Abstract
A substantial literature details the relationship between age-related changes to the hypothalamic-pituitary-adrenal (HPA) axis and deterioration of mnemonic functions that depend on the hippocampus. The relationship between adrenocortical status and other forms of memory that depend on the prefrontal cortex (PFC) is less well-understood in the context of advanced age. Here we characterized performance of young adult and aged F344 rats on a PFC-dependent working memory task and subsequently measured corticosterone levels over the diurnal cycle and during exposure to an acute stressor. Our analyses revealed that aged rats with better working memory mounted a greater corticosterone response during acute stress exposure than either young adults or age-matched rats with impaired working memory. We also observed that age-related elevation of basal corticosterone levels is not associated with working memory performance. Jointly, these data reveal that the HPA-mediated response to acute stress is positively associated with working memory in aging.
1. Introduction
Secretion of glucocorticoids mediated by the hypothalamic-pituitary-adrenal (HPA) axis is an essential physiological process that balances cellular energy requirements over the circadian cycle and also in response to stressful experiences (de Kloet et al., 2005; Herman et al., 2003; Keller-Wood and Dallman, 1984). Lower expression of glucocorticoid receptors (GR) in the aging hippocampus associates with dysregulated release of glucocorticoids and impaired spatial memory (Bizon et al., 2001; Issa et al., 1990; Montaron et al., 2006; Yau et al., 1995). GRs in the prefrontal cortex (PFC) also regulate HPA axis activity (Diorio et al., 1993; Radley et al., 2006a), but few studies have investigated the relationship between modulation of glucocorticoid secretion and age-related decline of PFC-dependent memory. Specifically, working memory, which involves the temporary maintenance of information used to guide current and future action, critically depends on PFC (Funahashi et al., 1993; McQuail et al., 2016; Sloan et al., 2006) and deteriorates with age across species (Bachevalier et al., 1991; Beas et al., 2013; Hernandez et al., 2017; Lamar and Resnick, 2004; Oscar-Berman and Bonner, 1985; Rapp and Amaral, 1989). In this study, we examined differences in circadian- and stress-associated glucocorticoid secretion between young and aged rats in relation to performance on a PFC-dependent working memory task.
2. Materials and Methods
2.1. Subjects
Male, Fischer 344 (F344) rats were acquired at ages of 4 mo (young adults, n= 11) or 20 mo (aged, n=16) from the Aging Rodent Colony maintained by Charles River Laboratories for the National Institute on Aging. All rats were housed in an AAALAC-accredited vivarium in the McKnight Brain Institute Building at the University of Florida in accordance with the rules and regulations of the University of Florida Institutional Animal Care and Use Committee and NIH guidelines. The facility was maintained at a consistent 25°C with a 12 h light/dark cycle (lights on at 0600 h) with free access to food and water except as noted below.
2.2. Delayed Response Testing
All rats were restricted to 85% of ad libitum fed weight and shaped to perform a delayed response test of working memory (Fig 1A). This task requires intact function of medial PFC (mPFC; McQuail et al., 2016; Sloan et al., 2006), the rodent homolog of the primate dorsolateral PFC. Behavioral testing was conducted in operant testing chambers (Coulbourn Instruments, Whitehall, PA, USA) using shaping procedures identical to those described previously (Beas et al., 2013; McQuail et al., 2016). In the testing phase of the task, each trial was comprised of 3 phases. In the “sample” phase, a single response lever (left or right, randomly counterbalanced within pairs of trials) was extended into the chamber. A lever press resulted in retraction of the lever and initiated a delay of 0, 2, 4, 8, 12, 18, or 24 s (presented in a randomized order in each block of 7 trials). During this “delay” phase, rats were required to nose-poke into the central food trough. The “choice” phase was initiated by the first such nose-poke emitted after the delay period expired. In the “choice” phase, both left and right levers were extended, and a press on the same lever presented during the sample phase (a correct response) resulted in retraction of both levers and delivery of a single food pellet. A response on the opposite lever (an incorrect response) resulted in the retraction of both levers and initiation of 5 s “timeout” during which the house light was extinguished. Rats were tested in one 40-minute session per day until achieving stable performance over 5 consecutive days. Stability was defined as <10% variation in performance at 18 and 24 s delays while completing no fewer than 70 trials per day.
Fig 1. Age-related decline of working memory.
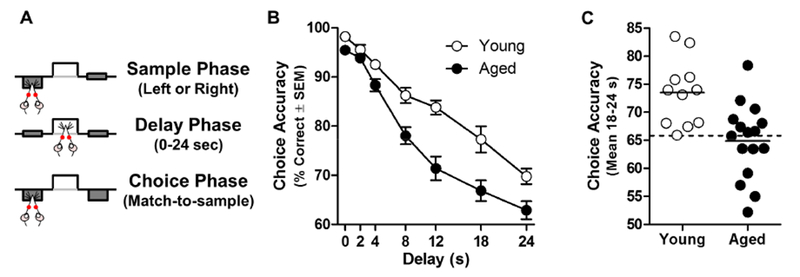
A: Schematic of a trial in the operant delayed response task used to assess working memory in rats. B: Delayed response choice accuracy of aged rats (n=16; black circles) is reduced relative to young (n=11; white circles), particularly at longer delays (age × delay interaction: p<.05). Data are mean % correct choices (y-axis) ± SEM plotted as a function of delay (in s; x-axis). C: Choice accuracy (averaged across 18 and 24 s delays) of individual young and aged rats. Within the aged population, some performed within the range of young (n=8; aged-unimpaired, AU) whereas the remaining aged rats performed below the range of young (n=8; aged-impaired, AI). Solid lines indicate group mean performance and dashed line indicates the lowest level of young performance.
2.3. Blood Collection and Corticosterone Measurement
After behavioral testing was complete, rats were returned to free feeding for a minimum of 2 weeks before blood collection. To evaluate circadian variation in corticosterone (CORT) levels, blood was obtained via tail bleed between 0700-0800 h (lights on at 0600 h) and between 1900-2000 (lights off at 1800 h; similar to time course used in Sapolsky et al., 1983). One week later, rats were subjected to a 60 minute period of restraint stress. Beginning between 0900 and 0930 h, rats were placed into Plexiglas restrainers and blood collected at 0, 15, 30 and 60 minutes following the onset of restraint and also at 120 minutes, or 60 minutes after being released and returned to home-cage (similar to time course used in Segar et al., 2009). Plasma CORT was assayed in duplicate using the ImmunChem™ Double Antibody Corticosterone 125I RIA Kit for rats and mice (MP Biomedicals, Orangeburg, NY, USA) as previously described (Daubert et al., 2014).
2.4. Statistical Analyses
The chief index of delayed response performance was choice accuracy, or the percentage of correct responses after each delay. Choice accuracy was analyzed using a two-way, mixed factors analysis of variance (ANOVA) testing age as a between-subject factor and delay as a repeated, within-subjects factor. Aged rats were classified into cognitive subgroups on the basis of whether choice accuracy of each rat at 18 and 24 s delays fell within (aged-unimpaired; AU) or below (aged-impaired; AI) the range of young adults. CORT concentration ([CORT]; ng/ml) was determined by fitting activity counts of unknown samples to a standard curve comprised of known [CORT] after accounting for non-specific binding (determined in the absence of primary anti-corticosterone). To account for non-normal distribution of circadian [CORT], non-parametric Mann-Whitney U or Kruskal-Wallis tests were used to compare [CORT] between ages or cognitive subgroups. To analyze [CORT] during/following restraint, age or cognitive subgroup was tested as a between-subject factor and time point as a repeated, within-subjects factor followed by Bonferroni post hoc comparisons at specific time points. Bivariate correlations were used to test the relationship between [CORT] and working memory in aged rats. All data are reported as the mean ± standard error (SEM) and p<0.05 was considered significant in all comparisons.
3. Results
Choice accuracy of aged rats was significantly impaired compared to young adults in a delay-dependent manner (effect of age: F(1,25)=27.735, p<.001; age × delay interaction: F(6, 150)=3.350, p=.010; Fig 1B). Using the average of performance at the longest delays (18 and 24 s) as an index of individual differences in accuracy, n=8 aged rats performed within the range of young (aged-unimpaired; AU), while n=8 rats performed below the range of young (aged-impaired; AI; Fig 1C).
Although [CORT] was not significantly different between age groups one hour into the dark phase (1900 h; U=82, p=.790), [CORT] one hour after the start of the light phase (0700h) was significantly higher in aged compared to young (U=148, p=.002). When the latter data were tested according to cognitive subgroups, a significant effect (χ2(2)=8.782, p=.012) was due to the fact that both AU (p=.046) and AI (p=.031) differed from young but not each other (p=1.0; Fig 2A).
Fig 2. Relationship between age-related changes to HPA axis function and working memory.
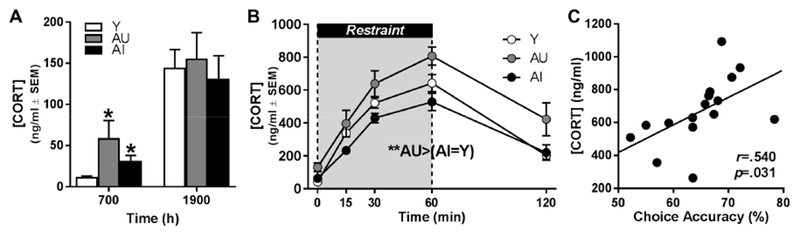
A: Plasma corticosterone concentration [CORT] was greater in aged-unimpaired (AU; n=8; gray bar) or aged-impaired (AI; n=8; black bar) compared to young adult (Y; n=11; white bar) at 0700 h (*p<.05 versus Y), but AU and AI were not significantly different from each other. Further, there was no difference between cognitive groups at 1900 h. Data are mean [CORT] (ng/ml; y-axis) ± SEM plotted as a function of time of day (h; x-axis). B: Secretion of CORT during and after 60 minutes of restraint stress was significantly different between cognitive groups (**p<.01). Inset: Post hoc comparisons determined that elevation of [CORT] was greater in the AU (gray circles) subgroup relative to both AI (black circles) and Y (white circles); AI and Y were not significantly different from each other. Data are mean [CORT] (ng/ml; y-axis) ± SEM plotted as a function of time following onset of restraint (min; x-axis); the shaded portion indicates the period of restraint. C: [CORT] at the conclusion of 60 min restraint stress (ng/ml; y-axis) is positively associated with choice accuracy (average % correct at 18 and 24 s delays; x-axis) in aged rats (n=16; black circles); solid line denotes line of best fit. Inset: Pearson’s r and p.
One hour of restraint stress induced a significant elevation of [CORT] (main effect of time point: (F(4,100)=111.897, p<.001) that was not significantly different between age groups (main effect of age: F(1,25)=.597, p=.447; age × time point interaction: F(4, 100)= 1.510, p=.205). In contrast, comparisons between cognitive subgroups revealed significant differences in [CORT] (main effect of subgroup: F(2,24)=6.815, p=.005; subgroup ×; time point interaction: F(8,96)=1.810, p=.084; Fig 2B). Post hoc comparisons determined that [CORT] was greater in AU compared to young (p=.037) and AI (p=.005) but young and AI did not differ from each other (p=.807). Bivariate correlations between maximal [CORT] observed after 60 minutes of restraint and working memory choice accuracy in aged rats revealed that higher [CORT] was significantly associated with better working memory performance (r=.540, p=.031; Fig 2C).
4. Discussion
The PFC is essential for working memory (Funahashi et al., 1993; McQuail et al., 2016; Sloan et al., 2006) and contributes to regulation of the HPA axis (Diorio et al., 1993; Radley et al., 2006a). Prior work shows that aging increases individual differences in corticosterone secretion stimulated during acute stress in F344 rats (Segar et al., 2009). The current data expand upon this finding, revealing that, within this enhanced variation, the neuroendocrine stress response among aged rats is positively associated with working memory ability. Although evidence expressly linking hormonal responses to acute stress and working memory in aging humans is limited, our present finding complements data showing that a greater cortisol response elicited by a psycho-social stressor associates with better working memory in older humans (Almela et al., 2014; Pulopulos et al., 2015). Significantly, this relationship is distinct from circadian modulation of cortisol that is also affected in aging (Evans et al., 2012, 2011; Geerlings et al., 2015). Consistent with prior work (Sapolsky, 1992; Sapolsky et al., 1983), basal corticosterone level is greater in aged rats. This difference was most evident during the inactive (i.e., light) phase of the day, and likely reflects diminished capacity to down-regulate corticosterone during rest. Alternatively, this difference could reflect a temporal shift in the circadian variation of corticosterone secretion, although others, sampling at more frequent intervals, have not observed such a shift in aged male, F344 rats (Morano et al., 1994; Sonntag et al., 1987). Regardless, changes in circadian modulation of corticosterone were not reliably associated with working memory. Together these data suggest multi-faceted changes in regulation of glucocorticoids with age. While elevations in basal corticosterone occur as a consequence of chronological age, it is specifically the ability to effectively mount a robust corticosterone response during a stressor that is strongly linked to working memory function.
Notably, these findings differ somewhat from a recent study in which elevated diurnal corticosterone in aged Sprague-Dawley rats was associated with impaired performance on a spatial delayed alternation working memory task (Anderson et al., 2014). It is not clear the degree to which methodological differences influence the specific conclusions of the aforementioned study and the present work. While HPA axis responses differ between F344 and Sprague-Dawley rats (Dhabhar et al., 1993, 1995, 1997; Uchida et al., 2008), corticosterone release stimulated by acute stress was not evaluated in Anderson et al (2014). It may also be consequential that the delayed spatial alternation task used by Anderson et al (2014) and other studies of age-related decline of working memory (Kang et al., 2013; Mizoguchi et al., 2009) is partially dependent on hippocampus (Aggleton et al., 1986). Consequently, hippocampal contributions to spatial working memory complicate the ability to fully ascribe deficits to PFC-dependent processes given that a plethora of previous work relates elevated corticosterone in aged rats to impaired performance on hippocampal-dependent memory tasks (Bizon et al., 2001; Issa et al., 1990; Montaron et al., 2006; Yau et al., 1995). The operant delayed response task used in the present study was specifically chosen because performance depends on the rodent mPFC but not hippocampus (Sloan et al., 2006). Collectively, the findings of this and other studies indicate that simply attenuating excessive glucocorticoid levels in older adults is unlikely to serve as an effective intervention to mitigate deleterious effects on cognition. Rather, effective interventions will likely involve restoration of precise HPA axis function that reinstates the appropriate dynamic regulation of glucocorticoids in synchrony with circadian cues and in response to stressors.
Chronic stress or long-term corticosterone treatment provokes remodeling of dendritic spines on PFC neurons, with “thin” spines comprising an especially susceptible subpopulation (Anderson et al., 2016; Goldwater et al., 2009; Gourley et al., 2013; Radley et al., 2006b, 2008, 2013). In aging individuals, loss of thin spines on PFC neurons associates with severity of cognitive impairment (Anderson et al., 2014; Dumitriu et al., 2010; Hao et al., 2007). The significance of thin spine loss may relate to their enriched content of ionotropic glutamate receptors of the NMDA-subtype (NMDARs; Kasai et al., 2003; Noguchi et al., 2005), which are essential for persistent firing activity of PFC neurons during cognitive task performance and accurate maintenance of memoranda in working memory stores (McQuail et al., 2016; Wang et al., 2013). Although we cannot infer causality in the present study, NMDARs are well-positioned to modulate the relationship between working memory and HPA axis via interactions with GRs in PFC neurons. On the one hand, regressive structural modifications could defend aging PFC neurons from NMDAR-mediated excitotoxicity that is exacerbated by increased glucocorticoid exposure resulting from diminished negative feedback on to HPA axis (Armanini et al., 1990; Li et al., 2018). On the other hand, mild, acute stress potentiates working memory and NMDAR synaptic currents through a GR-dependent mechanism (Yuen et al., 2009). Consequently, lower expression of NMDARs (McQuail et al., 2016; Piggott et al., 1992) and GRs (Bizon et al., 2001; Perlman et al., 2007) in the aging PFC could reflect homeostatic adjustments that protect neural viability at the expense of cognitive function. The current data highlight the need for mechanistic studies that investigate interactions between GRs and NMDARs in the aging PFC, with the goal to differentiate between processes that support cognition versus those that endanger neuron survival.
In conclusion, the current findings show that an augmented glucocorticoid response to stress is associated with maintained working memory function in aged rats. This relationship coincides with, but is distinct from, age-related increases in basal corticosterone. Notably, the increased corticosterone response to stress in aged rats with intact working memory exceeded that of young rats, indicating a distinct phenotype that does not simply reflect maintenance of physiological responses typical of young. Instead, this response may reflect an effective compensation for dysregulated neuroendocrine function in aging that protects appropriate signaling through mechanisms such as NMDARs that are critical for working memory. Future studies are needed to identify the mechanisms that drive these effects.
Highlights.
Aging impairs mPFC-dependent working memory in inbred, male F344 rats
Basal corticosterone is not associated with working memory in aging
Acute stress-induced corticosterone is associated with working memory in aging
Acknowledgements.
We thank Matthew Bruner, Caesar Hernandez, Helmut Hiller, Vicky Kelley and Shannon Wall for technical assistance. This work was supported by NIH grants F32AG051371 (to JAM) and R01AG029421 (to JLB), and by the McKnight Brain Research Foundation (to JLB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- Aggleton JP, Hunt PR, Rawlins JN, 1986. The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behav. Brain Res 19, 133–146. [DOI] [PubMed] [Google Scholar]
- Almela M, Hidalgo V, van der Meij L, Pulopulos MM, Villada C, Salvador A, 2014. A low cortisol response to acute stress is related to worse basal memory performance in older people. Front. Aging Neurosci 6, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Birnie AK, Koblesky NK, Romig-Martin SA, Radley JJ, 2014. Adrenocortical status predicts the degree of age-related deficits in prefrontal structural plasticity and working memory. J. Neurosci 34, 8387–8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Glanz RM, Johnson SB, Miller MM, Romig-Martin S, Radley JJ, 2016. Prolonged corticosterone exposure induces dendritic spine remodeling and attrition in the rat medial prefrontal cortex. J. Comp. Neurol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanini MP, Hutchins C, Stein BA, Sapolsky RM, 1990. Glucocorticoid endangerment of hippocampal neurons is NMDA-receptor dependent. Brain Res 532, 7–12. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Landis LS, Walker LC, Brickson M, Mishkin M, Price DL, Cork LC, 1991. Aged monkeys exhibit behavioral deficits indicative of widespread cerebral dysfunction. Neurobiol. Aging 12, 99–111. [DOI] [PubMed] [Google Scholar]
- Beas B, Setlow B, Bizon J, 2013. Distinct manifestations of executive dysfunction in aged rats. Neurobiol. Aging 34, 2164–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, Helm KA, Han J-S, Chun H-J, Pucilowska J, Lund PK, Gallagher M, 2001. Hypothalamic–pituitary–adrenal axis function and corticosterone receptor expression in behaviourally characterized young and aged Long–Evans rats. Eur. J. Neurosci 14, 1739–1751. [DOI] [PubMed] [Google Scholar]
- Daubert DL, Looney BM, Clifton RR, Cho JN, Scheuer DA, 2014. Elevated corticosterone in the dorsal hindbrain increases plasma norepinephrine and neuropeptide Y, and recruits a vasopressin response to stress. Am. J. Physiol. - Regul. Integr. Comp. Physiol 307, R212–R224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Joëls M, Holsboer F, 2005. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci 6, 463–475. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS, Spencer RL, 1997. Adaptation to prolonged or repeated stress--comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology 65, 360–368. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS, Spencer RL, 1993. Stress response, adrenal steroid receptor levels and corticosteroid-binding globulin levels--a comparison between Sprague-Dawley, Fischer 344 and Lewis rats. Brain Res 616, 89–98. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL, 1995. Differential activation of adrenal steroid receptors in neural and immune tissues of Sprague Dawley, Fischer 344, and Lewis rats. J. Neuroimmunol 56, 77–90. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ, 1993. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci 13, 3839–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WGM, Lou W, Rapp PR, Morrison JH, 2010. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J. Neurosci 30, 7507–7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P, Hucklebridge F, Loveday C, Clow A, 2012. The cortisol awakening response is related to executive function in older age. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol 84, 201–204. [DOI] [PubMed] [Google Scholar]
- Evans PD, Fredhoi C, Loveday C, Hucklebridge F, Aitchison E, Forte D, Clow A, 2011. The diurnal cortisol cycle and cognitive performance in the healthy old. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol 79, 371–377. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS, 1993. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: evidence for mnemonic “scotomas.” J. Neurosci 13, 1479–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerlings MI, Sigurdsson S, Eiriksdottir G, Garcia ME, Harris TB, Gudnason V, Launer LJ, 2015. Salivary cortisol, brain volumes, and cognition in community-dwelling elderly without dementia. Neurology 85, 976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, Morrison JH, 2009. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience 164, 798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Swanson AM, Koleske AJ, 2013. Corticosteroid-induced neural remodeling predicts behavioral vulnerability and resilience. J. Neurosci 33, 3107–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WGM, Lou W, Lasley BL, Hof PR, Morrison JH, 2007. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A 104, 11465–11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE, 2003. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo–pituitary–adrenocortical responsiveness. Front. Neuroendocrinol 24, 151–180. [DOI] [PubMed] [Google Scholar]
- Hernandez CM, Vetere LM, Orsini CA, McQuail JA, Maurer AP, Burke SN, Setlow B, Bizon JL, 2017. Decline of prefrontal cortical-mediated executive functions but attenuated delay discounting in aged Fischer 344 × Brown Norway hybrid rats. Neurobiol. Aging 60, 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa AM, Rowe W, Gauthier S, Meaney MJ, 1990. Hypothalamic-pituitary-adrenal activity in aged, cognitively impaired and cognitively unimpaired rats. J. Neurosci 10, 3247–3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Ling Q, Liu W, Lu B, Liu Y, He L, Liu J, 2013. Down-regulation of dorsal striatal RhoA activity and impairment of working memory in middle-aged rats. Neurobiol. Learn. Mem 103, 3–10. [DOI] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H, 2003. Structure–stability–function relationships of dendritic spines. Trends Neurosci 26, 360–368. [DOI] [PubMed] [Google Scholar]
- Keller-Wood ME, Dallman MF, 1984. Corticosteroid Inhibition of ACTH Secretion. Endocr. Rev 5, 1–24. [DOI] [PubMed] [Google Scholar]
- Lamar M, Resnick SM, 2004. Aging and prefrontal functions: dissociating orbitofrontal and dorsolateral abilities. Neurobiol. Aging 25, 553–558. [DOI] [PubMed] [Google Scholar]
- Li S-X, Han Y, Xu L-Z, Yuan K, Zhang R-X, Sun C-Y, Xu D-F, Yuan M, Deng J-H, Meng S-Q, Gao X-J, Wen Q, Liu L-J, Zhu W-L, Xue Y-X, Zhao M, Shi J, Lu L, 2018. Uncoupling DAPK1 from NMDA receptor GluN2B subunit exerts rapid antidepressant-like effects. Mol. Psychiatry 23, 597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuail JA, Beas BS, Kelly KB, Simpson KL, Frazier CJ, Setlow B, Bizon JL, 2016. NR2A-containing NMDARs in the prefrontal cortex are required for working memory and associated with age-related cognitive decline. J. Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi K, Shoji H, Tanaka Y, Maruyama W, Tabira T, 2009. Age-related spatial working memory impairment is caused by prefrontal cortical dopaminergic dysfunction in rats. Neuroscience 162, 1192–1201. [DOI] [PubMed] [Google Scholar]
- Montaron MF, Drapeau E, Dupret D, Kitchener P, Aurousseau C, Le Moal M, Piazza PV, Abrous DN, 2006. Lifelong corticosterone level determines age-related decline in neurogenesis and memory. Neurobiol. Aging 27, 645–654. [DOI] [PubMed] [Google Scholar]
- Morano MI, Vázquez DM, Akil H, 1994. The role of the hippocampal mineralocorticoid and glucocorticoid receptors in the hypothalamo-pituitary-adrenal axis of the aged Fisher rat. Mol. Cell. Neurosci 5, 400–412. [DOI] [PubMed] [Google Scholar]
- Noguchi J, Matsuzaki M, Ellis-Davies GCR, Kasai H, 2005. Spine-Neck Geometry Determines NMDA Receptor-Dependent Ca2+ Signaling in Dendrites. Neuron 46, 609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Bonner RT, 1985. Matching- and delayed matching-to-sample performance as measures of visual processing, selective attention, and memory in aging and alcoholic individuals. Neuropsychologia 23, 639–651. [DOI] [PubMed] [Google Scholar]
- Perlman WR, Webster MJ, Herman MM, Kleinman JE, Weickert CS, 2007. Age-related differences in glucocorticoid receptor mRNA levels in the human brain. Neurobiol. Aging 28, 447–458. [DOI] [PubMed] [Google Scholar]
- Piggott MA, Perry EK, Perry RH, Court JA, 1992. [3H]MK-801 binding to the NMDA receptor complex, and its modulation in human frontal cortex during development and aging. Brain Res 588, 277–286. [DOI] [PubMed] [Google Scholar]
- Pulopulos MM, Hidalgo V, Almela M, Puig-Perez S, Villada C, Salvador A, 2015. Acute stress and working memory in older people. Stress Amst. Neth 18, 178–187. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Anderson RM, Hamilton BA, Alcock JA, Romig-Martin SA, 2013. Chronic stress-induced alterations of dendritic spine subtypes predict functional decrements in an hypothalamo–pituitary–adrenal-inhibitory prefrontal circuit. J. Neurosci 33, 14379–14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Arias CM, Sawchenko PE, 2006a. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J. Neurosci 26, 12967–12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WGM, Liston C, Hof PR, McEwen BS, Morrison JH, 2006b. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb. Cortex N. Y. N 1991 16, 313–320. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR, 2008. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J. Comp. Neurol 507, 1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Amaral DG, 1989. Evidence for task-dependent memory dysfunction in the aged monkey. J. Neurosci 9, 3568–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, 1992. Do glucocorticoid concentrations rise with age in the rat? Neurobiol. Aging 13, 171–174. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS, 1983. The adrenocortical stress-response in the aged male rat: impairment of recovery from stress. Exp. Gerontol 18, 55–64. [DOI] [PubMed] [Google Scholar]
- Segar TM, Kasckow JW, Welge JA, Herman JP, 2009. Heterogeneity of neuroendocrine stress responses in aging rat strains. Physiol. Behav 96, 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan HL, Good M, Dunnett SB, 2006. Double dissociation between hippocampal and prefrontal lesions on an operant delayed matching task and a water maze reference memory task. Behav. Brain Res 171, 116–126. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Goliszek AG, Brodish A, Eldridge JC, 1987. Diminished diurnal secretion of adrenocorticotropin (ACTH), but not corticosterone, in old male rats: possible relation to increased adrenal sensitivity to ACTH in vivo. Endocrinology 120, 2308–2315. [DOI] [PubMed] [Google Scholar]
- Uchida S, Nishida A, Hara K, Kamemoto T, Suetsugi M, Fujimoto M, Watanuki T, Wakabayashi Y, Otsuki K, McEwen BS, Watanabe Y, 2008. Characterization of the vulnerability to repeated stress in Fischer 344 rats: possible involvement of microRNA-mediated down-regulation of the glucocorticoid receptor. Eur. J. Neurosci 27, 2250–2261. [DOI] [PubMed] [Google Scholar]
- Wang M, Yang Y, Wang C-J, Gamo NJ, Jin LE, Mazer JA, Morrison JH, Wang X-J, Arnsten AFT, 2013. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron 77, 736–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JL, Olsson T, Morris RG, Meaney MJ, Seckl JR, 1995. Glucocorticoids, hippocampal corticosteroid receptor gene expression and antidepressant treatment: relationship with spatial learning in young and aged rats. Neuroscience 66, 571–581. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z, 2009. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc. Natl. Acad. Sci. U. S. A 106, 14075–14079. [DOI] [PMC free article] [PubMed] [Google Scholar]


