Abstract
Introduction
The establishment of the influenza sentinel surveillance system in Kinshasa, Bas Congo, Maniema, Katanga, and Kasai Provinces allowed generation of important data on the molecular epidemiology of human influenza viruses circulating in the Democratic Republic of Congo (DRC). However, some challenges still exist, including the need for extending the influenza surveillance to more provinces. This study describes the pattern of influenza virus circulating in DRC during 2015.
Methodology
Nasopharyngeal swabs were collected from January to December 2015 from outpatients with influenza-like illness (ILI) and in all hospitalized patients with Severe Acute Respiratory Infection (SARI). Molecular analysis was done to determine influenza type and subtype at the National Reference Laboratory (NRL) in Kinshasa using real time reverse transcription-polymerase chain reaction (rRT-PCR). Analysis of antiviral resistance by enzyme inhibition assay and nucleotide sequencing was performed by the Collaborating center in the USA (CDC, Atlanta).
Results
Out of 2,376 nasopharyngeal swabs collected from patients, 218 (9.1%) were positive for influenza virus. Among the positive samples, 149 were characterized as influenza virus type A (Flu A), 67 as type B (Flu B) and 2 mixed infections (Flu A and B). Flu A subtypes detected were H3N2 and H1N1. The Yamagata strain of Flu B was detected among patients in the country. Individuals aged between 5 and 14 years accounted for the largest age group affected by influenza virus. All influenza viruses detected were found to be sensitive to antiviral drugs such as oseltamivar, zanamivir, peramivir and laninamivar.
Conclusion
The present study documented the possible involvement of both circulation of Flu A and B viruses in human respiratory infection in certain DRC provinces during 2015. This study emphasises the need to extend the influenza surveillance to other provinces for a better understanding of the epidemiology of influenza in DRC. It is envisioned that such a system would lead to improved disease control and patient management.
Introduction
Influenza is a respiratory infection caused by influenza viruses [1]. It spreads rapidly among susceptible individuals, particularly during seasonal epidemics or pandemics and imposes a considerable economic burden attributed to increased hospitalizations among others [1]. Human influenza viruses are members of the Orthomyxoviridae family. In humans, only influenza A and B viruses (Flu A and B) are of epidemiological interest [2]. In Africa, for many years, influenza epidemiology was mainly described in countries with temperate climates like South Africa and Morocco [3, 4] Presently, the situation appears to be changing as considerable data in tropical countries such as the Democratic Republic of Congo (DRC), Kenya and Zambia has been reported [5, 6, 7]. On the African continent, influenza causes severe illness and deaths in both temperate and tropical settings [8, 9]. Identification and characterization of circulating influenza viruses is essential to detect the emergence of antigenic drift variants causing influenza epidemics. The detection of antiviral resistance and identification of novel A strains with the potential to cause an influenza pandemic are also needed [10]. Thus, influenza surveillance provides a basis for selection of the virus strains to be included in the annual formulation of influenza vaccines [11, 12].
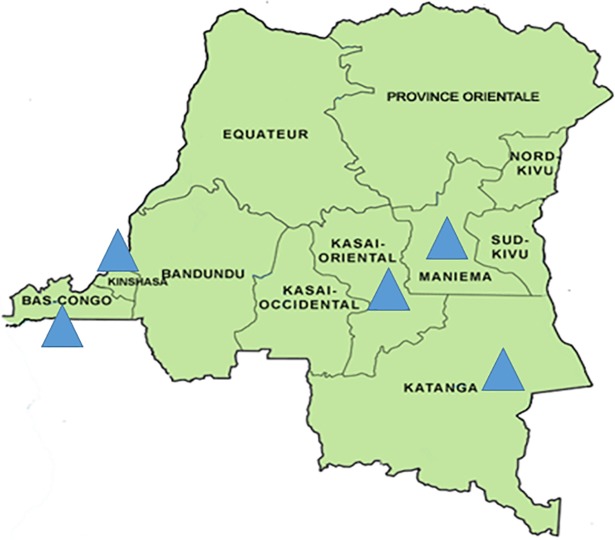
The Influenza Sentinel Surveillance System was established in the DRC in 2008 following the pandemic threat posed by the Asian-origin H5N1 highly pathogenic avian influenza virus. The surveillance was established in 11 urban and rural sentinel sites in five of the 11 provinces of the country (Fig 1). Those sites were selected on the basis of higher accessibility and affordability to patients, higher medical staff qualifications, and adequate specimen storage capacity. The surveillance system serves to monitor antiviral resistance, detect or identify novel influenza strains capable of causing a pandemic; to determine the epidemiology of influenza and other viral respiratory diseases; to characterize and monitor trends in disease and deaths from Severe Acute Respiratory Infection (SARI); to determine the proportions of influenza cases confirmed among patients hospitalized for SARI, to identify Influenza-like Illness (ILI) in outpatient, and to collect data with a view of understanding the disease burden in the country. This study was aimed at describing the pattern of influenza viruses circulating in DRC during 2015.
Fig 1. Map of Democratic Republic of Congo, the blue triangle represent provinces where flu surveillance is conducted.
Materials and methods
Ethics statement
The influenza sentinel surveillance protocol was adapted from World Health Organization (WHO) guidelines with support from the national influenza surveillance program at the DRC Ministry of Health. This protocol was implemented as part of routine public health surveillance by the Ministry of Health and was therefore considered a service and not subject to human subjects review. However, some of the authors had access to identifying informations of the patients who participated in the surveillance program.
Sample collection
Throat and/or nasal swabs obtained from patients with clinical evidence of ILI or SARI were collected using a cryovial which contained 3ml of viral transport medium and kept in the fridge (4–8°C) at the sentinel site, until they were packaged and transported on ice packs to The National Influenza Reference Laboratory (NIRL) in Kinshasa where they were aliquoted in tree different eppendorf tubes and kept frozen at −80°C before processing by real-time reverse-transcription polymerase chain reaction (rRT-PCR) assay. The NIRL, located at the Institut National de Recherche Biomédicale (INRB) is the only laboratory in DRC capable to perform influenza diagnostic by (rRT-PCR) and it has met the standards of the External Quality Assessment Program (EQAP) administered by WHO.
RNA extraction and amplification
RNA was extracted from 140 μL obtained from an aliquot of 1 ml (from one eppendorf tube) using the QIAmp Viral RNA mini kit (Qiagen) and amplified using AgPath One-Step rRT-PCR for influenza virus typing (Ambion, Applied Biosystems) with the ABI 7500 Fast PCR Systems (Life Technologies). Samples were first tested for influenza virus type A and B by rRT-PCR, followed by rRT-PCR subtyping for H1N1, H3N2, H5N1 and H7N9 in samples that were positive for Flu A while those positive to Flu B were tested for B victoria and B yamagata.
Assay for neuraminidase inhibitor susceptibility
Neuraminidase activity was measured using the fluorescent substrate, 2'- (4-methylumbelliferyl)-α-D-N-acetylneuraminic acid (MUNANA; Sigma) [13]. Briefly, 15 μl of virus was incubated with 30 μl of 100 μM MUNANA in 32.5 mM MES buffer pH 6.5 containing 4 mM CaCl2 for 1 hr at 37°C. The reaction was stopped by addition of 150 μl 0.14 M NaOH in 83% Ethanol and fluorescence of the released 4-methylumbelliferone was measured at excitation and emission wavelengths of 365 nm and 450 nm, respectively. The activity of each virus sample was titrated, by assaying serial twofold dilutions, and virus suspensions were adjusted to equivalent Neuraminidase activities, which fell in the linear portion of the activity curve. Each virus was preincubated for 30 minutes at 37°C with oseltamivar, zanamivir, peramivir and laninamivar at final concentrations of 5 μM-0.05pM, in serial 10-fold dilutions, Neuraminidase activity measured and the drug concentration that inhibited 50% of the neuraminidase activity (IC50) was determined [14].
Sequence analysis
Isolates from DRC were sent to the collaborating center in USA (CDC, Atlanta) for sequencing and genetic characterization as previously described by Zhou et al and Shepard et al [15, 16]. Multiple sequence alignments were carried out for each data set using the CLC Main Workbench 5.7.2 software. A phylogenetic tree was inferred from each resulting nt sequence alignment by the Maximum-likelihood (ML) algorithm implemented in the MEGA version 7.7.1 software under the best substitution model (model having the lowest Bayesian Information Criterion), transition/transversion (Ts/Tv) ratio and ML base composition estimated from the empirical dataset [17, 18, 19]. For the tree topology, only trees from distance based analysis, requiring lesser space, are presented. Distance based phylograms were reconstructed by the neighbor-joining method with the Kimura two-parameter method for computing evolutionary distances for genetic distance determination and pairwise deletion for gaps [19].
Results
Molecular detection of influenza virus types and subtypes
During the study period, a total of 2,376 nasopharyngeal swabs were collected from 11 sentinel sites and analysed by the DRC surveillance system team. Of the samples analysed, rRT-PCR assay revealed that 218 samples were positive for influenza virus, of which 149 were characterized as Flu A, 67 as Flu B and two mixed infections (Flu A and B) (Table 1). The Flu A and B viruses were subjected to more detailed characterization by rRT-PCR, which indicated that among the 149 Flu A detected: 107 were classified as H1N1, 38 as H3N2 and 4 samples were untypeable while all isolated Flu B were determined as belonging to the B/Yamagata lineage. It was also noted that the incidence of Flu A (H3N2) was remarkably higher in January 2015 and decreased quickly, then disappeared in the second semester of the year 2015 (Fig 2).
Table 1. Distribution of Flu type by age group in 2015.
| Flu type | Age group | 0–4 years |
5–14 years |
15–24 years |
25–40 years |
>40 years | Total |
|---|---|---|---|---|---|---|---|
| Type A | 42 (7.6%) | 65 (9%) | 14 (3.9%) | 17 (4.5%) | 11 (2.8%) |
149 (6.2%) |
|
| Type B | 20 (3.6%) | 26 (3.6%) | 8 (2.2%) | 9 (2.4%) | 4 (1%) |
67 (2.8%) | |
| Type A&B | 2 (0.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (0.9%) | |
| Negative | 484 (88.32%) | 624 (87.2%) |
331 (93.7%) |
345 (92.9%) |
374 (96.1%) |
2158 (90.8%) | |
| Total | 548 (100%) | 715 (100%) | 353 (100%) | 371 (100%) | 389 (100%) | 2376 (100%) | |
Fig 2. Frequency of type and subtype influenza virus reported by month during 2015.
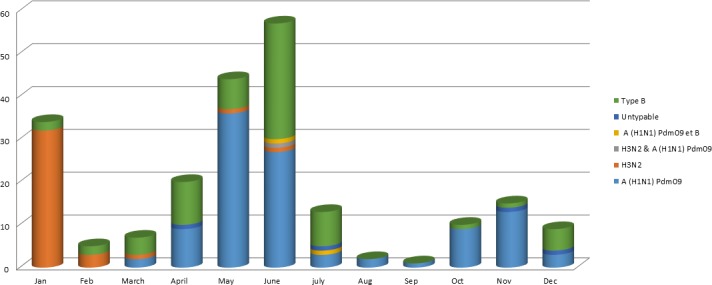
The present study revealed that the largest group of individuals affected by the disease was aged between 5 and 14 years, followed by those from 0 to 5 years (Table 1). During 2015, both Flu A and B co-circulated among human population, in the same province (Bas Congo, Kinshasa, Katanga) at same periods (Fig 2).
Phylogenetic analysis of A (H1N1) pdm09, A (H3N2) and B (Yamagata)
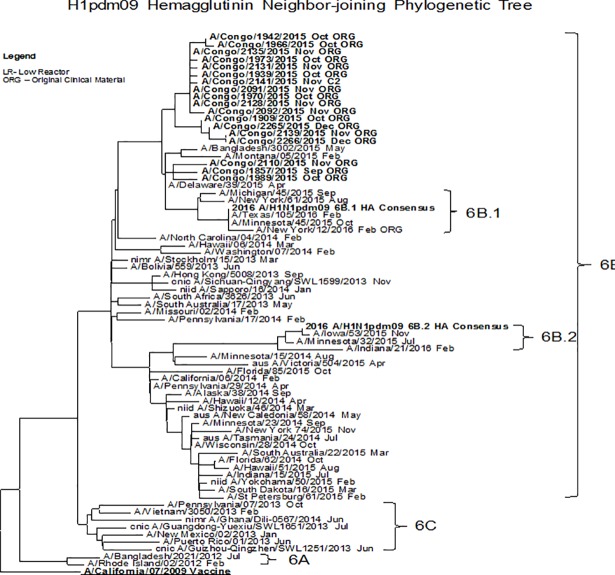
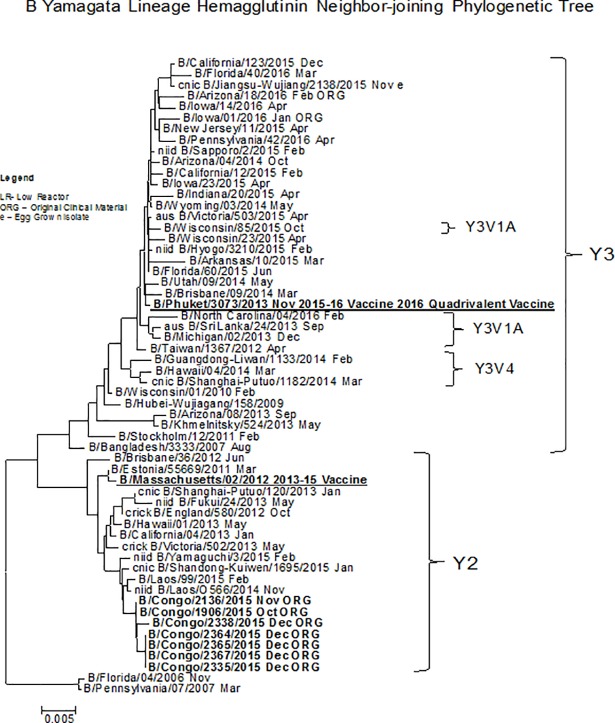
Multiple sequence alignments were carried out for each data set using the CLC Main Workbench 5.7.2 software and phylogenetic trees were constructed using neighbor-joining method [17, 18, 19]. Phylogenetic analysis of the hemagglutinin gene of Flu A (H1N1) viruses in DRC revealed that they were closely related to the 2009 pandemic virus (A/California/07/2009 like) and these viruses fell into genetic group 6B (Fig 3). On the other hand, the hemagglutinin gene of Flu A (H3N2) tested were phylogenetically related to A/Switzerland/9715293/2013. However, the Influenza B (Yamagata) provided by the DRC during 2015, were phylogenetically similar to B/Massachusetts/02/2012 2013–15 and belonged to genetic subgroup 3 (Fig 4). Analysis of susceptibility to the neuraminidase inhibitors exhibited normal sensitive to all antiviral drugs tested (oseltamivir, zanamivir, peramivir and laninamivir.) Table 2.
Fig 3. Representative hemagglutin Neighbor-joining phylogenetic tree of A (H1N1) pdm09 viruses collected in 2015.
The A/California/07/2009 (Underlined, bold) was used as reference in TREESUB. The consensus HA tree and the transcribed aminoacid substitutions were visualized in fig Tree. The scalebar represents the average number of nucleotide substitutions per site. Genetic groups 6B, 6C, 6A are depicted in the tree. DRC isolates (bold) clustered with viruses from genetic groups 6B.
Fig 4. Representative B yamagata Lineage hemagglutinin Neighbor-joining phylogenetic tree of B/Yamagata lineage viruses collected in 2015.
The B/Massachusetts/02/2012 (underlined, bold) was used as reference in TREESUB. The consensus HA tree and the transcribed aminoacid substitutions were visualized in fig tree. The scalebar represents the average number of nucleotide substitutions per site. Genetic groups Y3 and Y2 are depicted in the tree. DRC isolates (bold) clustered in the Y2 lineage.
Table 2. Influenza type, sub type and IC 50 values.
| LABIDx | Flu type and sub type | IC50xx (fold differencexxx) | |||
|---|---|---|---|---|---|
| Oseltamivir | Zanamivir | Peramivir | Laninamivir | ||
| 15GP0026 | Type A/ H3N2 | 0.17 (3) | 0.26 (1) | 0.09 (1) | 0.32 (1) |
| 15GP0001 | Type A/ H3N2 | 0.16 (1) | 0.28 (1) | 0.09 (1) | 0.36 (1) |
| 15GP0002 | Type A/ H3N2 | 0.17 (1) | 0.27 (1) | 0.08 (1) | 0.3 (1) |
| 15GP0004 | Type A/ H3N2 | 0.13 (1) | 0.24 (1) | 0.08 (1) | 0.28 (1) |
| 15GP0008 | Type A/ H3N2 | 0.15 (1) | 0.28 (1) | 0.09 (1) | 0.32 (1) |
| 15GP0015 | Type A/ H3N2 | 0.14 (1) | 0.25 (1) | 0.1 (1) | 0.4 (1) |
| 15 GP 1857 | Type A/ H1pdm09 | 0.16 (1) | 0.23 (1) | 0.06 (1) | 0.19 (1) |
| 15 GP 1942 | Type A/ H1pdm09 | 0.2 (1) | 0.21 (1) | 0.06 (1) | 0.17 (1) |
| 15 GP 1966 | Type A/ H1pdm09 | 0.18 (1) | 0.24 (1) | 0.07 (1) | 0.17 (1) |
| 15 GP 1970 | Type A/ H1pdm09 | 0.16 (1) | 0.18 (1) | 0.06 (1) | 0.24 (1) |
| 15 GP 1973 | Type A/ H1pdm09 | 0.2 (1) | 0.2 (1) | 0.07 (1) | 0.22 (1) |
| 15 GP 2091 | Type A/ H1pdm09 | 0.17 (1) | 0.16 (1) | 0.06 (1) | 0.17 (1) |
| 15 GP 1906 | Type B/ Yamagata | 10.32 (1) | 0.65 (1) | 0.3 (1) | 1.05 (1) |
| 15 GP 2136 | Type B/ Yamagata | 6.92 (1) | 0.5 (1) | 0.33 (1) | 0.82 (1) |
| 15 GP 2335 | Type B/ Yamagata | 8.25 (1) | 0.48 (1) | 0.25 (1) | 0.94 (1) |
| 15 GP 2338 | Type B/ Yamagata | 8.88 (1) | 0.57 (1) | 0.33 (1) | 1.11 (1) |
| 15 GP 2364 | Type B/ Yamagata | 12.95 (2) | 0.46 (1) | 0.3 (1) | 0.88 (1) |
| 15 GP 2365 | Type B/ Yamagata | 7.61 (1) | 0.44 (1) | 0.24 (1) | 0.83(1) |
| 15 GP 2367 | Type B/ Yamagata | 11.15 (1) | 0.46 (1) | 0.28 (1) | 0.9 (1) |
x = Laboratory number (identification)
xx = Generated in fluorescent NI assay
xxx = Compared with the mean IC50 for the drug by subtype for influenza viruses A, and by antigenic lineage for type B viruses
Discussion
Data from this study demonstrated that both Flu A (subtypes H3N2 and H1N1) and B (subtype yamagata) viruses co-circulated in the human population in DRC during 2015. Regarding Flu A, the genotype H3N2 was only predominant in January and February while H1N1 started to be predominant from March and covered the rest of the year 2015. The reason of the short- period spread of H3N2 over the country remain unknown but the most important is that vaccines were made for both of them (H1N1 and H3N2). Contrary to the Flu A profil, only one lineage was present. Indeed, only the B/Yamagata lineage was circulated in DRC from January to December 2015. Similarly, previous studies reported co-circulation of both types of influenza virus in many African countries, including Kenya, Senegal, South Africa, Tunisia and Uganda [20, 21]. Indeed, Flu A (H1N1) pdm09 viruses have continued to circulate worldwide since their emergence in 2009. Moreover, Flu A (H1N1) pdm09 outbreaks closely related to A/California/07/2009-like strains were reported in Asia, Europe, North America and several countries from Africa and Central or South America [22]. Thus, the A/California/07/2009-like virus has been the WHO recommended A(H1N1)pdm09 vaccine component since 2009, including vaccines for the 2016 influenza season in the Southern Hemisphere (SH) and the 2016–2017 influenza season in the Northern Hemisphere (NH) [22].
Genetic characterization revealed that the majority of Flu A(H3N2) viruses tested, were phylogenetically related to A/Switzerland/9715293/2013, the virus which was recommended by the WHO as the Flu A(H3N2) component for the 2015 SH as well as the 2015–2016 NH vaccine formulations [22]. Previous studies reported that both Flu B (B/Victoria/2/87 and B/Yamagata/16/88) lineages have continued to co-circulate, with B/Victoria-lineage viruses predominating in many countries [23, 24, 25]. However, our findings revealed that all Flu B were B/Yamagata and phylogenetically similar to B/Phuket/3073/2013, the second Flu B component of quadrivalent vaccines for the 2016 SH and 2016–2017 NH influenza seasons. The first quarter of the year 2015 was dominated with Flu A (H3N2) co circulating with some Flu B, from March to December 2015, Flu B continued to co circulate with Flu A H1N1pdm which reached its peak in November 2015 (Fig 2).
The emergence of resistance activity to antiviral drug was observed among Cameroonian A(H1N1) isolates in early 2008 and in other African countries [26, 27], while the analyses of of antiviral resistance by enzyme inhibition assay done in this study showed that the DRC isolates were highly sensitive to all antiviral drugs.
It is clear that DRC is one of the biggest African country. Unfortunately, the influenza surveillance system was not able to cover the whole country. Indeed, only 5 out of 11 provinces were selected for implementing influenza surveillance and this could not give the real picture of all Influenza strains circulating in DRC, which considered as a limitation of the data that we could obtain. Results from this study showed that no resistance to antiviral drugs have been detected in DRC samples and this could be because influenza antiviral drugs have never been used extensively in DRC.
Conclusion
This study documented the circulation of Flu A (H3N2 and H1N1) and Flu B (Yagamata) in five provinces under influenza surveillance. However, the influenza situation in other provinces remains largely unknown. So, there is a need to extend the surveillance in the remaining provinces for a better control and knowledge of circulating influenza strains.
Supporting information
(XLSX)
Acknowledgments
We thank Mr Baby Muyembe, Ms Naomi Mitonga and Mr Jean Claude Changa Changa from the Virology department of INRB for their invaluable technical assistance in this study.
Data Availability
The minimal dataset required to replicate the findings are contained within the paper and Supporting Information files. In addition, the virological data can be obtained from the World Health Organization, Influenza Department's FluNet (FluID@who.int) with no restriction to data access.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Gessner BD, Shindo N, Briand S. Seasonal influenza epidemiology in sub-Saharan Africa: a systematic review. Lancet Infect Dis. 2011, 11(3):223–35. 10.1016/S1473-3099(11)70008-1 [DOI] [PubMed] [Google Scholar]
- 2.Kamps BS, Hoffmann C, Preiser J Influenza report on Virology of Human Influenza. Available at www.influenzareport.com/ir/virol.htm. Accessed 17th March 2013. 2006
- 3.McAnerney JM, Cohen C, Moyes J, Besselaar TG, Buys A, Schoub BD, et al. Twenty-five years of outpatient influenza surveillance in South Africa, 1984–2008. J Infect Dis. 2012, 206 (Suppl 1): S153–S158. 10.1093 [DOI] [PubMed] [Google Scholar]
- 4.Barakat A, Ihazmad H, Benkaroum S, Cherkaoui I, Benmamoun A, Youbi M, et al. El (2011): Influenza surveillance among outpatients and inpatients in Morocco, 1996–2009. PLoS One. 2011, 6: e24579– 10.1371/journal.pone.0024579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muyembe Tamfum JJ, Nkwembe E, Bi Shamamba SK, Bankoshi F, Ilunga BK, Katz KA, et al. Sentinel surveillance for influenza-like illness, severe acute respiratory illness, and laboratory-confirmed influenza in Kinshasa, Democratic Republic of Congo, 2009–2011. J Infect Dis. 2012, 206 Suppl 1:S36–40. 10.1093/infdis/jis537 [DOI] [PubMed] [Google Scholar]
- 6.Katz MA, Lebo E, Emukule G, Njuguna HN, Aura B, Cosmas L, et al. Epidemiology, seasonality, and burden of influenza and influenza-like illness in urban and rural Kenya, 2007–2010. J Infect Dis. 2012, 206 (Suppl 1): S53–S60. 10.1093/infdis/jis530 [DOI] [PubMed] [Google Scholar]
- 7.Theo A, Liwewe M, Ndumba I, Mupila Z, Tambatamba B, Mutemba C, et al. (2012) Influenza Surveillance in Zambia, 2008–2009. J Infect Dis. 2012, 206: S173–S177. 10.1093/infdis/jis599 [DOI] [PubMed] [Google Scholar]
- 8.Dawood FS, Iuliano AD, Reed C, Meltzer MI, Shay DK, Cheng PY, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis. 2012, 12: 687–695. 10.1016/S1473-3099(12)70121-4 [DOI] [PubMed] [Google Scholar]
- 9.Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. 2011, 378: 1917–1930. 10.1016/S0140-6736(11)61051-9 [DOI] [PubMed] [Google Scholar]
- 10.Hilleman MR. Realities and enigmas of human viral influenza: pathogenesis, epidemiology and control. Vaccine. 2002, 20 (25–26): 3068–3087. 10.1016/S0264-410X(02)00254-2 [DOI] [PubMed] [Google Scholar]
- 11.WHO. Recommended composition of influenza virus vaccines for use in the 2008–2009 influenza season. Wkly Epidemiol Rec. 2008, 83 (9): 81–87. [PubMed] [Google Scholar]
- 12.WHO. Recommended composition of influenza virus vaccines for use in the 2009 southern hemisphere influenza season. Wkly Epidemiol Rec. 2008, 83 (41): 366–372. [PubMed] [Google Scholar]
- 13.Potier M, Mameli L, Belisle M, Dallaire L, Melancon SB. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-alpha-D-N-acetylneuraminate) substrate. Anal Biochem. 1979, 94: 287–296. 10.1016/0003-2697(79)90362-2 [DOI] [PubMed] [Google Scholar]
- 14.Wetherall N, Trivedi T, Zeller J, Hodges-Savola C, McKimm-Breschkin J, Zambon M, Hayden F. Evaluation of Neuraminidase Enzyme Assays Using Different Substrates To Measure Susceptibility of Influenza Virus Clinical Isolates to Neuraminidase Inhibitors: Report of the Neuraminidase Inhibitor Susceptibility Network. J Clin Microbiol. 2003, 41: 742–750. 10.1128/JCM.41.2.742-750.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou B, Wentworth D Influenza A virus molecular virology techniques. Methods Mol. Biol. 2012, 865: 175–192. 10.1007/978-1-61779-621-0_11 [DOI] [PubMed] [Google Scholar]
- 16.Shepard SS, Meno S, Bahl J, Wilson MM, Barnes J, Neuhaus E. Viral deep Sequencing needs an adaptive approach: IRMA, the iterative refinement meta-assembler. BMC Genomics. 2016, 17: 708 10.1186/s12864-016-3030-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saitou N, and Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 1987, 4(4), 406–425 10.1093/oxfordjournals.molbev.a040454 [DOI] [PubMed] [Google Scholar]
- 18.Gascuel O. BIONJ: An improved version of the NJ algorithm based on a simple model of sequence data. Molecular Biology and Evolution 1997, 14 685–695. 10.1093/oxfordjournals.molbev.a025808 [DOI] [PubMed] [Google Scholar]
- 19.Studier JA, Keppler KJ (. A note on the neighbor-joining algorithm of Saitou and Nei. Molecular Biology and Evolution 1988, 5(6) 729–731. 10.1093/oxfordjournals.molbev.a040527 [DOI] [PubMed] [Google Scholar]
- 20.WHO. Recommended composition of influenza virus vaccines for use in the 2008–2009 influenza season. Wkly Epidemiol Rec.2008, 83 (9): 81–87.Google Scholar [PubMed] [Google Scholar]
- 21.WHO. Recommended composition of influenza virus vaccines for use in the 2009 southern hemisphere influenza season. Wkly Epidemiol Rec. 2008, 83 (41): 366–372 [PubMed] [Google Scholar]
- 22.Influenza Division report, Center for Disease Control and Prevention 2016 (Altanta-USA).
- 23.Hirve S, Newman LP, Paget J, Azziz-Baumgartner E, Fitzner J, Bha N, et al. Influenza seasonality in the tropics and subtropics when to vaccinate. Plos One. 2016, 11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beckett CG, Kasasih H, Ma’roef C, Listiyaningsih E, Elyazar IR, Wuryadi S, et al. Influenza surveillance in Indonesia. 1999–2003. Clin. Infect Dis. 2004, 39: 443–449. 10.1086/422314 [DOI] [PubMed] [Google Scholar]
- 25.Gachara G, Ngeranwa J, Magara JM, Simwa JM, Wango PW, Lifumo SM, et al. influenza virus strain in Nairobi, Kenya. J. clin Virol. 2006, 35(1): 117–118. 10.1016/j.jcv.2005.10.004 [DOI] [PubMed] [Google Scholar]
- 26.Besselaar TG, Naidoo D, Buys A, Gregory V, McAnerney JM, Manamela JM, et al. Widespread oseltamivir resistance in influenza A viruses (H1N1), South Africa. Emerg Infect Dis. 2008, 14 (11): 1809–1810. 10.3201/eid1411.080958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Njouom R, Sadeuh Mba SA, Noah Noah D, Gregory V, Collins P, Cappy P, et al. Hay Circulation of human influenza viruses and emergence of Oseltamivir-resistant A(H1N1) viruses in Cameroon, Central Africa. BMC Infectious Diseases 2010, 10:56 10.1186/1471-2334-10-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
The minimal dataset required to replicate the findings are contained within the paper and Supporting Information files. In addition, the virological data can be obtained from the World Health Organization, Influenza Department's FluNet (FluID@who.int) with no restriction to data access.