Abstract
The polymerization of functional monomers provides direct access to functional polymers without need for postpolymerization modification; however, monomer synthesis can become a bottleneck of this approach. New methods that enable rapid installation of functionality into monomers for living polymerization are valuable. Here, we report the three-step convergent synthesis (two-step longest linear sequence) of a divalent exo-norbornene imide capable of efficient coupling with various nucleophiles and azides to produce diversely functionalized branched macromonomers optimized for ring-opening metathesis polymerization (ROMP). In addition, we describe an efficient iterative procedure for the synthesis of tri-and tetra-valent branched macromonomers. We demonstrate the use of these branched macromonomers for the synthesis of Janus bottlebrush block copolymers as well as for the generation of bottlebrush polymers with up to three conjugated small molecules per repeat unit. This work significantly expands the scalability and diversity of nanostructured macromolecules accessible via ROMP.
Graphical Abstract
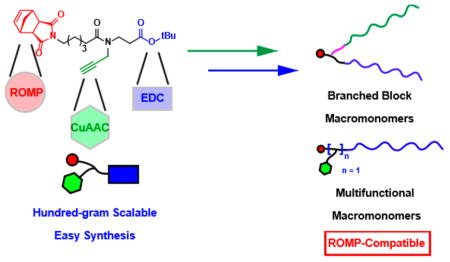
Recent years have witnessed major advances in the fields of macromolecular design and synthesis thanks to the interplay between catalysis, organic synthesis, and polymer chemistry.1 Bottlebrush polymers have garnered particularly extensive interest due to the development of novel methods for their synthesis and to their advantageous properties.2 Ring-opening metathesis polymerization (ROMP) of norbornene-based macromonomers (MMs) is a powerful strategy for the synthesis of complex functional bottlebrush polymers with diverse functions and applications.1b,c,3 Multiple functionalities can be installed into such polymers via copolymerization of different MMs, the combination of MMs with small molecule monomers, or by the use of branched MMs that carry several functional species within a single MM; the latter allows for the syntheses of highly uniform multivalent branched bottlebrush polymers (BBPs).4 For example, we have established a first-generation branched norbornene MM precursor (G1, Scheme 1A) that contains two orthogonal functional sites: an alkyne for copper-catalyzed alkyne azide cycloaddition (CuAAC) and a carboxylic acid for carbodiimide coupling, which enables the synthesis of bivalent MMs with two functional domains (e.g., polymers, drug molecules, imaging agents, and ligands).5 A six-carbon spacer between the tertiary amide branch point was found to be critical for the ROMP of MMs with bulky groups (e.g., polymers and/or drug molecules) to high conversion. This strategy has provided BBPs and related polymers with applications that span multiple disciplines (e.g., self-assembly, drug delivery, and molecular imaging).4c,d,6
Scheme 1.
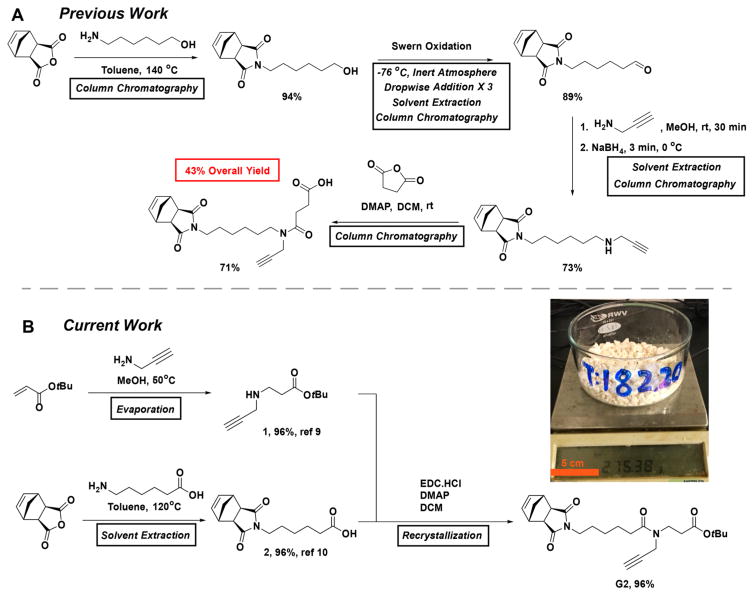
(A) Previous Synthesis of G1;8 (B) This work: Synthesis of G2
Though G1 works exceptionally well for ROMP of complex MMs, it suffers three key drawbacks: (1) its synthesis involves a four-step linear sequence that requires multiple chromatographic purifications, which severely limits its scalability, (2) it is a viscous oil that is difficult to handle in neat form, and (3) it can only be conjugated to two functional species. In an effort to translate our BBPs to practical applications that require large quantities of material, an improved route to a G1 equivalent was required. In addition, a facile method to expand the valency of our branched MMs would provide an approach to increasing the loading of functional species, for example, drugs and imaging agents, within BBPs.
Herein, we describe a three-step convergent synthesis (two-step longest linear sequence) that provides a novel norbornene alkyne-branch-COOtBu derivative (G2, Scheme 1B) in 92% yield on the 100 g scale (compared to 43% for the 0.1 g scale synthesis of G1).5 G2 differs from G1 by the placement of a single carbonyl group as well as the presence of a tert-butyl ester. The latter makes G2 a readily handled powder rather than a viscous oil. We show that branched MMs prepared from G2 are excellent substrates for BBP synthesis via ROMP. We also introduce an iterative synthesis of G2 derivatives with two and three alkynes, which provides access to novel tri- and tetravalent MMs and BBPs. This work establishes an efficient synthetic route to multivalent norbornene derivatives that significantly advance the scale and diversity of functional polymers that are accessible by ROMP.
The synthesis of G2 (Scheme 1B, see Supporting Information (SI) for detailed procedures) commenced with the preparation of alkyne 17 and carboxylic acid 28 both in 96% yield following literature procedures. Notably, 1 and 2 were isolated as pure compounds following rotary evaporation and liquid–liquid extraction, respectively; no chromatography was needed. Standard carbodiimide-mediated amidation followed by recrystallization or silica gel chromatography gave the desired compound G2 as an easy-to-handle white solid (96% yield from recrystallization, Scheme 1B, inset). Altogether, the route shown in Scheme 1B provided G2 in 92% yield over three steps (compared to 43% typical overall yield and four chromatographic purifications for G1).
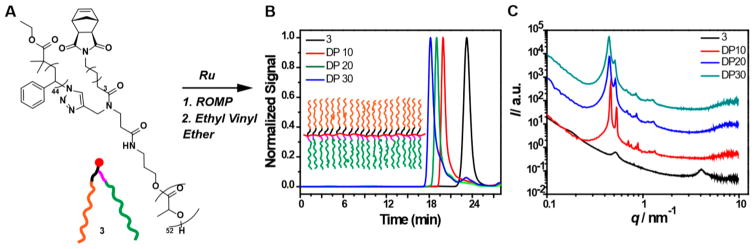
To confirm that G2 was suitable for ROMP of demanding MMs, we prepared its polystyrene (PS)-branch-polylactide (PLA) derivative 3 (Figure 1A; see SI for details).4d,5 ROMP using various ratios of 3 to Grubbs third-generation bis-pyridyl complex (Ru) provided PS-branch-PLA Janus BBPs with variable degrees of polymerization (DP): 10, 20, and 30. In each case, a very high conversion of 3 was achieved, as determined by gel permeation chromatography (GPC, Figure 1B and Table S1). Small-angle X-ray scattering (SAXS) was used to study the bulk morphology of 3 and its corresponding Janus BBPs of varied DP (Figure 1C). Samples were cast from THF solutions and annealed at 145 °C under vacuum for 6 h (see SI). Remarkably, while 3 was disordered, the three Janus BBPs prepared from it displayed a bicontinuous gyroid morphology (domain spacing, d = 13.7, 14.1, and 14.3 nm for DP 10, 20, and 30, respectively). The observation of robust assembly for Janus BBPs derived from a disordered diblock MM (i.e., 3) is consistent with our previous studies,4d which revealed that preorganization of block copolymers on the side chains of bottlebrush polymers can drive self-assembly at χN values (where χ is the Flory–Huggins interaction parameters and N is the DP) below the threshold for assembly of the analogous linear block copolymer. Notably, in our previous work, we only observed the bicontinuous gyroid morphology for polydime-thylsiloxane-branch-poly(tert-butyl acrylate) Janus BBPs. Though PS- and PLA-based MMs have been mainstays of bottlebrush block copolymer assembly,5,9 to our knowledge, the results presented here provide the first examples of the bicontinuous gyroid morphology for this composition.
Figure 1.
(A) Structure and graft-through ROMP of PS-branch-PLA MM 3. (B) GPC traces for 3 and Janus bottlebrush copolymers of varied DP. (C). SAXS profiles for 3 and Janus bottlebrush copolymers of varied DP.
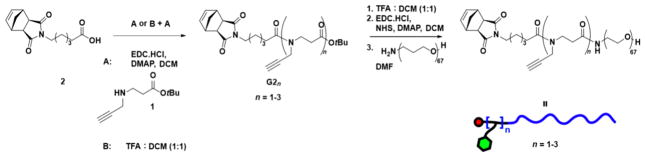
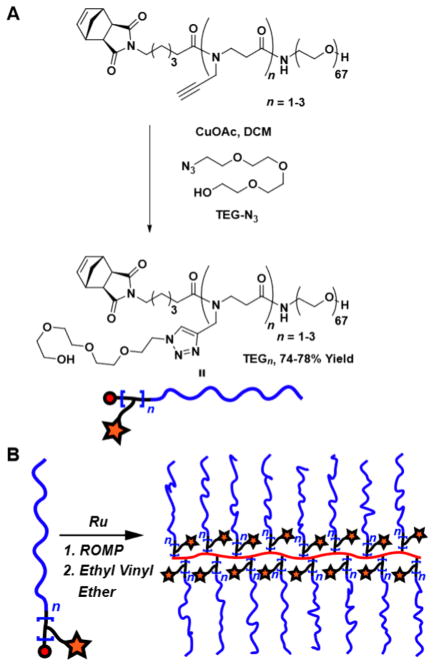
Having shown that G2 is a suitable precursor for the synthesis of branched MMs that are applicable in the context of ROMP, we sought to expand its valency beyond two. We surmised that G2 derivatives with greater than one alkyne could be prepared by iterative coupling of 1 onto G2. In the event, G2 was exposed to trifluoroacetic acid (TFA) and the resulting carboxylic acid was coupled to 1 to generate bis-alkyne G22 in 79% yield (Scheme 2). Repetition of this sequence provided tris-alkyne G23 in 54% yield. Coupling of 3 kDa monoamine-terminated polyethylene glycol (PEG) to parent compound G2 as well as multialkynes G22 and G23 followed by conjugation of azido-triethylene glycol (TEG-N3) afforded a set of novel branched MMs: mono-, bis-, and tris-TEG MMs (TEG1, TEG2, and TEG3, respectively; Scheme 3A).
Scheme 2.
Synthesis of Bis- and Tris-Alkynes G22 and G23
Scheme 3.
(A) Synthesis of TEGn and chexn (n = 1, 2, or 3); (B) Schematic for Graft-through ROMP of Multivalent MMs
1H NMR spectra for TEGn (where n = 1, 2, or 3) MMs are provided in Figure 2A. The norbornene proton resonance is observed at ~6.25 ppm (Figure 2A, purple box), while resonances for the triazole protons and the methylene protons alpha to the triazole are observed at ~7.80 (Figure 2A, blue box) and ~4.5, respectively (Figure 2A, red box). Integration of these sets of resonances confirms that TEG1, TEG2, and TEG3 possess one, two, and three triazoles, respectively. Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry (MS) further supported the proposed structures of TEGn (Figures S32, S34, and S36). Exposure of 40 equiv of TEGn to 1 equiv of Ru provided BBPs with ≥90% conversion of TEGn for all n values (Figure 2B). The GPC trace for TEG1 was very similar to that of a control polymer with identical side chains prepared from G1 (Figure S42), which supports the notion that G2 is a suitable replacement for G1. Finally, GPC traces of BBPs prepared via ROMP of TEG3 using varied TEG3:Ru values from 10 to 50 displayed progressively shorter retention times and high conversions (Figure 2C and Table S2); this data suggest that this MM is an excellent substrate for ROMP.
Figure 2.
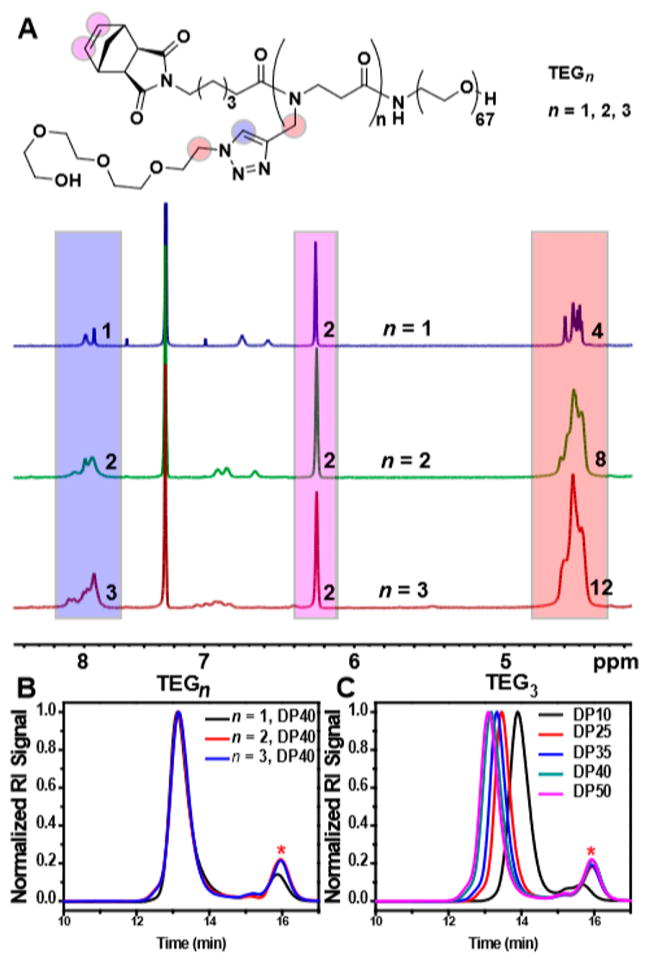
(A) 1H NMR spectra for TEGn (n = 1, 2, or 3). Integrals of key peaks are provided. CDCl3; ω/2π = 400 MHz. (B) GPC traces for multivalent bottlebrush polymers prepared from TEGn with target DP = 40. (C) GPC traces for bottlebrush polymers derived from TEG3 with various target DP values; *residual TEGn.
In conclusion, we have demonstrated the scalable synthesis of a norbornene-based MM that contains functional groups for convenient orthogonal conjugation to small molecules and polymers. In addition, MMs containing one, two, or three alkynes were conjugated to azido-tetraethylene glycol; this approach increases the mass fraction of the conjugated species significantly (from 6.1 to 15.5% w/w for TEG). ROMP of these MMs proceeded in very high conversion in all cases. In the future, we expect to exploit the modularity and scalability of this system, as well as the enhanced loading capacity of multivalent MMs, for applications in combination drug delivery, molecular imaging, and self-assembly.
Supplementary Material
Acknowledgments
We thank the NIH (1R01CA220468-01) and the U.S. Air Force Office of Scientific Research (FA9550-14-1-0292) for support of this work. H.V.-T.N. thanks the NSF for a Graduate Research Fellowship. Z.H. thanks the CSC for a visiting Ph.D. student fellowship. The research used resources of APS, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory (Contract No. DE-AC02-06CH11357), and the CMS beamline (11-BM) of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory (Contract No. DE-SC0012704).
Footnotes
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmacro-lett.8b00201.
Experimental protocols and characterization data (PDF).
References
- 1.(a) Hawker CJ, Wooley KL. The Convergence of Synthetic Organic and Polymer Chemistries. Science. 2005;309:1200–1205. doi: 10.1126/science.1109778. [DOI] [PubMed] [Google Scholar]; (b) Matyjaszewski K, Tsarevsky NV. Nanostructured Functional Materials Prepared by Atom Transfer Radical Polymerization. Nat Chem. 2009;1:276–288. doi: 10.1038/nchem.257. [DOI] [PubMed] [Google Scholar]; (c) Iha RK, Wooley KL, Nystrom AM, Burke DJ, Kade MJ, Hawker CJ. Applications of Orthogonal “Click Chemistries in the Synthesis of Functional Soft Materials. Chem Rev. 2009;109:5620–5686. doi: 10.1021/cr900138t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kamber NE, Jeong W, Waymouth RM, Pratt RC, Lohmeijer BGG, Hedrick JL. Organocatalytic Ring-Opening Polymerization. Chem Rev. 2007;107:5813–5840. doi: 10.1021/cr068415b. [DOI] [PubMed] [Google Scholar]
- 2.(a) Rzayev J. Molecular Bottlebrushes: New Opportunities in Nanomaterials Fabrication. ACS Macro Lett. 2012;1:1146–1149. doi: 10.1021/mz300402x. [DOI] [PubMed] [Google Scholar]; (b) Sheiko SS, Sumerlin BS, Matyjaszewski K. Cylindrical Molecular Brushes: Synthesis, Characterization, and Properties. Prog Polym Sci. 2008;33:759–785. [Google Scholar]; (c) Lee HI, Pietrasik J, Sheiko SS, Matyjaszewski K. Stimuli-responsive Molecular Brushes. Prog Polym Sci. 2010;35:24–44. [Google Scholar]; (d) Verduzco R, Li X, Pesek SL, Stein GE. Structure, Function, Self-assembly, and Applications of Bottlebrush Copolymers. Chem Soc Rev. 2015;44:2405–2420. doi: 10.1039/c4cs00329b. [DOI] [PubMed] [Google Scholar]
- 3.(a) Xia Y, Olsen BD, Kornfield JA, Grubbs RH. Efficient Synthesis of Narrowly Dispersed Brush Copolymers and Study of Their Assemblies: the Importance of Side Chain Arrangement. J Am Chem Soc. 2009;131:18525–18532. doi: 10.1021/ja908379q. [DOI] [PubMed] [Google Scholar]; (b) Xia Y, Kornfield JA, Grubbs RH. Efficient Synthesis of Narrowly Dispersed Brush Polymers via Living Ring-Opening Metathesis Polymerization of Macromonomers. Macromolecules. 2009;42:3761–3766. [Google Scholar]; (c) Cheng C, Khoshdel E, Wooley K. L One-Pot Tandem Synthesis of a Core–shell Brush Copolymer from Small Molecule Reactants by Ring-opening Metathesis and Reversible Addition–fragmentation Chain Transfer (Co)polymerizations. Macromolecules. 2007;40:2289–2292. [Google Scholar]; (d) Li Z, Zhang K, Ma J, Cheng C, Wooley KL. Facile Syntheses of Cylindrical Molecular Brushes by a Sequential RAFT and ROMP “Grafting-through” Methodology. J Polym Sci, Part A: Polym Chem. 2009;47:5557–5563. doi: 10.1002/pola.23626. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Li Z, Ma J, Cheng C, Zhang K, Wooley KL. Synthesis of Hetero-grafted Amphiphilic Diblock Molecular Brushes and Their Self-assembly in Aqueous Medium. Macromolecules. 2010;43:1182–1184. [Google Scholar]; (f) Teo YC, Xia Y. Importance of Macromonomer Quality in the Ring-opening Metathesis Polymerization of Macromonomers. Macromolecules. 2015;48:5656–5662. [Google Scholar]; (g) Radzinski SC, Foster JC, Chapleski RC, Jr, Troya D, Matson JB. Bottlebrush Polymer Synthesis by Ring-opening Metathesis Polymerization: the Significance of the Anchor Group. J Am Chem Soc. 2016;138:6998–7004. doi: 10.1021/jacs.5b13317. [DOI] [PubMed] [Google Scholar]; (h) Lu X, Tran TH, Jia F, Tan X, Davis S, Krishnan S, Amiji MM, Zhang K. Providing Oligonucleotides with Steric Selectivity by Brush-polymer-assisted Compaction. J Am Chem Soc. 2015;137:12466–12469. doi: 10.1021/jacs.5b08069. [DOI] [PubMed] [Google Scholar]; (i) Lu X, Jia F, Tan X, Wang D, Cao X, Zheng J, Zhang K. Effective Antisense Gene Regulation via Noncationic, Polyethylene Glycol Brushes. J Am Chem Soc. 2016;138:9097–9100. doi: 10.1021/jacs.6b05787. [DOI] [PubMed] [Google Scholar]; (j) Jia F, Lu X, Wang D, Cao X, Tan X, Lu H, Zhang K. Depth-profiling the Nuclease Stability and the Gene Silencing Efficacy of Brush-architectured Poly(ethylene glycol)–DNA Conjugates. J Am Chem Soc. 2017;139:10605–10608. doi: 10.1021/jacs.7b05064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Sveinbjornsson BR, Weitekamp RA, Miyake GM, Xia Y, Atwater HA, Grubbs RH. Rapid Self-assembly of Brush Block Copolymers to Photonic Crystals. Proc Natl Acad Sci U S A. 2012;109:14332–14336. doi: 10.1073/pnas.1213055109. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Miyake GM, Piunova VA, Weitekamp RA, Grubbs RH. Precisely Tunable Photonic Crystals from Rapidly Self-assembling Brush Block Copolymer Blends. Angew Chem, Int Ed. 2012;51:11246–11248. doi: 10.1002/anie.201205743. [DOI] [PubMed] [Google Scholar]; (c) Barnes JC, Bruno PM, Nguyen HVT, Liao L, Liu J, Hemann MT, Johnson JA. Using an RNAi Signature Assay To Guide the Design of Three-drug-conjugated Nanoparticles with Validated Mechanisms, In Vivo Efficacy, and Low Toxicity. J Am Chem Soc. 2016;138:12494–12501. doi: 10.1021/jacs.6b06321. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kawamoto K, Zhong M, Gadelrab KR, Cheng LC, Ross CA, Alexander-Katz A, Johnson JA. Graft-through Synthesis and Assembly of Janus Bottlebrush Polymers from A-branch-B Diblock Macromonomers. J Am Chem Soc. 2016;138:11501–11504. doi: 10.1021/jacs.6b07670. [DOI] [PubMed] [Google Scholar]; (e) Li Y, Tabak-Christian L, Fuan VLF, Zou J, Cheng C. Crosslinking-Induced Morphology Change of Latex Nanoparticles: a Study of RAFT-mediated Polymerization in Aqueous Dispersed Media Using Amphiphilic Double-brush Copolymers as Reactive Surfactants. J Polym Sci, Part A: Polym Chem. 2014;52:3250–3259. [Google Scholar]; (f) Zou J, Jafr G, Themistou E, Yap Y, Wintrob ZAP, Alexandridis P, Ceacareanu AC, Cheng C. pH-sensitive Brush Polymer-drug Conjugates by Ring-opening Metathesis Copolymerization. Chem Commun. 2011;47:4493–4495. doi: 10.1039/c0cc05531j. [DOI] [PubMed] [Google Scholar]; (g) Radzinski SC, Foster JC, Scannelli SJ, Weaver JR, Arrington KJ, Matson JB. Tapered Bottlebrush Polymers: Cone-shaped Nanostructures by Sequential Addition of Macromonomers. ACS Macro Lett. 2017;6:1175–1179. doi: 10.1021/acsmacrolett.7b00724. [DOI] [PubMed] [Google Scholar]; (h) Unsal H, Onbulak S, Calik F, Er-Rafik M, Schmutz M, Sanyal A, Rzayev J. Interplay between Molecular Packing, Drug Loading, and Core Cross-linking in Bottlebrush Copolymer Micelles. Macromolecules. 2017;50:1342–1352. [Google Scholar]; (i) Onbulak S, Rzayev J. Synthesis and One-dimensional Assembly of Cylindrical Polymer Nanoparticles Prepared from Tricomponent Bottlebrush Copolymers. J Polym Sci, Part A: Polym Chem. 2017;55:3868–3874. [Google Scholar]
- 5.Johnson JA, Lu YY, Burts AO, Xia Y, Durrell AC, Tirrell DA, Grubbs RH. Drug-loaded, Bivalent-bottle-brush Polymers by Graft-through ROMP. Macromolecules. 2010;43:10326–10335. doi: 10.1021/ma1021506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Li Y, Zou J, Das BP, Tsianou M, Cheng C. Well-defined Amphiphilic Double-brush Copolymers and Their Performance as Emulsion Surfactants. Macromolecules. 2012;45:4623–4629. [Google Scholar]; (b) Li Y, Themistou E, Zou J, Das BP, Tsianou M, Cheng C. Facile Synthesis and Visualization of Janus Double-brush Copolymers. ACS Macro Lett. 2012;1:52–56. doi: 10.1021/mz200013e. [DOI] [PubMed] [Google Scholar]; (c) Liu J, Burts AO, Li Y, Zhukhovitskiy AV, Ottaviani MF, Turro NJ, Johnson JA. Brush-first Method for the Parallel Synthesis of Photocleavable, Nitroxide-labeled Poly(ethylene glycol) Star Polymers. J Am Chem Soc. 2012;134:16337–16344. doi: 10.1021/ja3067176. [DOI] [PubMed] [Google Scholar]; (d) Burts AO, Liao L, Lu YY, Tirrell DA, Johnson JA. Brush-first and Click: Efficient Synthesis of Nanoparticles that Degrade and Release Doxorubicin in Response to Light. Photochem Photobiol. 2014;90:380–385. doi: 10.1111/php.12182. [DOI] [PubMed] [Google Scholar]; (e) Burts AO, Gao AX, Johnson JA. Brush-first Synthesis of Core-photo-degradable Miktoarm Star Polymers via ROMP: Towards Photo-responsive Self-assemblies. Macromol Rapid Commun. 2014;35:168–173. doi: 10.1002/marc.201300618. [DOI] [PubMed] [Google Scholar]; (f) Liao L, Liu J, Dreaden EC, Morton SW, Shopsowitz KE, Hammond PT, Johnson JA. A Convergent Synthetic Platform for Single-nanoparticle Combination Cancer Therapy: Ratiometric Loading and Controlled Release of Cisplatin, Doxorubicin, and Camptothecin. J Am Chem Soc. 2014;136:5896–5899. doi: 10.1021/ja502011g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Gao AX, Liao L, Johnson JA. Synthesis of Acid-labile PEG and PEG-doxorubicin-conjugate Nanoparticles via Brush-first ROMP. ACS Macro Lett. 2014;3:854–857. doi: 10.1021/mz5004097. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Sowers MA, McCombs JR, Wang Y, Paletta JT, Morton SW, Dreaden EC, Boska MD, Ottaviani MF, Hammond PT, Rajca A, Johnson JA. Redox-responsive Branched-bottlebrush Polymers for in vivo MRI and Fluorescence Imaging. Nat Commun. 2014;5:5460. doi: 10.1038/ncomms6460. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Nguyen HVT, Chen Q, Paletta JT, Harvey P, Jiang Y, Zhang H, Boska MD, Ottaviani MF, Jasanoff A, Rajca A, Johnson JA. Nitroxide-based Macromolecular Contrast Agents with Unprecedented Transverse Relaxivity and Stability for Magnetic Resonance Imaging of Tumors. ACS Cent Sci. 2017;3:800. doi: 10.1021/acscentsci.7b00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy O, Faure S, Thery V, Didierjean C, Taillefumier C. Cyclicβ-Peptoids. Org Lett. 2008;10:921–924. doi: 10.1021/ol7030763. [DOI] [PubMed] [Google Scholar]
- 8.Patel PR, Kiser RC, Lu YY, Fong E, Ho WC, Tirrell DA, Grubbs RH. Synthesis and Cell Adhesive Properties of Linear and Cyclic RGD Functionalized Polynorbornene Thin Films. Biomacromolecules. 2012;13:2546–2553. doi: 10.1021/bm300795y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Gu W, Huh J, Hong SW, Sveinbjornsson BR, Park C, Grubbs RH, Russell R. Self-assembly of Symmetric Brush Diblock Copolymers. ACS Nano. 2013;7:2551–2558. doi: 10.1021/nn305867d. [DOI] [PubMed] [Google Scholar]; (b) Macfarlane RJ, Kim B, Lee B, Weitekamp RA, Bates CM, Lee SF, Chang AB, Delaney KT, Fredrickson GH, Atwater HA, Grubbs RH. Improving Brush Polymer Infrared One-dimensional Photonic Crystals via Linear Polymer Additives. J Am Chem Soc. 2014;136:17374–17377. doi: 10.1021/ja5093562. [DOI] [PubMed] [Google Scholar]; (c) Lin TP, Chang AB, Luo SX, Chen HY, Lee B, Grubbs RH. Effects of Grafting Density on Block Polymer Self-assembly: from Linear to Bottlebrush. ACS Nano. 2017;11:11632–11641. doi: 10.1021/acsnano.7b06664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.