Abstract
Cannabis is the most commonly used illicit drug in the U.S., and craving for cannabis is related to cannabis use. Exercise has been demonstrated to reduce craving for substances. To examine the effects of exercise on cannabis craving, we conducted a 3-week, within-subject crossover experiment. Young-adult men (n = 35) and women (n = 11), age 18-25 years (M = 20.76, SD = 1.68), who regularly (≥ 3×/week) used cannabis participated in a cue exposure paradigm to stimulate craving. After each of three separate craving inductions, they completed a 10-minute bout of exercise that varied in intensity (rest, moderate, vigorous). Craving was assessed before and after the induction, immediately following the exercise, and at three 10-minute intervals (total of 30-minutes). Results of condition-specific, repeated measures ANOVAs showed non-significant reductions in immediate post-exercise craving for the moderate and vigorous conditions. We used latent growth modeling to examine the trajectory of craving rebound during the 30 minutes following exercise and explored the effect of baseline weekly cannabis use in predicting craving rebound. Within 30-minutes post-exercise, craving rebounded for both the moderate, F(3, 135) = 9.10, p < 0.01, and vigorous, F(3, 135) = 3.48, p < 0.05, conditions. We found that among cannabis users reporting larger quantities of typical weekly cannabis use, craving rebounded more quickly following vigorous than moderate exercise, b = 0.02, SE = 0.02, 95% CI [0.00, 0.06]. The findings suggest that moderate exercise may be useful for reducing craving, particularly among those who use larger quantities of cannabis.
Keywords: cannabis craving, cannabis use, marijuana craving, exercise, young-adults
Cannabis is the most commonly used illicit drug by young adults in the U.S., with 19.6% of 18-25 year olds reporting current (past month) use and 8.6% reporting daily or almost daily use in the past month (Azofeifa et al., 2016). Using cannabis at higher frequencies and in large quantities is associated with both physical and psychological consequences, including respiratory problems, cognitive problems, and dependence (Hasin, 2018; National Academies of Sciences, Engineering, and Medicine [NAS], 2017). Among the factors that situationally contribute to cannabis use is craving, which is described as the subjective experience of a deep desire or intense urge to use cannabis (Rosenberg, 2009; Tiffany, 2001; Tiffany & Wray, 2012).
Craving is associated with the motivation to use cannabis (Weiss, 2005) and is considered a symptom of cannabis use disorder (CUD; American Psychiatric Association, 2013). Among dependent cannabis users, craving also has been linked to both withdrawal (Allsop, Norberg, Copeland, Fu, & Budney, 2011), and relapse (Bottlender & Soyka, 2004; Lowman, Hunt, Litten, & Drummond, 2000; Weiss, 2005). Exposure to cannabis cues has been used to successfully induce craving in laboratory settings (Gray, LaRowe, & Upadhyaya, 2008; Gray, LaRowe, Watson, & Carpenter, 2011; Lundahl & Greenwald, 2016; Lundahl & Johanson, 2011). In a recent meta-analysis, cue exposure paradigms were shown to have moderate effects on self-reported cannabis craving (Norberg, Kavanagh, Olivier, & Lyras, 2016). Regardless of the specific method used, the ability to experimentally induce cannabis craving provides opportunities to examine approaches for reducing such craving, particularly those approaches that have been used to reduce craving for other substances. The results of such studies have implications for understanding and initially testing strategies that could serve as effective interventions to reduce cannabis craving and related use. One such approach, for which additional research is needed, is the examination of various aspects of the role of physical activity/exercise on cannabis craving.
Engaging in physical activity, even as little as 60-150 minutes per week, has numerous physical and psychological benefits, including with regard to substance use and misuse (U.S. Department of Health & Human Services [DHHS], 2008). For example, studies of physical activity and nicotine/smoking cigarettes have consistently found that exercise aids in the reduction of withdrawal symptoms and desire to smoke (Daniel, Cropley, & Fife-Shaw, 2006; Marcus et al., 2005; Taylor & Katomeri, 2007; Ussher, Nunziata, Cropley, & West, 2001; Van Rensburg, Elibero, Kilpatrick, & Drobes, 2013). There also is evidence that the intensity of physical activity is associated with reduced craving for tobacco. Daniel and colleagues (2004) found that different intensity levels of exercise had differential effects on reducing the desire to use tobacco; specifically, moderately intense exercise was more effective compared to low-intensity and no exercise.
The effects of exercise also have been examined in interventions for alcohol use disorders. For example, moderate exercise was found to improve mood, reduce anxiety, and reduce alcohol craving in the context of a 12-week intervention for alcohol dependent adults (Brown, Prince, Minami, & Abrantes, 2016). Acute bouts of exercise also have produced short-term reductions in alcohol craving (Ussher, Sampuran, Doshi, West, & Drummond, 2004). Although the number of studies is relatively small, a recent review of this literature concluded that there is promising evidence for including exercise in alcohol treatment (Manthou et al., 2016).
Exercise/physical activity is relatively accessible, can take many forms, and is affordable. To date, exercise has been shown to be effective in reducing alcohol and nicotine craving and misuse. It also may have implications for reducing cannabis use. Buchowski and colleagues (2011) examined the relationship between physical activity/exercise and cannabis craving/use. Their results demonstrated a significant reduction in average craving score over the course of ten exercise sessions. Earlier, Pate and colleagues (1996) showed that failure to engage in physical activity/exercise was associated with cannabis use among adolescents and young adults, suggesting that physical activity may be protective against cannabis use for these age groups. Most recently, exercise showed initial promise as a component in interventions for reducing cannabis craving and related misuse (Brellenthin & Koltyn, 2016). To better understand this potential, we conducted an experimental study in which we examined the effects of exercise on short-term changes in cannabis craving.
The Current Study
In the current preliminary study, we used a within-subject, crossover design to examine the effect of exercise on experimentally-induced cannabis craving. Specifically, we induced cannabis craving through a standardized craving induction procedure prior to each of three separate sessions of exercise that varied in intensity (cf. Gray et al., 2008). To examine the effects of exercise intensity (rest, moderate, vigorous) on cannabis craving, we assessed cannabis craving at multiple times during the study. Specifically, craving assessments occurred immediately prior to and after completion of the craving induction, during and after the exercise session, and for 30-minutes post-exercise (in 10-minute intervals).
The study was designed to examine the effects of different intensities of exercise on cannabis craving. First, we hypothesized that the craving induction would produce increased cannabis craving, regardless of the exercise condition. We also hypothesized that there would be a dose-response effect of exercise intensity on craving scores, such that higher intensity exercise would produce lower craving scores immediately following the exercise. We were interested in the duration of the effect of exercise on craving. We conducted exploratory analyses to examine the duration of participants’ reports of craving immediately following exercise through 30-minutes (in 10-minute increments) post-exercise (cf. Daniel et al., 2006; Ussher et al., 2004; Van Rensburg et al., 2013).
Even among regular users of cannabis, there is a continuum of typical weekly use. We explored whether typical weekly cannabis use influenced craving levels during the time (30 minutes) just following exercise. Specifically, we examined the relationship between typical weekly cannabis use and post-exercise craving rebound, which we conceptualized as the slope of the post-exercise cannabis craving scores at 10-, 20-, and 30-minutes post-exercise.
Method
Participants
Participants were 46 young-adult men (n = 35; 76%) and women (n = 11; 24%) who regularly (≥ 3×/week) used cannabis and agreed to participate in a 3-week study of cannabis use and daily life. As presented in Table 1, their mean age was 20.76 years (SD = 1.68), nearly one third (32.6%) were minorities and 76.1% were college students. In terms of history of cannabis use, the mean age of first use was 15.80 (SD = 2.94) years. Most (67.4%) were daily cannabis users, and the sample reported using 13.26 (SD = 10.72) joints per week. Typical method of using cannabis was bong/bowl (65.2%). About one-half (54.3%) reported spending $100 to $200 per month to buy cannabis. The participants reported that they exercised 7.39 (SD = 8.34) hours during a typical week and 50 reported that they engaged in physical activity on 5 to 7 days per week.
Table 1.
Demographic Information and Sample Characteristics
| Demographic Information | % | M | SD |
|---|---|---|---|
| Gender | |||
| Men | 73.9 | ||
| Women | 26.1 | ||
| Age (in years) | 20.76 | 1.68 | |
| Education (in years) | 14.37 | 1.65 | |
| Ethnicity | |||
| African American/Minority | 32.6 | ||
| European American | 67.4 | ||
| Student Status | |||
| Student | 76.1 | ||
| Non-Student | 23.9 | ||
| Cannabis Variables | |||
| Age of First Use | 15.80 | 2.94 | |
| Typical Weekly Quantity (standard joints) | 13.26 | 10.72 | |
| Typical Pattern of Use | |||
| Daily user (at least once/day) | 67.4 | ||
| 5-6 days per week | 23.9 | ||
| 3-4 days per week | 8.7 | ||
| Typical Method of Using Cannabis | |||
| Bowl/Bong | 65.2 | ||
| Blunt | 28.2 | ||
| Other (Joint, One-hitter, Vaporizer) | 6.6 | ||
| Marijuana Problems Index (MPI total; possible maximum score = 92) | 36.30 | 10.30 | |
| Monthly dollars (USD) spent on Cannabis | |||
| <$100 | 19.6 | ||
| $100-$200 | 54.3 | ||
| >$200 | 26.1 | ||
| Exercise Variables | |||
| Typical weekly Hours Exercised | 7.39 | 8.34 | |
| Typical Pattern of Exercise | |||
| 5-7 days per week | 50.0 | ||
| 1-4 days per week | 43.5 | ||
| 0 days per week | 6.5 |
Note: N = 46.
Study eligibility criteria were: 1) age 18-25 years; 2) use of cannabis at least 3×/week; 3) at least 5th grade education; 4) no history of substance use treatment or long-term psychiatric treatment; 5) no evidence of current alcohol or drug use disorder; 6) no current criminal justice involvement (e.g., probation); 7) Body Mass Index (BMI) < 30 kg/m2; and, 8) no medical contraindications to exercise (e.g., heart condition). This study was approved by the University at Buffalo, SUNY Institutional Review Board. Each participant received up to $300 USD for participating in all aspects of the research.
Recruitment and phone screening
We used print advertisements in community and college newspapers as well as posted flyers to recruit young adults in the Buffalo, New York, metropolitan area. The ads read, “Do you use marijuana?” We conducted phone screenings to assess whether the 353 individuals who responded to the ads met our eligibility criteria. Key questions in the phone screen included age, physical health (e.g., BMI, medical conditions), mental health history, typical use of licit and illicit drugs, and problem drug use (DAST-10; Skinner, 1982). Based on the phone screening, 74 men and women (21% of interested individuals) initially were found eligible to participate in the study.
In-person screening
The 74 individuals who passed the phone screen were invited to an in-person appointment where they were further screened for eligibility, including verification of self-reported substance use and physical heath. To verify current substance use, we conducted a urine drug screen in which participants provided unsupervised urine samples, which were instantly analyzed using the OnTrak TesTcup from Roche Diagnostics Corporation (Indianapolis, IN). This diagnostic test simultaneously detects drugs or drug metabolites in urine, particularly amphetamines (1,000 ng/ml), cocaine metabolite (i.e., benzoylecgonine; 300 ng/ml), THC (i.e., cannabis; 50 ng/ml) and morphine (i.e., opiates; 300 ng/ml). Nine individuals failed the urine drug tests (6 tested negative for THC, 3 tested positive for amphetamines). Urine samples from female participants were separately tested using an over-the-counter pregnancy test; one participant was excused due to a positive pregnancy test. We later excluded one participant whose blood pressure rating was elevated just prior to an exercise session and one participant who failed to comply with the experimental procedures. In total, 28 individuals were excluded, leaving us with a final sample of 46 men and women (see Figure 1). There were no significant differences in age, sex, student status, or employment status between the 46 study participants and the 28 excluded individuals.
Figure 1.
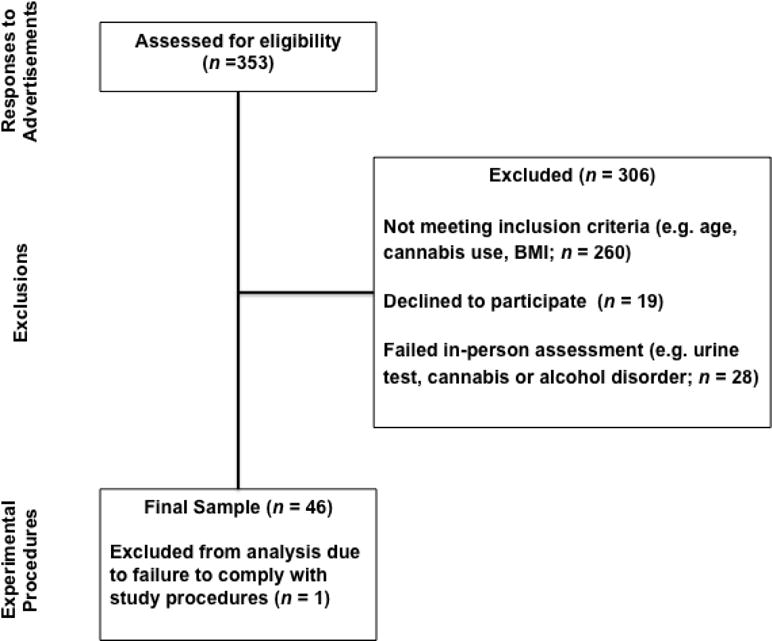
Flow from Respondents to Participation in Experimental Procedures
Pre-exercise screening
Based on American College of Sports Medicine (ACSM; 2010) guidelines, participants with systolic blood pressure ≥ 140 and/or diastolic blood pressure ≥ 90 were not permitted to exercise. As previously noted, one participant was excused from the study due to a high blood pressure readings. At the start of each exercise session, participants completed the 7-item Physical Activity Readiness Questionnaire (PAR-Q; Chisholm et al., 1978; Thomas, Reading, & Shephard, 1992) as a safety screening measure. No participants were excluded based on their PAR-Q responses. We also reviewed each participant’s responses to seven supplemental questions about whether they had engaged in behaviors such as substance use (licit and illicit drugs), exercise, and eating along with the timing of such behavior(s), prior to the appointments that included exercise. The seven behaviors were placed in the rows of a grid and six different time-periods were listed in the columns. The time-periods ranged from “within the last hour” (most recent) to “13-24 hours ago” (most distant). Self-reports indicated that participants tended to adhere to the 24-hour abstention from using cannabis and had not engaged in other behaviors that could negatively impact their completion of the exercise session. Our approach is in line with procedures used in previous research involving short-term abstention from substances (Muraven, Collins, & Nienhaus, 2002; Taylor, Ussher, & Faulkner, 2007).
Design and Procedure
The study used a within-subjects crossover design in which each participant completed three different (counterbalanced) exercise conditions held at the research site, on three different days, each separated by at least three days. We ran participants individually in order to standardize experimental procedures related to the design of the study and to maintain confidentiality regarding use of cannabis. Participants were instructed to refrain from using cannabis and other substances for 24 hours prior to each exercise session. We used a behavior X time grid to collect self-reports that were used to verify the 24-hour period of abstention from using substances. We also administered an alcohol breath test to ensure that each participant’s blood alcohol level (BAL) was zero. No positive BALs were registered. We did not objectively verify the short-term abstention from cannabis, because unlike alcohol, the metabolites of THC (the psychoactive ingredient in cannabis) have a relatively long excretion half-life (from hours through several days), given factors associated with their pharmacology and metabolism (Wong, Brady, & Li, 2014). Specifically, cannabis is metabolized slowly (Sharma, Murthy, & Bharath, 2012), and so there are challenges related to using urine to test brief abstention from cannabis (Gray et al., 2008).
Each participant attended a total of five appointments, conducted at the University at Buffalo’s Center for Health Research (CHR). The first appointment included a fitness test on a cycle ergometer, completion of background questionnaires, and 45 minutes of training on how to use research cell phones and accelerometers for real-time data collection (not described here). Each participant was randomly assigned to the order in which he/she would experience each of the three exercise conditions. The three exercise conditions were: 1) a no exercise/rest control condition of sitting quietly for 10 minutes while listening to classical music and viewing neutral images on a computer; 2) a moderate-intensity, 10-minute exercise condition riding a cycle ergometer; and, 3) a vigorous-intensity, 10-minute exercise condition riding a cycle ergometer.
At each subsequent appointment (numbers 2, 3, 4), the order of cannabis craving induction and assessment procedures was as follows: 1) assessment of pre-induction cannabis craving; 2) exposures to cannabis cues to induce craving (total 4 ½ minutes); 3) cannabis craving assessment, pre-exercise; 4) 10-minute exercise condition; 5) cannabis craving assessment, immediately post-exercise; 6) cannabis craving assessment, 10-minute post-exercise; 7) cannabis craving assessment, 20-minute post-exercise; 8) cannabis craving assessment, 30-minute post-exercise. See Figure 2. Appointment 5 consisted of questionnaire completion (not described here), computerized behavioral economic tasks (not described here) and final payment.
Figure 2.
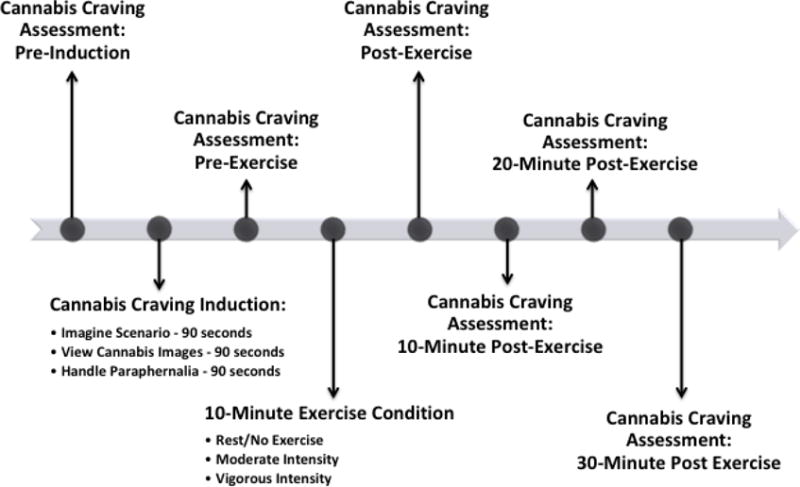
Cannabis Craving Induction and Assessment Procedures During Each Exercise Session
Screening Measures
Cannabis Use Problems Identification Test
(CUPIT; Bashford, Flett, & Copeland, 2010). The CUPIT is a 16-item measure of problems related to cannabis use during the past 12-months. The items focus on patterns of cannabis use, cannabis problems, and difficulties refraining from using cannabis. Questions use a multiple-choice format, in which response options varied. Individuals who scored 20 or more points were not eligible to participate.
Drug Abuse Screening Test
(DAST-10; Skinner, 1982). This brief version of the 28-item original DAST (Skinner, 1982) was used to identify problems due to illicit drug use. The items (e.g., Do you use more than one drug at a time?) all use Yes/No response options. All affirmative responses were given 1 point, and the total point value was used as an indicator of the severity of problems. Individuals who scored 3 or more points were not eligible to participate.
Short Michigan Alcohol Screening Test
(S-MAST; Selzer, Vinokur, & van Rooijen, 1975). The S-MAST is a 13-item diagnostic test for problem alcohol use. The items (e.g., Are you able to stop drinking when you want to?) all use Yes/No response options. All affirmative responses were given a point of 1, and the total point value was used as an indicator of the severity of problems. Individuals who scored 3 or more points were not eligible to participate.
Background Measures Administered at Appointment 1
At the initial in-person appointment, participants completed a battery of computerized psychometrically-sound questionnaires. The measures presented below were directly related to describing our sample and providing background information (see Table 1). Questionnaire order was counterbalanced across participants (cf. Collins, Bradizza, & Vincent, 2007; Collins, Vincent, Yu, Liu, & Epstein, 2014).
General Information Questionnaire
(GIQ; Collins, Lapp, Emmons, & Isaac, 1990). This 37-item self-report measure assessed background information including demographic characteristics (e.g., age, sex) and use of alcohol (the Daily Drinking Questionnaire (DDQ); Collins, Park, & Marlatt, 1985) and other substances.
Marijuana Use Questionnaire
(MUQ; Collins et al., 2014). Based on a review of existing cannabis use questionnaires (e.g., Marijuana Smoking History Questionnaire, Bonn-Miller & Zvolensky, 2009), this 23-item self-report questionnaire assesses various aspects of typical cannabis use, including: a) method of using cannabis (e.g., blunt, bong); b) pattern and context of use (alone vs. with others); c) frequency and quantity of past cannabis use; and d) symptoms of cannabis abuse and dependence. To standardize reports of cannabis use, all participants, regardless of smoking method (e.g., joint, blunt), were trained to report the quantity of cannabis they smoked in terms of the number of average-sized joints they could have rolled, with one average-sized joint defined as ½ gram of cannabis.
Marijuana Acquisition and Use Patterns Questionnaire
(MAUQ; Collins et al., 2014). This 16-item, semi-structured, in-person interview provided a detailed assessment of cannabis users’ smoking methods and use patterns, as well as their purchasing behavior (e.g., money spent on cannabis). Items from this questionnaire were used to describe cannabis use patterns.
Marijuana Craving Questionnaire – Short Form
(MCQ - SF; Heishman, et al., 2009). The 12-item MCQ-SF is based on the original 47-item MCQ (Heishman, Singleton, & Liguori, 2001). The 12-item measure was developed by selecting the three highest loading items that represent each of the four craving dimensions (i.e., compulsivity, emotionality, expectancy, purposefulness) assessed by the MCQ. The MCQ-SF was found to be a reliable and valid measure of the dimensions of marijuana craving (Heishman et al., 2009). In the current study, the MCQ-SF was found to be internally consistent; alpha = .87.
Marijuana Problems Index
(MPI; Simons & Carey, 2006). The MPI is a 23-item measure of psychological, social, occupational, and physical problems that may result from cannabis use. Items (e.g., Went to work or school high.) are rated on a 5-point scale (0 = Never to 4 = More than 10 times) as to their occurrence during the past 12 months. Total MPI scores were computed by summing all items, with higher scores indicating more frequent cannabis problems. Cronbach’s alpha for the current sample was 0.86 (cf. Simons & Carey, 2006).
Self-Administered Timeline Followback
(S-TLFB; Collins, Kashdan, Koutsky, Morsheimer, & Vetter, 2008). The S-TLFB is a self-administered, retrospective report of daily cannabis use quantity and frequency during each of the past 30 days. Quantity of cannabis was reported as average-sized joints. This measure was used to create the typical weekly cannabis use variable.
Overview of the Exercise Sessions
At the start of each exercise session, each participant completed the following questionnaire.
Amended Physical Activity Readiness Questionnaire
(PAR-Q; Chisholm et al., 1978; Thomas, Reading, & Shephard, 1992). The PAR-Q is a 7-item, Yes/No, self-assessment of physical health (e.g., dizziness, chest pain) in the context of exercise. To further assess exercise readiness, we added eight questions about current medications, medical symptoms (difficulty breathing) and other health issues (e.g., pregnancy). At the start of each exercise session we also assessed past 24-hour use of licit and illicit substances.
Exercise Conditions
During each of the three exercise conditions, participants wore a heart rate monitor and transmitter belt (POLAR WearLink® + transmitter, Polar Electro Inc., Lake Success, NY, www.polar.com) around his or her chest throughout the exercise sessions for continual monitoring by staff. The control condition consisted of 10 minutes of rest, during which the participant sat quietly without access to cell phone, internet, or reading materials. To reduce boredom, participants watched neutral mood (e.g., scenery) images on a computer screen while listening to soft classical music. The two exercise conditions involved moderate and vigorous intensity exercise on a cycle ergometer. Exercise intensity was determined using the Karvonen formula (Karvonen, Kentala, & Mustala, 1957) and was consistent with American College of Sports Medicine (ACSM; 2010) guidelines. It was set at resting heart rate (assessed at the first appointment) plus 45-55% (moderate intensity) or 70-80% (vigorous intensity) of each individual’s heart rate reserve. Heart rate reserve (maximal heart rate – resting heart rate) was calculated using Robergs & Landwehr’s (2002) age-predicted maximal heart rate formula [220 – age (in years)].
Participants completed a 3-minute warm-up on a cycle ergometer (Monark 828E Ergomedic fitness cycle, Medco Supply Company, Chicago, IL) immediately prior to starting the 10-minute exercise session. During the warm-up, the resistance (kilopounds or Kp) was gradually increased from a starting point of 0.5, in order to meet and maintain the participant’s target heart rate range for the given exercise condition (moderate- or vigorous-intensity). The maximum Kp during the exercise sessions was 1.0 to 1.5 for women and 1.5 to 2.0 for men, and maximum RPM was 100 for both genders. We recorded participants’ heart rate at 30-second intervals during the exercise sessions and participants’ self-reports of exercise intensity (Borg Rating of Perceived Exertion Scale [RPE]; Borg, 1982) at 3-minute intervals. Contact with research staff was kept to a minimum. At the end of the 10-minute exercise session, the participant performed a 2-3 minutes cool down and stretching exercises.
Cannabis Craving Induction
Based on previous research, we used multiple sensory modalities to stimulate participants’ desire to use cannabis (cf. Gray et al., 2008; Singleton, Trotman, Zavahir, Taylor, & Heishman, 2002). The procedure consisted of instructing participants to: 1) close their eyes and listen to a recorded 60-second cannabis smoking scenario while imagining that they were in the scene, then continue to imagine themselves smoking cannabis for an additional 30 seconds (cf. Tiffany & Hakenewerth, 1991); 2) view cannabis-related images (9 images, 10 seconds each); and, 3) handle cannabis-related objects (e.g., glass pipe) for 90 seconds. The order of tasks (i.e., imagine scenario, view cannabis images, then handle paraphernalia) was fixed across conditions. The cannabis craving procedure closely paralleled that used by Gray et al. (2008) in terms of the nature and order of the tasks and the duration of the craving induction (90 seconds per task for a total of 4½ minutes).
Cannabis Craving Assessment During Exercise Sessions
The experimental procedures required repeated measurement of cannabis craving in a laboratory setting (cf., Rosenberg, 2009). We focused on craving as involving the subjective experience of a deep desire or intense urge to use cannabis (Rosenberg, 2009; Tiffany, 2001; Tiffany & Wray, 2012). Thus, the measure used to assess cannabis craving consisted of four items pertaining to urge/desire to use cannabis. Participants were instructed to indicate the number (Likert scale; 0 = “Not at all”, and 9 = “Very much”) that “best describes how you are feeling at this moment” for each of four statements. Each statement ended with the words, “right now”: 1) I need to smoke marijuana; 2) I have an urge to smoke marijuana; 3) I want to smoke marijuana; and 4) I have a strong desire to smoke marijuana. The first statement (I need to smoke marijuana) was taken from the 12-item Marijuana Craving Questionnaire Short Form (MCQ-SF; Heishman et al., 2009). The remaining three items were similar to those used in previous studies of cue-induced craving for cannabis (cf. Lundahl & Greenwald, 2016; Lundahl & Johanson, 2011). The participants completed the same four items at each of the seven different assessments that occurred before, during, and after the cannabis craving induction and exercise session (see Figure 1). Our 4-item cannabis craving measure was internally consistent (Cronbach’s alpha = 0.96 to 0.97) across the seven assessments.
Other researchers have made the case that a short measure of desire/urge is the most useful approach to measuring drug craving. For example, Kozlowski and colleagues compared different versions of a multi-faceted measure of urge to smoke cigarettes and showed that a 3-item scale was the preferred approach for measuring cigarette urge/craving (Kozlowski, Pillitteri, Sweeney, Whitfield, & Graham, 1996). We sought additional evidence of the usefulness of our brief (4-item) measure of cannabis craving by comparing the three statements adapted from Lundahl and colleagues (2011; 2016; i.e., items #2, #3, and #4, above) with the 12-item MCQ-SF (Heishman et al., 2009), which already included our item #1. For our sample, Cronbach’s alpha for the baseline MCQ-SF was 0.87 while the alpha for the three craving items, also administered at baseline, was 0.85. Additionally, we found a strong correlation between the total scores on our brief 4-item craving measure and the MCQ-SF, r = 0.79, n = 46, p < 0.01. These results provide evidence that our 4-item craving measure was both internally consistent and valid.
Statistical Considerations/Analysis Plans
Craving induction/manipulation check
Because the craving induction occurred before the experimental exercise sessions, we averaged the mean craving scores across all three exercise conditions. We used a paired samples t-test to compare the mean craving scores just prior to the craving induction with the mean craving scores just after the induction, prior to the exercise session. The results will provide evidence as to whether the craving induction was successful in increasing craving scores.
Effects of Exercise on Cannabis Craving1,2
Changes in craving pre- to post-exercise
In order to examine whether there was a dose-response effect for exercise intensity on craving reduction, we used a repeated measures analysis of variance (RM-ANOVA) to compare the change in pre- to post-exercise craving across the three exercise conditions (rest, moderate, vigorous). Given the within-subjects design and related loss of independence in our sample, the exercise condition was the repeated variable in our model.
Changes in craving immediately post-exercise to 30-minutes post-exercise
In order to compare the post-exercise craving scores within each condition, we performed separate RM-ANOVAs in which time was the repeated variable. The time variable was based on post-exercise craving assessments taken: 1) immediately after completing the exercise session, 2) 10-minutes post-exercise, 3) 20-minutes post-exercise, and 4) 30-minutes post-exercise. Consistent with the study design and hypotheses, the three exercise conditions were treated as separate to allow us to examine within-person change as opposed to change between groups.
Typical weekly cannabis use and effects of exercise on craving rebound
We used Structural Equation Modeling (SEM) to explore the association between typical weekly cannabis use and effects of exercise on craving rebound. Embedded in our SEM was a Latent Growth Model (LGM) that allowed us to model the trajectory of post-exercise craving over the 30-minute period during which it was measured. LGM was used for its flexibility in measuring an individual’s growth trajectory and differences over time (Duncan & Duncan, 2009). The slopes derived from the LGMs, which we operationalized as representing craving rebound, were then used as outcome variables. We used typical weekly cannabis use (assessed at baseline) to predict the slopes (see Figure 3).
Figure 3. Post-Exercise Craving Rebound and Association with Typical Weekly Cannabis Use.

1. Craving rebound (i.e., the slope of the cannabis craving scores immediately following exercise, 10-minutes, 20-minutes, and 30-minutes following exercise).
2. Impact of typical weekly cannabis use on craving rebound.
To determine the best trajectory to model the craving rebound slopes, we compared our sample data against four possible trajectories for each experimental condition: 1) a No Growth model (intercept only); 2) a Linear Growth model (time score: 0, 1, 2, 3); 3) a Log Growth model (time score: 0, 0.69, 1.09, 1.38); and, 4) a Quadratic Growth model (time score: 0, 1, 4, 9). The best fitting model for each condition was chosen using the following three fit indices and will be described in the results section: 1) the root mean square error of approximation (RMSEA; Steiger, 1990); 2) the comparative fit index (CFI; Bentler, 1990); and, 3) the standardized root mean square residual (SRMR; McDonald & Ho, 2002).
Because the sampling distributions of our growth models were positively skewed, traditional statistical testing and formulation of confidence interval may not be accurate (Neal & Simons, 2007). To address this issue, we employed a bias-corrected (BC) bootstrapping method of determining confidence intervals, which were more appropriate for the data. BC bootstrapping resamples the data (here, 5,000 times) to create a representative sampling distribution that is able to accommodate asymmetrical distributions. The confidence intervals are formulated by taking the area under the curve of the sampling distribution. BC bootstrapped confidence intervals provide the most reliable estimates in smaller samples (Hayes & Scharkow, 2013) and so are included in our growth models.
Results
Manipulation Check/Craving Induction
We conducted a paired samples t-test to compare self-reported craving before and after the craving-induction protocol. There was a significant increase in craving score from the assessment just prior to the induction (M = 2.28, SD = 0.31) to the post-induction assessment (M = 3.21, SD = 0.34); t(45) = −7.32, p < 0.01. See Table 2 for mean craving scores over time by condition. These results support our hypothesis that the craving induction would significantly increase craving scores.
Table 2.
Mean Ratings of Cannabis Craving by Exercise Condition
| Exercise Condition | Pre-Induction | Pre-Exercise | Post-Exercise | 10-Minute Post-Exercise | 20-Minute Post-Exercise | 30-Minute Post-Exercise |
|---|---|---|---|---|---|---|
| Rest | 2.41(2.33) | 3.43(2.38) | 2.57(2.29) | 2.57(2.28) | 2.63(2.34) | 2.65(2.44) |
| Moderate | 2.27(2.35) | 3.07(2.48) | 2.06(2.32) | 2.44(2.30) | 2.67(2.45) | 2.71(2.49) |
| Vigorous | 2.16(2.25) | 3.13(2.47) | 1.87(2.10) | 2.22(2.22) | 2.39(2.21) | 2.44(2.21) |
Note: N = 46. Means (SDs) of 4-item Marijuana Craving measure
Effects of Exercise on Cannabis Craving
Changes in craving pre- to post-exercise
We conducted a RM-ANOVA to examine the dose-response effect of exercise condition (rest, moderate, vigorous) on reductions in craving scores. We found no significant differences in the change from pre- to post-exercise craving among the three conditions, F(2, 90) = 1.66, p > 0.10. These results indicate that exercise intensity did not influence the level of reduction in self-reported craving. Thus, our hypothesis was not supported.
Changes in craving immediate post-exercise to 30-minutes post-exercise
Results of an RM-ANOVA showed a significant increase in the post-exercise craving scores over time for both the moderate, F(3, 135) = 9.10, p < 0.01 and vigorous exercise conditions, F(3, 135) = 3.48, p < 0.05, but not for the rest condition. Specifically, in the moderate condition the craving score increased from 2.06 immediately post-exercise to 2.71 at the 30-minute post-exercise assessment. In the vigorous condition, the craving score increased from 1.87 immediately post-exercise to 2.44 at the 30-minute post-exercise assessment. These results indicate that the effects of exercise dissipated and craving rebounded over the course of the 30 minutes after completing the exercise condition.
Typical weekly cannabis use and effects of exercise on craving rebound
We used structural equation modeling (SEM) to explore the relationship between typical weekly cannabis use and cannabis craving rebound. We constructed a separate model for each exercise condition (rest, moderate, vigorous). Within each SEM, we embedded an LGM to explore whether typical weekly cannabis use predicted post-exercise craving rebound (i.e., craving score changes across 30-minutes post-exercise, assessed at 10-minute intervals). We determined that the best fitting trajectory models were No Growth for the rest condition (RMSEA = 0.00, CFI = 1.00, SRMR = 0.01), and Log Growth for both the moderate (RMSEA = 0.00, CFI = 1.00, SRMR = 0.01) and vigorous conditions (RMSEA = 0.10, CFI = 0.99, SRMR = 0.03). We used these models in all subsequent analyses. Only in the vigorous condition did we find an effect. Specifically, we found that cannabis users reporting larger quantities of typical weekly cannabis use at baseline, exhibited a steeper slope (i.e., quicker return to initial craving score) following vigorous exercise, b = 0.02, SE = 0.02, 95% CIs [0.00, 0.06].
Discussion
In this preliminary study, we successfully used exposure to cannabis cues (e.g., imagery, paraphernalia) to significantly increase craving in young adults who were regular (≥ 3×/week) cannabis users. Consistent with previous research, the craving induction procedure successfully elevated craving (e.g., Gray et al., 2008; Gray et al, 2011; Lundahl & Greenwald, 2016; Lundahl & Johanson, 2011; Singleton et al., 2002). Thus, as hypothesized, we found that experimental exposure to cannabis cues produced increases in craving that are analogous to what might occur in real-world situations encountered by cannabis users.
Effects of Exercise on Cannabis Craving
To examine the role of exercise intensity in reducing craving, we used a within-subjects design in which participants engaged in separate sessions involving three different levels of exercise (rest, moderate, vigorous), in randomized order, following the induction of cannabis craving. We were not able to demonstrate a significant reduction in cannabis craving immediately following the exercise sessions.
Although the lack of support for a dose-response effect of exercise intensity on craving was unexpected, it may reflect phenomena that have been described in exercise interventions for substance use and misuse. Recent reviews of research on the role of exercise in reducing substance use craving, mostly in the context of interventions for dependent individuals, have described mixed findings (Linke & Ussher, 2015; Taylor et al., 2007; Wang, Wang, Wang, Li, & Zhou, 2014). Possible reasons cited for these mixed results include, an insufficient duration of exercise during the intervention and the shorter length of the intervention (Linke & Ussher, 2015; Taylor et al., 2007). Our exercise sessions were of relatively short duration (10-minutes) therefore may not have lasted long enough to be effective. Linke and Ussher (2015) also suggested that just the inclusion of a control group when comparing craving responses to different exercise intensities, would make the differences between the varying intensities statistically similar. By this reasoning, our inclusion of a control (i.e., rest) condition, may have contributed to the failure to find significance.
Our subject selection criteria also may have influenced our findings. Our sample consisted of young adults who regularly used cannabis, but were not dependent on cannabis. Previous studies that illustrated a significant effect of exercise on cannabis craving studied cannabis-dependent samples (cf. Buchowski et al., 2011; Lundahl & Greenwald, 2016; Lundahl & Johanson, 2011). It could be that dependent samples respond differently to exercise interventions compared to nondependent samples, such as were included in the current study.
To explore the nature of the craving response following exercise, we assessed craving for 30-minutes (in 10-minute increments) following each exercise session, and ran separate RM-ANOVAs for each condition. Our results showed an immediate, though non-significant reduction in craving following both moderate and vigorous exercise. However, the initial reduction in craving as a result of exercise faded, and craving levels approached the pre-induction level by the 30-minute post-exercise assessment. It may be useful to design interventions that capitalize on the initial reductions in craving shortly after exercise. There was no significant change in craving over time following the no exercise/rest condition, meaning that craving remained elevated when participants were sedentary. This suggests that less active/sedentary cannabis users who experience craving when exposed to cannabis cues in real life may continue to experience craving beyond the initial point of exposure.
Craving Rebound
To expand on our findings that craving returned to initial levels during the time following exercise, we examined whether typical cannabis use (assessed at baseline) would predict the trajectory of the rebound (i.e., return to initial levels) in craving scores. Using SEM with embedded growth models, we found that for cannabis users reporting larger quantities of typical weekly cannabis use at baseline, 10-minutes of vigorous exercise produced a faster rate of craving rebound than did 10-minutes of moderate exercise. Stated in another way, moderate exercise may have a helped to maintain reductions in cannabis craving. One practical implication of this finding is that moderate exercise interventions to reduce cannabis craving may be more promising than interventions that use vigorous exercise. Also, even among nondependent cannabis users, typical levels of cannabis use may influence responses to exercise. We suggest that future research should include consideration of the role of typical use patterns. These suggestions are consistent with previous research that examined the role of exercise intensity in reducing craving and/or substance use in noncannabis using samples. For example, Brown et al. (2016) found that moderate-intensity exercise reduced alcohol craving. Daniel et al. (2004) found that moderate-intensity exercise was more effective than low-intensity exercise or no exercise for reducing craving for cigarettes. Future research on the effects of exercise on cannabis craving should examine the efficacy of moderate-intensity exercise for reducing cannabis craving and related cannabis use. Analogous to our recommendation regarding the role of typical levels of cannabis use, future research also should examine the role of background levels of typical exercise in the response to exercise interventions for reducing cannabis craving.
Limitations and Future Directions
There are some limitations to this proof-of-concept study that likely impact on the generalizability of the findings. Such limitations are related to factors that include: the participants’ demographic characteristics; the physical fitness of the sample; and, the length of the exercise session.
The study was conducted with a small, predominantly male, sample of young-adults who regularly used cannabis, but were not dependent on cannabis. Although prevalence data indicates that cannabis is used by young-adult men more than young-adult women (Azofeifa et al., 2016), the small number of female participants means that we cannot comment on the role of gender in any aspects of this study. Similarly, the small sample sizes of the different ethnic groups mean that we cannot comment on the role of ethnicity in any aspects of this study (see Table 1). The age (young adults, aged 18 to 25 years) of the participants limits the generalizability of the findings to older samples of cannabis users.
The sample’s physical-fitness and the brief length of the exercise sessions also lessen the generalizability of our findings. To lessen the level of fatigue experienced by the participants who were instructed to engage in exercise sessions of varying intensities, we recruited and screened for physically-fit young-adults who had a BMI < 30. As a result, our sample consisted of individuals who regularly engaged in physical activity in their daily lives. Other studies (e.g., Buchowski et. al., 2011) have tested the effects of exercise on sedentary or minimally-active cannabis users. As stated previously, it would be useful to examine the role of typical exercise pattern as a possible contributor to individual’s responses to exercise interventions designed to reduce cannabis craving and use. In addition, our moderate and vigorous exercise sessions lasted only 10-minutes; short in comparison to exercise interventions (see Wang et al., 2014). Given the participants’ relative fitness and the short (10-minute) duration of the exercise sessions, it is possible that the bouts of moderate and vigorous exercise were within the scope of our participants’ normal daily activity level. Thus, we cannot comment on the likely response to longer durations of exercise and/or effects on individuals who are more sedentary in their daily lives.
The findings of this preliminary study provide the basis for future research to further examine the role of exercise, particularly moderate exercise, in reducing cannabis craving and use. To increase the generalizability of our initial findings, researchers should expand their samples by recruiting and testing a larger number of participants, including a larger proportion of females. Researchers also should recruit participants that represent a wider range of ages, ethnicities, fitness levels, as well as typical exercise and cannabis use patterns. Such expansion would allow researchers to test for differences among subgroups, thereby maximizing the potential to tailor exercise interventions for specific populations, including cannabis-dependent users. Future research also should examine the effects of the length and/or number of exercise sessions over intervention periods of different durations, as has been done in previous research on alcohol and tobacco use (e.g., Brown et al., 2016; Daniel et al., 2006; Manthou et al., 2016; Marcus et al., 2005; Taylor & Katomeri, 2007; Ussher et al., 2004; Van Rensburg et al., 2013).
Public significance statement.
Cannabis cues (images, paraphernalia) can increase craving and thereby promote use in real-world contexts. Our findings suggest that regular users of cannabis who encounter such cues might find moderate exercise helpful for initially reducing craving and maintaining that reduction over a period of up to 30 minutes.
Footnotes
Given the within-subjects design, we also conducted the analyses using Structural Equation Modeling (SEM) and comparative effect sizes. These analyses achieved results that are similar to the ANOVA-based analyses, which we describe here for ease of presentation and interpretation.
We tested for both time and order effects of the data to rule out the possibility that the craving scores were a function of either the repetition of similar experimental procedures or the order in which the exercise conditions were administered. No meaningful differences were found for either time or order.
Contributor Information
Sandy D. Wilson, Doctoral Candidate, Department of Community Health and Health Behavior, University at Buffalo, State University of New York, 3435 Main Street, 825 Kimball Tower, Buffalo, New York 14214-8028, Direct phone: 716-829-5623, Fax: 716-829-6040.
R. Lorraine Collins, Professor, Department of Community Health and Health Behavior, University at Buffalo, State University of New York
Mark A. Prince, Assistant Professor, Department of Psychology, Colorado State University
Paula C. Vincent, Research Assistant Professor, Department of Community Health and Health Behavior, University at Buffalo, State University of New York
References
- Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The Cannabis Withdrawal Scale development: Patterns and predictors of cannabis withdrawal and distress. Drug and Alcohol Dependence. 2011;119:123–129. doi: 10.1016/j.drugalcdep.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Thompson WR, Gordon NF, Pescatello LS, editors. American College of Sports Medicine (ACSM) ACSM’s guidelines for exercise testing and prescription. 8th. Chicago, IL: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Azofeifa A, Mattson ME, Schauer G, McAfee T, Grant A, Lyerla R. National estimates of marijuana use and related indicators – National Survey on Drug Use and Health, United States, 2002-2014. Morbidity and Mortality Weekly Report Surveillance Summaries. 2016;65:1–25. doi: 10.15585/mmwr.ss6511a1. [DOI] [PubMed] [Google Scholar]
- Bashford J, Flett R, Copeland J. The cannabis use problems identification test (CUPIT): Development, reliability, concurrent and predictive validity among adolescents and adults. Addiction. 2010;105:615–625. doi: 10.1111/j.1360-0443.2009.02859.x. [DOI] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107(2):238. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Bonn-Miller MO, Zvolensky MJ. An evaluation of the nature of marijuana use and its motives among young adult active users. The American Journal of Addictions. 2009;18:409–416. doi: 10.1080/10550490903077705. [DOI] [PubMed] [Google Scholar]
- Borg GA. Psychophysical bases of perceived exertion. Medicine and Science in Sports and Exercise. 1982;14:377–381. [PubMed] [Google Scholar]
- Bottlender M, Stoyka M. Impact of craving on alcohol relapse during, and 12 months following, outpatient treatment. Alcohol & Alcoholism. 2004;39:357–361. doi: 10.1093/alcalc/agh073. [DOI] [PubMed] [Google Scholar]
- Brellenthin AG, Koltyn KF. Exercise as an adjunct treatment for cannabis use disorder. The American Journal of Drug and Alcohol Abuse. 2016;42:481–489. doi: 10.1080/00952990.2016.1185434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Prince MA, Minami H, Abrantes AM. An exploratory analysis of changes in mood, anxiety and craving from pre-to post-single sessions of exercise, over 12 weeks, among patients with alcohol dependence. Mental Health and Physical Activity. 2016;11:1–6. doi: 10.1016/j.mhpa.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchowski MS, Meade NN, Charboneau E, Park S, Dietrich MS, Cowan RL, Martin PR. Aerobic exercise training reduces cannabis craving and use in non-treatment seeking cannabis-dependent adults. PloS ONE. 2011;6(3):e17465. doi: 10.1371/journal.pone.0017465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm DM, Collis ML, Kulak LL, Davenport W, Gruber N, Stewart G. PAR-Q Validation Report: The evaluation of a self-administered pre-exercise screening questionnaire for adults. Victoria: Canada: BC Ministry of Health and Health and Welfare 1978 [Google Scholar]
- Collins RL, Bradizza CM, Vincent PC. Young adult malt liquor drinkers: Prediction of alcohol problems and marijuana use. Psychology of Addictive Behaviors. 2007;21:138–146. doi: 10.1037/0893-164X.21.2.138. [DOI] [PubMed] [Google Scholar]
- Collins RL, Kashdan TB, Koutsky JR, Morsheimer ET, Vetter CJ. A self-administered timeline follow-back to measure variations in underage drinkers’ alcohol intake and binge drinking. Addictive Behaviors. 2008;33:196–200. doi: 10.1016/j.addbeh.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RL, Lapp WM, Emmons KM, Isaac LM. Endorsement and strength of alcohol expectancies. Journal of Studies on Alcohol. 1990;51:336–342. doi: 10.15288/jsa.1990.51.336. [DOI] [PubMed] [Google Scholar]
- Collins RL, Parks GA, Marlatt GA. Social determinants of alcohol consumption: The effects of social interaction and model status on the self-administration of alcohol. Journal of Consulting and Clinical Psychology. 1985;53:189–200. doi: 10.1037/0022-006X.53.2.189. [DOI] [PubMed] [Google Scholar]
- Collins RL, Vincent PC, Yu J, Liu L, Epstein LH. A behavioral economic approach to assessing demand for marijuana. Experimental and Clinical Psychopharmacology. 2014;22:211–221. doi: 10.1037/a0035318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel J, Cropley M, Ussher M, West R. Acute effects of a short bout of moderate versus light intensity exercise versus inactivity on tobacco withdrawal symptoms in sedentary smokers. Psychopharmacology. 2004;174:320–326. doi: 10.1007/s00213-003-1762-x. [DOI] [PubMed] [Google Scholar]
- Daniel JZ, Cropley M, Fife-Schaw C. The effect of exercise in reducing desire to smoke and cigarette withdrawal symptoms is not caused by distraction. Addiction. 2006;101:1187–1192. doi: 10.1111/j.1360-0443.2006.01457.x. [DOI] [PubMed] [Google Scholar]
- Duncan TE, Duncan SC. The ABC’s of LGM: An introductory guide to latent variable growth curve modeling. Social and Personality Psychology Compass. 2009;3(6):979–991. doi: 10.1111/j.1751-9004.2009.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, LaRowe SD, Upadhyaya HP. Cue reactivity in young marijuana smokers: A6preliminary investigation. Psychology of Addictive Behaviors. 2008;22:582–586. doi: 10.1037/a0012985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, LaRowe SD, Watson NL, Carpenter MJ. Reactivity to in vivo marijuana cues among cannabis-dependent adolescents. Addictive Behaviors. 2011;36:140–143. doi: 10.1016/j.addbeh.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS. US epidemiology fo cannais use and associated problems. Neropsychopharmacology. 2018;43:195–212. doi: 10.1038/npp.2017.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, Scharkow M. The relative trustworthiness of inferential tests of the indirect effect in statistical mediation analysis: Does method really matter? Psychological Science. 2013;24(10):1918–1927. doi: 10.1177/0956797613480187. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA. Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug and Alcohol Dependence. 2009;102:35–40. doi: 10.1016/j.drugalcdep.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Liguori A. Marijuana Craving Questionnaire: Development and initial validation of a self- report instrument. Addiction. 2001;96:1023–1034. doi: 10.1046/j.1360-0443.2001.967102312.x. [DOI] [PubMed] [Google Scholar]
- Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Annales Medicinae Experimentalis et Biologiae Fenniae. 1957;35:307–315. [PubMed] [Google Scholar]
- Kozlowski LT, Pillitteri JL, Sweeney CT, Whitfield KE, Graham JW. Asking questions about urges or cravings for cigarettes. Addictive Behaviors. 1996;10(4):248–260. [Google Scholar]
- Linke SE, Ussher M. Exercise-based treatments for substance use disorders: Evidence, theory, and practicality. The American Journal of Drug and Alcohol Abuse. 2015;41(1):7–15. doi: 10.3109/00952990.2014.976708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowman C, Hunt WA, Litten RZ, Drummond DC. Research perspectives on alcohol craving: An overview. Addiction. 2000;95:S45–S54. doi: 10.1080/09652140050111636. [DOI] [PubMed] [Google Scholar]
- Lundahl LH, Greenwald MK. Magnitude and duration of cue-induced craving for marijuana in volunteers with cannabis use disorder. Drug and Alcohol Dependence. 2016;166:143–149. doi: 10.1016/j.drugalcdep.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundahl LH, Johanson CE. Cue-induced craving for marijuana in cannabis-dependent adults. Experimental and Clinical Psychopharmacology. 2011;19:224–230. doi: 10.1037/a0023030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthou E, Georgakouli K, Fatouros IG, Gianoulakis C, Theodorakis Y, Jamurtas AZ. Role of exercise in the treatment of alcohol use disorders (Review) Biomedical Reports. 2016;4:535–545. doi: 10.3892/br.2016.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus BH, Lewis BA, Hogan J, King TK, Albrecht AE, Bock B, Parisi AF, Niaura R, Abrams DB. The efficacy of moderate-intensity exercise as an aid for smoking cessation in women: A randomized controlled trial. Nicotine & Tobacco Research. 2005;7:871–880. doi: 10.1080/14622200500266056. [DOI] [PubMed] [Google Scholar]
- McDonald RP, Ho MHR. Principles and practice in reporting statistical equation analyses. Psychological Methods. 2002;7(1):64–82. doi: 10.1037/1082-989x.7.1.64. [DOI] [PubMed] [Google Scholar]
- Muraven M, Collins RL, Neinhaus K. Self-control and alcohol restraint: An initial application of the self-control strength model. Psychology of Addictive Behaviors. 2002;16(2):113. doi: 10.1037//0893-164x.16.2.113. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (NAS) The health effects of cannabis and cannabinoids: The current state of evidence and recommendations for research. Washington, DC: National Academies Press; 2017. [PubMed] [Google Scholar]
- Neal DJ, Simons JS. Inference in regression models of heavily skewed alcohol use data: A comparison of ordinary least squares, generalized linear models, and bootstrap resampling. Psychology of Addictive Behaviors. 2007;21(4):441–452. doi: 10.1037/0893-164X.21.4.441. [DOI] [PubMed] [Google Scholar]
- Norberg MM, Kavanagh DJ, Olivier J, Lyras S. Craving cannabis: A meta-analysis of self-report and pyschophysiological cue-reactivity studies. Addiction. 2016;111:1923–1934. doi: 10.1111/add.13472. [DOI] [PubMed] [Google Scholar]
- Pate RR, Heath GW, Dowda M, Trost SG. Associations between physical activity and other health behaviors in a representative sample of US adolescents. American Journal of Public Health. 1996;86:1577–1581. doi: 10.2105/ajph.86.11.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robergs RA, Landwehr R. The surprising history of the “HRmax=220-age” equation. Journal of Exercise Physiology Online. 2002;5:1–10. [Google Scholar]
- Rosenberg H. Clinical and laboratory assessment of the subjective experience of drug craving. Clinical Psychology Review. 2009;29:519–534. doi: 10.1016/j.cpr.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, van Rooijen MA. A self-administered short Michigan alcoholism screening test (SMAST) Journal of Studies on Alcohol. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. http://dx.doi.org/10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Sharma P, Murthy P, Bharath MS. Chemistry, metabolism, and toxicology of cannabis: Clinical implications. Iranian Journal of Psychiatry. 2012;7(4):149–156. [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Carey KB. An affective and cognitive model of marijuana and alcohol problems. Addictive Behaviors. 2006;31:1578–1592. doi: 10.1016/j.addbeh.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Singleton EG, Trotman AJ, Zavahir M, Taylor RC, Heishman SJ. Determination of the reliability and validity of the marijuana craving questionnaire using imagery scripts. Experimental and Clinical Psychopharmacology. 2002;10:47–53. doi: 10.1037//1064-1297.10.1.47. [DOI] [PubMed] [Google Scholar]
- Skinner HA. The drug abuse screening test. Addictive Behaviors. 1982;7:363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Steiger JH. Structural model evaluation and modification: An interval estimation approach. Multivariate Behavioral Research. 1990;25(2):173–180. doi: 10.1207/s15327906mbr2502_4. [DOI] [PubMed] [Google Scholar]
- Taylor AH, Katomeri M. Walking reduces cue-elicited cigarette cravings and withdrawal symptoms, and delays ad libitum smoking. Nicotine Tobacco Research. 2007;9:1183–90. doi: 10.1080/14622200701648896. [DOI] [PubMed] [Google Scholar]
- Taylor AH, Ussher MH, Faulkner G. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: A systematic review. Addiction. 2007;102(4):534–543. doi: 10.1111/j.1360-0443.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionnaire (PAR-Q) Canadian Journal of Sport Sciences. 1992;17:338–345. [PubMed] [Google Scholar]
- Tiffany ST. Craving. In: Carson-DeWitt R, editor. Encyclopedia of Drugs, Alcohol, & Addictive Behavior. 2nd. Vol. 1. New York: Macmillan Reference USA; 2001. pp. 354–357. [Google Scholar]
- Tiffany ST, Hakenewerth DM. The production of smoking urges through imaginery manipulation: Psychological and verbal manifestations. Addictive Behaviors. 1991;16:389–400. doi: 10.1016/0306-4603(91)90047-l. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Wray JM. The clinical significance of drug craving. Annals of the New York Academy of Sciences. 2012;1248:1–17. doi: 10.1111/j.1749-6632.2011.06298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (DHHS) Physical Activity Has Many Health Benefits. 2008 Retrieved from: www.health.gov/paguidelines.
- Ussher M, Sampuran AK, Doshi R, West R, Drummond DC. Acute effect of a brief bout of exercise on alcohol urges. Society for the Study of Addiction. 2004;99:1542–1547. doi: 10.1111/j.1360-0443.2004.00919.x. [DOI] [PubMed] [Google Scholar]
- Ussher M, Nunziata P, Cropley M, West R. Effect of a short bout of exercise on tobacco withdrawal symptoms and desire to smoke. Psychopharmacology. 2001;158:66–72. doi: 10.1007/s002130100846. [DOI] [PubMed] [Google Scholar]
- Van Rensburg KJ, Elibero A, Kilpatrick M, Drobes DJ. Impact of aerobic exercise intensity on craving and reactivity to smoking cues. Experimental and Clinical Psychopharmacology. 2013;21:196–203. doi: 10.1037/a0032768. [DOI] [PubMed] [Google Scholar]
- Wang D, Wang Y, Wang Y, Li R, Zhou C. Impact of physical exercise on substance use disorders: A meta-analysis. PloS ONE. 2014;9(10):e110728. doi: 10.1371/journal.pone.0110728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F. Neurobiology of craving, conditioned reward and relapse. Current Opinion in Pharmacology. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Wong K, Brady JE, Li G. Establishing legal limits for driving under the influence of marijuana. Injury Epidemiology. 2014;1:26. doi: 10.1186/s40621-014-0026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


