Abstract
Common pollutants such as heavy metals and cadmium is among those with high environmental concerns. In vivo studies had shown that cadmium (Cd) causes oocyte degeneration and embryo mortality, and lowers pregnancy rates in mammals. However, there is limited information available about direct effects of Cd on oocyte maturation and/or embryo development. This study was aimed to investigate if Cd has any effect on the oocyte maturation and/or embryo development in vitro. Bovine COCs were collected from the slaughter house and cultured for 24 h in serum-free media only (Controls) or supplemented with 0.2, 2.0 and 20.0 μM CdCl2. At 24 h cumulus cell expansion was assessed in all COCs. COCs were either denuded and stained for determination of nuclear maturation or fertilized for assessment of subsequent embryo development. Cd at the lowest concentration (0.2 μM) did not affect any of the parameters studied. However, at higher concentrations (2.0 and 20.0 μM) it significantly (P < 0.05) reduced the percentage of fully-expanded COCs and significantly (P < 0.05) increased the percentage of partially and/or non-expanded COCs compared to controls and 0.2 μM. Cadmium at higher concentrations (2.0 and 20.0 μM) also significantly (P < 0.01) reduced the percentage of oocytes reaching metaphase II stage compared to controls and 0.2 μM. Post-fertilization cleavage rate in presumptive zygotes and blastocyst development significantly (P < 0.05) reduced 0.2, 2.0 and 20.0 μM CdCl2 compared to the controls (0.0 μM). In conclusion, these results suggest that Cd had direct detrimental effects on the bovine oocyte maturation and its developmental competence.
Keywords: Blastocyst, Bovine oocyte, Cadmium, Embryo development, Maturation, Zygote
1. Introduction
The possible exposure of humans and animals to many industrial chemicals and pesticides has been a growing concern over the last decade for both the scientific community and the general public. Several studies have suggested that these environmental contaminants could adversely affect reproductive functions and embryo abnormalities in animal populations [1], [2], [3], [4], [5], [6]. Cadmium (Cd; atomic number 48; relative atomic mass 112.40) is one of the most toxic environmental and industrial heavy metals because of its long half-life (15–30 years) and its widespread occurrence. Relevant industrial Cd-emitting processes include the combustion of fossil fuels, leachate from landfill sites, run-off agricultural land, mining and smelting operations. Electroplating and manufacture of pigments, plastics, plastic stabilizers and nickel-Cd batteries also produce Cd as a by-product [6]. Moreover, it is also produced by the gradual process of erosion and abrasion of rocks and soils which are caused by events such as forest fires and volcanic eruptions [7], [8].
The real problem with Cd is that it is not eliminated from ecosystems, mainly because of very long half-life but enters the food chain through environmental contamination of soil and is bioaccumulated [9]. Cadmium is excreted at a low rate from the body and is accumulated over time in various body organs including reproductive organs. Various sources of Cd exposure are reported, such as occupational, ecosystem-linked, diet-associated and non-occupational sources [10]. In cows and ewes, effects on various systems have been reported due to Cd present in feed and water [11], [12]. Industrial cadmium (pesticides, batteries, tobacco, rubber processing) is strongly implicated in human and animal poisoning and its low-level exposure is a strong reproductive toxicant to humans too. Physiological concentrations of Cd in blood from cattle reared around different industrial/urban areas have been reported to range from 0.03 to 0.12 µg/mL [13], whereas in humans these values are 2.9 ± 2.5 mg/mL (human blood) [14], and 6.73 ± 0.31 ng/mL (follicular fluid) [15]. In several species, long-term exposure to Cd causes organ damage or functional deficiency [16] and in female mammals Cd affects ovarian function both directly and indirectly [17], [18], [19].
Oocyte maturation is crucial for nuclear maturation, successful fertilization and embryo development [20], [21]. Oocyte maturation is a process during which the oocyte acquires its intrinsic ability to support the subsequent stages of development in a stepwise manner, ultimately reaching to the activation of embryonic genome. This process involves complex and distinct, although linked, events of nuclear and cytoplasmic maturation [22]. Nuclear maturation involves resumption of meiosis, progression to metaphase II (MII), and the accompanying cytoplasmic changes that prepare the oocyte for fertilization and subsequent embryonic development [23].
Cadmium being an environmental contaminant has been reported to be toxic, carcinogenic [24], [25] and mutagenic [25]. Cadmium chloride has been shown to be toxic to the cumulus oocyte complexes [26]. Oocyte development and associated events have been disrupted by Cd administration in different species [6]. The role of Cd in suppressing FSH-induced cumulus expansion in oocyte–cumulus complexes (OCC) isolated from large antral porcine follicles has been described by Mlynarcikova et al. [21]. High concentrations of Cd completely suppressed oocyte maturation and also significantly suppressed an integral component of expanded cumulus cells in porcine oocytes [27].
There is limited number of studies done on the effect of Cd on the processes of maturation and fertilization of the oocyte. Therefore, the aim of this study was, to investigate, in vitro effect of Cd on the bovine oocyte maturation, (IVM) fertilization (IVF), and subsequent embryo development.
2. Materials and methods
2.1. Chemicals and reagents
All chemicals and reagents were purchased from Sigma Chemical Company unless otherwise stated.
2.2. Experiments
Water soluble Cadmium chloride (CdCl2) was used as a source of Cadmium (Cd). Four different concentrations 0.0 µM, 0.2 µM, 2.0 µM and 20 µM CdCl2 were added to serum-free oocyte maturation medium (M-199). In this study, a total of 1081 Cumulus-oocyte complexes (COCs) (grade 1; with homogenous ooplasm and more than four complete layers of cumulus cells) were used. COCs (n: 331) were used for checking nuclear maturation and 360 COCs were used for fertilization and/or cleavage rate of presumptive zygotes.
In the 1st experiment, effect of increasing concentrations of ‘Cd’ on oocyte nuclear maturation was studied. A total of 331 COCs were used in three independent repeats, allocating about 25–30 oocytes per replicate (0 µM Control, 0.2 µM, 2.0 µM and 20 µM CdCl2). Cumulus cell expansion was recorded after 24 h of culture in all 1081 oocytes, and assessment of nuclear maturation of oocytes was done by fixation and staining.
In the 2nd experiment, effect of increasing concentrations of ‘Cd’ in the oocyte maturation medium was studied on the cleavage rate and blastocyst formation. A total of 360 oocytes were used in three independent repeats, divided into four treatments having about 20–25 oocytes per replicate. Oocytes were matured in the serum-free medium supplemented with either 0 µM controls, or 0.2, or 2.0, or 20 µM CdCl2. The matured oocytes were fertilized using frozen semen. Cleavage rate was recorded on day 2 of culture, and blastocyst formation was recorded on day 8 of culture.
2.3. Collection of ovaries and oocytes
Cow ovaries collected from a local slaughter house were transported to the laboratory, within 2 h after slaughtering, in a thermos bottle containing 1× PBS at 37–38 °C. The ovaries were washed with fresh 1X PBS several times immediately after arrival in the laboratory. Cumulus-oocyte complexes (COCs) were aspirated from follicles of 3–8 mm in diameter with an 18-gauge needle using 10 mL syringe. The grade 1 COCs were selected under a stereomicroscope and washed two times in M-199 supplemented with 25 µM HEPES and 0.4% (w/v) BSA.
2.4. In vitro maturation
Oocytes were matured in four-well dishes (NUNC, Thermo Fisher Scientific, Loughborough, Leicestershire, UK) containing 20 µL serum-free maturation medium (M-199 supplemented with 0.6% (w/v) fatty acid-free BSA, 10 mg/mL FSH (Follitropin; Bioniche Animal Health, Belleville, Ontario, Canada), 10 mg/ml LH (Leutropin; Bioniche Animal Health), 10 mg/mL oestradiol and 10 mg/mL gentamycin) for 24 h at 38.5 °C under 5% CO2 in humidified air [28].
2.5. Assessment of cumulus cell expansion
Expansion of cumulus cells was recorded after 24-h of maturation under a stereomicroscope as described previously [29]. Levels of expansion recorded were a). Fully expanded (all cumulus cells were loosened or spreaded), b). Partially expanded (only outer layers of cells were loosened or spreaded) and c). Not expanded.
2.6. Assessment of stage of nuclear maturation of oocytes by staining
Nuclear maturation of oocytes was assessed by staining of oocytes as previously described Marei et al. [29]. All oocytes were denuded and placed on a slide holding the cover slip supported by four droplets of vaseline/paraffin mixture (40:1). Fixation of denuded oocytes was done by placing the slides in acetic acid:methanol fixative (1:3 v/v) for at least 48 h. For staining, aceto-orcein stain (1% orcein in 45% acetic acid) was used. Nuclear maturation of the oocytes was determined according to the morphology of the nuclear material under phase contrast microscope (Leica, Milton Keynes, UK).
2.7. In vitro fertilization
Oocytes matured in vitro with maturation medium containing 0, 0.2, 2.0 and 20 µM CdCl2 were fertilized with frozen semen (gift from Genus ABS, Nantwich, Cheshire, UK) from a single bull as previously described [18]. Sperms were selected by swim up for 45 min at 38.5 °C under 5% CO2 in humidified air in Calcium-free medium. After that, supernatant was centrifuged at 300g at 20 °C and pellet was re-suspended in fertilization medium (Tyrode’s albumin-lactate-pyruvate media supplemented with 0.6% (w/v) fatty acid-free BSA, 1 mg/mL heparin, 50 ng/mL adrenaline and 50 ng/mL hypotaurine). For removing or de-aggregating cumulus cells, COCs were gently pipetted leaving only few layers of cumulus cells. Nearly 30 of these COCs were washed with oocyte wash medium once and then transferred into 400 mL of fertilization medium containing 1 × 106 sperm/mL. These cultures were incubated for 18 h at 38.5 °C in a humidified incubator of 5% CO2 in air.
2.8. Embryo culture
After gentle pipetting, presumptive zygotes were denuded from cumulus cells and cultured in 500 mL of synthetic oviductal fluid medium containing amino acids, sodium citrate and myoinositol [30] supplemented with 0.4% (w/v) fatty acid-free BSA at 38.5 °C in a humidified incubator with 5% O2, 5% CO2 and 90% N2. This was considered as day 1 (fertilization as day 0) of culture and continued up to day 8 for the formation of blastocyst. Cleavage rate and blastocyst development was measured on day 2 and 8 of culture, respectively.
2.9. Statistical analysis
In all the experiments, the data were from at least three independent repeats. Binominal data from cumulus expansion, oocyte nuclear maturation, cleavage, and blastocyst rates were converted into percentages and are presented as Means ± SEM. The data on cumulus expansion were analysed by Chi-square, whereas the data on oocyte nuclear maturation, cleavage rate and blastocyst development were checked for equal variances and normal distribution before being subjected to ANOVA using SPSS 20 (version 20, SPSS Inc., Chicago, IL, USA). Differences were considered significant at 5% probability level.
3. Results
Table 1 gives a summary of the effects of Cd on the expansion of oocyte cumulus cells, nuclear maturation, post-fertilization cleavage and blastocyst development rate.
Table 1.
The effects of different concentrations of cadmium (Cd) on the expansion of oocyte cumulus cells, nuclear maturation, cleavage rate and Blastocyst Development.
| Cadmium | Cumulus Cell Expansion |
Nuclear Maturation |
Embryo Development |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose | Full (%) | Partial (%) | None (%) | GV (%) | GVBD (%) | MI (%) | AI (%) | TI (%) | MII (%) | Cleavage Rate (%) | Blastocyst (%) |
| 0.0 µM | 92.9 ± 3.2a | 5.1 ± 2.2a | 1.9 ± 1.3a | 1.4 ± 1.4a | 0.0 ± 0.0a | 2.9 ± 2.9a | 1.4 ± 1.4a | 2.9 ± 3.0a | 91.2 ± 5.0a | 75.0 ± 5.7a | 19.0 ± 3.5a |
| 0.2 µM | 86.3 ± 3.3a | a10.9±2.2a,b | 2.7 ± 1.6a | 3.6 ± 1.8a | 0.0 ± 0.0a | 1.8 ± 1.8a | 0.0 ± 0.0a | 8.0 ± 1.4a,b | 86.6 ± 4.0a | 54.0 ± 4.5a | 10.0 ± 2.0b |
| 2.0 µM | 50.3 ± 12.9b | 28.3 ± 8.6,a,b | 21.4 ± 6.2a | 3.9 ± 0.8a | 3.0 ± 3.0a | 6.4 ± 3.2a | 0.8 ± 0.8a | 17.1 ± 3.7a,b | 68.7 ± 3.4b | 32.0 ± 4.6c | 6.0 ± 1.5b,c |
| 20 µM | 14.6 ± 7.7c | 33.0 ± 8.5b | 52.4 ± 9.4b | 6.7 ± 3.4a | 0.0 ± 0.0a | 10.9 ± 3.5a | 0.0 ± 0.0a | 19.6 ± 5.3b | 62.8 ± 1.4b | 15.0 ± 4.1d | 1.0 ± 0.0c |
The values are given as Mean ± SEM. Values with different superscripts within a column differ significantly (P < 0.05; Cumulus cell expansion and embryo development) (P < 0.01; Nuclear maturation).
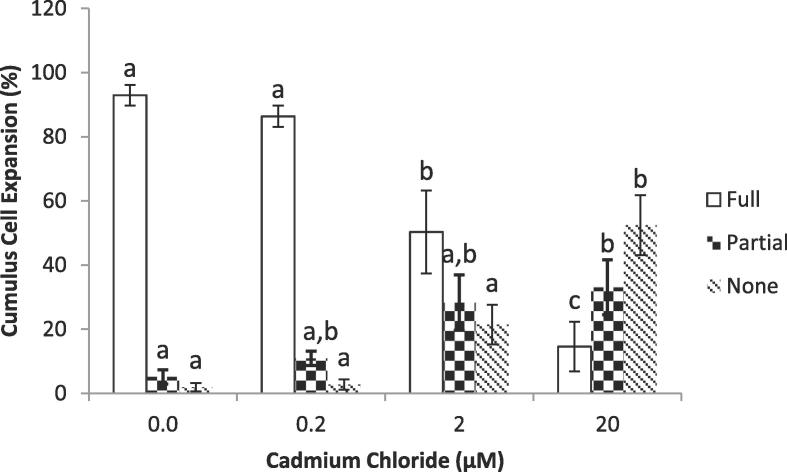
3.1. Cumulus cell expansion
Fig. 1 shows percentage of COCs with different levels of expansion of cumulus cells around the oocytes as affected by different concentrations of Cd. Cadmium (Cd) at 2.0 and 20.0 µM significantly (P < 0.05) reduced the percentage of fully-expanded COCs compared to controls and 0.2 µM. However, no difference was observed between the controls and 0.2 µM Cd. Moreover, percentage of oocytes with partial or no expansion of cumulus cells significantly (P < 0.05) increased when treated with 2.0 and 20.0 µM compared to controls and 0.2 µM, whereas no differences were observed between the controls (0.0 µM CdCl2) and lowest concentration group (0.2 µM CdCl2).
Fig. 1.
The effect of different concentrations of Cadmium (Cd) on the percentage of Cumulus cell expansion. The bars (Mean ± SEM) with different letters within the same expansion category are significantly (P < 0.05) different.
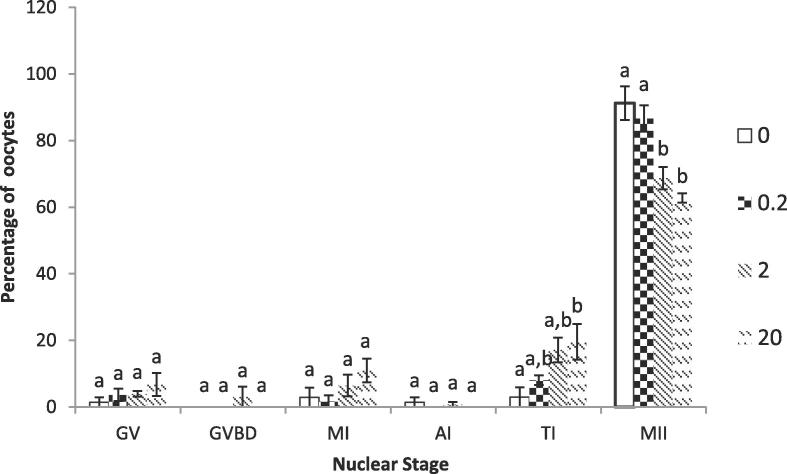
3.2. Nuclear maturation
Cadmium at 2.0 and 20.0 µM significantly (P < 0.01) reduced the percentage of oocytes reaching metaphase II stage compared to controls and 0.2 µM Cd. Both 2 and 20 µM CdCl2 were also significantly different with respect to Telophase I but only from control group (0.0 µM CdCl2). Percentages of oocytes in other stages of nuclear maturation (GV, GVBD, MI and AI) were similar among different treatment groups (Fig. 2).
Fig. 2.
The effect of different concentrations of CdCl2 (0.0, 0.2, 2.0, 20.0 µM) on the percentage of oocytes at different stages of nuclear maturation. The bars (Mean ± SEM) with different letters within a certain stage of nuclear maturation are significantly (P < 0.01) different.
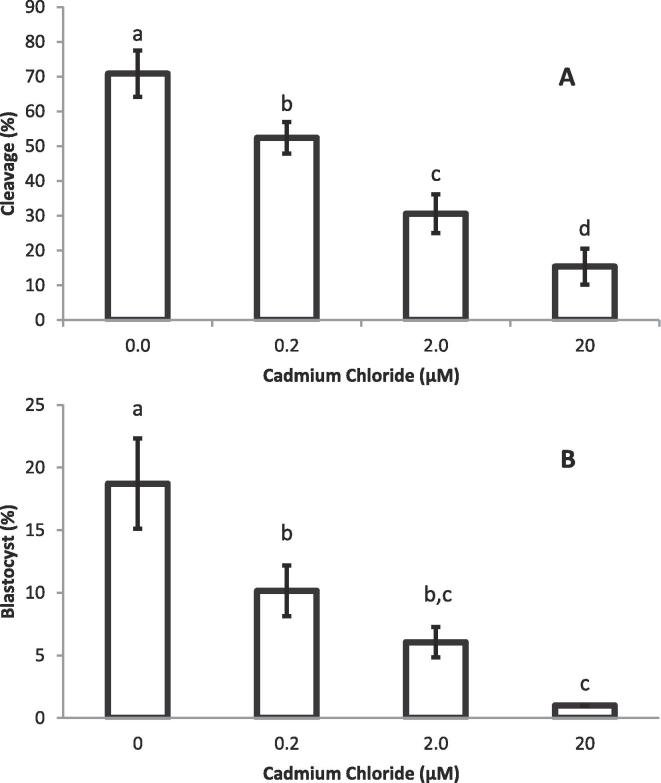
3.3. Embryo development
Post-fertilization cleavage rate in presumptive zygotes (Fig. 3A) and blastocyst development (Fig. 3B) were significantly (P < 0.05) different between the controls (0.0 μM) and the Cd-treated groups.
Fig. 3.
The effect of different concentrations of CdCl2 (0.0, 0.2, 2.0, 20.0 µM) on the% cleavage rate of presumptive zygotes (A) and % Blastocyst development (B). The bars (Mean ± SEM) with different letters are significantly (P < 0.05) different.
4. Discussion
Animal populations in the world are exposed daily to varying levels of environmental contaminants causing adverse affects on reproductive, endocrine, and immune functions. Common pollutants are heavy metals and pesticides. The toxic heavy metals of high environmental concern are cadmium, lead, arsenic, and mercury [12]. The results of the present study have shown a significant adverse effect of cadmium on the in vitro bovine oocyte maturation and embryo development. Our results are in consistent with a number of studies reported earlier. For example, female Wistar rats treated subcutaneously with a single concentration of 0.02, 0.04, 0.06 or 0.08 mmol CdCl2 per kg body weight showed a decreased (P < 0.001) in oocyte maturation rate with a metaphase II (MII) rate of 86.6%, 77.6%, 43%, 4.3% for 0.02, 0.04, 0.06 or 0.08 mmol CdCl2, respectively [31]. Nandi et al. [12] investigated the effects of nine concentrations (0, 0.005, 0.05, 0.5, 1.0, 1.5, 2.5, 5, and 10 µg/ml) of cadmium on buffalo oocyte viability, morphological abnormities, maturation, and embryonic development in vitro. Cadmium was found to have a dose-dependent effect on the viability, morphological abnormalities, maturation, cleavage and morula/blastocyst yield, and blastocyst hatching. A significant decline in viability of oocytes was observed at 1.0 mg/mL cadmium compared to the control group. The concentration of cadmium causing 100% oocyte death (1-day culture) was 18 µg/mL. Cadmium at 1.0 µg/mL caused a significant reduction in the maturation of oocytes compared to the lower concentrations.
Cadmium chloride was quite potent to reduce oocyte nuclear maturation in vitro. At 1.48 µM CdCl2 the percentage of the oocytes that reached MII stage was already reduced to 50% compared to the control [32]. Moreover, Cadmium chloride has been reported to be toxic to the in vitro process of bovine oocyte maturation in a concentration-dependent manner [26]. In experiments with the ovine oocytes, Leoni et al. [33] found that maturation rate was significantly affected at 2 and 20 µM CdCl2, with a metaphase II (MII) rate of 63.8% and 32.0%, respectively when compared with controls (96.8%). Both Cd concentrations used also reduced the numbers of fertilized oocytes in culture (25.9% and 4.7%, respectively; control rate, 76.1%) and increased the rate of oocyte degeneration (16.0 and 60.6%, control rate: 4.3%) (22). De et al. [34] reported that two-cell embryos of the mouse were remarkably resistant to Cd, but toxicity increased with development, and morulae readily degenerated after Cd exposure.
It is clear from the results of this and the above-mentioned studies that Cadmium at physiological concentrations can negatively affect oocyte maturation, fertilization and subsequent embryo development. This negative effect observed in our study may be because of Cadmium’s ability to suppress hyaluronic acid synthesis and therefore cumulus cell expansion, as has been reported in porcine oocytes [9], [15] or because of its cytotoxic effect as in the case of the bovine cumulus cells (CCs) [35]; the denuded oocytes (without cumulus cells) became less sensitive to Cd than cumulus-enclosed oocytes, indicating higher Cd-induced cytotoxicity on CCs than the oocyte [35]. It is also a possibility that there might be more than one mechanism in operation at the same time. In addition, Cd may exert its effect by interacting with membrane transporters involved in the uptake of nutritive metals, such as calcium, iron, zinc, copper and manganese, through a process called “ionic mimicry” or alternatively by depleting cell protein sulfhydryl reserves and interfering with normal protein folding [11] or by inducing cumulus-oocyte mitochondria over-activity and oxidative damage as has been reported in case of ovine COCs [36]. However, it was not the aim of this study to investigate how Cd may achieve its effect on IVM of bovine oocytes and subsequent IVF and embryo development and therefore, is a matter for further research.
5. Conclusions
Cadmium chloride inhibited the bovine oocyte growth and development in vitro. Our results showed that cadmium at higher concentrations (2 and 20 µM CdCl2) reduce the in vitro bovine oocyte cumulus expansion, nuclear maturation, the cleavage rate and blastocyst development. These results suggest that Cadmium has detrimental effects on the bovine oocyte maturation and its developmental competence.
Acknowledgments
Acknowledgement
We are thankful to the Turkish Council of Higher Education for the financial support to Assoc. Prof. Yaşar AKAR for his visit to the Reproduction laboratory of the Royal Veterinary College, London, UK.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- 1.Colborn T., vom Saal F.S., Soto A.M. Developmental effects of endocrine disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sumpter J.P., Jobling S. Vitellogenesis as a biomarker for estrogenic contamination of the aquatic environment. Environ Health Perspect. 1995;103(Suppl 7):173–178. doi: 10.1289/ehp.95103s7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toppari J., Larsen J.C., Christiansen P., Giwercman A., Grandjean P., Guillette L.J., Jr. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. 1996;104(Suppl 4):741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vos J.G., Dybing E., Grem H.A., Ladefoged O., Lambré C., Tarazona J.V. Health effects of endocrine disrupting chemicals on wildlife, with special reference to the European situation. Crit Rev Toxicol. 2000;30:71–133. doi: 10.1080/10408440091159176. [DOI] [PubMed] [Google Scholar]
- 5.Foster P.M.D., Mylchreest E., Gaido K.W., Sar M. Effects of phthalate esters on the developing reproductive tract of male rats. Hum Reprod Update. 2001;7:231–235. doi: 10.1093/humupd/7.3.231. [DOI] [PubMed] [Google Scholar]
- 6.Thompson J., Bannigan J. Cadmium: toxic effects on the reproductive system and the embryo. Reprod Toxicol. 2008;25:304–315. doi: 10.1016/j.reprotox.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Marcano L.B.C., Carruyo I.M., Montiel X.M., Morales C.B., De Soto M. Effect of cadmium on cellular viability in two species of microalgae (Scenedesmus sp. and Dunaliella viridis) Biol Trace Elem Res. 2009;130:86–93. doi: 10.1007/s12011-009-8316-y. [DOI] [PubMed] [Google Scholar]
- 8.Cannino G., Ferruggia E., Luparello C., Rinaldi A.M. Cadmium and mitochondria. Mitochondrion. 2009;9:377–384. doi: 10.1016/j.mito.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Sugita M., Tsuchiya K. Estimation of variation among individuals of biological half time of cadmium calculated from accumulation data. Environ Res. 1995;68:31–37. doi: 10.1006/enrs.1995.1005. [DOI] [PubMed] [Google Scholar]
- 10.Flora S.J.S., Pachauri V., Saxena G. Arsenic, cadmium and lead. In: Gupta R.C., editor. Reproductive and developmental toxicology. Elsevier Inc; London: 2011. pp. 415–438. [Google Scholar]
- 11.Bires J., Maracek I., Bartko P., Biresova M., Weissova T. Accumulation of trace elements in sheep and the effects upon qualitative and quantitative ovarian changes. Vet Hum Toxicol. 1995;37:349–356. [PubMed] [Google Scholar]
- 12.Nandi S., Gupta P.S.P., Selvaraju S., Roy S.C., Ravindra J.P. Effects of exposure to heavy metals on viability, maturation, fertilization, and embryonic development of buffalo (Bubalus bubalis) oocytes in vitro. Arch Environ Contam Toxicol. 2010;58:194–204. doi: 10.1007/s00244-009-9342-7. [DOI] [PubMed] [Google Scholar]
- 13.Patra R.C., Swarup D., Sharma M.C., Naresh R. Trace mineral profile in blood and hair from cattle environmentally exposed to lead and cadmium around different industrial Units. J Vet Med A. 2006;53:511–517. doi: 10.1111/j.1439-0442.2006.00868.x. [DOI] [PubMed] [Google Scholar]
- 14.Angerer P., Kessel R., Bencze K., Tewordt M., Mauermayer R., Friesen A. The cadmium content of human tissues from biopsies. Zentralbl Bakteriol Mikrobiol Hyg B. 1988;187:18–30. [PubMed] [Google Scholar]
- 15.Zenzes M.T., Krishnan S., Krishnan B., Zhang H., Casper R.F. Cadmium accumulation in follicular fluid of women in in vitro fertilization-embryo transfer is higher in smokers. Fertil Steril. 1995;64:599–699. doi: 10.1016/s0015-0282(16)57799-1. [DOI] [PubMed] [Google Scholar]
- 16.Sharara F.I., Seifer D.B., Flaws J.A. Environmental toxicants and female reproduction. Fertil Steril. 1998;70:613–622. doi: 10.1016/s0015-0282(98)00253-2. [DOI] [PubMed] [Google Scholar]
- 17.Paksy K., Varga B., Horvath E., Tatrai R., Ungvary G. Acute effects of cadmium on preovulatory serum FSH, LH and prolactin levels and on ovulation and ovarian hormone secretion in oestrus rats. Reprod Toxicol. 1989;3:241–247. doi: 10.1016/0890-6238(89)90018-x. [DOI] [PubMed] [Google Scholar]
- 18.Paksy K., Varga B., Naray M., Kiss I., Folly G., Ungvary G. Uptake and distribution of Cd in the ovaries, adrenals and pituitary in pseudopregnant rats. Effects of Cd on progesterone levels. Environ Res. 1990;51:83–89. doi: 10.1016/s0013-9351(05)80184-7. [DOI] [PubMed] [Google Scholar]
- 19.Wier P.J., Miller R.K., Maulik D., Sant’Agnese P.A. Toxicity of cadmium in the perfused human placenta. Toxicol Appl Pharmacol. 1990;105:156–171. doi: 10.1016/0041-008x(90)90367-4. [DOI] [PubMed] [Google Scholar]
- 20.Luz J.V., Alcântara-Neto A.S., Batista R.I.T.P., Souza J.M.G., Teixeira D.I.A., Melo L.M. Expression of CD44 in sheep oocytes and preimplantation embryos. Genet Mol Res. 2012;11(2):799–809. doi: 10.4238/2012.April.3.2. [DOI] [PubMed] [Google Scholar]
- 21.Mlynarcikova A., Fickova M., Scsukova S. Ovarian intrafollicular processes as a target for cigarette smoke components and selected environmental reproductive disruptors. Endocr Regul. 2005;39:20–31. [PubMed] [Google Scholar]
- 22.Ferreira E.M., Vireque A.A., Adona P.R., Meirelles F.V., Ferriani R.A., Navarro P.A.A.S. Cytoplasmic maturation of bovine oocytes: structural and biochemical modifications and acquisition of developmental competence. Theriogenology. 2009;71:836–848. doi: 10.1016/j.theriogenology.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 23.Anas M.K.I., Suzuki C., Yoshioka K., Iwamura S. Effect of mono-(2-ethylhexyl) phthalate on bovine oocyte maturation in vitro. Reprod Toxicol. 2003;17:305–310. doi: 10.1016/s0890-6238(03)00014-5. [DOI] [PubMed] [Google Scholar]
- 24.Koudrine A.V. Trace elements and apoptosis. J Trace Elem Med Biol. 1998;12:65–76. doi: 10.1016/S0946-672X(98)80028-X. [DOI] [PubMed] [Google Scholar]
- 25.Schenk B., Weimer M., Bremerc S., Burg B.V.D., Cortvrindte R., Freybergerf A. The ReProTect feasibility study, a novel comprehensive in vitro approach to detect reproductive toxicants. Reprod Toxicol. 2010;30:200–218. doi: 10.1016/j.reprotox.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Luciano A.M., Franciosi F., Lodde V., Corbani D., Lazzari G., Crotti G. Transferability and inter-laboratory variability assessment of the in vitro bovine oocyte maturation (IVM) test within ReProTect. Reprod Toxicol. 2010;30(1):81–88. doi: 10.1016/j.reprotox.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Vrsanska S., Nagyova E., Mlynarcikova A., Fickova M., Kolena J. Components of cigarette smoke inhibit expansion of oocyte–cumulus complexes from porcine oocytes. Physiol Res. 2003;52:383–387. [PubMed] [Google Scholar]
- 28.Fouladi-Nashta A.A., Campbell K.H.S. Dissociation of oocyte nuclear and cytoplasmic maturation by the addition of insulin in cultured bovine antral follicles. Reproduction. 2006;131(3):449–460. doi: 10.1530/rep.1.00581. [DOI] [PubMed] [Google Scholar]
- 29.Marei W.F., Wathes D.C., Fouladi-Nashta A.A. The effect of linolenic acid on bovine oocyte maturation and development. Biol Reprod. 2009;81:1064–1072. doi: 10.1095/biolreprod.109.076851. [DOI] [PubMed] [Google Scholar]
- 30.Holm P., Booth P.J., Schmidt M.H., Greve T., Callesen H. High bovine blastocyst development in a static in vitro production system using sofaa medium supplemented with sodium citrate and myo-inositol with or without serum-proteins. Theriogenology. 1999;52(4):683–700. doi: 10.1016/S0093-691X(99)00162-4. [DOI] [PubMed] [Google Scholar]
- 31.Pisa J., Cibulka J., Ptácek M. Effect of subcutaneous application of a single cadmium dose on oocyte maturation in vitro. Physiol Bohemoslov. 1990;39(2):185–190. [PubMed] [Google Scholar]
- 32.Van Woudenberg A.B., Grollers-Mulderij M., Snel C., Jeurissena N., Stieruma R., Wolterbeek A. The bovine oocyte in vitro maturation model: a potential tool for reproductive toxicology screening. Reprod Toxicol. 2012;34:251–260. doi: 10.1016/j.reprotox.2012.05.098. [DOI] [PubMed] [Google Scholar]
- 33.Leoni G., Bogliolo L., Deiana G., Berlinquer F., Rosati I., Pintus P.P. Influence of cadmium exposure on in vitro ovine gamete dysfunction. Reprod Toxicol. 2002;16(4):371–377. doi: 10.1016/s0890-6238(02)00040-0. [DOI] [PubMed] [Google Scholar]
- 34.De S.K., Paria B.C., Dey S.K., Andrews G.K. Stage-specific effects of cadmium on preimplantation embryo development and implantation in the mouse. Toxicology. 1993;80:13–25. doi: 10.1016/0300-483x(93)90073-2. [DOI] [PubMed] [Google Scholar]
- 35.Lazzari G., Tessaro I., Crotti G., Galli C., Hoffman S., Bremer S. Development of an in vitro test battery for assessing chemical effects on bovine germ cells under the ReProTect umbrella. Toxicol Appl Pharmacol. 2008;233:360–370. doi: 10.1016/j.taap.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 36.Martino N.A., Marzano G., Mangiacotti M., Miedico O., Sardanelli A.M., Gnoni A. Exposure to cadmium during in vitro maturation at environmental nanomolar levels impairs oocyte fertilization through oxidative damage: a large animal model study. Reprod Toxicol. 2017;69:132–145. doi: 10.1016/j.reprotox.2017.02.005. [DOI] [PubMed] [Google Scholar]