Abstract
Solid tumors are complex three-dimensional (3D) networks of cancer and stromal cells within a dynamic extracellular matrix. Monolayer cultures fail to recapitulate the native microenvironment and therefore are poor candidates for pre-clinical drug studies and studying pathways in cancer. The tissue engineering toolkit allows us to make models that better recapitulate the 3D architecture present in tumors. Moreover, the role of the mechanical microenvironment, including matrix stiffness and shear stress from fluid flow, is known to contribute to cancer progression and drug resistance. We review recent developments in tissue engineered tumor models with a focus on the role of the biomechanical forces and propose future considerations to implement to improve physiological relevance of such models.
Keywords: ex vivo tumor model, matrix stiffness, shear stress, tissue engineering
Graphical abstract
Introduction
Cancer arises due to genetic aberrations and the initial expansion of neoplastic cells. However, solid tumor progression and metastasis is facilitated by the niche hosting the cancerous cells. This niche, known as the tumor microenvironment, is a complex three-dimensional (3D) network of stromal, vascular, and immune cells within a dynamic extracellular matrix (ECM) [1]. Yet, pre-clinical drug testing is carried out in two-dimensional monolayer cultures for their ease and efficiency despite flat polystyrene being a poor mimic for the tumor microenvironment [2]. The development of 3D cell culture methods has led to several engineered systems in natural and synthetic scaffolds that better recapitulate cell-cell and cell-ECM interactions than traditional monolayer culture [3–5].
Of growing interest is the role of physical changes due to biomechanical forces in tumor progression as cells use mechanotransduction systems to sense and respond to external forces [6,7]. Two major changes observed in solid tumors are increased ECM stiffness and altered interstitial fluid flow [8]. Matrix metalloproteinases (MMPs) heavily remodel the ECM, which leads to extensive crosslinking of collagen type I fibrils. The changes in stiffness and architecture have a direct effect on processes involving cell-ECM contacts such as migration and numerous downstream effects via integrin-mediated signaling cascades including those related to the cell cycle and differentiation [6]. As such, stiffness and integrin signaling contribute to cancer resistance to chemotherapeutics. The specific mechanisms of known mechanosensitive pathways have been reviewed in depth elsewhere [9–11]. Abnormal fluid flow leads to an uneven distribution of soluble growth factors and nutrients; the resultant shear stress remodels the tumor vasculature, and like changes to ECM stiffness, activates integrin-mediated signaling pathways that alter cell proliferation and differentiation [8,12]. Fluid flow also facilitates metastasis by carrying cancer cells away from the primary tumor to distant sites through the blood stream or lymphatic system.
The tissue engineering paradigm of generating tissues through a combination of scaffolds, cells, and biochemical factors provides a large toolkit for modeling these physical changes observed in cancer. 3D tissue engineered tumors have great potential to be used for pre-clinical drug screening and understanding pathways in cancer, particularly changes in gene and protein expression that lead to outcomes such as malignant phenotype, acquired drug resistance, and metastatic potential. Some examples of tissue engineered tumor models that investigate the role of the mechanical microenvironment are listed in Table 1. Here we briefly address materials selection for tissue engineered tumor models and then highlight recent work that has employed tissue engineering principles to model the biomechanical forces present in solid tumors. We end with a discussion of the direction in which tumor modeling should progress in the future.
Table 1.
Examples of 3D cancer models that evaluate the mechanical forces in the tumor microenvironment.
| Model | Mechanical environment |
Outcomes |
|---|---|---|
| Ovarian cancer spheroids in gelatin methacrylamide [15] | Substrate stiffnesses: 0.7–16.5 kPa |
|
| HCC on collagen-coated polyacrylamide [29–32] | Substrate stiffnesses: 1–10 kPa and 6–16 kPa | Increasing stiffness |
| ES in electrospun PCL [37] | Flow-induced shear stress: 1.7–17.0 cPa | Increasing shear stress
|
| ES in porous collagen and hyaluronic acid [41] | Unconfined dynamic compression (1% or 10% strain) | Combination of matrix strain and interstitial flow
|
| Colorectal cancer co-cultured with fibroblasts in decellularized jejunum [47] | Flow-induced shear stress: 3.0–5.0 mPa (average 3.34 mPa) | Flow conditions
|
| MSCs grown in breast cancer-conditioned media in porous mineralized poly(lactic-co-glycolic acid) [48] | Dynamic compression (10% peak strain) | Compressive loading
|
Materials selection in tissue engineered tumor models
As in any tissue engineering application, materials selection is an important factor to consider in the design of engineered tumor models. Natural ECM materials are desirable because they better recapitulate the structure and function of native tissue. Indeed, Matrigel, a basement membrane protein extract derived from Engelbreth-Holm-Swarm sarcoma in mice, has long been used as a substrate for 3D stem cell and organoid culture, but it suffers from batch-to-batch variability in composition and is not easily modified [13,14]. Natural materials are also generally expensive and using them on a large scale is cost-prohibitive. On the other hand, semi-synthetic and synthetic materials have risen in popularity for their tunability in fabrication methods; physicochemical properties including stiffness, degradability, and presentation of adhesive ligands; and low cost. For example, poly(ethylene glycol) (PEG) and gelatin methacrylamide hydrogels of variable stiffnesses have been shown to be suitable for ovarian cancer spheroid culture [15,16], and mechanically dynamic PEG hydrogels have been shown to support intestinal stem cell expansion and subsequent differentiation and organoid formation as mediated by yes-associated protein 1 (YAP1) [17].
An additional concern regarding scaffold materials in cancer models is that even with all other parameters held constant, the material itself may induce differential secretion of factors such as cytokines and thus, affect outcomes, including gene and protein expression, of 3D culture or co-culture experiments [18]. Comparisons of different biomaterials used in tumor models have been reviewed in depth elsewhere [4,5]. The remainder of this review will address models in which modulation of substrate stiffness, shear stress, or dynamic mechanical loading have revealed important aspects of tumor progression and drug resistance.
ECM stiffness
Cell adhesion to the surrounding matrix is dependent upon the mechanical properties of the ECM, which have a direct consequence on cell morphology. As shown in Figure 1, glioblastoma multiforme (GBM) cells in soft PEG hydrogels tend to spread out early and form extensive networks over two weeks of culture, whereas GBM cells in stiff hydrogels appear spindle-like and grow long, thin actin-rich protrusions over the culture period [19]. It has also been well documented that cancer cells exhibit increased resistance to drugs in response to independently increasing matrix stiffness and adhesive ligand density in biomaterials-based in vitro tumor models [20,21]. Changes in matrix biophysical properties are also known to elicit a malignant phenotype or upregulate genes, such as vascular endothelial growth factor, MMP-2, MMP-9, and hypoxia-inducible factor 1, that are markers of a malignant phenotype [20,22]. Recent developments in tissue engineered tumor modeling include systems that incorporate multiple decoupled properties into a single platform to show how factors such as adhesive ligand density, degradability by proteolysis, and matrix stiffness affect cancer progression and drug response [23,24]. Synergistic effects of ECM stiffness and adhesive ligand density in the design of drug screening platforms were demonstrated by Zustiak et al., who employed alginate-based 3D scaffolds with or without the cell adhesive peptide Arg-Gly-Asp (RGD) in a model of GBM [25]. Human GBM cells were more resistant to cytotoxic compounds on stiffer gels; this phenomenon was disrupted by the presence of RGD but regained when integrin-based cell-matrix interactions were inhibited.
Figure 1.
U87 glioblastoma cell morphology in soft and stiff PEG hydrogels [19]. (A) Cells in soft hydrogels spread starting on day 3 and form extended networks of protrusions over 14 days, while cells in stiff hydrogels spread starting on day 7 and have protrusions that get longer over the culture period. Scale bar = 50 µm. (B) H&E staining and actin (red) and nuclei (blue) staining on soft and stiff hydrogels on day 21 show that cells in soft hydrogels are fibroblast-like and cell spheroids in stiff hydrogels have actin-rich protrusions at the periphery. Scale bar = 75 µm. Reprinted with permission from [19]. Copyright 2014 American Chemical Society.
One of the challenges of using native ECM materials is the difficulty of decoupling biophysical properties such as pore size and substrate stiffness. Cassereau et al. designed a 3D tension bioreactor system that manipulates the stiffness of collagen I hydrogels without altering the pore size, structure, or composition of the ECM [26]. Moreover, this system allowed them to make hydrogels with a stiffness gradient (0.12 kPa/mm) and in studies with oncogenically transformed mammary epithelial cell organoids, consistent with previous findings [27], found that increasing ECM stiffness potentiates tumor cell migration. Not only does ECM stiffness generally increase with tumor stage and grade, but stiffness gradients within a tumor can enable cancer cell migration through durotaxis, and metastasized cancer cells tend to be attracted to tissues with excessive ECM crosslinking and strong stiffness differential gradients [27,28]. Though its current iteration lacks much of the biochemical factors in the native tumor microenvironment, the bioreactor system is advantageous for its ability to examine a well-defined graded response of cancer cells to stiffness alone, and while not demonstrated in this study, it has potential to elucidate the specific mechanisms that enable tumors to progress and metastasize in response to ECM stiffness.
Development of targeted therapies requires a deep understanding of the pathways involved in cancer progression, yet the mechanisms by which tumors respond to their mechanical environment are not yet fully understood. Thus, recent studies in engineered tumor models have also focused on elucidating the signaling cascades that are impacted by changes in ECM stiffness, especially those found in common forms of cancer, such as hepatocellular carcinoma (HCC), or highly malignant and aggressive cancers like GBM [29–34]. As far as tissue engineering is concerned, these platforms are simplistic, but effective. For instance, collagen type-I coated polyacrylamide gels are popular substrates for mechanistic studies with HCC progression. Pang et al. demonstrated for the first time that increasing ECM stiffness contributes to a malignant phenotype in HCC by modulating response through the β1 integrin/FAK/Rho GTPase signal transduction pathway and activation of transforming growth factor-β1 [31]. An outstanding example demonstrating the efficacy of collagen-coated polyacrylamide as a model for HCC was done by You et al. [32]. In 2006, a landmark study by Engler et al. showed that matrix elasticity directs mesenchymal stem cell lineage [35]. Given that poor differentiation is indicative of a more aggressive disease and unfavorable clinical outcomes, You et al. investigated whether matrix stiffness-mediated effects on stemness are observed in HCC [32]. They found that increasing matrix stiffness upregulates the expression of Nanog and SOX2, which are related to stemness. Moreover, higher matrix stiffness activates the Akt/mTOR/SOX2 pathway—known to be an indicator of poor prognosis in HCC patients—as mediated by β1 integrin, and thus links this signaling pathway to HCC stemness. This connection was validated when the stemness properties of HCC cells on stiff matrices were reduced after treatment with rapamycin, a mTOR-specific inhibitor. The limitation of using collagen-coated polyacrylamide as a platform for tumor modeling is that the cells may not be fully embedded in a 3D environment. Models of HCC could be improved by encapsulating cells within the ECM material and through co-culture with cells of the stromal compartment such as hepatic stellate cells. Nevertheless, these studies are commendable for having isolated the role of matrix stiffness in HCC.
Further elucidation of factors that vary with tissue stiffness can be gleaned from analysis of genomic, proteomic, and transcriptomic datasets of 3D functional tissue [11,36]. These analyses can reveal correlations between the different components within a tissue and can be used to better develop hypotheses that can be tested in model systems and inform our understanding of the molecular mechanisms by which cells and tissues respond to their mechanical environment. They are particularly relevant for cancer models in tissues, such as bone, where the microscopic and macroscopic mechanical properties differ by orders of magnitude.
Fluid flow and dynamic mechanical stimulation
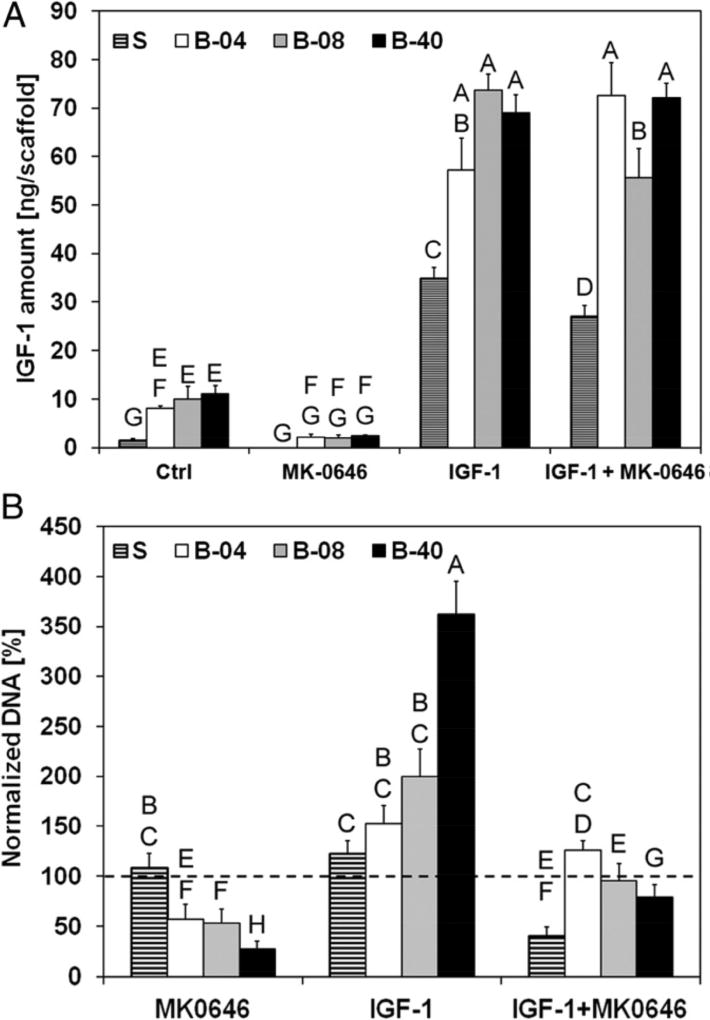
Designing tumor models and culture systems that introduce shear stress or other forms of mechanical loading to cells will prove useful for studying shear stress-dependent sensitivity of cancer cells to pathway-targeted therapies. In a 3D model of Ewing’s sarcoma (ES) on electrospun poly(ε-caprolactone) scaffolds cultured in a flow perfusion bioreactor developed in our laboratory, mechanical stimulation by flow-derived shear stress promoted the upregulated the production of insulin-like growth factor-1 (IGF-1) as shown in Figure 2 [37]. We also observed a shear stress-dependent response to dalotuzumab and resistance to the IGF-1 receptor blockade. We further demonstrated that the inclusion of stromal cells, modeled by co-culture of ES cells with mesenchymal stem cells (MSCs), activate interleukin-6 and Stat3; the latter interferes with the equilibrium of IGF-1 and its antagonists secreted by MSCs [38].
Figure 2.
Effect of flow-derived shear stress on Ewing sarcoma cell sensitivity to dalotuzumab (MK-0646), a humanized monoclonal antibody inhibitor of human IGF-1R. Santoro et al. measured IGF-1 ligand secretion and drug sensitivity in ES cells cultured under static (S) conditions or different flow rates in a bioreactor (B-04, 0.04 mL/min; B-08, 0.08 mL/min; B-40, 0.40 mL/min) [37]. (A) IGF-1 per scaffold in conditioned media after 10 days of culture. Culture of ES cells in flow conditions stimulates autocrine IGF-1 secretion. Error bars represent SD (n = 3). (B) ES cell viability after 3 days of culture followed by 7 days of exposure to dalotuzumab, IGF-1, or both. Cell viability is presented as the DNA content of the treatment group normalized to the DNA content of the untreated group. Dotted line represents 100% baseline. Higher shear stress leads to resistance to dalotuzumab activity and competitive binding between IGF-1 and dalotuzumab favors IGF-1, but convective transport under high flow rate increases availability of the drug to cells. Error bars represent SD (n = 6). Levels not connected by the same letter are statistically different (P < 0.05). Image used with permission from the authors as required for images included in articles published in the Proceedings of the National Academy of Sciences USA.
Advances in 3D printing technology allow for scaffolds of more complex architecture that are comparable to native tissue [39]. Our laboratory used extrusion-based 3D printing to generate scaffolds of poly(propylene fumarate) with a pore size gradient that mimics the heterogeneous permeability of solid tumors. ES cells cultured in these scaffolds under flow conditions experienced different shear stresses depending on the pore size and the orientation of the pore size gradient. Consistent with previous results, shear stress upregulated IGF-1 secretion; we also found that the direction of the pore size gradient affected the amount of IGF-1 secreted [40]. Further studies to design scaffolds with more relevant structures in combination with bioreactor culture will enhance the biomimicry of tissue engineered tumor models and their utility as platforms to study cancer biology and pre-clinical drug testing.
Recently, the role of more complex mechanical stimulation on tumor drug sensitivity has been explored. Marturano-Kruik et al. applied unconfined, dynamic compressive loading to ES cells in 3D porous matrices composed of collagen I and hyaluronic acid [41]. The combination of tissue strain and resultant fluid shear stress within the scaffold pores was shown to promote ERK1/2-dependent RUNX2 expression that is not seen in 2D and static 3D cultures. Notably, activation of the ERK1/2-RUNX2 signal transduction pathway reduced the efficacy of the receptor tyrosine kinase inhibitor sorafenib by increasing cell proliferation and decreasing apoptosis, indicating that this pathway could be a promising therapeutic target in ES. This study is a strong example of how tissue engineering tools can effectively model the complex mechanical characteristics of the tumor microenvironment and elucidate novel pathways to target in drug development.
An additional component of the tumor microenvironment that is currently under active investigation using 3D models is the tumor vasculature. Current models of tumor angiogenesis suffer from oversimplicity and are carried out in static conditions [42,43]. Yet, shear stress regulates angiogenesis [12], and we see a large research gap in models of angiogenesis that incorporate shear forces. Microfluidic platforms, discussed further below, have the capacity to model vasculature with flow-induced shear [44,45], but larger scale models still lack in this area.
Modeling metastasis
A small but growing number of model systems have been developed to investigate the process of tumor metastasis and test drugs for advanced tumor stages. A study by Fuhrmann et al. is worth noting for its use of a shear-generating spinning disk device to examine the adhesive state and metastatic potential of mammary epithelial cell lines [46]. At concentrations of Ca2+ and Mg2+ like those found in stromal conditions, metastatic cell lines display heterogeneity and changes in focal adhesion dynamics that result in a decrease in adhesion strength. The mechanism of focal adhesion cation sensitivity is not yet known, but this study supports the concept that adhesion strength could be used instead of biomarkers as a biophysical metric to identify metastatic cells within a tumor. Though it lacks a 3D scaffold and therefore, physiologically relevant architecture, the spinning disk platform elucidates the contribution of shear forces to metastasis in a way that was previously unknown and informs us of conditions worth considering in the development of future models for studying metastasis.
More relevant models of tumor metastasis address the role of both mechanical stimulation and stromal cells in the metastatic niche. Nietzer et al. generated a 3D model of colorectal cancer in a bioreactor that fostered cell growth and metastasis-like tissue formation [47]. Human SW480 colon cancer cells cultured on a scaffold derived from decellularized porcine jejunum exhibited proliferation that correlated well with primary colon cancer in all stages (as defined by UICC I-IV) and both upregulation of E-cadherin and downregulation of vimentin expression, indicative of the induction of the mesenchymal-to-epithelial transition and the formation of secondary tumors. In co-culture with primary fibroblasts, the cancer cells formed tumor-like aggregates and remodeled the scaffold, an effect that was even more pronounced under conditions of flow-induced shear stress. Treating the cells with 5-fluorouracil, a standard therapy for treating colon cancer, decreased the number of tumor cells in the presence of fibroblasts, demonstrating the importance of including stromal components along with shear stress in model systems. 3D cultures of the bone microenvironment under dynamic mechanical loading have also been used to elucidate cell interactions involved in bone metastases. Lynch et al. found that bone marrow-derived MSCs grown under dynamic compressive loading in mineralized 3D scaffolds with media conditioned by dynamically-loaded MDA-MB231 breast cancer cells differentiate osteogenically and express higher levels of osteopontin [48]. Though this study was limited in that only osteopontin was measured as a marker for bone matrix and further work is required to understand all the components involved in the formation of osteolytic bone metastases characteristic of breast cancer, these results suggest that mechanical stimulation is a major factor that aids breast cancer cells in signaling to MSCs to create a pro-metastatic environment. Future work in similar models should be extended to examine paracrine signaling in both directions (not just tumor to stroma), as well as direct cell-cell contact in establishing the pro-metastatic niche.
Microfluidic tools are popular in tissue engineering for making organ-on-a-chip systems that can recapitulate physiological conditions of native tissue fluid flow, biochemical factor concentration gradients, and cell crosstalk with a high degree of control; different organs-on-a-chip can be interconnected to increase the complexity of these systems [44,45]. As such, microfluidic platforms have gained traction as candidates for modeling cancer metastasis and tumor vasculature. For instance, breast cancer spheroids in a microfluidic device containing 3D ECM to mimic the vascular environment display shear-dependent spheroid size, and co-culture of spheroids with activated fibroblasts promote metastasis by altering cancer cell migration rate, distance, and proliferation [49].
In a remarkable study, Lee et al. used a simple microfluidics device to demonstrate the role of wall shear stress in stimulating YAP1 to promote cancer cell migration [50]. This occurs through ROCK-triggered YAP1 activation through the LIMK-cofilin signaling pathway. Interestingly, only YAP1 and not its paralog TAZ, enhanced cell motility. They also identified 36 genes whose expression was modified by YAP1 activity in response to shear stress. Many of these genes are known to regulate the hallmarks of cancer described by Hanahan and Weinberg in 2000 [51], and with the data, suggest that there is a network of YAP1-dependent genes that lead to invasive cancer cell behavior fundamental to metastasis in response to shear stress levels in the lymphatic system. Microfluidic tumor models are inherently small in volume, so they are limited in their capacity to recapitulate phenomena that occur in tumors on the macroscopic scale. However, they have proven invaluable to tumor modeling as they provide strong evidence that mechanical stimuli in the tumor microenvironment contribute to cancer progression and thus, underscore the need to include biomechanical forces when designing relevant tumor models.
The future of tissue engineered tumor models
Tissue engineering techniques have helped contribute to the paradigm shift from studying cancer in monolayer culture to studying cancer in systems that reflect the complex network of cells and ECM in a tumor. Tissue engineering will continue to promote a better understanding of cancer biology and improved platforms for testing chemotherapeutics through the design of physiologically relevant ex vivo models of solid tumors. We now recognize that the mechanical microenvironment of tumors is significantly different than that of normal tissue and it must be addressed in order to fully understand tumor progression and drug response [52]. Here we have highlighted recent models that employ tissue engineering strategies to demonstrate the significance of ECM stiffness, flow-derived shear stress, and mechanical loading in models of solid tumors.
Recognizing that the tumor microenvironment is dynamic and constantly shifts as cancer progresses, moving forward, ex vivo tumor models should address not only the forces exerted by the mechanical microenvironment, but also the influence of other cellular components comprising the tumor [38,47,49]. Of particular interest is the role of immune cells in the tumor progression. In 2011, Hanahan and Weinberg added to their list of hallmarks the ability of cancer cells to avoid immune destruction as an emerging hallmark and tumor-promoting inflammation as an enabling characteristic of cancer pathogenesis [51,53]. The following year Swartz and Lund proposed that synergy between lymphatic drainage and fluid flow-induced mechanotransduction alters the immune microenvironment and promotes tumor immune escape [54]. Springer and Fischbach have reviewed the role of ECM properties and mechanotransduction on macrophage phenotype and biomaterials systems that model these interactions as it pertains to the tumor microenvironment [55].
A rapidly expanding field that has great potential to aid in the design of ex vivo tumor models with greater cellular and architectural complexity is 3D bioprinting, in which 3D printing techniques are employed to generate tissues from biomaterials, cells, and growth factors. The breadth of printable materials and cell-compatible printing techniques available—reviewed in depth elsewhere—provide an array of options for recreating the cellular heterogeneity and ECM of the tumor microenvironment [56,57]. Tumor-like tissues generated via direct assembly have been shown to provide a 3D environment that enhances cell proliferation, MMP expression, and chemoresistance compared to 2D culture [58]. In addition, bioprinting has been used in conjunction with other scaffold fabrication methods to create unique co-culture conditions that emphasize the role of stroma in tumor cell survival and resistance to drugs [59]. Yet, challenges remain in bioprinting, including the lack of standardization of printers, limitations in resolution of architectural features, difficulty in scalability, and the need to optimize printing conditions that maintain material integrity and cell viability, especially when printing multiple cell types [56].
Although 3D models are superior mimics of the tumor microenvironment in comparison to monolayer cultures, they have not been fully adopted in the drug development industry because there is no standard method of 3D cell culture. But, if we are to accurately recapitulate the tumor microenvironment without the need for costly animal models that fail to reflect human disease, then we should continue moving in the direction of designing models that are comprehensive rather than reductionist with regards to architecture as well as physicochemical and biochemical cues. Current models demonstrate the effect of one or two components of interest on disease progression. We can take advantage of the plethora of tissue engineering tools and 3D cell culture techniques at our disposal to generate more complex systems that address tumor heterogeneity to model cancer pathology.
Tissue engineering provides a toolkit for modeling the tumor microenvironment
Tumor models with biomechanical forces more fully replicate cancer pathology
Future tumor models should combine mechanical environment and stromal compartments
Acknowledgments
We acknowledge support towards the development of tissue engineered tumor models from the National Institutes of Health (R01 CA180279 and P41 EB023833).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- 1.Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 2.Lamhamedi-Cherradi SE, Santoro M, Ramammoorthy V, Menegaz BA, Bartholomeusz G, Iles LR, Amin HM, Livingston JA, Mikos AG, Ludwig JA. 3D tissue-engineered model of Ewing's sarcoma. Adv Drug Deliv Rev. 2014;79–80:155–171. doi: 10.1016/j.addr.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8:839–845. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 4.Fong EL, Harrington DA, Farach-Carson MC, Yu H. Heralding a new paradigm in 3D tumor modeling. Biomaterials. 2016;108:197–213. doi: 10.1016/j.biomaterials.2016.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu L, Mooney DJ. Biomaterials and emerging anticancer therapeutics: engineering the microenvironment. Nat Rev Cancer. 2016;16:56–66. doi: 10.1038/nrc.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 7.Przybyla L, Muncie JM, Weaver VM. Mechanical control of epithelial-to-mesenchymal transitions in development and cancer. Annu Rev Cell Dev Biol. 2016;32:527–554. doi: 10.1146/annurev-cellbio-111315-125150. [DOI] [PubMed] [Google Scholar]
- 8.Shieh AC. Biomechanical forces shape the tumor microenvironment. Ann Biomed Eng. 2011;39:1379–1389. doi: 10.1007/s10439-011-0252-2. [DOI] [PubMed] [Google Scholar]
- 9.Spill F, Reynolds DS, Kamm RD, Zaman MH. Impact of the physical microenvironment on tumor progression and metastasis. Curr Opin Biotechnol. 2016;40:41–48. doi: 10.1016/j.copbio.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leight JL, Drain AP, Weaver VM. Extracellular matrix remodeling and stiffening modulate tumor phenotype and treatment response. Annu Rev Cancer Biol. 2017;1:313–334. [Google Scholar]
- 11.Cho S, Irianto J, Discher DE. Mechanosensing by the nucleus: From pathways to scaling relationships. J Cell Biol. 2017;216:305–315. doi: 10.1083/jcb.201610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain RK, Martin JD, Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng. 2014;16:321–346. doi: 10.1146/annurev-bioeng-071813-105259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benton G, Arnaoutova I, George J, Kleinman HK, Koblinski J. Matrigel: from discovery and ECM mimicry to assays and models for cancer research. Adv Drug Deliv Rev. 2014;79–80:3–18. doi: 10.1016/j.addr.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886–1890. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- 15.Kaemmerer E, Melchels FP, Holzapfel BM, Meckel T, Hutmacher DW, Loessner D. Gelatine methacrylamide-based hydrogels: an alternative three-dimensional cancer cell culture system. Acta Biomater. 2014;10:2551–2562. doi: 10.1016/j.actbio.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 16.Loessner D, Stok KS, Lutolf MP, Hutmacher DW, Clements JA, Rizzi SC. Bioengineered 3D platform to explore cell-ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials. 2010;31:8494–8506. doi: 10.1016/j.biomaterials.2010.07.064. [DOI] [PubMed] [Google Scholar]
- 17.Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordonez-Moran P, Clevers H, Lutolf MP. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539:560–564. doi: 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- 18.Regier MC, Montanez-Sauri SI, Schwartz MP, Murphy WL, Beebe DJ, Sung KE. The Influence of Biomaterials on Cytokine Production in 3D Cultures. Biomacromolecules. 2017;18:709–718. doi: 10.1021/acs.biomac.6b01469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C, Tong X, Yang F. Bioengineered 3D brain tumor model to elucidate the effects of matrix stiffness on glioblastoma cell behavior using PEG-based hydrogels. Mol Pharmaceutics. 2014;11:2115–2125. doi: 10.1021/mp5000828. [DOI] [PubMed] [Google Scholar]
- 20.Chang FC, Tsao CT, Lin A, Zhang M, Levengood SL, Zhang M. PEG-chitosan hydrogel with tunable stiffness for study of drug response of breast cancer cells. Polymers (Basel) 2016;8 doi: 10.3390/polym8040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zustiak SP. The role of matrix compliance on cell responses to drugs and toxins: towards predictive drug screening platforms. Macromol Biosci. 2015;15:589–599. doi: 10.1002/mabi.201400507. [DOI] [PubMed] [Google Scholar]
- 22.Pedron S, Harley BA. Impact of the biophysical features of a 3D gelatin microenvironment on glioblastoma malignancy. J Biomed Mater Res A. 2013;101:3404–3415. doi: 10.1002/jbm.a.34637. [DOI] [PubMed] [Google Scholar]
- 23.Enemchukwu NO, Cruz-Acuna R, Bongiorno T, Johnson CT, Garcia JR, Sulchek T, Garcia AJ. Synthetic matrices reveal contributions of ECM biophysical and biochemical properties to epithelial morphogenesis. J Cell Biol. 2016;212:113–124. doi: 10.1083/jcb.201506055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher SA, Anandakumaran PN, Owen SC, Shoichet MS. Tuning the microenvironment: Click-crosslinked hyaluronic acid-based hydrogels provide a platform for studying breast cancer cell invasion. Adv Funct Mater. 2015;25:7163–7172. [Google Scholar]
- 25.Zustiak SP, Dadhwal S, Medina C, Steczina S, Chehreghanianzabi Y, Ashraf A, Asuri P. Three-dimensional matrix stiffness and adhesive ligands affect cancer cell response to toxins. Biotechnol Bioeng. 2016;113:443–452. doi: 10.1002/bit.25709. [DOI] [PubMed] [Google Scholar]
- •26.Cassereau L, Miroshnikova YA, Ou G, Lakins J, Weaver VM. A 3D tension bioreactor platform to study the interplay between ECM stiffness and tumor phenotype. J Biotechnol. 2015;193:66–69. doi: 10.1016/j.jbiotec.2014.11.008. The authors created a tension bioreactor that alters the stiffness of collagen hydrogels without changing the pore size or structure and used the bioreactor to generate stiffness gradients and study breast cancer phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oudin MJ, Weaver VM. Physical and chemical gradients in the tumor microenvironment regulate tumor cell invasion, migration, and metastasis. Cold Spring Harb Symp Quant Biol. 2016;81:189–205. doi: 10.1101/sqb.2016.81.030817. [DOI] [PubMed] [Google Scholar]
- 29.Dong Y, Xie X, Wang Z, Hu C, Zheng Q, Wang Y, Chen R, Xue T, Chen J, Gao D, et al. Increasing matrix stiffness upregulates vascular endothelial growth factor expression in hepatocellular carcinoma cells mediated by integrin beta1. Biochem Biophys Res Commun. 2014;444:427–432. doi: 10.1016/j.bbrc.2014.01.079. [DOI] [PubMed] [Google Scholar]
- 30.You Y, Zheng Q, Dong Y, Wang Y, Zhang L, Xue T, Xie X, Hu C, Wang Z, Chen R, et al. Higher matrix stiffness upregulates osteopontin expression in hepatocellular carcinoma cells mediated by integrin beta1/GSK3beta/beta-catenin signaling pathway. PLoS One. 2015;10:e0134243. doi: 10.1371/journal.pone.0134243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pang M, Teng Y, Huang J, Yuan Y, Lin F, Xiong C. Substrate stiffness promotes latent TGF-beta1 activation in hepatocellular carcinoma. Biochem Biophys Res Commun. 2017;483:553–558. doi: 10.1016/j.bbrc.2016.12.107. [DOI] [PubMed] [Google Scholar]
- ••32.You Y, Zheng Q, Dong Y, Xie X, Wang Y, Wu S, Zhang L, Wang Y, Xue T, Wang Z, et al. Matrix stiffness-mediated effects on stemness characteristics occurring in HCC cells. Oncotarget. 2016;7:32221–32231. doi: 10.18632/oncotarget.8515. The authors demonstrate that matrix stiffness upregulates expression of genes related to stemness in hepatocellular carcinoma and activates the Akt/mTOR/SOX2 pathway as mediated by the β1 integrin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao SS, Dejesus J, Short AR, Otero JJ, Sarkar A, Winter JO. Glioblastoma behaviors in three-dimensional collagen-hyaluronan composite hydrogels. ACS Appl Mater Interfaces. 2013;5:9276–9284. doi: 10.1021/am402097j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao SS, Lannutti JJ, Viapiano MS, Sarkar A, Winter JO. Toward 3D biomimetic models to understand the behavior of glioblastoma multiforme cells. Tissue Eng Part B Rev. 2014;20:314–327. doi: 10.1089/ten.teb.2013.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 36.Discher DE, Smith L, Cho S, Colasurdo M, Garcia AJ, Safran S. Matrix mechanosensing: From scaling concepts in 'omics data to mechanisms in the nucleus, regeneration, and cancer. Annu Rev Biophys. 2017;46:295–315. doi: 10.1146/annurev-biophys-062215-011206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •37.Santoro M, Lamhamedi-Cherradi SE, Menegaz BA, Ludwig JA, Mikos AG. Flow perfusion effects on three-dimensional culture and drug sensitivity of Ewing sarcoma. Proc Natl Acad Sci U S A. 2015;112:10304–10309. doi: 10.1073/pnas.1506684112. The authors show how shear stress alters IGF-1R signaling and drug response in Ewing sarcoma grown in a 3D environment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •38.Santoro M, Menegaz BA, Lamhamedi-Cherradi SE, Molina ER, Wu D, Priebe W, Ludwig JA, Mikos AG. Modeling stroma-induced drug resistance in a tissue-engineered tumor model of Ewing sarcoma. Tissue Eng Part A. 2017;23:80–89. doi: 10.1089/ten.tea.2016.0369. Expanding upon the work in reference [37], this study investigates the contribution of stromal cells in Ewing sarcoma drug resistance through co-culture of ES with MSCs under flow-induced shear stress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. J Biol Eng. 2015;9:4. doi: 10.1186/s13036-015-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trachtenberg JE, Santoro M, Williams C, Piard CM, Smith BT, Placone JK, Menegaz BA, Molina ER, Lamhamedi-Cherradi S-E, Ludwig JA, et al. Effects of shear stress gradients on Ewing sarcoma cells using 3D printed scaffolds and flow perfusion. ACS Biomater Sci Eng. 2017;4:347–356. doi: 10.1021/acsbiomaterials.6b00641. [DOI] [PubMed] [Google Scholar]
- ••41.Marturano-Kruik A, Villasante A, Yaeger K, Ambati SR, Chramiec A, Raimondi MT, Vunjak-Novakovic G. Biomechanical regulation of drug sensitivity in an engineered model of human tumor. Biomaterials. 2018;150:150–161. doi: 10.1016/j.biomaterials.2017.10.020. A comprehensive study demonstrating that dynamic mechanical loading applied to a 3D model of Ewing sarcoma alters the ERK1/2-RUNX2 signaling pathway and response to sorafenib through a combination of matrix strain and flow-induced shear stress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chwalek K, Bray LJ, Werner C. Tissue-engineered 3D tumor angiogenesis models: potential technologies for anti-cancer drug discovery. Adv Drug Deliv Rev. 2014;79–80:30–39. doi: 10.1016/j.addr.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Song HH, Park KM, Gerecht S. Hydrogels to model 3D in vitro microenvironment of tumor vascularization. Adv Drug Deliv Rev. 2014;79–80:19–29. doi: 10.1016/j.addr.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhise NS, Ribas J, Manoharan V, Zhang YS, Polini A, Massa S, Dokmeci MR, Khademhosseini A. Organ-on-a-chip platforms for studying drug delivery systems. J Control Release. 2014;190:82–93. doi: 10.1016/j.jconrel.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsai HF, Trubelja A, Shen AQ, Bao G. Tumour-on-a-chip: microfluidic models of tumour morphology, growth and microenvironment. J R Soc Interface. 2017;14 doi: 10.1098/rsif.2017.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuhrmann A, Banisadr A, Beri P, Tlsty TD, Engler AJ. Metastatic state of cancer cells may be indicated by adhesion strength. Biophys J. 2017;112:736–745. doi: 10.1016/j.bpj.2016.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •47.Nietzer S, Baur F, Sieber S, Hansmann J, Schwarz T, Stoffer C, Hafner H, Gasser M, Waaga-Gasser AM, Walles H, et al. Mimicking Metastases Including Tumor Stroma: A New Technique to Generate a Three-Dimensional Colorectal Cancer Model Based on a Biological Decellularized Intestinal Scaffold. Tissue Eng Part C Methods. 2016;22:621–635. doi: 10.1089/ten.tec.2015.0557. The authors demonstrate the importance of including stromal cells and fluid flow to model metastasis in colon cancer in a scaffold of decellularized intestine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••48.Lynch ME, Chiou AE, Lee MJ, Marcott SC, Polamraju PV, Lee Y, Fischbach C. Three-dimensional mechanical loading modulates the osteogenic response of mesenchymal stem cells to tumor-derived soluble signals. Tissue Eng Part A. 2016;22:1006–1015. doi: 10.1089/ten.tea.2016.0153. The authors demonstrate that paracrine factors secreted by mechanically loaded breast cancer cells upregulates osteogenesis in mechanically loaded MSCs. This points to evidence that mechanical stimuli-mediated tumor-to-stroma to signaling plays a role in generating sites for bone metastasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mina SG, Huang P, Murray BT, Mahler GJ. The role of shear stress and altered tissue properties on endothelial to mesenchymal transformation and tumor-endothelial cell interaction. Biomicrofluidics. 2017;11:044104. doi: 10.1063/1.4991738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •50.Lee HJ, Diaz MF, Price KM, Ozuna JA, Zhang S, Sevick-Muraca EM, Hagan JP, Wenzel PL. Fluid shear stress activates YAP1 to promote cancer cell motility. Nat Commun. 2017;8:14122. doi: 10.1038/ncomms14122. The authors use a microfluidics device to show that shear stress similar to the lymphatic system activates YAP1 and modulates the expression of 36 genes downstream of YAP1 including many related to the hallmarks of cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 52.Nagelkerke A, Bussink J, Rowan AE, Span PN. The mechanical microenvironment in cancer: How physics affects tumours. Semin Cancer Biol. 2015;35:62–70. doi: 10.1016/j.semcancer.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 54.Swartz MA, Lund AW. Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat Rev Cancer. 2012;12:210–219. doi: 10.1038/nrc3186. [DOI] [PubMed] [Google Scholar]
- 55.Springer NL, Fischbach C. Biomaterials approaches to modeling macrophage-extracellular matrix interactions in the tumor microenvironment. Curr Opin Biotechnol. 2016;40:16–23. doi: 10.1016/j.copbio.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Albritton JL, Miller JS. 3D bioprinting: improving in vitro models of metastasis with heterogeneous tumor microenvironments. Dis Model Mech. 2017;10:3–14. doi: 10.1242/dmm.025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Samavedi S, Joy N. 3D printing for the development of in vitro cancer models. Curr Opin Biomed Eng. 2017;2:35–42. [Google Scholar]
- 58.Zhao Y, Yao R, Ouyang L, Ding H, Zhang T, Zhang K, Cheng S, Sun W. Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication. 2014;6:035001. doi: 10.1088/1758-5082/6/3/035001. [DOI] [PubMed] [Google Scholar]
- 59.Park TM, Kang D, Jang I, Yun WS, Shim JH, Jeong YH, Kwak JY, Yoon S, Jin S. Fabrication of in vitro cancer microtissue array on fibroblast-layered nanofibrous membrane by inkjet printing. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18112348. [DOI] [PMC free article] [PubMed] [Google Scholar]