism among childhood cancer survivors, whereas the pituitary dose-response for hypothyroidism at very low thyroid doses (< 1 Gy) may approximate the radiation dose-dependence for central hypothyroidism. Radiation-related risk remained elevated more than 25 years after exposure, highlighting the importance of long-term surveillance for both types of hypothyroidism. Results of this study illustrate how irradiation of one anatomic site may influence the risk of adverse outcomes typically associated with radiation-induced dysfunction in a distant organ. Although the risk of hypothyroidism due to radiotherapy predominates over that due to chemotherapy among childhood cancer survivors, our results also suggest that certain types of chemotherapy may increase the risk.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Intramural Research Program of the U.S. National Institutes of Health (NIH), National Cancer Institute Division of Cancer Epidemiology and Genetics. The Childhood Cancer Survivor Study is supported by the National Cancer Institute (CA55727, G.T. Armstrong, Principal Investigator) and the Lance Armstrong Foundation grant 147149. Support to St. Jude Children’s Research Hospital is also provided by the Cancer Center Support (CORE) grant (CA21765, C. Roberts, Principal Investigator) and the American Lebanese-Syrian Associated Charities (ALSAC).
FOOTNOTES
“Thyroid dose-response” as used here refers to dose-response for hypothyroidism rather than dose-response for thyroid damage. We do not suggest that the dose-response for thyroid damage depends on dose to the pituitary.
The diagnosis of primary hypothyroidism is based on an elevated serum TSH level accompanied by low serum free T4 (fT4) in clinical hypothyroidism and low to low-normal fT4 in subclinical hypothyroidism (10, 26). In central hypothyroidism, serum TSH levels typically are low or within the normal range, with low fT4 levels in clinical disease and low-normal fT4 in subclinical disease (10, 26).
REFERENCES
- 1.National Research Council. Health risks from exposure to low levels of ionizing radiation. BEIR VII Phase 2. Washington, DC: National Academies Press, 2006. [PubMed] [Google Scholar]
- 2.UNSCEAR. Sources, effects and risks of ionizing radiation. UNSCEAR 2013 Report Volume II Scientific Annex B: Effects of radiation exposure of children. http://www.unscear.org/docs/reports/2013/UNSCEAR_2013_Report_Annex_B_Children .pdf, 2013.
- 3.Hancock SL, Cox R, McDougall R: Thyroid diseases after treatment of Hodgkin’s disease. N Engl J Med 1991; 29;325:599–605. [DOI] [PubMed] [Google Scholar]
- 4.Hancock SL, McDougall IR, Constine LS. Thyroid abnormalities after therapeutic external irradiation. Int J Radiat Biol Oncol Biol Phys 1995; 31:1165–70. [DOI] [PubMed] [Google Scholar]
- 5.Sklar C, Whitton J, Mertens A, Stovall M, Green D, Maria N, et al. Abnormalities of the thyroid in survivors of Hodgkin’s disease: data from the Childhood Cancer Survivor Study. J Clin Endocrinol Metab 2000; 85:3227–32. [DOI] [PubMed] [Google Scholar]
- 6.Chow EJ, Friedman DL, Stovall M, Yasui Y, Whitton JA, Robison LL, et al. : Risk of thyroid dysfunction and subsequent thyroid cancer among survivors of acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer 2009; 53:432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ron E, Brenner A. Non-malignant thyroid diseases after a wide range of radiation exposures. Radiat Res 2010; 174:877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veiga LHS, Lubin JH, Anderson H, de Vathaire F, Tucker M, Bhatti P, et al. A pooled analysis of thyroid cancer incidence following radiotherapy for childhood cancer. Radiat Res 2012; 178:365–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veiga LHS, Holmberg E, Anderson H, Pottern L, Sadetzki S, Adams MJ, et al. Thyroid cancer after childhood exposure to external radiation: an updated pooled analysis of 12 studies. Radiat Res, 2016; 185:473–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lubin JH, Adams MJ, Shore R, Holmberg E, Schneider AB, Hawkins M, et al. Thyroid cancer following childhood low-dose radiation exposure: a pooled analysis of nine cohorts. J Clin Endocrinol Metab 2017;102:2575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhandare N, Kennedy L, Malyapa RS, Morris CG, Mendenhall WM. Primary and central hypothyroidism after radiotherapy for head-and-neck tumors. Int J Radiat Oncol Biol Phys 2007; 68:1131–9. [DOI] [PubMed] [Google Scholar]
- 12.Yamada M, Mori M. Mechanisms related to the pathophysiology and management of central hypothyroidism. Endocrinol Metab 2008; 4:683–94. [DOI] [PubMed] [Google Scholar]
- 13.Brabant G, Toogood AA, Shalet SM, Frobisher C, Lancashire ER, Reulen RC, et al. Hypothyroidism following childhood cancer therapy -- an underdiagnosed complication. Int J Cancer 2012; 130:1145–50. [DOI] [PubMed] [Google Scholar]
- 14.Chemaitilly W, Li Z, Huang S, Ness KK, Clark KL, Green DM, et al. Anterior hypopituitarism in adult survivors of childhood cancers treated with cranial radiotherapy: a report from the St Jude Lifetime Cohort Study. J Clin Oncol 2015; 33:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clement SC, Schouten-van Meeteren AYN, Boot AM, Claahsen-van der Grinton HL, Granzen B, Sen Han K, et al. , Prevalence and risk factors for early endocrine disorders in childhood brain tumor survivors: a nationwide multicenter study. J Clin Oncol 2016; 34:4362–70. [DOI] [PubMed] [Google Scholar]
- 16.Leisenring WM, Mertens AC, Armstrong GT, Stovall MA, Neglia JP, Lanctot JQ, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol 2009; 27:2319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robison LL, Armstrong GT, Boice JD, Chow EJ, Davies SM, Donaldson SS, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol 2009; 27:2308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stovall M, Weathers R, Kasper C, Smith SA, Travis L Ron E, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res 2006; 166:141–57. [DOI] [PubMed] [Google Scholar]
- 19.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999; 18:695–706. [DOI] [PubMed] [Google Scholar]
- 20.Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks. The Stata Journal 2004; 4:103–12. [Google Scholar]
- 21.Darzy KH. Radiation-induced hypopituitarism. Curr Opin Endocrinol Diabetes Obes 2013; 20:342–53. [DOI] [PubMed] [Google Scholar]
- 22.Follin C, Erfuth EM. Long-term effect of cranial radiotherapy on pituitary-hypothalamus area in childhood acute lymphoblastic leukemia survivors. Curr Treat Options in Oncol 2016; 17:50. doi: 10.1007/s11864-016-0426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Littley MD, Shalet SM, Beardwell CG. Radiation and hypothalamic-pituitary function. Baillieres Clin Endocrinol Metab. 1990; 4:147–75. [DOI] [PubMed] [Google Scholar]
- 24.Sklar CA, Wolden S. Therapy for pediatric brain tumors and the risk of growth hormone deficiency. J Clin Oncol 2011; 29:4743–4. [DOI] [PubMed] [Google Scholar]
- 25.Littley MD 1, Shalet SM, Beardwell CG, Robinson EL, Sutton ML Radiation-induced hypopituitarism is dose-dependent . Clin Endocrinol. 1989, 31:363–73. [DOI] [PubMed] [Google Scholar]
- 26.Bhandare N, Kennedy L, Malyapa RS, Morris CG, Mendenhall WM. Hypopituitarism after radiotherapy for extranodal head and neck cancers in pediatric patients. Am J Clin Oncol 2008; 31:567–72. [DOI] [PubMed] [Google Scholar]
- 27.Darzy KH, Shalet SM. Hypopituitarism following radiotherapy. Pituitary. 2009; 12:40–50. [DOI] [PubMed] [Google Scholar]
- 28.Darzy KH, Shalet SM. Hypopituitarism following radiotherapy revisited. Endocr Dev 2009; 15:1–24. [DOI] [PubMed] [Google Scholar]
- 29.Pietrzk M, Kozik MB. The influence of the antimitotic drug CCNU on the neurosecretion of rat hypothalamo-hypophyseal system. Folia Histochem Cytobio 1985; 23:27–32. [PubMed] [Google Scholar]
- 30.Green DM, Kawashima T, Stovall M, Leisenring W, Sklar C, Mertens AC, et al. Fertility of female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol 2009; 27:2677–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Santen HM, Vulsma T, Dijkraaf MG, Blumer RME, Heinen R, Jaspers MWM, et al. No damaging effects of chemotherapy in addition to radiotherapy on the thyroid axis in young adult survivors of childhood cancer. J Clin Endocrinol Metab 2003; 88:3657–63. [DOI] [PubMed] [Google Scholar]
- 32.Rose SR, Schreiber RE, Kearney NS, Lustig RH, Danish RK, Burghen GA, et al. Hypothalamic dysfunction after chemotherapy. J Pediatr Endocrinol Metab 2004; 17:55–66. [DOI] [PubMed] [Google Scholar]
- 33.Aoki Y, Belin RM, Clickner R, Jeffries R, Phillips L, Mahaffey KR. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES) 1999–2002). Thyroid 2007; 17:1211–23. [DOI] [PubMed] [Google Scholar]
- 34.Mertens AC, Walls RS, Taylor L, Mitby PA, Whitton J, Inskip PD, et al. Characteristics of childhood cancer survivors predictive of their successful tracing. J Clinical Epidemiol 2004; 57:933–44. [DOI] [PubMed] [Google Scholar]
- 35.Ness KK, Leisenring W, Goodman P Kawashima T, Mertens AC, Oeffinger KC, et al. Assessment of selection bias in clinic-based populations of childhood cancer survivors: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer 2009; 52:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.