Abstract
Aging is a major risk factor for developing postoperative cognitive dysfunction (POCD). Neuroinflammatory processes, which can play a causal role in the etiology of POCD, are potentiated or primed as a function of aging. Here we explored whether exposure to a microorganism with immunoregulatory and anti-inflammatory properties, Mycobacterium vaccae NCTC11659 (M. vaccae), could ameliorate age-associated neuroinflammatory priming. Aged (24 mos) and adult (3 mos) male F344XBN rats were immunized with heat-killed M. vaccae (3 injections, once per week) prior to undergoing a laparotomy or anesthesia control procedure. Aged, but not young rats, showed post-operative learning/memory deficits in a fear conditioning paradigm. Importantly, M. vaccae immunization protected aged rats from these surgery-induced cognitive impairments. M. vaccae immunization also shifted the aged pro-inflammatory hippocampal microenvironment towards an anti-inflammatory phenotype. Furthermore, M. vaccae immunization reduced age-related hyperinflammatory responses in isolated hippocampal microglia. Overall, our novel data suggest that M. vaccae can induce an anti-inflammatory milieu in the aged brain and thus mitigate the neuroinflammatory and cognitive impairments induced by surgery.
Keywords: aging, microglia, neuroimmune, POCD, T cells, surgical stress
1. Introduction
Life expectancy continues to rise worldwide. Although humans are living longer, they are not necessarily healthier than before: older individuals (> 60 years) account for approximately one-quarter of the global burden of disease (Prince et al., 2015). Mental, behavioral, and neurological disorders have increased substantially in the elderly population in recent years (Prince et al., 2015). Indeed, aging is a significant risk factor for cognitive decline in response to hospitalization and surgery (Wilson et al., 2012), and age is the most salient predictor for the development of post-operative cognitive dysfunction (POCD) (Monk et al., 2008; Steinmetz et al., 2009). POCD is characterized by impairments in memory, concentration, and information processing following surgery and these impairments significantly impact quality of life and increase the risk of disability and mortality (Steinmetz et al., 2009).
Amplified neuroinflammatory responses to challenge occur with normal aging and likely contribute to POCD vulnerability. In humans, inflammation is positively associated with the development of POCD (Burkhart et al., 2010; Hudetz et al., 2011). Factors that potentiate neuroinflammatory responses, such as opioids (e.g. fentanyl) (Watkins et al., 2009), increase the risk for developing delirium and POCD (Burkhart et al., 2010) whereas anti-inflammatory strategies (e.g. ketamine) reduce the risk for the development of POCD (Hudetz et al., 2009). There is also substantial evidence from animal models that age-related changes in neuroinflammatory dynamics are causal factors in the development of POCD. Peripheral immune stimuli (e.g., infection or surgery) induce potentiated neuroinflammatory and behavioral changes in aged mice and rats (Barrientos et al., 2012b; Cao et al., 2010; Chen et al., 2008; Fonken et al., 2016; Godbout et al., 2005; Rosczyk et al., 2008; Terrando et al., 2010). For example, whereas adult (3 mos) rats rapidly recover cognitive function after a laparotomy procedure, aged (24 mos) rats exhibit prolonged post-surgery cognitive deficits (4+ days) (Barrientos et al., 2012b). Elevations in hippocampal interleukin (IL)-1β, a cytokine that is considered a master regulator of neuroinflammation (Basu et al., 2004), likely mediate these cognitive impairments following surgery. Several lines of evidence demonstrate that aged rodents exhibit protracted elevations in IL-1β post-surgery and that blocking IL-1β signaling with pharmacological or transgenic approaches can prevent surgery-induced cognitive deficits (Barrientos et al., 2012b; Cibelli et al., 2010). Indeed, IL-1β, as well as other pro-inflammatory mediators, negatively impact neural mechanisms (e.g., long-term potentiation (LTP)) that are involved in memory formation (Lynch, 2010).
Importantly, aged but not senescent animals do not necessarily exhibit declines in cognition or increased pro-inflammatory cytokines under steady-state conditions (Barrientos et al., 2006). Rather, in aged animals, the neuroimmune response to immune challenge (e.g., surgery or infection) is potentiated; a phenomenon termed “priming” (Cox et al., 2012; Fonken et al., 2016). Age-associated inflammatory priming also occurs at the cellular level, in that microglia show potentiated responses to immune stimuli ex vivo (Fonken et al., 2016; Frank et al., 2010). Primed inflammatory responses may develop with aging due to a combination of exogenous (environmental pollutants, pathogens, injury) and endogenous (changes in glucocorticoids, accumulation of danger signals, etc.) factors that accumulate over the lifespan (reviewed in (Fonken et al., 2018b).
Overly sterile conditions are one unique modern environmental divergence that may modulate the immune system. The hygiene or “old friends” hypothesis posits that a lack of exposure to micro- and macro-organisms that have evolved over time to program the immune system during development may cause it to become dysregulated (Rook, 2013). This hypothesis does not suggest that exposure to infections or outright pathogens is beneficial, but rather, that lack of exposure to environmental and symbiotic organisms that engage the immune system can be detrimental to immune function. This modern decrease in the occurrence of immune stimulation during development, relative to the microbiome with which the species evolved, applies both to humans and laboratory rodents. Mounting evidence suggests that the reintroduction of immunoregulatory micro-and macro-organisms in an overly “clean” environment can quell immune sensitization (reviewed in (Lowry et al., 2016)). For example, Mycobacterium vaccae (M. vaccae), a fast-growing and widely distributed species of saprophytic bacteria found in soil (Rook et al., 2004), can modify immune responses in both humans and rodents (Groschel et al., 2014). Immunizing adult rats with a heat-killed preparation of M. vaccae can protect against stress-elicited, primed, hyperactive immune responses and accompanying stress-induced behavioral impairments (Reber et al, 2016; Frank et al, 2018). Of note, heat-killed bacteria have the same peripheral anti-inflammatory impact as do live bacteria (Laudanno et al., 2006). Importantly, aged animals that are underexposed to “old friends” may be particularly vulnerable to impaired immunoregulation (Rook et al., 2013). Thus, here we hypothesized that treating aged rats with M. vaccae prior to undergoing a surgical procedure (i.e., laparotomy), which served as a peripheral immune challenge, would protect against primed neuroinflammatory responses and memory deficits. In agreement with this hypothesis, our results suggest that M. vaccae pre-immunization can inhibit the neuroinflammatory and behavioral changes induced by laparotomy in aged rats. Moreover, the protective effects of M. vaccae were associated with increased hippocampal expression of markers for anti-inflammatory microglia and regulatory T cells. These findings are of particular significance since M. vaccae NCTC 11659 can be given to humans (Groschel et al., 2014).
2. Methods
2.1. Animals
Adult (3 mos) and aged (24 mos) male F344XBN F1 rats were used in these experiments. Rats of this age and strain were selected to study healthy, non-neurodegenerative aging (these rats live for > 30 mos)(Barrientos et al., 2006). Aged rats that had overt health issues such as tumors were excluded from experiments. Rats were received from NIA and pair-housed (52 cm L × 30 cm W × 21 cm H) with an age-matched conspecific. Food and water were available ad libitum and rats were maintained at an ambient temperature of 22±2°C on a 12:12 light cycle with lights on at 0700. Upon arrival, rats were acclimated for 7+ days prior to any experimental manipulation. All experimental procedures were conducted in accordance with ARRIVE guidelines and were approved by the University of Colorado Boulder Institutional Animal Care and Use Committee. Efforts were made to minimize animal use and discomfort.
2.2. Experimental design
2.2.1. Experiment 1: Does M. vaccae immunization protect aged rats against surgery-induced memory impairments?
Adult and aged rats received three subcutaneous injections of M. vaccae or vehicle spaced one week apart (i.e., one injection per week for three weeks). Five days following the final injection of M. vaccae, rats underwent a laparotomy or sham surgery. Three days post-surgery rats received a contextual fear conditioning pre-exposure paradigm, to evaluate hippocampal-dependent memory (Barrientos et al., 2012b; Barrientos et al., 2006). This experiment started with an n = 6 per group (48 rats total) and three rats were excluded from the final analyses because they developed tumors (1 aged-vehicle-sham, 1 aged-vehicle-laparotomy, and 1 aged-M. vaccae-laparotomy). The pre-exposure paradigm separates the construction of the conjunctive representation of the conditioning context from the association of that representation with the shock. This behavioral procedure allows for detection of memory impairments more selective to the hippocampus than does a standard fear-conditioning paradigm (Barrientos et al., 2006). On the first day of the paradigm (3 days post-laparotomy), rats were placed in a novel clear plastic conditioning chamber (26 cm length × 21 cm width × 24 cm height) with a removable floor of stainless steel rods (1.5 mm diameter) that was placed inside a white ice chest that was open to the front. Rats were exposed to the context six times over the course of 6 h during the light phase. The duration of the first context exposure was 5 min and subsequent exposures lasted 40 sec. Three days later, rats were placed in the conditioning context and immediately received a 2 sec 1.5 mA shock (in context for < 5 sec). 24 h following the immediate shock, animals were placed back into the conditioning context or a control context and freezing behavior was assessed, as an index of contextual memory, over a 6-min period. Freezing behavior was scored by a condition-blind observer. 24 h following fear conditioning testing, tissue was collected. This experiment is outlined in Figure 1A.
Figure 1. M. vaccae prevents laparotomy-induced cognitive impairments in aged rats.
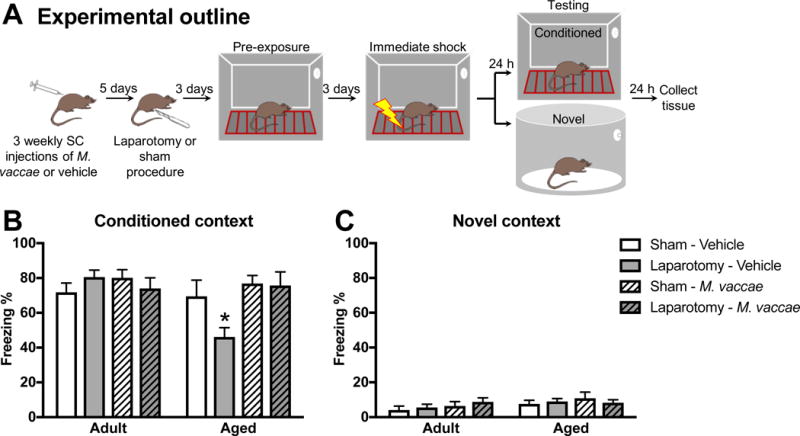
(A) Experimental outline: aged and young rats received subcutaneous M. vaccae injections once per week for three weeks. Five days following the final injection, rats underwent a laparotomy or sham procedure. Three days post-surgery, rats underwent training in a pre-exposure fear-conditioning paradigm. Freezing behavior in the (B) conditioned and (C) novel context of the fear-conditioning paradigm is presented as percent of total time (6 min) freezing (Freezing %). Results were analyzed using a 2 × 2 × 2 ANOVA with age, surgery, and M. vaccae treatment as between-subjects factors (n = 5-6/group). Data are expressed as mean ± SEM. *differs from all other groups, p < 0.05.
2.2.2. Experiment 2: Does M. vaccae immunization shift the inflammatory profile of aged rats post-surgery?
Adult and aged rats were immunized with M. vaccae or vehicle (3 subcutaneous injections, once per week). Five days following the final injection of M. vaccae, rats underwent laparotomy or sham surgery. This experiment started with n = 7 per group; two aged rats were excluded from the final analyses due to tumors (1 aged-vehicle-sham and 1 aged-M. vaccae laparotomy) and one adult-M. vaccae-laparotomy rat was excluded from RNA analyses due to poor quality RNA (housekeeping gene expression also indicated sample was a significant outlier z = 4.4). Three days post-surgery, hippocampal tissue was collected following a PBS perfusion. This time-point post-surgery was selected for assessment of inflammatory mediators because it corresponds to the time post-surgery when conditioning to the context occurred in Experiment 1. Surgery-induced inflammatory mediators can interfere with subsequent memory consolidation.
2.2.3. Experiment 3: Can M. vaccae immunization reduce inflammatory priming in microglia isolated from aged rats?
Aged rats were immunized with M. vaccae or vehicle (3 subcutaneous injections, once per week). Five days following the final injection, hippocampal tissue was collected following a PBS perfusion. Highly pure microglia were isolated (see section 2.7.) from whole hippocampus and stimulated ex vivo with lipopolysaccharide (LPS) for 2 h (n = 4/group), which elicits a pro-inflammatory immune response in a concentration-dependent manner (Frank et al., 2006).
2.3. M. vaccae preparation and delivery
Rats were treated with a heat-killed M. vaccae suspension as previously described (Frank et al., 2018; Reber et al., 2016). Rats received either subcutaneous injections of 0.1 mg whole heat-killed M. vaccae suspension (10 mg/ml sterile stock solution diluted to 1 mg/ml with sterile borate-buffered saline; NCTC 11659 strain, batch ENG 1, provided by Bio Elpida, Lyon, France) or vehicle (sterile borate-buffered saline). Rats received three injections, each spaced 1 week apart (see experimental outline in Fig 1A). No adverse physiological or behavioral consequences of M. vaccae were observed: there were no overt signs of sickness behavior and M. vaccae did not affect weight gain in adult or aged rats (data not shown).
2.4. Laparotomy procedure
This subcostal laparotomy procedure was developed to model human abdominal exploratory surgery by Martin et al and has previously been used in our laboratory (Barrientos et al., 2012b; Martin et al., 2004; Martin et al., 2005). The surgery was performed under aseptic conditions. Rats were anesthetized with halothane anesthesia, the abdominal region was shaved, and the surgical site was cleaned with 70% EtOH followed by surgical scrub. A 3-cm vertical incision was made through the skin, muscle wall, and abdominal wall approximately 0.5 cm below the lower right rib. The surgical opening and viscera were then manipulated. Approximately 10 cm of intestine was exteriorized and vigorously rubbed for 30 sec. The intestines were then placed back into the rat peritoneal cavity and the muscle and abdominal wall were sutured separately using sterile chromic gut sutures (3-0, PS-2, Ethicon). The skin was closed using reflex clips (9 mm, WPI) and a triple antibiotic ointment was applied (CVS). For the sham procedure, rats were anesthetized, shaved, and prepped in the same manner as laparotomy rats, but no incision was made. Sham and laparotomy procedures occurred in parallel: the halothane anesthesia delivery line was bi-furcated such that a sham rat and laparotomy rat were maintained on the same concentration of anesthesia and exposed to anesthesia for the same duration (~25 min).
2.5. Tissue collection
Animals were given a lethal intraperitoneal injection of sodium pentobarbital (Fatal-plus; 150 mg/kg). After rats were completely unresponsive (as assessed by pedal reflex), they were transcardially perfused with ice-cold phosphate buffered saline (0.9% saline) for 3 min to remove peripheral immune leukocytes from the central nervous system (CNS) vasculature. Brains were rapidly extracted, placed on ice, and the hippocampus was dissected out. Hippocampus was collected given that this brain region is particularly vulnerable to age-associated neuroinflammatory priming (Barrientos et al., 2009). For experiments involving measurement of in vivo cytokine mRNA expression, hippocampus was flash frozen in liquid nitrogen and stored at −80 °C. For experiments involving ex vivo LPS stimulation of isolated hippocampal microglia, hippocampal microglia were immediately isolated.
2.6. ELISA
Hippocampal samples were sonicated on ice using a tissue extraction reagent (Invitrogen; Catalog #: FNN0071) supplemented with protease inhibitor cocktail (Sigma-Aldrich; Catalog #: P2714). Homogenates were centrifuged (14,000×g for 10 min at 4 °C) and supernatants collected and stored at −20 °C. Total protein was quantified using a Bradford assay. An ELISA for rat IL-1β (R&D systems; Catalog #: RLB00) was run according to the manufacturer’s instructions and IL-1β protein levels normalized to total protein (pg IL-1β/100 μg total protein).
2.7. Microglia isolations and ex vivo treatments
Hippocampal microglia were isolated using a Percoll density gradient as previously described (Frank et al., 2006). Rats were PBS-perfused, brains were removed, and the hippocampus was dissected out on ice. The hippocampus was homogenized in 3 mL of 0.2% glucose in 1X Dulbecco’s Phosphate Buffered Saline (DPBS). The hippocampal homogenate was passed through a 40-μm filter and the filter was rinsed with an additional 2 mL of DPBS. Cells were pelleted in 5-ml falcon tubes at 1000 g for 10 min at 22 °C and then the supernatant was poured off. A Percoll (GE Healthcare) gradient was created by re-suspending the pellet in 2 mL of 70% isotonic Percoll (isotonic Percoll consists of 10:1 Percoll:10X DPBS; 100% isotonic Percoll is then diluted with 1X DPBS), followed by a layer of 2 mL 40% isotonic Percoll and topped with 1 mL DPBS. The gradient was spun at 1200 g for 30 min at 22 °C with no acceleration or brake. Myelin debris was removed and then microglia were extracted from the 40%/70% interface. Microglia were washed in DPBS and pelleted at 1000 g for 10 min at 22 °C. Microglia were resuspended in media (filtered Dulbecco’s Modified Eagle Medium (DMEM (Gibco) +10% fetal bovine serum (FBS)) and microglia concentration and viability were determined by trypan blue exclusion. Microglia were plated at a density of 8,000 cells/100 μL in a 96-well v-bottom plate. To assess microglia cytokine responsiveness, cells were challenged ex vivo with lipopolysaccharide (LPS; E. coli serotype 0111:B4; Sigma-Aldrich) at a concentration of 10 or 100 ng/mL or media alone at 37 °C, 5% CO2. The LPS concentrations, incubation time, and cell density were based on previous publications from our laboratory (Frank et al., 2010; Frank et al., 2006). After 2 h, plates were centrifuged at 1000 g for 10 min at 4 °C to pellet cells and wells were aspirated. Cells were washed with 1X DPBS, centrifuged at 1000 g for 10 min at 4 °C, and RNA was isolated using a CellsDirect Kit (Invitrogen; Catalog #: 11739-010) according to the manufacturer’s instructions.
2.8. qPCR
Rat primers were previously designed using Genbank at the National Center for Biotechnology Information (NCBI), the Operon Oligo Analysis Tool, and the Basic Local Alignment Search Tool at NCBI and obtained from Invitrogen. Primers were designed to span exon/exon boundaries and thus exclude amplification of genomic DNA. Primer specificity was verified by melt curve analysis. Primers included Arg1 (F: CTACCTGCTGGGAAGGAAG and R: GTCCTGAAAGTAGCCCTGTC), β-actin (F: TTCCTTCCTGGGTATGGAAT and R: GAGGAGCAATGATCTTGATC), CD200 (F: CTCTCTATGTACAGCCCATAG and R: GGGAGTGACTCTCAGTACTAT), CD206 (F: AATGGGTGCCTCCCTGGTTT and R: AGGGTCACCCGTTTTCCAGT), CD3 (F: GCCAGGAAGAGTATGAAGTC and R: GAAATCCTCTAGCACCAGGT), FOXP3 (F: TTATCCAGCCTGCCTCAGAC and R: TAGGAGTTCTGAGCCCTTGG), IL-1β (F: CCTTGTGCAAGTGTCTGAAG and R: GGGCTTGGAAGCAATCCTTA), IL-4 (F: CAACAAGGAACACCACGGAG and R: GGTGCAGCTTCTCAGTGAGT) IL-6 (F: AGAAAAGAGTTGTGCAATGGCA and R: GGCAAATTTCCTGGTTATATCC), IL-10 (F: GGACTTTAAGGGTTACTTGGG and R: AGAAATCGATGACAGCGTCG), and MHCII (F: AGCACTGGGAGTTTGAAGAG and R: AAGCCATCACCTCCTGGTAT). RNA was extracted from hippocampal homogenates using TRIZOL reagent and 2 μg of RNA was reversed transcribed to cDNA using Superscript II (Invitrogen; Catalog #: 18064014) according to the manufacturer’s instructions. RNA was isolated from microglia and reversed transcribed to cDNA using SuperScript III CellsDirect cDNA Synthesis System (Invitrogen; Catalog #: 18080-300). PCR amplification of cDNA was performed using the Quantitect SYBR Green PCR Kit (Qiagen; Catalog # 204145) with a MyiQ Single-Color Real-Time PCR Detection System (BioRad, Hercules, CA, USA). Gene expression was run in duplicate and analyzed with the 2^ΔΔCt method relative to β-actin. There were no group differences in β-actin expression.
2.9. Statistical analysis
All data are presented as mean ± standard error of the mean (SEM). Data were analyzed with StatView and Prism 7 (GraphPad Software, La Jolla, CA). Data were analyzed using analysis of variance (ANOVA) with age, surgery, and M. vaccae treatment as the between-subjects factors (2 × 2 × 2). For the ex vivo microglia experiment, results were analyzed with 2 × 3 ANOVAs with M. vaccae and LPS as the independent variables. F values are reported for each ANOVA and serve as the criteria for post hoc analysis (Tukey’s HSD). Threshold for statistical significance was set at two-tailed p < 0.05.
3. Results
3.1. M. vaccae immunization prevented memory deficits in aged rats following surgery
Young (3 mos) and aged (24 mos) rats received three subcutaneous injections of M. vaccae or vehicle, each injection spaced 7 days apart (experiment outlined in Fig 1A). Five days following the final injection of M. vaccae rats underwent a laparotomy or sham procedure (described in 2.4). After a 3-day recovery period rats were tested in a pre-exposure fear conditioning paradigm (described in 2.2.1). Freezing behavior is used as an index of memory in this task as freezing is a dominant fear response in rats. There were no differences in baseline freezing (prior to conditioning) between the groups (data not shown, p > 0.05). During the testing portion of the fear conditioning paradigm, there was a three-way interaction for freezing behavior in the conditioned context (age × M. vaccae × surgery: F1,36 = 5.7, p < 0.05, Fig 1B). In agreement with previous findings, aged vehicle-treated rats that underwent a laparotomy procedure had reduced freezing in the context where they previously received a foot shock (conditioned context), which is suggestive of an impairment in memory (post hoc, p < 0.05). Indeed, postoperative aged rats showed a 25% reduction in freezing compared to aged rats that underwent the sham procedure; however, this freezing deficit was completely abolished by M. vaccae treatment. Aged rats that were immunized with M. vaccae prior to a laparotomy procedure displayed comparable freezing to aged sham animals (post hoc, p > 0.05). There were no differences in freezing behavior in adult rats, or between any of the groups in the novel context (Fig 1B&C). Thus, postoperative memory deficits in aged rats were completely ameliorated by M. vaccae immunization.
3.2. M. vaccae immunization altered neuroinflammatory responses in aged rats that underwent a laparotomy
Young and aged rats received three subcutaneous injections of M. vaccae or vehicle, each injection spaced seven days apart. Five days following the final injection with M. vaccae, rats underwent a laparotomy or sham procedure and tissue was collected three days later (experiment outlined in Fig 2A). Previous work indicates that age-related POCD in rats is dependent on increases in hippocampal IL-1β (Barrientos et al., 2012b). In agreement with these findings, aged rats that received a laparotomy procedure had elevated IL-1β mRNA expression (age × surgery: F1,45 = 5.3, p < 0.05; Fig 2B). The three-way interaction of age × surgery × M. vaccae was not statistically significant (p = 0.1); however, treatment with M. vaccae, reduced IL-1β mRNA (main effect of M. vaccae: F1,45 = 6.5, p < 0.05). Hippocampal IL-1β protein was regulated in a similar manner as IL-1β mRNA (Fig 2C). Aged vehicle-treated rats that underwent a laparotomy procedure had enhanced IL-1β protein and M. vaccae immunization protected against the increase in IL-1β. Age- and surgery-induced elevations in NFKBIA mRNA expression (age: F1,45 = 13.9, surgery: F1,45 = 8.8, p < 0.05) were also ameliorated by M. vaccae pretreatment (M. vaccae: F1,45 = 4.3, p < 0.05; Fig 2D).
Figure 2. The hippocampal pro-inflammatory environment in aged rats was abrogated by M. vaccae treatment.
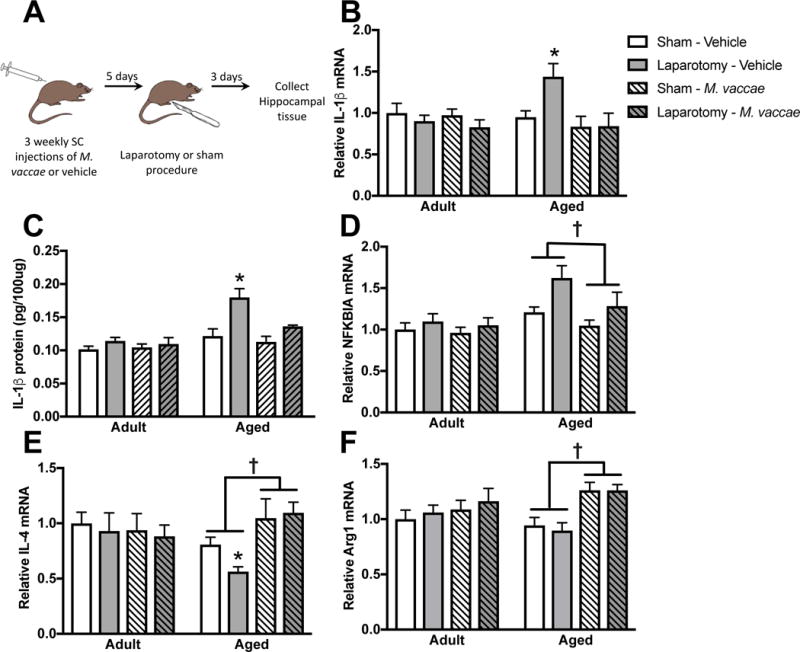
(A) Experimental outline. Hippocampal IL-1β (B) gene and (C) protein expression were elevated in aged rats that underwent a laparotomy procedure and reduced by M. vaccae treatment. (D) NFKBIA was also elevated by surgery in aged rats and reduced by M. vaccae. M. vaccae treatment upregulated (E) IL-4 and (F) Arginase1 mRNA expression. Results were analyzed using a 2 × 2 × 2 ANOVA with age, surgery, and M. vaccae treatment as between-subjects factors (n = 6-7/group). Data are expressed as mean ± SEM. *differs from all other groups, †effect of M. vaccae, p < 0.05.
M. vaccae can buffer against the pro-inflammatory effects of stress by upregulating anti-inflammatory pathway genes in the periphery (IL-10 and transforming growth factor beta [TGFβ]) and central nervous system (IL-4) (Reber et al, 2016; Frank et al, 2018). Thus, here we evaluated whether M. vaccae immunization induces an anti-inflammatory immunophenotype in the aged brain. Overall, IL-10 mRNA was expressed at a low level and was not detectable in a number of samples (spread equally throughout the groups). In PCR samples that did amplify before 35 cycles, there was an age-related reduction in IL-10 mRNA expression (F1,34 = 9.1, see Table 1). However, M. vaccae did not significantly upregulate IL-10 mRNA expression. TGF-β was not significantly regulated by age, surgery, or M. vaccae treatment (Table 1). In contrast, IL-4 mRNA expression was suppressed in the hippocampus of aged rats. M. vaccae treatment was protective against age-associated decrements in IL-4 (age × M. vaccae: F1,45 = 7.2, p < 0.05; Fig 2E): M. vaccae increased IL-4 expression in the hippocampus of aged, but not young adult rats (post hoc, p < 0.05).
Table 1.
Relative gene expression. Gene expression was run in duplicate and analyzed with the 2^ΔΔCt method relative to β-actin.
| Group | Adult | Aged | ||||||
|---|---|---|---|---|---|---|---|---|
| Vehicle | M. vaccae | Vehicle | M. vaccae | |||||
| Sham | Laparotomy | Sham | Laparotomy | Sham | Laparotomy | Sham | Laparotomy | |
| Gene | Three days post-laparotomy (behaviorally naïve rats) | |||||||
| Arg1 | 1.00 ± 0.08 | 1.06 ± 0.07 | 1.09 ± 0.08 | 1.16 ± 0.11 | 0.94 ± 0.07 | 0.9 ± 0.07 | 1.26 ± 0.07 | 1.26 ± 0.05 |
| CD200L | 1.00 ± 0.08 | 1.02 ± 0.07 | 0.98 ± 0.06 | 0.99 ± 0.11 | 0.82 ± 0.06 | 0.79 ± 0.05 | 0.94 ± 0.06 | 1.03 ± 0.12 |
| CD206 | 1.00 ± 0.09 | 0.81 ± 0.09 | 0.88 ± 0.15 | 0.94 ± 0.11 | 0.59 ± 0.04 | 0.49 ± 0.06 | 0.69 ± 0.04 | 0.75 ± 0.08 |
| CD-4 | 1.00 ± 0.07 | 0.85 ± 0.05 | 0.92 ± 0.07 | 0.82 ± 0.07 | 2.00 ± 0.14 | 1.80 ± 0.17 | 1.8 ± 0.14 | 1.80 ± 0.05 |
| IL-4 | 1.00 ± 0.10 | 0.93 ± 0.16 | 0.94 ± 0.15 | 0.88 ± 0.10 | 0.81 ± 0.07 | 0.56 ± 0.04 | 1.05 ± 0.17 | 1.10 ± 0.10 |
| IL-10 | 1.00 ± 0.17 | 0.79 ± 0.16 | 1.02 ± 0.19 | 0.96 ± 0.31 | 0.50 ± 0.15 | 0.43 ± 0.11 | 0.70 ± 0.13 | 0.62 ± 0.23 |
| IL-1β | 1.00 ± 0.12 | 0.90 ± 0.07 | 0.97 ± 0.08 | 0.83 ± 0.09 | 0.95 ± 0.08 | 1.44 ± 0.15 | 0.83 ± 0.13 | 0.84 ± 0.16 |
| Foxp3 | 1.00 ± 0.10 | 1.09 ± 0.10 | 1.18 ± 0.12 | 1.16 ± 0.09 | 1.14 ± 0.14 | 1.11 ± 0.09 | 1.52 ± 0.23 | 1.55 ± 0.19 |
| NFKBIA | 1.00 ± 0.08 | 1.10 ± 0.10 | 0.96 ± 0.07 | 1.05 ± 0.09 | 1.21 ± 0.06 | 1.62 ± 0.15 | 1.05 ± 0.07 | 1.28 ± 0.17 |
| MHCII | 1.00 ± 0.12 | 0.73 ± 0.08 | 0.95 ± 0.14 | 0.73 ± 0.07 | 4.41 ± 0.29 | 3.98 ± 0.48 | 2.94 ± 0.19 | 2.84 ± 0.37 |
| TGF-β | 1.00 ± 0.08 | 0.84 ± 0.06 | 0.94 ± 0.06 | 0.82 ± 0.14 | 1.04 ± 0.04 | 0.93 ± 0.06 | 0.89 ± 0.05 | 0.97 ± 0.05 |
| Eight days post-laparotomy (behavior rats) | ||||||||
| Arg1 | 1.00 ± 0.06 | 0.81 ± 0.09 | 0.90 ± 0.11 | 0.80 ± 0.09 | 0.71 ± 0.08 | 0.69 ± 0.06 | 0.90 ± 0.07 | 1.07 ± 0.07 |
| IL-4 | 1.00 ± 0.17 | 1.02 ± 0.07 | 1.19 ± 0.14 | 1.06 ± 0.10 | 0.71 ± 0.09 | 0.61 ± 0.10 | 1.06 ± 0.11 | 1.04 ± 0.07 |
| IL-1β | 1.00 ± 0.14 | 1.19 ± 0.09 | 1.07 ± 0.08 | 1.10 ± 0.05 | 1.11 ± 0.14 | 0.95 ± 0.13 | 1.13 ± 0.14 | 0.87 ± 0.09 |
IL-4 induces anti-inflammatory (M2) polarization in peripheral macrophages (Koning et al., 2010) as well as cluster of differentiation 200 (CD200) in the CNS (Lyons et al., 2007a), which inhibits pro-inflammatory activation in microglia through its cognate receptor cluster of differentiation 200 receptor 1 (CD200R1) expressed on microglia (Gorczynski et al., 2004). Thus, we also evaluated several markers typical of M2 cells (arginase 1 (Arg1) and cluster of differentiation 206 (CD206)) and CD200. Arg1 and CD206 were reduced in aged rats and significantly upregulated by M. vaccae treatment (age × M. vaccae, p < 0.05; Fig 2F and Table 1). There were also age-related reductions in CD200 (age: F1,45 = 7.7, p < 0.05), but no compensation by treatment with M. vaccae (p > 0.05; Table 1). There was no effect of surgery or three-way interaction on Arg1, CD206, or CD200 gene expression. Of note, tissue for gene analyses was collected 8 days after the final M. vaccae injection so it is possible that upregulation of additional markers of alternative activation might occur at acute (earlier) post-injection time points.
To determine whether M. vaccae had persistent effects on neuroinflammatory phenotype, hippocampi were examined from rats that underwent fear conditioning (13 days following the final injection of M. vaccae). At this later time post-M. vaccae and -laparotomy, IL-4 and Arg1 were also significantly upregulated in the hippocampus of M. vaccae immunized rats (F1,36 = 7.6, p < 0.05; Table 1). There were no differences in IL-1β at this later time point (p > 0.05, Table 1). Thus, M. vaccae treatment in aged rats elicits an anti-inflammatory shift in the CNS milieu that persists for at least ~2 weeks post-immunization.
3.3. Aged rats increased T cell markers in the hippocampus
The peripheral anti-inflammatory effects of M. vaccae have been largely attributed to effects on T cells (Reber et al., 2016). However, T cells are not routinely detected in the brain parenchyma as access is tightly regulated by the blood-brain barrier. Here, we show that mRNA expression for the pan T cell marker, CD3, is upregulated in the hippocampus of aged rats and reduced by M. vaccae immunization (age × M. vaccae F1,45 = 8.6, < 0.05; Fig 3A). CD4, a marker for helper T cells, was upregulated in the hippocampus of aged rats (age: F1,45 = 90.9, p < 0.05; Fig 3B). Furthermore, Forkhead Box P3 (FOXP3) mRNA expression, which directs development and function of regulatory T cells, was increased by M. vaccae treatment in aged rats (age: F1,45 = 5.2, M. vaccae: F1,45 = 4.7, p < 0.05; Fig 3C). This may indicate that M. vaccae treatment shifts the population of T cells gaining access to the CNS.
Figure 3. T cell markers are regulated by aging and M. vaccae.
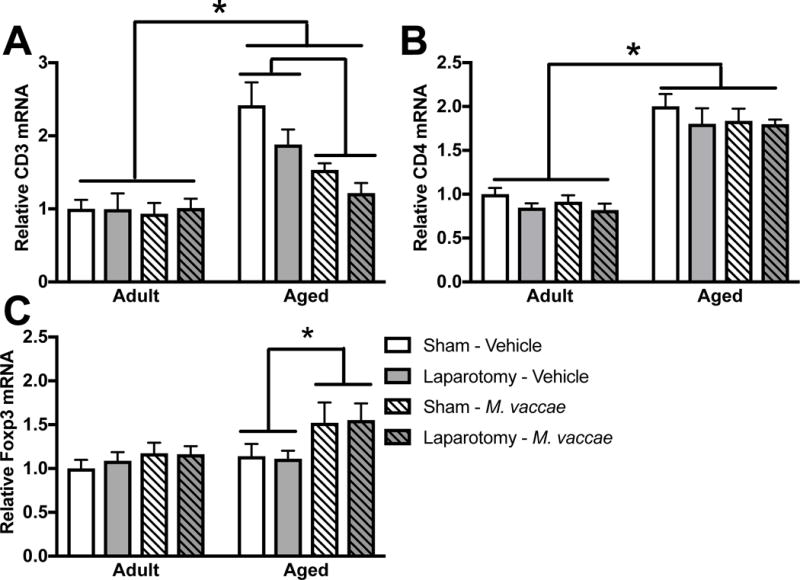
(A) CD3 mRNA expression was upregulated in the hippocampus of aged as compared to young rats and reduced by M. vaccae immunization. (B) CD4 mRNA expression was upregulated in the aged rat hippocampus and (B) Foxp3 mRNA expression was elevated in aged rats by M. vaccae treatment. Results were analyzed using a 2 × 2 × 2 ANOVA with age, surgery, and M. vaccae treatment as between-subjects factors (n = 6-7/group). Data are expressed as mean ± SEM. *p < 0.05.
3.4. M. vaccae immunization dampened inflammatory responses of aged microglia ex vivo
Microglia are considered the predominant innate immune cell of the CNS (Ransohoff and Perry, 2009) and a key cellular substrate of aging-induced neuroinflammatory priming (Barrientos et al., 2012a). Thus, the present experiment examined whether in vivo M. vaccae immunization in aged rats reduces ex vivo microglia reactivity. To test this possibility, microglia were isolated from the hippocampus of aged rats five days after the final injection of M. vaccae and directly exposed to an immune challenge (outlined in Fig 4A). It is important to note here that in the findings presented thus far, laparotomy served as an immune challenge in vivo. In the present experiment, microglia were directly exposed to an immune challenge ex vivo to determine whether M. vaccae treatment in vivo blunts microglia priming. In this case, LPS served as the immune challenge instead of laparotomy given our prior findings that the microglial pro-inflammatory response to LPS ex vivo is potentiated in aged animals (Frank et al., 2010). Also, it is important to note that young animals were not included here given the lack of an M. vaccae effect on anti-inflammatory processes in these animals. Microglia were plated ex vivo and challenged with media alone (0 ng LPS control) or LPS (10 and 100 ng). M. vaccae immunization blunted aged microglia reactivity to LPS as indicated by IL-1β mRNA expression (M. vaccae × LPS: F2,18 = 3.7, p < 0.05; Fig 4B) and IL-6 mRNA expression (LPS: F2,18 = 30.7, M. vaccae: F1,18 = 8.7, p < 0.05; Fig 4C). M. vaccae also reduced NFKBIA increases caused by LPS treatment (M. vaccae × LPS: F2,18 = 4.6, p < 0.05, Fig 4D).
Figure 4. Microglia isolated from aged rats are less inflammatory following in vivo M. vaccae treatment.
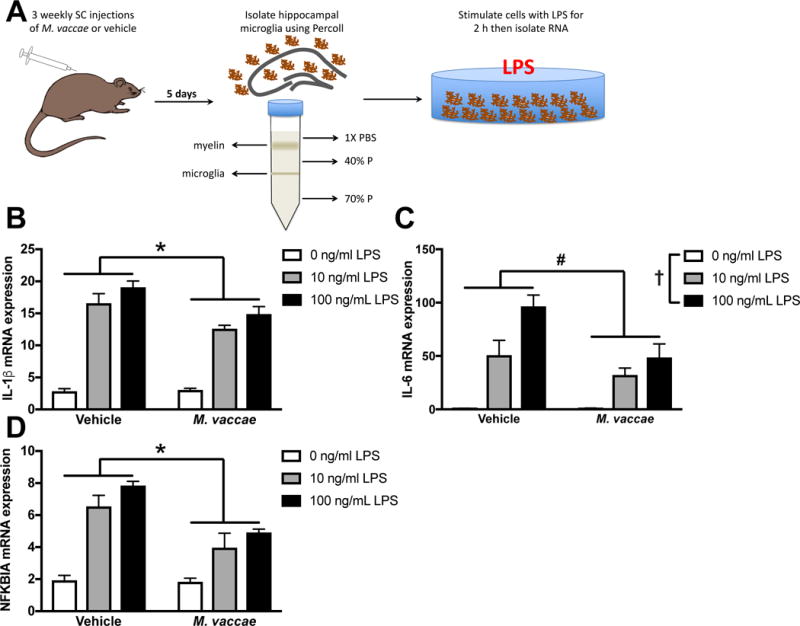
(A) Experimental design: aged rats received subcutaneous M. vaccae injections once per week for three weeks. Five days following the final injection, microglia were isolated from the hippocampus and stimulated with LPS. (B) IL-1β, (C) IL-6, and (D) NFKBIA mRNA expression were reduced in microglia isolated from aged M. vaccae-treated rats compared to vehicle-treated rats. Results were analyzed using a 2 × 3 ANOVA with age and LPS as the independent variables (n = 4/group). Data are expressed as mean ± SEM. *LPS × M. vaccae interaction, #main effect of M. vaccae, †main effect of LPS; p < 0.05 in all cases.
4. Discussion
Aged individuals are vulnerable to developing cognitive impairments following surgery (Barrientos et al., 2012b; Monk et al., 2008; Steinmetz et al., 2009) and cognitive decline post-surgery appears dependent on a pro-inflammatory neuroenvironment (Barrientos et al., 2012b). Indeed, consistent with our prior findings (Barrientos et al., 2012b), the present results demonstrate that a laparotomy procedure induced impairments in hippocampal-dependent memory, concomitant with increased hippocampal pro-inflammatory cytokines in aged, but not young rats. This finding is consistent with a number of prior reports demonstrating that the neuroimmune microenvironment of aged animals becomes sensitized to diverse inflammatory stimuli including E. coli infection and traumatic brain injury (Barrientos et al., 2015). For this reason, treatment strategies that induce a prolonged desensitization of the neuroimmune microenvironment might be particularly effective at mitigating the heightened neuroinflammatory effects of immune challenges in aged animals. Here we focused on a microbial-based treatment strategy (M. vaccae), which buffers against pro-inflammatory conditions in the periphery (Reber et al., 2016) and has prolonged anti-inflammatory effects (~12 weeks) (Zuany-Amorim et al., 2002). It is important to note that the effects of M. vaccae on neuroimmune processes have largely been unexplored. Thus, here we tested the hypothesis that a microbial treatment prior to surgery could re-direct the CNS immune system towards an anti-inflammatory phenotype and prevent surgery-induced neuroinflammatory increases and cognitive dysfunction. Our findings suggest that M. vaccae pretreatment protects against surgery-induced cognitive impairments in aged rats. Furthermore, M. vaccae treatment shifted the aged hippocampus from a pro-inflammatory milieu (increased IL-1β and NFKBIA) towards an anti-inflammatory phenotype (reduced IL-1β and NFKBIA and increased IL-4 and Arg1). Our results also indicate a possible role for microglia and T cells in mediating the benefits of M. vaccae treatment.
M. vaccae may abrogate neuroinflammatory priming in aged rats through upregulating IL-4 signaling. In agreement with previous findings, aged rats had reduced hippocampal IL-4 mRNA expression (Maher et al., 2005; Nolan et al., 2005) and IL-4 was further suppressed in aged rats by a surgical challenge (Li et al., 2017). Reductions in hippocampal IL-4 in aged rats have previously been associated with cognitive deficits and impairments in LTP (Li et al., 2017; Lyons et al., 2007b; Maher et al., 2005; Nolan et al., 2005). Central administration of IL-4 is able to reverse inflammation-associated deficits in aged animals (Li et al., 2017; Lyons et al., 2007b; Maher et al., 2005; Nolan et al., 2005). Here, we show that M. vaccae treatment induced a prolonged (8 and 13 days post-M. vaccae) upregulation in IL-4 mRNA expression in the hippocampus of aged rats. Importantly, IL-4 acts as an anti-inflammatory agent on innate immune cells including microglia and can help resolve an inflammatory response (Gadani et al., 2012). IL-4 can induce an alternative activation state (M2) in macrophages and microglia (Gadani et al., 2012). In support of this, Arg1, which is a traditional marker for M2 macrophages/microglia, was also elevated by M. vaccae.
Consistent with its induction of an anti-inflammatory milieu (i.e., increased IL-4) in the hippocampus of aged animals, M. vaccae treatment also abrogated the potentiating effect of surgery on pro-inflammatory factors in the aged hippocampus. NFKBIA expression was elevated in aged rats that underwent surgery but reduced by M. vaccae treatment. NFKBIA expression is a general indicator of activity of the canonical inflammatory transcription factor NF-κB, such that NFKBIA is induced by NF-κB to provide negative feedback inhibition of NF-κB function (Sun et al., 1993). In parallel, IL-1β mRNA and protein expression were upregulated in the hippocampus of aged rats compared to young rats 3 days following laparotomy. M. vaccae treatment blocked the surgery-induced protein and mRNA increase of this pro-inflammatory cytokine in aged animals. Pro-inflammatory changes following laparotomy, and specifically elevations in IL-1β, are essential for cognitive deficits in this laparotomy model: blocking IL-1β signaling with an intracisterna magna (ICM) injection of IL1 receptor antagonist (IL1-RA) can prevent cognitive deficits in aged rats (Barrientos et al., 2012b). The laparotomy-elicited pro-inflammatory response likely resolves by 8 days post-surgery as the elevation in hippocampal IL-1β mRNA expression was not evident in tissue collected 8 days following laparotomy in rats that underwent behavioral testing. It is possible, however, that the stress of behavioral testing disrupted IL-1β signaling at this time point. Here, we focused on what is considered the “master” pro-inflammatory cytokine IL-1β (Basu et al., 2004), which is thought to set in motion a cascade of downstream inflammatory processes. Our previous work indicates that aging-induced priming is not restricted to IL-1β signaling, but more generally increases a number of pro-inflammatory pathways in response to immune stimulation (Fonken et al., 2016). This is consistent with the notion that IL-1β signaling is a “gatekeeper” of inflammation (Dinarello, 2011).
M. vaccae could facilitate anti-inflammatory changes in the CNS via several potential mechanisms. First, M. vaccae could communicate anti-inflammatory signals through the vagus nerve. A precedent for this idea extends from findings that inflammatory mediators such as cytokines are detected by afferent vagal neurons in the nodose and jugular ganglia that signal to the nucleus of the solitary tract and other brain stem structures (reviewed in (Pavlov and Tracey, 2012)). This afferent signal is essential for the communication of pro-inflammatory signals to the CNS (Watkins et al., 1995). Whether anti-inflammatory signals are similarly communicated, however, is less well established. Interestingly, the vagus nerve also mediates gut-brain axis signaling (Breit et al., 2018). Subcutaneous injections of M. vaccae induce changes in the gut microbiota, suggesting that M. vaccae may also signal the vagus via this indirect pathway (Reber et al., 2016). Second, it is possible that M. vaccae blunts the peripheral inflammatory response at the surgical site and thus prevents the communication of an enhanced signal to the brain. This explanation is unlikely, however; aged rats tend to exhibit dampening of peripheral immune responses with age (Rawji et al., 2016). Previous research has demonstrated that inflammatory priming is specific to the CNS in aged rats (Barrientos et al., 2009). Third, M. vaccae-derived metabolites, such as triacylglycerols, long-chain saturated fatty acid polyesters, or their fatty acid derivatives (Agusti et al., 2008) may cross the blood-brain barrier. Fourth, peripheral immune cells, such as macrophages or T cells, may traffic into the CNS or signal through the meningeal compartment. Although T cells do not typically access the CNS under healthy conditions, there is evidence that aged animals exhibit a gradual enhancement in T cell homing to the brain (Gemechu and Bentivoglio, 2012). Indeed, here we show that the pan T cell marker, CD3 was upregulated in the aged hippocampus and reduced by M. vaccae immunization. CD4, which is a glycoprotein found on the surface of helper T cells was also upregulated in the hippocampus of aged rats (CD4 expression was not altered by M. vaccae). Furthermore, M. vaccae may shift the phenotype of the T cell population as M. vaccae increased hippocampal expression of FOXP3, a marker of regulatory T cells (Tregs). Importantly, the peripheral anti-inflammatory effects of M. vaccae require Tregs (Reber et al., 2016). T cells can modulate innate immune cells in the CNS (Walsh et al., 2014; Xie et al., 2015) and redirecting the population of T cells that access the CNS promotes a reparative environment in other contexts. Future studies could explore how peripheral M. vaccae alters T cell density and phenotype in the CNS.
Microglia likely underlie exaggerated immune responses in the aged brain, as aged microglia ex vivo show amplified responses to inflammatory stimuli (Fonken et al., 2016; Frank et al., 2010). The present results demonstrate that M. vaccae, as compared to vehicle treatment, induced a considerable reduction in the pro-inflammatory response of microglia isolated from aged rats. These results suggest that M. vaccae immunization shifts the balance of neuroimmune microenvironment towards an anti-inflammatory milieu resulting in desensitization of microglia to pro-inflammatory stimuli. Further, these findings are consistent with the induction of IL-4 by M. vaccae in the aged hippocampus. For instance, microglia exposed to IL-4 take on a more reparative “alternatively activated” phenotype (Butovsky et al., 2006; Kigerl et al., 2009). Interestingly, microglia from aged mice are not as responsive to ex vivo IL-4 treatment (Fenn et al., 2012) and previous work suggests that microglia isolated from aged mice have impaired IL-4 signaling following infection or injury (Fenn et al., 2014; Fenn et al., 2012). We found that M. vaccae upregulated hippocampal IL-4 in aged rats for at least 13 d post-treatment, and that hippocampal microglia from M. vaccae-treated aged rats were less responsive to inflammatory stimulus ex vivo. Thus, M. vaccae treatment may be a novel strategy to rescue decrements in the IL-4 system in aged animals.
One limitation to this study is that it did not include female rats as aged F344xBN female rats were not available through the NIA colony. There are sex differences in the neuroimmune system (Osborne et al., 2018), including sex differences in microglia priming in other contexts such as stress (Fonken et al., 2018a). Furthermore, mild cognitive impairments develop more rapidly in women (Lin et al., 2015) and women are at great risk for developing Alzheimer’s disease (Altmann et al., 2014). Thus, determining whether M. vaccae is protective against cognitive decline in females is an important future direction.
5. Conclusions
Taken together, these results indicate that M. vaccae pretreatment can prevent pathological neuroinflammation and cognitive impairments in aged rats that undergo a surgical challenge. In aged rats, prior M. vaccae immunization was able to rescue surgery-elicited memory deficits. In parallel, M. vaccae boosted expression of anti-inflammatory and regulatory T cell markers in the aged hippocampus, suggesting that it induces a persistent beneficial immune shift in the CNS. Further, microglia from M. vaccae-treated aged hippocampus displayed dampened neuroinflammatory priming.
Our data highlight the beneficial immunomodulatory role of M. vaccae in a model of aging-elicited neuroinflammatory priming. M. vaccae could improve other conditions caused or exacerbated by neuroinflammatory priming, and future studies could address whether delivering M. vaccae after neuroinflammatory activation can still be protective. In addition, our data suggest that neuroinflammatory priming with age could be improved by exploring other potential microbial treatments (Williamson et al., 2016). One remarkable strength of the treatment regimen employed herein is that the delivery route is minimally invasive (subcutaneous), yet able to modulate neuroinflammation in the hippocampus and ameliorate related behavioral deficits. Future studies should reveal whether M. vaccae and other micro-/macroorganism treatments improve neuroinflammatory pathology, how this peripheral-to-central signaling occurs, and whether these treatments are viable immunomodulators in humans.
Highlights.
Aged, but not young rats, show post-operative learning/memory deficits.
Prophylactic immunization with M. vaccae prevents cognitive deficits in aged rats.
M. vaccae likely prevents cognitive impairments by shifting the neuroimmune environment.
M. vaccae immunization may signal the CNS via T cells.
Acknowledgments
This research was supported by NIH grant R21MH116263 to C.A.L., L.F.K., M.G.F., and S.F.M, NIH grant R01MH108523 to M.G.F., L.R.W., and S.F.M., and a NARSAD Young Investigator Grant to L.K.F. The authors thank University of Colorado Boulder Office of Animal Resources staff for excellent animal care.
Abbreviations
- Arg1
arginase 1
- CD200
cluster of differentiation 200
- CD200R
cluster of differentiation 200 receptor
- CD206
cluster of differentiation 206, also known as mannose receptor (MR)
- CNS
central nervous system
- DMEM
Dulbecco’s Modified Eagle Medium
- DPBS
Dulbecco’s Phosphate Buffered Saline
- FBS
fetal bovine serum
- FOXP3
forkhead box P3
- ICM
intracisterna magna
- IL
interleukin
- IL1-RA
IL1 receptor antagonist
- LPS
lipopolysaccharide
- LTP
long-term potentiation
- NFKBIA
nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha
- PBS
phosphate-buffered saline
- POCD
postoperative cognitive dysfunction
- TGFβ
transforming growth factor beta
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agusti G, Astola O, Rodriguez-Guell E, Julian E, Luquin M. Surface spreading motility shown by a group of phylogenetically related, rapidly growing pigmented mycobacteria suggests that motility is a common property of mycobacterial species but is restricted to smooth colonies. Journal of bacteriology. 2008;190(20):6894–6902. doi: 10.1128/JB.00572-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann A, Tian L, Henderson VW, Greicius MD, Alzheimer’s Disease Neuroimaging Initiative, I. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. 2014;75(4):563–573. doi: 10.1002/ana.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun. 2009;23(1):46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Watkins LR, Maier SF. Aging-related changes in neuroimmune-endocrine function: implications for hippocampal-dependent cognition. Hormones and behavior. 2012a;62(3):219–227. doi: 10.1016/j.yhbeh.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Hein AM, Frank MG, Watkins LR, Maier SF. Intracisternal interleukin-1 receptor antagonist prevents postoperative cognitive decline and neuroinflammatory response in aged rats. J Neurosci. 2012b;32(42):14641–14648. doi: 10.1523/JNEUROSCI.2173-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27(5):723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Kitt MM, Watkins LR, Maier SF. Neuroinflammation in the normal aging hippocampus. Neuroscience. 2015;309:84–99. doi: 10.1016/j.neuroscience.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Krady JK, Levison SW. Interleukin-1: a master regulator of neuroinflammation. J Neurosci Res. 2004;78(2):151–156. doi: 10.1002/jnr.20266. [DOI] [PubMed] [Google Scholar]
- Breit S, Kupferberg A, Rogler G, Hasler G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front Psychiatry. 2018;9:44. doi: 10.3389/fpsyt.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhart CS, Dell-Kuster S, Gamberini M, Moeckli A, Grapow M, Filipovic M, Seeberger MD, Monsch AU, Strebel SP, Steiner LA. Modifiable and nonmodifiable risk factors for postoperative delirium after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2010;24(4):555–559. doi: 10.1053/j.jvca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31(1):149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Cao XZ, Ma H, Wang JK, Liu F, Wu BY, Tian AY, Wang LL, Tan WF. Postoperative cognitive deficits and neuroinflammation in the hippocampus triggered by surgical trauma are exacerbated in aged rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(8):1426–1432. doi: 10.1016/j.pnpbp.2010.07.027. [DOI] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22(3):301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS, Maze M. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68(3):360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox FF, Carney D, Miller AM, Lynch MA. CD200 fusion protein decreases microglial activation in the hippocampus of aged rats. Brain Behav Immun. 2012;26(5):789–796. doi: 10.1016/j.bbi.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. A clinical perspective of IL-1beta as the gatekeeper of inflammation. Eur J Immunol. 2011;41(5):1203–1217. doi: 10.1002/eji.201141550. [DOI] [PubMed] [Google Scholar]
- Fenn AM, Hall JC, Gensel JC, Popovich PG, Godbout JP. IL-4 signaling drives a unique arginase+/IL-1beta+ microglia phenotype and recruits macrophages to the inflammatory CNS: consequences of age-related deficits in IL-4Ralpha after traumatic spinal cord injury. J Neurosci. 2014;34(26):8904–8917. doi: 10.1523/JNEUROSCI.1146-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Henry CJ, Huang Y, Dugan A, Godbout JP. Lipopolysaccharide-induced interleukin (IL)-4 receptor-alpha expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain Behav Immun. 2012;26(5):766–777. doi: 10.1016/j.bbi.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Frank MG, Gaudet AD, D’Angelo HM, Daut RA, Hampson EC, Ayala MT, Watkins LR, Maier SF. Neuroinflammatory priming to stress is differentially regulated in male and female rats. Brain Behav Immun. 2018a doi: 10.1016/j.bbi.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Frank MG, Gaudet AD, Maier SF. Stress and aging act through common mechanisms to elicit neuroinflammatory priming. Brain, Behavior, and Immunity. 2018b doi: 10.1016/j.bbi.2018.07.012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Frank MG, Kitt MM, D’Angelo HM, Norden DM, Weber MD, Barrientos RM, Godbout JP, Watkins LR, Maier SF. The Alarmin HMGB1 Mediates Age-Induced Neuroinflammatory Priming. J Neurosci. 2016;36(30):7946–7956. doi: 10.1523/JNEUROSCI.1161-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Watkins LR, Maier SF. Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. Journal of neuroimmunology. 2010;226(1–2):181–184. doi: 10.1016/j.jneuroim.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Fonken LK, Dolzani SD, Annis JL, Siebler PH, Heinze JD, Schmidt D, Watkins LR, Maier SF, Lowry CA. Immunization with Mycobacterium vaccae induces an anti-inflammatory milieu in the CNS: attenuation of stress-induced microglial priming, alarmins and anxiety-like behavior. Brain, Behavior, and Immunity. 2018 doi: 10.1016/j.bbi.2018.05.020. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: immunophenotypic and functional characteristics. Journal of neuroscience methods. 2006;151(2):121–130. doi: 10.1016/j.jneumeth.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Gadani SP, Cronk JC, Norris GT, Kipnis J. IL-4 in the brain: a cytokine to remember. J Immunol. 2012;189(9):4213–4219. doi: 10.4049/jimmunol.1202246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemechu JM, Bentivoglio M. T Cell Recruitment in the Brain during Normal Aging. Front Cell Neurosci. 2012;6:38. doi: 10.3389/fncel.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19(10):1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Gorczynski R, Chen Z, Kai Y, Lee L, Wong S, Marsden PA. CD200 is a ligand for all members of the CD200R family of immunoregulatory molecules. J Immunol. 2004;172(12):7744–7749. doi: 10.4049/jimmunol.172.12.7744. [DOI] [PubMed] [Google Scholar]
- Groschel MI, Prabowo SA, Cardona PJ, Stanford JL, van der Werf TS. Therapeutic vaccines for tuberculosis–a systematic review. Vaccine. 2014;32(26):3162–3168. doi: 10.1016/j.vaccine.2014.03.047. [DOI] [PubMed] [Google Scholar]
- Hudetz JA, Gandhi SD, Iqbal Z, Patterson KM, Pagel PS. Elevated postoperative inflammatory biomarkers are associated with short- and medium-term cognitive dysfunction after coronary artery surgery. J Anesth. 2011;25(1):1–9. doi: 10.1007/s00540-010-1042-y. [DOI] [PubMed] [Google Scholar]
- Hudetz JA, Iqbal Z, Gandhi SD, Patterson KM, Byrne AJ, Hudetz AG, Pagel PS, Warltier DC. Ketamine attenuates post-operative cognitive dysfunction after cardiac surgery. Acta Anaesthesiol Scand. 2009;53(7):864–872. doi: 10.1111/j.1399-6576.2009.01978.x. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29(43):13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning N, van Eijk M, Pouwels W, Brouwer MS, Voehringer D, Huitinga I, Hoek RM, Raes G, Hamann J. Expression of the inhibitory CD200 receptor is associated with alternative macrophage activation. J Innate Immun. 2010;2(2):195–200. doi: 10.1159/000252803. [DOI] [PubMed] [Google Scholar]
- Laudanno O, Vasconcelos L, Catalana J, Cesolari J. Anti-inflammatory effect of bioflora probiotic administered orally or subcutaneously with live or dead bacteria. Dig Dis Sci. 2006;51(12):2180–2183. doi: 10.1007/s10620-006-9175-4. [DOI] [PubMed] [Google Scholar]
- Li Z, Liu F, Ma H, White PF, Yumul R, Jiang Y, Wang N, Cao X. Age exacerbates surgery-induced cognitive impairment and neuroinflammation in Sprague-Dawley rats: the role of IL-4. Brain Res. 2017;1665:65–73. doi: 10.1016/j.brainres.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Lin AK, Choudhury KR, Rathakrishnan BG, Marks DM, Petrella JR, Doraiswamy PM. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimer’s & Dementia: Translational Research & Clinical Interventions. 2015;1(2):103–110. doi: 10.1016/j.trci.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA, Smith DG, Siebler PH, Schmidt D, Stamper CE, Hassell JE, Jr, Yamashita PS, Fox JH, Reber SO, Brenner LA, Hoisington AJ, Postolache TT, Kinney KA, Marciani D, Hernandez M, Hemmings SM, Malan-Muller S, Wright KP, Knight R, Raison CL, Rook GA. The Microbiota, Immunoregulation, and Mental Health: Implications for Public Health. Curr Environ Health Rep. 2016;3(3):270–286. doi: 10.1007/s40572-016-0100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA. Age-related neuroinflammatory changes negatively impact on neuronal function. Front Aging Neurosci. 2010;1:6. doi: 10.3389/neuro.24.006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons A, Downer EJ, Crotty S, Nolan YM, Mills KH, Lynch MA. CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. J Neurosci. 2007a;27(31):8309–8313. doi: 10.1523/JNEUROSCI.1781-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons A, Griffin RJ, Costelloe CE, Clarke RM, Lynch MA. IL-4 attenuates the neuroinflammation induced by amyloid-beta in vivo and in vitro. J Neurochem. 2007b;101(3):771–781. doi: 10.1111/j.1471-4159.2006.04370.x. [DOI] [PubMed] [Google Scholar]
- Maher FO, Nolan Y, Lynch MA. Downregulation of IL-4-induced signalling in hippocampus contributes to deficits in LTP in the aged rat. Neurobiol Aging. 2005;26(5):717–728. doi: 10.1016/j.neurobiolaging.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC. Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology. 2004;101(1):191–203. doi: 10.1097/00000542-200407000-00030. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Kahn WR, Eisenach JC. Abdominal surgery decreases food-reinforced operant responding in rats: relevance of incisional pain. Anesthesiology. 2005;103(3):629–637. doi: 10.1097/00000542-200509000-00028. [DOI] [PubMed] [Google Scholar]
- Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108(1):18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- Nolan Y, Maher FO, Martin DS, Clarke RM, Brady MT, Bolton AE, Mills KH, Lynch MA. Role of interleukin-4 in regulation of age-related inflammatory changes in the hippocampus. The Journal of biological chemistry. 2005;280(10):9354–9362. doi: 10.1074/jbc.M412170200. [DOI] [PubMed] [Google Scholar]
- Osborne BF, Turano A, Schwarz JM. Sex differences in the neuroimmune system. Current Opinion in Behavioral Sciences. 2018;23:118–123. doi: 10.1016/j.cobeha.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex–linking immunity and metabolism. Nature reviews Endocrinology. 2012;8(12):743–754. doi: 10.1038/nrendo.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince MJ, Wu F, Guo Y, Gutierrez Robledo LM, O’Donnell M, Sullivan R, Yusuf S. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385(9967):549–562. doi: 10.1016/S0140-6736(14)61347-7. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annual review of immunology. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Rawji KS, Mishra MK, Michaels NJ, Rivest S, Stys PK, Yong VW. Immunosenescence of microglia and macrophages: impact on the ageing central nervous system. Brain. 2016;139(Pt 3):653–661. doi: 10.1093/brain/awv395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber SO, Siebler PH, Donner NC, Morton JT, Smith DG, Kopelman JM, Lowe KR, Wheeler KJ, Fox JH, Hassell JE, Jr, Greenwood BN, Jansch C, Lechner A, Schmidt D, Uschold-Schmidt N, Fuchsl AM, Langgartner D, Walker FR, Hale MW, Lopez Perez G, Van Treuren W, Gonzalez A, Halweg-Edwards AL, Fleshner M, Raison CL, Rook GA, Peddada SD, Knight R, Lowry CA. Immunization with a heat-killed preparation of the environmental bacterium Mycobacterium vaccae promotes stress resilience in mice. Proc Natl Acad Sci U S A. 2016;113(22):E3130–3139. doi: 10.1073/pnas.1600324113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook GA. Regulation of the immune system by biodiversity from the natural environment: an ecosystem service essential to health. Proc Natl Acad Sci U S A. 2013;110(46):18360–18367. doi: 10.1073/pnas.1313731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook GA, Lowry CA, Raison CL. Microbial ‘Old Friends’, immunoregulation and stress resilience. Evol Med Public Health. 2013;2013(1):46–64. doi: 10.1093/emph/eot004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosczyk HA, Sparkman NL, Johnson RW. Neuroinflammation and cognitive function in aged mice following minor surgery. Experimental gerontology. 2008;43(9):840–846. doi: 10.1016/j.exger.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS, Group I Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110(3):548–555. doi: 10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]
- Sun SC, Ganchi PA, Ballard DW, Greene WC. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science. 1993;259(5103):1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010;107(47):20518–20522. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JT, Zheng J, Smirnov I, Lorenz U, Tung K, Kipnis J. Regulatory T cells in central nervous system injury: a double-edged sword. J Immunol. 2014;193(10):5013–5022. doi: 10.4049/jimmunol.1302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Goehler LE, Relton JK, Tartaglia N, Silbert L, Martin D, Maier SF. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci Lett. 1995;183(1–2):27–31. doi: 10.1016/0304-3940(94)11105-r. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Rice KC, Maier SF. The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends in pharmacological sciences. 2009;30(11):581–591. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson LL, McKenney EA, Holzknecht ZE, Belliveau C, Rawls JF, Poulton S, Parker W, Bilbo SD. Got worms? Perinatal exposure to helminths prevents persistent immune sensitization and cognitive dysfunction induced by early-life infection. Brain Behav Immun. 2016;51:14–28. doi: 10.1016/j.bbi.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Hebert LE, Scherr PA, Dong X, Leurgens SE, Evans DA. Cognitive decline after hospitalization in a community population of older persons. Neurology. 2012;78(13):950–956. doi: 10.1212/WNL.0b013e31824d5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Choudhury GR, Winters A, Yang SH, Jin K. Cerebral regulatory T cells restrain microglia/macrophage-mediated inflammatory responses via IL-10. Eur J Immunol. 2015;45(1):180–191. doi: 10.1002/eji.201444823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuany-Amorim C, Manlius C, Trifilieff A, Brunet LR, Rook G, Bowen G, Pay G, Walker C. Long-term protective and antigen-specific effect of heat-killed Mycobacterium vaccae in a murine model of allergic pulmonary inflammation. J Immunol. 2002;169(3):1492–1499. doi: 10.4049/jimmunol.169.3.1492. [DOI] [PubMed] [Google Scholar]


