Abstract
Ultrasound is currently the only noninvasive technology able to completely diagnose and manage kidney stones.
Introduction
Kidney stones currently affect 1 in 11 Americans over their lifetime (Scales et al., 2012) and the prevalence is rising. Although often asymptomatic in the kidney, a stone can cause debilitating pain, nausea, and/or changes in urination as it obstructs urine flow in the passage from the kidney to the bladder. The focus of treatment for kidney stones in the emergency room is to manage the pain, although about 20% of patients are admitted for an urgent surgical procedure to release urine backup (generally the source of the pain; Ghani et al., 2014). If stones do not pass naturally (usually occurring when the size is >5 mm), at least one surgery must be performed to remove the stone. Once a patient has had a kidney stone, there is a 40% chance of having another symptomatic stone within 5 years (Worcester and Coe, 2008). Emergency room visits coupled with the various management options and monitoring for kidney stones puts the estimated annual cost in the United States at over $10 billion (Litwin and Saigal, 2012). The goal of this article is to report on the acoustical principles behind new techniques and technologies for the diagnosis and management of kidney stones with ultrasound.
Background
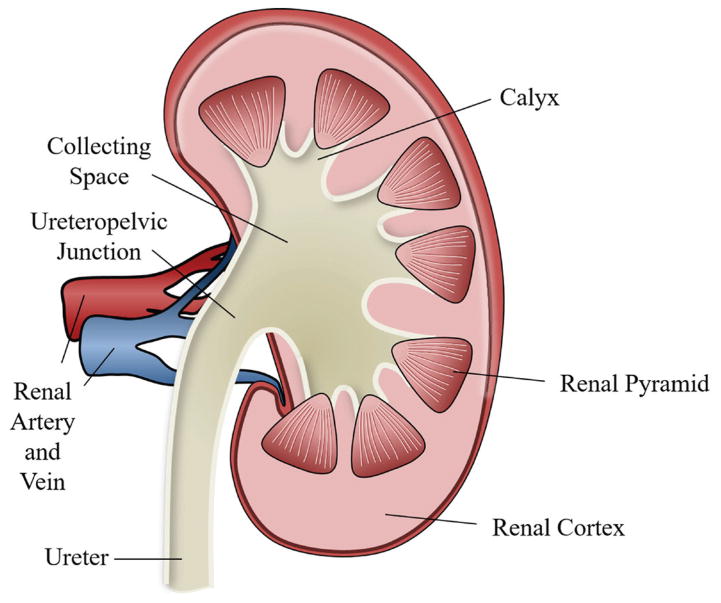
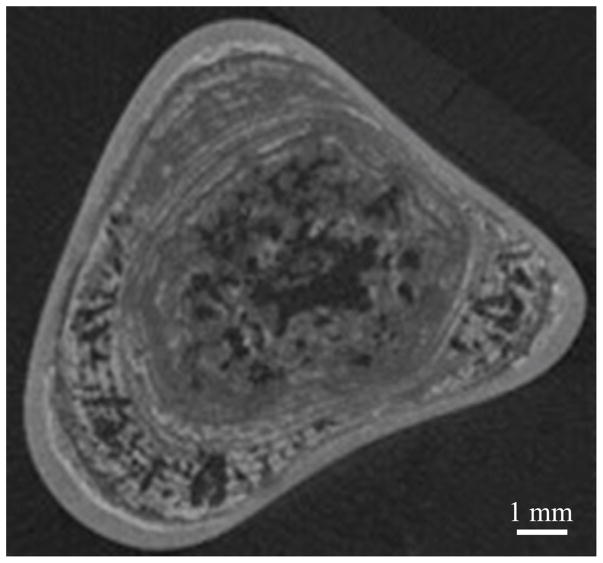
Kidneys are bean-shaped organs (Figure 1) located just below the rib cage on either side of the spine. Each about the size of a fist, kidneys filter over a liter of blood per minute, extracting water and metabolic waste to form urine. In normal kidneys, microscopic crystals form in supersaturated urine and pass harmlessly from the body; however, when these crystals adhere to structures in the kidney, stones begin to form. Stones can form not only in the urine-filled collecting space of the kidney but also in the 200- to 300-μm-diameter ducts that filter the waste from the blood in the cortex and renal pyramids. Kidney stones are very heterogeneous structures composed of inorganic crystals held together by an organic protein matrix, as shown in the X-ray microcomputed tomography (μCT) image of a stone in Figure 2 (Williams et al., 2010). The most common type of kidney stone is calcium oxalate, which accounts for approximately 80% of stones, at least in the United States (Worcester and Coe, 2008). Other types of stones include calcium phosphate, uric acid, struvite, and cystine. Except for struvite stones that form from the metabolic by-product of some infectious bacteria, it is not clear what causes stones to form, although genetics, diet, and hydration all contribute.
Figure 1.
Diagram showing key structures in a cross section of a human kidney. The top of the figure is toward the head and the artery and vein point toward the center of the body. Filtration and fluid extraction occurs in the renal cortex and renal pyramids from blood that enters via the renal artery and, after filtration, exits via the renal vein. The filtrate is then transferred through tubules in the pyramids to the collecting space in the calyx. Urine then passes down the ureter to the bladder.
Figure 2.
Center microcomputed tomography (μCT) slice of a calcium oxalate monohydrate (COM) kidney stone. Gray structures in the image show the inorganic crystals; dark or radiolucent regions are indicative of the organic protein matrix and may contain pockets or trapped gas or microbubbles. Image courtesy of James C. Williams, Jr.
Interest by the National Aeronautics and Space Administration and the Department of Defense
The National Aeronautics and Space Administration (NASA) and the Department of Defense (DoD) have a unique interest in portable and noninvasive technologies for the diagnosis and management of kidney stones (Simon et al., 2016). Astronauts are at an increased risk of forming stones because of dehydration, stasis, and bone demineralization. There have been more than 32 stones observed postflight in US astronauts (Sibonga and Pietrzyk, 2017), and one Russian cosmonaut was preparing for emergency deorbit from space when his stone and the mission critical crisis passed. Should a stone become symptomatic on the International Space Station, the current operational protocol includes administration of pharmaceuticals and emergency evacuation to Earth. However, as astronauts venture farther from Earth, emergency evacuation is unfeasible because transit increases from hours to days, weeks, or months. Urinary stones are also a significant military medical concern because they result in lost duty days and medical evacuations (average 60 per year) and are brought on by environmental stressors common to military training and operational settings (Armed Forces Health Surveillance Branch, 2011). Specifically, the risk of dehydration is high for an unacclimated soldier in a warm climate, and the mean interval from deployment to a symptomatic stone is 93 days (Evans and Costabile, 2005). For both NASA and DoD, clinical technologies used to diagnose and manage stones are too large or complex, thus necessitating evacuation. Therefore, NASA and DoD have supported the development of portable, noninvasive technologies to diagnose and treat kidney stones in the field or in flight.
Kidney Stone Detection
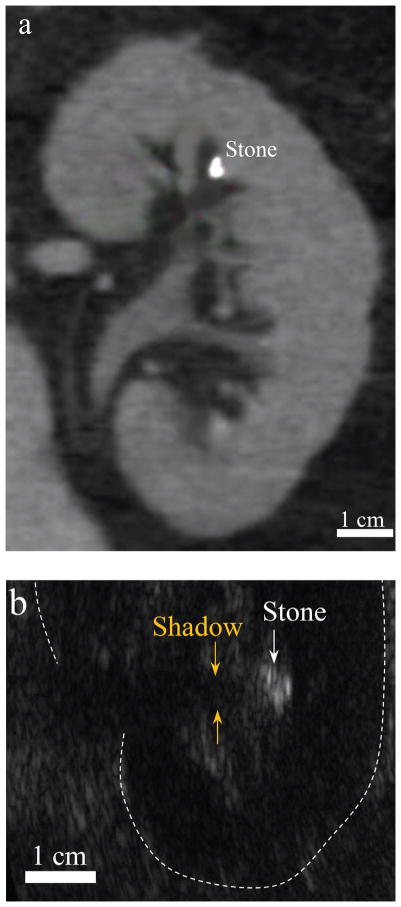
To advise patients on management options for kidney stones, physicians would like to know the size, composition, number, and location of the stones. X-ray computed tomography (CT), which combines X-ray images taken at different angles to produce cross-sectional images or slices is considered the gold standard for diagnosing stones. CT images of stones require little interpretation for physicians to (1) identify the stone position and kidney structures, (2) predict something about stone composition or susceptibility to breakage based on the intensity of the stone, and (3) accurately size the stone (Figure 3a). However, CT also exposes patients to ionizing radiation that increases the risk of developing cancer and limits its use for routine monitoring (Fwu et al., 2013).
Figure 3.
Imaging modalities commonly used to detect kidney stones: computed tomography (CT; a) and ultrasound (b) in two different human subjects. In the CT slice, the stone appears brighter than the surrounding tissue and kidney structures can be clearly identified. In the ultrasound image, the stone appears brighter than the surrounding tissue with a posterior acoustic shadow, which is the ideal case of stone imaging with ultrasound, Image is oriented with the ultrasound probe on the right-hand side of the page. CT image courtesy of Barbrina Dunmire.
In most studies, ultrasound is reported as less sensitive than CT for detecting stones; however, a recent study demonstrated ultrasound as statistically equivalent to CT for diagnosing stones in the emergency room (Smith-Bindman et al., 2014). Yet few surgeons will go to surgery based on ultrasound images alone (Ganesan et al., 2017). Ultrasound (Figure 3b) is less commonly used to detect kidney stones in the United States because of the high availability of CT and the dependence of ultrasound on the skills and interpretation of the user. In the ideal ultrasound image, kidney stones appear as the brightest object in the image, with a posterior acoustic shadow due to the high acoustic impedance of the stones compared with the surrounding soft tissue. However, the stones do not always appear appreciably brighter than the surrounding tissues because the surface curvature or small size of a stone (relative to the ultrasound wavelength) do not necessarily scatter sound directly back to the transducer.
Another challenge in detecting stones with ultrasound is that commercial ultrasound scanners are optimized to detect subtle changes in soft tissue properties, resulting in poor contrast and resolution when imaging stones. Improvements can be made to optimize kidney stone detection. For example, ultrasound machines compress the voltage range of the received signals to display the intensity of the backscattered signal in a limited number of grayscale levels. Rather than map linearly, the voltage is mapped to intensity on a curve that, for most machines, accentuates the difference between the weak signals reflected from tissue and compresses all stronger reflections to the top few bins of brightness. Altering the compression curve to accentuate the strong stone reflections can make stones easier to detect but sacrifices sensitivity to differentiate soft tissues. Similarly, averaging images made from several angles smooths the image, which is desirable for tissues but washes out the strong contrast of the stone and its shadow. These improvements have already been shown to reduce stone size overestimation by 1.6 ± 1.0 mm (May et al., 2016a) compared with overestimates as high as 3.9 ± 1 mm with clinical ultrasound (Sternberg et al., 2016). Size accuracy was further improved, with a difference of only 0.8 ± 0.6 mm (compared with CT), by measuring the width of the posterior acoustic shadow rather than the width of the stone in the image (May et al., 2016a). The reason a stone itself appears large but the shadow does not is not well understood.
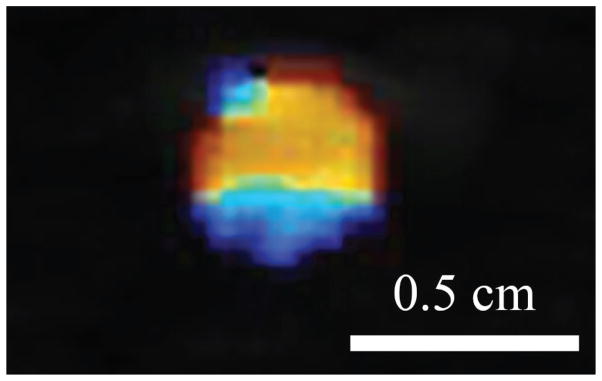
Another way to make a stone more conspicuous in an image is to use the Doppler ultrasound “twinkling artifact” (Rahmouni et al., 1996; Figure 4). In Doppler ultrasound, an ensemble of ultrasound pulses is transmitted and the variability in the backscattered pulses of that ensemble is evaluated through correlation to detect changes that are displayed as color overlaid on a grayscale image. Twinkling occurs when each pulse in the ensemble differs in a nondeterministic way, causing the ultrasound machine to highlight the stone with a mosaic of changing colors. If pulses differ deterministically from a phase or time lag due to the target moving away from the probe as in blood flow, a solid color representing flow velocity appears on the screen (Kremkau, 2010). Whereas stones in grayscale ultrasound images are on average no brighter than the brightest background tissue, the signal strength on a twinkling stone is on average 37 times stronger (Cunitz et al., 2017). Although twinkling can make stones very conspicuous, not all stones twinkle, and at times, modern machines show twinkling on image features that are not stones (Masch et al., 2016).
Figure 4.
The color Doppler ultrasound twinkling artifact highlights kidney stones with a mosaic of colors.
To make twinkling a reliable detection technique, it is important to understand why twinkling occurs. Machine jitter, radiation force that moves the stone, and internal reverberations within the stone have all been proposed to cause twinkling. The oscillation of cavitation bubbles trapped in stone surface crevices could also cause the backscattered signal to differ between each pulse of the ensemble. This hypothesis was recently tested by exposing stones to elevated static pressure that compresses bubbles and drives them into solution because solubility increases with pressure in accordance with Henry’s law. In a water bath, twinkling on stones was found to be suppressed by elevated hydrostatic pressure. Similarly, improved wetting of the stone surface with alcohol to remove bubbles also suppressed twinkling (Lu et al., 2013). The spectral Doppler signatures of twinkling on stones resemble those from gas-filled ultrasound contrast agents (Bruce et al., 2016), and microbubbles entrenched on the stone surface have now been directly observed with a high-speed camera after enlarging the bubbles with a modified lithotripter pulse (Simon et al., 2017). Future work includes determining the distribution and contents of the surface cavitation bubbles as well as modeling the effect of elevated pressures on the resonance of crevice bubbles, which may help elucidate why only some stones twinkle.
Kidney Stone Management
The preferred management for kidney stones, when small enough, is observation to see if the stone will pass on its own. The likelihood of spontaneous stone passage depends on stone size: 80% of small stones less than 5 mm in diameter pass spontaneously, but less than 30% of stones greater than 6 mm in diameter pass spontaneously (Ueno et al., 1977). Some medications that are thought to facilitate spontaneous stone passage are available, but their effectiveness is controversial (Somani et al., 2016).
If a stone does not pass or is accompanied by complications such as infection or obstruction, more proactive management options such as ureteroscopy, shock wave lithotripsy (SWL), and percutaneous nephrolithotomy (PCNL) are employed. In ureteroscopy, a flexible ureteroscope with fiberoptic capabilities is inserted through the urethra, bladder, and ureter and into the kidney. Lasers inserted through the ureteroscope are then used to pulverize the stone to fragments that are passable or can be extracted.
Introduced in the 1980s (Chaussy et al., 1980), SWL is the only completely noninvasive treatment for kidney stones. In SWL, an electrohydraulic, electromagnetic, or piezoelectric source outside the patient generates shock waves that are focused on the kidney stone to shatter it into small fragments so they can pass through the urinary tract (Bailey et al., 2006).
Ureteroscopy and SWL are used in nearly equal proportion and account for over 90% of cases (Bach and Buchholz, 2011). PCNL is employed in rarer, more complex cases involving patients with many or very large stones and entails inserting a 1-cm-diameter tube into the kidney through the back. All procedures have associated injury to the kidney or ureter and risks of complications that research and patient selection can minimize.
Kidney Stone Fragmentation with Ultrasound
In SWL, shock waves act to break apart a stone through two dominant mechanisms: elastic waves and acoustic cavitation (Zhu et al., 2002; Sapozhnikov et al., 2007). Although elastic waves generate large dynamic tensile stresses within the stone body, producing fractures, acoustic cavitation bubbles in the fluid surrounding the stone grow and collapse against the surface, causing hydrodynamic jetting, surface erosion, and pitting. Researchers have sought to improve lithotripters by enhancing stress waves or cavitation. For instance, it has been suggested that a broader focus can achieve better shear wave production in a stone and enhance fractures (Eisenmenger, 2001). Others have proposed altering the waveform of the shock wave to better localize cavitation around the stone (Sokolov et al., 2001). However, these bubbles remain in the fluid after a shock and can rapidly multiply into a large cloud (Pishchalnikov et al., 2006). The result is attenuation of subsequent shocks and shielding of the acoustic energy from reaching the stone, leading to diminished fragmentation (Paterson et al., 2002). This effect limits the rate at which physicians operate a lithotripter to 1–2 shocks per second (to give bubbles time to dissolve), with clinical results showing increased safety and effectiveness at slower rates (Pace et al., 2005).
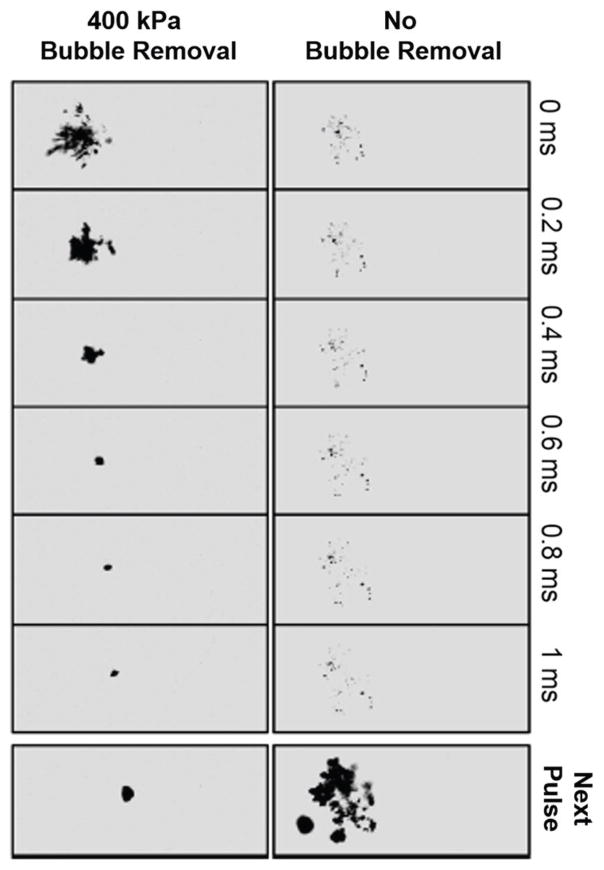
One exciting technology that may solve this problem is acoustic bubble coalescence (Duryea et al., 2014a,b). With a relatively weak pulse of ultrasound, bubbles on the stone can be made to linearly oscillate rather than violently expand and collapse. When two bubbles oscillate near each other, there can be a significant force on each bubble due to their radiated fields, known as the secondary Bjerknes forces (the primary Bjerknes force describes the radiation force on a bubble from a standing wave; Bjerknes, 1906). Under the condition that both bubbles are oscillating in phase, the secondary Bjerknes force causes them to be attracted. In a cloud, this leads to bubbles translating toward each other and coalescing when a weak acoustic field is applied. In experiments, Duryea et al. (2014b) demonstrated this effect with SWL-generated bubble clouds, reducing a shock-induced bubble cluster to a single bubble (Figure 5) and substantially improving stone fragmentation efficiency.
Figure 5.
Bubble cloud development with and without coalescence caused by low-intensity ultrasound. A bubble cluster is formed by an intense ultrasound pulse similar to a lithotripsy shock wave. Without the bubble removal ultrasound pulse (right), the majority of bubbles visible at t = 0 ms remain at t − 1 ms, and subsequent lithotripter pulses (bottom) grow these remaining bubbles, shielding the stone from the effects of the shock wave. With the bubble removal ultrasound pulse (left), a weak (400-kPa) ultrasound pulse arrives at the bubble cluster at t = 0 ms and lasts for 1 ms. The ultrasound pulse causes bubbles in the cluster to merge together or coalesce, such that at 1 ms only a single bubble remains. Reprinted with permission from Duryea et al. (2014b) ©IEEE.
Additionally, in SWL, breathing and patient motion cause a high number of shocks to miss the stone, reducing the efficiency of stone fragmentation while increasing tissue injury. Timing the shocks based on the patient’s respiratory cycle has been shown to improve stone fragmentation but provides no information as to the actual location of the stone. Ultrasound-based techniques that use multiple anatomical features (such as organ contour with vectors relating to the stone position; Koizumi et al., 2011) often outperform algorithms that simply track stone position as the imaging plane changes with respiratory motion. Programming the lithotripter to only fire when the stone is at the focus or motorizing the lithotripter to move with the stone has not been adopted clinically but has the potential to improve stone hit rate by more than 50% (Cleveland et al., 2004; Sorensen et al., 2012).
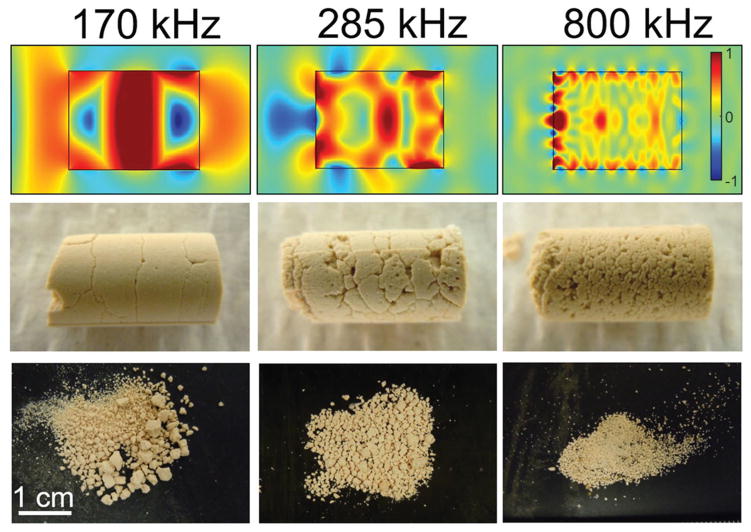
Another experimental method is burst wave lithotripsy (BWL) that uses sinusoidal focused ultrasound bursts rather than shocks to fragment stones (Maxwell et al., 2015). Operating in the range of 100 kHz to 1 MHz, focused ultrasound bursts are delivered to a stone to produce stresses and cause fractures. Similar to SWL, the dominant mechanisms of fragmentation are thought to be elastic waves in the stone and acoustic cavitation; however, BWL has some notable unique characteristics that differ from fragmentation in SWL. In particular, elastic wave models for BWL show evidence that surface waves play a particularly important role in producing initial stone fracture. Due to the sinusoidal nature of the pulse, the surface waves form periodic stresses along the stone surface. The spatial period is related to the frequency and the speed of sound for the surface waves that, for a medium such as a kidney stone, is close to the transverse sound speed of the material. The stresses from surface waves diminish as they extend into the stone and are limited to about one wavelength from the surface. The periodicity and limited depth of stresses created by sinusoidal pulsing in BWL leads to a distinctive fracture pattern and the generation of relatively uniform fragment sizes (Figure 6). Furthermore, the fragment sizes can be controlled by changing the wavelength of the ultrasound. The fragments can thus be made uniformly small so that they will pass spontaneously and asymptomatically from the urinary tract. Such a feature could lead to a significant improvement in lithotripsy success.
Figure 6.
Top: Normalized transient stress in model stones directly after impingement of ultrasound bursts of 170, 285, and 800 kHz. Middle: Corresponding fractures formed in artificial cylindrical stones in a water tank after a short exposure to burst wave lithotripsy (BWL) at each frequency. Bottom: Corresponding fragments produced at each frequency. Reprinted with permission from Maxwell et al. (2015) ©The Journal of Urology.
Repositioning Stones with Ultrasonic Propulsion
Fragments must pass once broken by SWL, BWL, or ureteroscopy. Because of gravity and kidney anatomy, fragments tend to collect and dwell in the lower half of the kidney after a procedure. To solve this problem, ultrasonic propulsion is being developed using acoustic radiation force to reposition kidney stones in the urinary tract (May et al., 2016b). In ultrasonic propulsion, a transducer is placed against the skin and ultrasound imaging is performed simultaneously with the same transducer or with a transducer that includes separate imaging and therapy components. The stone is then seen to move in real time.
There are conceivably many clinical uses for repositioning stones, including moving a painful obstructing stone, relocating a stone before breaking it, and expelling stone fragments during SWL or BWL to assess when the stone is fully comminuted. The acoustic radiation force (F) applied to the stone by ultrasonic propulsion can be estimated as the power (W) divided by the sound speed (c) of the fluid. The force stems from the acoustic energy being absorbed or reflected by the stone. If all the energy is reflected 180° directly back to the source, the force doubles because the energy was not only temporarily absorbed but reflected in the opposite direction. Properties of the stone, such as density and sound speed, affect the degree of reflection, and the stone material’s attenuation coefficient describes the degree of absorption. However, stone material properties are less variable and therefore have less impact on the net force than do stone shape and, particularly, mass (Chuong et al., 1993; Cleveland and McAteer, 2007). Assuming an absorbed or reflected power of 1 W is reasonable and is about 1/200 of the power for a maximum diagnostic pulse average intensity of 190 W/cm2 encountering a large-stone cross section of ~1 cm2. The estimated displacement for a pulse duration of 0.1 s is 0.7 cm, which is similar to the hop that is observed in experiments (Janssen et al., 2017).
Thus it is reasonable that average intensities below the maximum limit for diagnostic ultrasound could be used to move kidney stones, although a more accurate calculation of the radiation force becomes more complicated. Sapozhnikov and Bailey (2013) describe a model that simulates an arbitrary beam (ultrasound is usually focused) on an elastic spherical stone. Interestingly, this model shows that an ultrasound beam 20% broader than the stone imparts the most force on the stone, although one might expect any energy not directly impacting the stone to be wasted. The wave passing across or over the stone appears to couple energy into the stones primarily as shear waves in a process similar to that described for breaking stones (Sapozhnikov et al., 2007).
An ultrasonic propulsion system has been built and tested in humans, and stones were observed to reposition within the kidney. Stones were successfully repositioned in 14 of 15 subjects. The main clinically relevant findings were that 4 of 6 patients with postlithotripsy fragments passed collectively over 30 fragments; in 4 subjects, what appeared on clinical imaging to be one large stone requiring surgery was in fact a pile of passable small fragments; and 1 subject with a 1-cm obstructing stone felt relief from the stone movement procedure (Harper et al., 2016). Based on the observation of debris collections, the probe was redesigned to generate a longer pulse and a broader beam to sweep the stones like a leaf blower (Janssen et al., 2017). That system is now very similar in design to the BWL probes and has been shown to have synergy with BWL. Especially in the confined space of the kidney or ureter, BWL may crack the stone, but ultrasound propulsion separates the broken fragments from the main stone.
Conclusions
Significant advancements have been made in kidney stone detection and management with ultrasound. Changes in image-processing techniques have improved the appearance and sizing of stones with grayscale ultrasound, and the addition of twinkling has the potential to make stones easier to detect. Revisions to current technologies such as stone tracking and acoustic bubble coalescence as well as new technologies including ultrasonic propulsion and BWL have the potential to change the current clinical practice for managing kidney stones. The end result is a suite of technologies that may diagnose and manage kidney stones on Earth or in space with fewer complications and faster relief for those who experience kidney stones.
Acknowledgments
We acknowledge support from NASA Grant NCC 9–58, National Space Biomedical Research Institute (NSBRI), and Grants DK-43881 and DK-104854, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH). We thank our colleagues Barbrina Dunmire (University of Washington), Dr. Alexander Duryea (Histosonics, Inc.), and Dr. James C. Williams, Jr. (Indiana University) for the figures presented.
Biography

Adam D. Maxwell received his BS degree in electrical engineering from the University of Washington in 2006 and MS degree in electrical engineering in 2007 and PhD in biomedical engineering in 2012 from the University of Michigan. He joined the Department of Urology at the University of Washington as a postdoctoral fellow in 2012 and is now a research assistant professor in the same department. His present research is focused on developing new ultrasound-based technologies for kidney stone management.
Julianna C. Simon received her BS degree from Washington State University in 2009 and PhD from the University of Washington (UW) in 2013, both in bioengineering. She received a National Space Biomedical Research Institute First Award Postdoctoral Fellowship and continued working at the UW, switching her research focus from the mechanisms of tissue injury by focused ultrasound to the etiology of the ultrasound twinkling artifact for kidney stone detection. In January 2017, Dr. Simon joined the Pennsylvania State University as an assistant professor in acoustics where she is starting a biomedical acoustics laboratory.
Michael R. Bailey received his BS degree in 1991 from Yale University and MS and PhD degrees in 1994 and 1997, respectively, from the University of Texas at Austin, all in mechanical engineering. He is currently a senior principal engineer at the Applied Physics Laboratory and associate professor of mechanical engineering and adjunct associate professor of urology at the University of Washington. His research interests include cavitation, managing urinary stones, high-intensity focused ultrasound, and ultrasound imaging. He received the R. Bruce Lindsay Award in 2004 and became a Fellow of the Acoustical Society of America in 2007. He has served as the Biomedical Acoustics Technical Committee chair and on the Executive Council.
Contributor Information
Julianna C. Simon, Graduate Program in Acoustics, Pennsylvania State University, 201E Applied Science Building, University Park, Pennsylvania 16802, USA
Adam D. Maxwell, Center for Industrial and Medical, Ultrasound, Applied Physics Laboratory and Department of Urology, University of Washington, 1013 NE 40th Street, Seattle, Washington 98105, USA
Michael R. Bailey, Center for Industrial and Medical, Ultrasound, Applied Physics Laboratory;, Department of Mechanical Engineering; and Department of Urology, University of Washington, 1013 NE 40th Street, Seattle, Washington 98105, USA
References
- Armed Forces Health Surveillance Center. Urinary stones, active component, U.S. armed forces, 2001–2010. Medical Surveillance Monthly Report. 2011;18(12):9–12. [Google Scholar]
- Bach C, Buchholz N. Shock wave lithotripsy for renal and ureteric stones. European Urology Supplements. 2011;10:423–432. doi: 10.1016/j.eursup.2011.07.004. [DOI] [Google Scholar]
- Bailey MB, McAteer JA, Pishchalnikov YA, Hamilton MF, Colonius T. Progress in lithotripsy research. Acoustics Today. 2006;2(2):18–29. doi: 10.1121/1.2961131. [DOI] [Google Scholar]
- Bjerknes VFK. Fields of Force. Columbia University Press; New York: 1906. [Google Scholar]
- Bruce M, Cunitz B, Tremblay-Darveau C, Simon J, Dunmire B, Bailey M. Observations of the Doppler twinkling artifact of kidney stones are bubble related. The 21st European Symposium on Ultrasound Contrast Imaging Abstract Book; Rotterdam, The Netherlands. January 21–22, 2016; 2016. pp. 72–74. [Google Scholar]
- Chaussy C, Brendel W, Schmiedt E. Extracorporeally induced destruction of kidney stones by shock waves. The Lancet. 1980;316(8207):1265–1268. doi: 10.1016/S0140-6736(80)92335-1. [DOI] [PubMed] [Google Scholar]
- Chuong CJ, Zhong P, Preminger GM. Acoustic and mechanical properties of renal calculi: Implications in shock wave lithotripsy. Journal of Endourology. 1993;7(6):437–444. doi: 10.1089/end.1993.7.437. [DOI] [PubMed] [Google Scholar]
- Cleveland RO, McAteer JA. The physics of shock wave lithotripsy. In: Smith AD, Badlani GH, Bagley DH, Clayman RV, et al., editors. Smith’s Textbook on Endourology. B. C. Decker, Inc; Hamilton, ON, Canada: 2007. pp. 313–332. [Google Scholar]
- Cleveland RO, Anglade R, Babayan RK. Effect of stone motion on in vitro comminution efficiency of Storz Modulith SLX. Journal of Endourology. 2004;18(7):629–633. doi: 10.1089/end.2004.18.629. [DOI] [PubMed] [Google Scholar]
- Cunitz BW, Dunmire B, Sorensen MD, Haider YA, Thiel J, May PC, Liu Z, Bailey M, Dunmire B, Bruce M. Quantification of renal stone contrast with ultrasound in human subjects. Journal of Endourology. 2017 doi: 10.1089/end.2017.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duryea AP, Roberts WW, Cain CA, Tamaddoni HA, Hall TL. Acoustic bubble removal to enhance SWL efficacy at high shock rate: An in vitro study. Journal of Endourology. 2014a;28(1):90–95. doi: 10.1089/end.2013.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duryea AP, Tamaddoni HA, Cain CA, Roberts WW, Hall TL. Removal of residual nuclei following a cavitation event using low-amplitude ultrasound. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 2014b;61(10):1619–1626. doi: 10.1109/TUFFC.2014.006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenmenger W. The mechanisms of stone fragmentation in ESWL. Ultrasound in Medicine and Biology. 2001;27(5):683–693. doi: 10.1016/S0301-5629(01)00345-3. [DOI] [PubMed] [Google Scholar]
- Evans K, Costabile RA. Time to development of symptomatic urinary calculi in a high risk environment. Journal of Urology. 2005;173:858–861. doi: 10.1097/01.ju.0000152578.07262.1c. [DOI] [PubMed] [Google Scholar]
- Fwu CW, Eggers PW, Kimmel PL, Kusek JW, Kirkali Z. Emergency department visits: Use of imaging and drugs for urolithiasis have increased in the United States. Kidney International. 2013;83(3):479–486. doi: 10.1038/ki.2012.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan V, De S, Greene D, Torricelli FCM, Monga M. Accuracy of ultrasonography for renal stone detection and size determination: Is it good enough for management decisions? BJU International. 2017;119(3):464–469. doi: 10.1111/bju.13605. [DOI] [PubMed] [Google Scholar]
- Ghani KR, Roghmann F, Sammon JD, Trudeau V, Sukumar S, Rahbar H, Kumar R, Karakiewicz PI, Peabody JO, Menon M, Sun M, Trinh QD. Emergency department visits in the United States for upper urinary tract stones: Trends in hospitalization and charges. The Journal of Urology. 2014;191(1):90–96. doi: 10.1016/j.juro.2013.07.098. [DOI] [PubMed] [Google Scholar]
- Harper JD, Cunitz BW, Dunmire B, Lee FC, Sorensen MD, Hsi RS, Thiel J, Wessells H, Lingeman JE, Bailey MR. First in human clinical trial of ultrasonic propulsion of kidney stones. The Journal of Urology. 2016;195(4):956–964. doi: 10.1016/j.juro.2015.10.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen KM, Brand TC, Cunitz BW, Wang YN, Simon JC, Starr F, Liggitt HD, Thiel J, Sorensen MD, Harper JD, Bailey MR, Dunmire B. Safety and effectiveness of a longer focal beam and burst duration in ultrasonic propulsion for repositioning urinary stones and fragments. Journal of Endourology. 2017;31(8):793–799. doi: 10.1089/end.2017.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi N, Seo J, Lee D, Funamoto T, Nomiya A, Yoshinaka K, Sugita N, Homma Y, Matsumoto Y, Mitsuishi M. Robust kidney stone tracking for a non-invasive ultrasound theragnostic system-Servoing performance and safety enhancement. IEEE Conference on Robotics and Automation; Shanghai, China. May 9–13, 2011; 2011. pp. 2443–2450. [DOI] [Google Scholar]
- Kremkau FW. Sonography: Principles and Instruments. 8. Elsevier/Saunders; St. Louis, MO: 2010. [Google Scholar]
- Litwin M, Saigal C, editors. Urologic Diseases in America. US Department of Health and Human Services; US Government Printing Office; Washington, DC: 2012. NIH Publication No. 12-7865. [DOI] [Google Scholar]
- Lu W, Sapozhnikov OA, Bailey MR, Kaczkowski PJ, Crum LA. Evidence for trapped surface bubbles as the cause for the twinkling artifact in ultrasound imaging. Ultrasound in Medicine and Biology. 2013;39(6):1026–1038. doi: 10.1016/j.ultrasmedbio.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masch WR, Cohan RH, Ellis JH, Dillman JR, Rubin JM, Davenport MS. Clinical effectiveness of prospectively reported sonographic twinkling artifact for the diagnosis of renal calculus in patients without known urolithiasis. American Journal of Roentgenology. 2016;206(2):326–331. doi: 10.2214/AJR.15.14998. [DOI] [PubMed] [Google Scholar]
- Maxwell AD, Cunitz BW, Kreider W, Sapozhnikov OA, Ryan S, Hsi RS, Harper JD, Bailey MR, Sorensen MD. Fragmentation of urinary calculi in vitro by burst wave lithotripsy. The Journal of Urology. 2015;193(1):338–344. doi: 10.1016/j.juro.2014.08.009.fragmentation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PC, Haider Y, Dunmire B, Cunitz BW, Thiel J, Liu Z, Bruce M, Bailey MR, Sorensen MD, Harper JD. Stone-mode ultrasound for determining renal stone size. Journal of Endourology. 2016a;30(9):958–962. doi: 10.189/end.2016.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PC, Bailey MR, Harper JD. Ultrasonic propulsion of kidney stones. Current Opinion in Urology. 2016b;26(3):264–270. doi: 10.1097/MOU.0000000000000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace KT, Ghiculete D, Harju M, Honey RJ University of Toronto Lithotripsy Associates. Shock wave lithotripsy at 60 or 120 shocks per minute: A randomized, double-blind trial. The Journal of Urology. 2005;174(2):595–599. doi: 10.1097/01.ju.0000165156.90011.95. [DOI] [PubMed] [Google Scholar]
- Paterson RF, Lifshitz DA, Lingeman JE, Evan AP, Connors BA, Fineberg NS, Williams JC, Jr, McAteer JA. Stone fragmentation during shock wave lithotripsy is improved by slowing the shock wave rate: Studies with a new animal model. The Journal of Urology. 2002;168(5):2211–2215. doi: 10.1016/S0022-5347(05)64357-1. [DOI] [PubMed] [Google Scholar]
- Pishchalnikov YA, McAteer JA, Williams JC, Pishchalnikova IV, Vonderhaar RJ. Why stones break better at slow shockwave rates than at fast rates: In vitro study with a research electrohydraulic lithotripter. Journal of Endourology. 2006;20(8):537–541. doi: 10.1089/end.2006.20.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmouni A, Bargoin R, Herment A, Bargoin N, Vasile N. Color Doppler twinkling artifact in hyperechoic regions. Radiology. 1996;199(1):269–271. doi: 10.1148/radiology.199.1.8633158. [DOI] [PubMed] [Google Scholar]
- Sapozhnikov OA, Bailey MR. Radiation force of an arbitrary acoustic beam on an elastic sphere in a fluid. The Journal of the Acoustical Society of America. 2013;133(2):661–676. doi: 10.1121/1.4773924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapozhnikov OA, Maxwell AD, MacConaghy B, Bailey MR. A mechanistic analysis of stone fracture in lithotripsy. The Journal of the Acoustical Society of America. 2007;0(2):1190–1202. doi: 10.1121/1.2404894. [DOI] [PubMed] [Google Scholar]
- Scales CD, Jr, Smith AC, Hanley JM, Saigal CS. Prevalence of kidney stones in the United States. European Urology. 2012;0(1):160–165. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibonga JD, Pietrzyk R. Technical Report, Human Research Program. National Aeronautics and Space Administration; Houston, TX: 2017. Evidence Report: Risk of Renal Stone Formation. [Google Scholar]
- Simon JC, Dunmire B, Sorensen MD, Bailey MR. Developing complete ultrasonic management of kidney stones for spaceflight. Journal of Space Safety Engineering. 2016;3(2):50–57. doi: 10.1016/S2468-8967(16)30018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JC, Sapozhnikov OA, Kreider W, Breshock M, Williams JC, Jr, Bailey M. The role of trapped bubbles in kidney stone detection with the color Doppler ultrasound twinkling artifact. Physics in Medicine and Biology. 2017 doi: 10.1088/1361-6560/aa9a2f. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Bindman R, Aubin C, Bailitz J, Bengiamin RN, et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. The New England Journal of Medicine. 2014;371(12):1100–1110. doi: 10.1056/NEJMoa1404446. [DOI] [PubMed] [Google Scholar]
- Sokolov DL, Bailey MR, Crum LA. Use of a dual-pulse lithotripter to generate a localized and intensified cavitation field. The Journal of the Acoustical Society of America. 2001;110(3):1685–1695. doi: 10.1121/1.1394221. [DOI] [PubMed] [Google Scholar]
- Somani BK, Aboumarzouk O, Traxer O, Baard J, Kamphuis G, de la Rosette J. Medical expulsive therapy for ureteral stones: Where do we go from here? Nature Reviews: Urology. 2016;13(10):608–612. doi: 10.1038/nrurol.2016.146. [DOI] [PubMed] [Google Scholar]
- Sorensen MD, Bailey MR, Shah AR, Hsi RS, Paun M, Harper JD. Quantitative assessment of shockwave lithotripsy accuracy and the effect of respiratory motion. Journal of Endourology. 2012;26(8):1070–1074. doi: 10.1089/end.2012.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg KM, Eisner B, Larson T, Hernandez N, Han J, Pais VM. Ultrasonography significantly overestimates stone size when compared to low-dose, noncontrast computed tomography. The Journal of Urology. 2016;95:67–71. doi: 10.1016/j.urology.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Ueno A, Kawamura T, Ogawa A, Hisao T. Relation of spontaneous ureteral calculi to size. Urology. 1977;10(6):544–546. doi: 10.1016/0090-4295(77)90097-8. [DOI] [PubMed] [Google Scholar]
- Williams JC, McAteer JA, Evan AP, Lingeman JE. Microcomputed tomography for analysis of urinary calculi. Urological Research. 2010;38(6):477–484. doi: 10.1007/s00240-010-0326-x. [DOI] [PubMed] [Google Scholar]
- Worcester EM, Coe FL. Nephrolithiasis. Primary Care. 2008;35(2):369–391. doi: 10.1016/j.pop.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Cocks FH, Preminger GM, Zhong P. The role of stress waves and cavitation in stone comminution in shock wave lithotripsy. Ultrasound in Medicine and Biology. 2002;28(5):661–671. doi: 10.1016/S0301-5629(02)00506-9. [DOI] [PubMed] [Google Scholar]