Abstract
Background
Studies have shown select associations between cardiovascular risk factors and dementia, but most focus on Alzheimer’s disease (AD).
Objective
We enhance these works by evaluating the relationship between the presence of cardiovascular risk factors and the rate of cognitive decline, measured using the Mini-Mental State Examination (MMSE) and Clinical Dementia Rating Sum of Boxes (CDR-SUM) on four common dementia subtypes (AD, dementia with Lewy bodies (DLB), frontotemporal dementia (FTD), and vascular dementia (VaD), as well as non-demented elderly individuals (normal)).
Methods
We used generalized linear mixed models with random intercepts to account for correlation at the patient and center levels for each dementia subtype adjusting for time since initial visit, baseline cognitive score, age, and demographic factors. The cardiovascular risk factors evaluated included body mass index, diabetes, years smoking, atrial fibrillation, hypertension, and hypercholesterolemia.
Results
Patients diagnosed with AD (n=1899), DLB (n=65), FTD (n=168), or VaD (n=13); or lacked cognitive impairment (normal) (n=3583) were evaluated using data from the National Alzheimer’s Coordinating Centers. Cardiovascular risk factors were associated with select dementia subtypes including AD and FTD. Using MMSE and CDR-SUM, recent or active hypertension and hypercholesterolemia were associated with slower cognitive decline for AD patients, while higher body mass index and years smoking were associated with slower cognitive decline for FTD patients. However, several cardiovascular factors demonstrated associations with more rapid cognitive decline.
Conclusion
These results demonstrate disease specific associations and can provide clinicians guidance on predicted cognitive changes at the group level using information about cardiovascular risk factors.
Keywords: Alzheimer’s disease, dementia with Lewy bodies, frontotemporal dementia, neuropsychological tests, vascular risk factors, body mass index
1. INTRODUCTION
Dementia progressively deteriorates the quality of life through decreased functioning and cognition. Many studies have investigated factors that contribute to decline in functional and mental capabilities [1–5]. In these studies, functional and cognitive capacities have been evaluated for different dementia subtypes, and analyses have indicated that changes may vary by subtype. Interest has also extended into understanding cognitive decline in mixed dementia [5, 6]. Furthermore, studies have shown specific associations of cardiovascular risk factors to these subtypes [7, 8].
The relationship between cardiovascular disease and dementia is of particular interest as both have increased risk with age and many studies have identified associations between cardiovascular risk factors and the risk of dementia, especially for Alzheimer’s disease (AD) [9–27]. In a previous study, it was determined differential associations between late-life cardiovascular factors and neurodegenerative diseases exist for multiple dementia subtypes [7]. The presence of cardiovascular risk factors was evaluated in individuals who had a diagnosis of probable AD, possible AD, vascular dementia (VaD), dementia with Lewy bodies (DLB), frontotemporal dementia (FTD), Parkinson’s disease, progressive supranuclear palsy, or corticobasal degeneration. Compared to the non-demented group, body mass index (BMI) of patients with probable AD and DLB was found to be significantly lower. Other studies have also identified relationships between cardiovascular factors and various disease pathologies, such as obesity or heart failure leading to a higher risk of AD [21, 28]. Identifying these relationships are important as multiple studies have suggested pharmacological and non-pharmacological therapies can improve vascular health and delay the progression of dementia. Physical activity has been demonstrated to improve brain plasticity and increase resilience to AD [29] while pharmacological therapies and nutritional interventions can be used to decrease the risk of obesity and delay the onset of AD [30].
These findings support the differential relationship of specific diseases to cardiovascular factors and presents the question of how cardiovascular factors may be related to cognition for dementia patients. A recent study evaluated the relationship of cognitive decline (measured by the Mini-Mental State Examination (MMSE) [31] score and Clinical Dementia Rating Scale Sum of Boxes (CDR-SUM) [32, 33]) and cardiovascular risk factors, such as diabetes mellitus, hypertension, hypercholesterolemia, smoking, and being overweight on subjects diagnosed with AD or DLB [8]. The analysis revealed overweight AD patients had slower cognitive decline while hypertension and being overweight were also related to slower cognitive decline for DLB patients. The study indicates that cardiovascular risk factors are associated to cognition but was limited in the diagnoses that were evaluated.
The objective of the current study was to determine whether the rate of cognitive decline was affected by late life cardiovascular risk factors, using two measures of cognition, MMSE and CDR-SUM, across multiple dementia subtypes including AD, DLB, VaD, and FTD as well as non-demented elderly individuals (normal).
2. METHODS
2.1. Study Participants
The data for this study were obtained from the National Alzheimer’s Coordinating Center (NACC) Uniform Data Set (UDS) [34–36]. This database contains standardized clinical evaluations, and detailed descriptions have been previously published [34–36]. Information, including demographics, diagnoses, vascular conditions, and cognitive status, are stored in the UDS. The UDS began in 2005, and patients were evaluated annually thereafter. Our analysis includes information from UDS visits conducted between June 2005 and May 2016 for patients enrolled in 34 Alzheimer’s Disease Center’s (ADCs). Research using the NACC database was approved by the Institutional Review Board at the University of Washington for patients enrolled in ADCs in the United States. Figure 1 displays a flow chart of the inclusion and exclusion criteria for the study.
Figure 1.
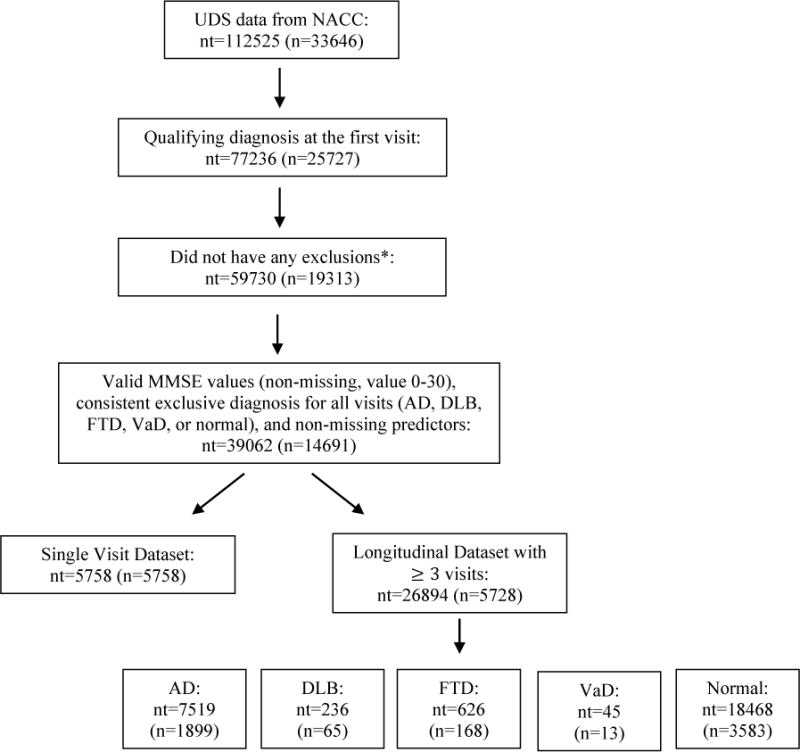
Flowchart of inclusion and exclusion criteria, n=number of subjects, nt=total number of visits/observations.
* Excluded if diagnosis of vitamin B12 deficiency, thyroid disease, alcohol abuse, other substance abuse, Down syndrome, Huntington disease, traumatic brain injury, hydrocephalus, central nervous system neoplasm, bipolar disorder, schizophrenia, anxiety, delirium, post-traumatic stress disorder, other major psychiatric illnesses, cognitive impairment due to alcohol abuse, cognitive impairment due to systemic disease/medical illness, cognitive impairment due to medications, brain cancer or CDR sum of boxes score greater than 16 at first qualifying visit
Only patients with a single clinical diagnosis of AD, DLB, FTD, or VaD, were included along with non-demented elderly individuals lacking a neurodegenerative disease diagnosis (normal). Patients were considered to have a diagnosis of AD if the presumptive etiologic diagnosis was probable AD or possible AD [37]. Due to the small sample size for FTD, the FTD subtypes were collapsed into one group and were comprised of patients with progressive supranuclear palsy, corticobasal degeneration, as well as other variants of FTD. The first visit in which a patient had one of the above qualifying diagnoses was considered the baseline visit. Visits prior to the baseline visit were removed, and visits following the qualifying visit were renumbered. In an interest to target the cognitive decline related to dementia, cases of mild cognitive impairment (MCI) were removed. For patients that were diagnosed with MCI and successively developed dementia, the visits were numbered starting at the first qualifying diagnosis of that specific dementia subtype (AD, DLB, FTD or VaD). If patients alternated between statuses of MCI and dementia during their visits, they were excluded from the study. Only individuals with a consistent diagnosis for all visits were evaluated. Individuals with mixed or changing diagnoses during the study period were excluded.
Furthermore, as done previously [7], a patient was excluded if, at first qualifying visit, he or she had a diagnosis of vitamin B12 deficiency, thyroid disease, alcohol abuse, other substance abuse, Down syndrome, Huntington disease, traumatic brain injury, hydrocephalus, central nervous system neoplasm, bipolar disorder, schizophrenia, anxiety, delirium, post-traumatic stress disorder, other major psychiatric illnesses, cognitive impairment due to alcohol abuse, cognitive impairment due to systemic disease/medical illness, cognitive impairment due to medications, and brain cancer. The Mini-Mental State Examination (MMSE) [31] and Clinical Dementia Rating Sum of Boxes (CDR-SUM) [32, 33], assessed at each of the visits, were evaluated as measures of cognitive ability. It is important to note that the CDR-SUM is a composite score that measures daily functioning in addition to cognitive ability. Subjects that had a CDR-SUM score greater than 16 at the beginning of the study were also excluded to remove patients with severe dementia, as similar to Gill et al. [4]. Visits where the MMSE or CDR-SUM was not recorded were excluded from the analysis. The number of months from the first visit was included in the model to account for unequally spaced visits due to missing values. A minimal follow up of two visits after the baseline visit were required for patients in the longitudinal dataset.
2.2. Cardiovascular Factors
Cardiovascular risk factors were evaluated for each patient at the annual visit to the ADC. The cardiovascular data considered for this study were body mass index (BMI), years smoking, atrial fibrillation, diabetes, hypertension, and hypercholesterolemia. Cardiovascular disease status was assessed by a clinician based on the subject report, medical records, and structured interviews, using guidelines from NACC [36]. BMI (weight (kg) divide by height (m) squared) and the number of years the patient had smoked were continuous variables. Atrial fibrillation, diabetes, hypertension, and hypercholesterolemia were categorized as recent/active, remote/inactive, and absent. A status of recent/active indicated that the condition occurred within the past year or required active management. Remote/inactive indicated that the condition occurred more than one year in the past but was resolved or was not currently being treated. The absent status indicated that the condition had never been present in the patient. In this study, the effect of having a recent/active diagnosis for cardiovascular risk factors was evaluated compared to remote/inactive and absent diagnoses.
2.3. Statistical Analysis
The statistical analyses were performed using the SAS statistical software (Version 9.4, SAS Institute Inc.). The means and proportions of demographic characteristics and cardiovascular risk factors were compared across groups using analysis of variance (ANOVA) for continuous variables and the test for difference of proportions for categorical variables. Post hoc analyses for individual comparisons were also performed using a Bonferroni adjustment on the Type I error rate. To account for the correlation between the subjects within the same ADC and repeated measurements on each subject, we fit generalized linear mixed models. We evaluated the effect of cardiovascular risk factors (BMI, years smoking, atrial fibrillation, diabetes, hypertension, and hypercholesterolemia) while adjusting for age, sex, race, education level, the number of months from the first visit (time), and baseline cognitive score. Interaction terms between the cardiovascular risk factors and time were included in the model to assess the effect of the cardiovascular risk factor on changes in cognition by month. Random effects were used to account for underlying differences between individuals and ADCs. A generalized linear mixed model was fit for each of the dementia subtypes (AD, DLB, FTD, VaD), as well as the normal population. A significance level of 0.05 was used to evaluate the impact of each factor. The MMSE, a measure of cognitive ability, and CDR-SUM, a composite measure of cognitive and daily living ability, were both selected as outcomes and evaluated for comparison. The possible MMSE scores range from 0 to 30, where a score of 0 indicates severe cognitive impairment and a score of 30 indicates no cognitive impairment. CDR-SUM scores typically range from 0 to 18, although in our study the scores range from 0 to 16 as patients with severe dementia were excluded. The CDR-SUM is a total score of dementia ratings for six areas including memory, orientation, judgment and problem-solving, community affairs, home and hobbies, and personal care. Each category is rated on a scale of 0 to 3 where 0 indicates no dementia and 3 indicates severe dementia. Clinical Dementia Rating Sum of Boxes scores of 0 reflect no cognitive impairment while scores closer to 18 suggest severe cognitive impairment.
3. RESULTS
3.1. Descriptive Statistics
The final data set contained 32,652 observations for 11,486 patients over 34 ADCs. Of these subjects, 5,758 had single visits. The remaining 5,728 patients (longitudinal dataset) collectively had 26,894 visits over the course of the study. Table 1 and Supplemental Table 1 display summaries of the demographic and clinical characteristics for the subjects at baseline in the longitudinal sample and total sample, respectively. Overall, the average number of visits per patient in the longitudinal data set was 4.7 visits (min = 3, max = 10). The average time between the first and last visit for patients with more than 1 visit was 51.7 months (min = 15, max = 114).
Table 1.
Participant characteristics at baseline for longitudinal sample ( 2 visits); age, education, body mass index (BMI), years smoking, Mini-Mental State Examination (MMSE), number of visits, time between first and last visit listed as average (standard deviation).
| Characteristics | AD n=1899 |
DLB n=65 |
FTD n=168 |
VaD n=13 |
Normal n=3583 |
p-value** |
|---|---|---|---|---|---|---|
| Age | 74.9 (9.2) |
71.0 (7.7) |
64.2 (8.4) |
73.1 (9.3) |
70.3 (9.6) |
<0.0001 |
| Sex, female, % | 50.1 | 13.9 | 30.4 | 30.8 | 65.6 | <0.0001 |
| Race, % | 0.0004 | |||||
| White | 85.3 | 95.4 | 92.9 | 76.9 | 81.5 | |
| Black or African American | 9.8 | 3.1 | 2.4 | 15.4 | 13.9 | |
| American Indian or Alaska Native | 0.4 | 0.0 | 0.0 | 0.0 | 0.2 | |
| Native Hawaiian or Pacific Islander | 0.0 | 0.0 | 0.0 | 0.0 | 0.0* | |
| Asian | 1.9 | 0.0 | 3.0 | 0.0 | 1.8 | |
| Multiracial | 2.7 | 1.5 | 1.8 | 7.7 | 2.6 | |
| Education | 14.7 (3.5) |
15.0 (3.4) |
15.1 (2.9) |
15.2 (3.2) |
15.8 (2.9) |
<0.0001 |
| BMI | 26.1 (4.3) |
26.9 (3.8) |
28.0 (5.0) |
28.8 (8.8) |
27.4 (5.3) |
<0.0001 |
| Years smoking | 10.2 (15.2) |
11.6 (15.3) |
10.0 (14.6) |
21.5 (26.5) |
9.3 (14.2) |
0.0096 |
| Atrial fibrillation, recent/active, % | 4.4 | 9.2 | 5.4 | 7.7 | 3.6 | 0.0964 |
| Diabetes, recent/active, % | 8.7 | 6.2 | 8.3 | 30.8 | 9.0 | 0.0745 |
| Hypertension, recent/active, % | 46.6 | 38.5 | 33.3 | 84.6 | 45.5 | 0.0004 |
| Hypercholesterolemia, recent/active, % | 50.4 | 56.9 | 44.1 | 53.9 | 44.7 | 0.0005 |
| MMSE | 22.4 (4.6) |
23.7 (4.2) |
23.6 (5.7) |
23.9 (4.5) |
29.1 (1.2) |
<0.0001 |
| CDR-SUM | 4.9 (2.7) |
4.9 (2.9) |
4.6 (3.2) |
4.8 (3.0) |
0.0* (0.2) |
<0.0001 |
| Number of visits | 4.0 (1.2) |
3.6 (1.0) |
3.7 (1.0) |
3.5 (0.7) |
5.2 (2.0) |
<0.0001 |
| Months between first and last visit | 40.1 (17.0) |
35.6 (13.7) |
37.7 (16.9) |
36.9 (12.0) |
58.8 (25.8) |
<0.0001 |
: due to rounding, percentage is 0.0
: p-value calculated using analysis of variance (ANOVA) for continuous variables and test for difference of proportions for categorical variables across all the groups
At baseline, the FTD group was significantly younger on average compared to the other four diagnosis groups. The DLB group had a significantly lower percent of females compared to normal and AD patients, which was expected as DLB is noted to occur more frequently in men [38, 39]. AD patients had significantly lower education levels compared to normal patients, although they had comparable education levels to DLB, FTD, and VaD patients. AD and DLB patients had the lowest BMI at baseline. In addition, patients with a diagnosis of VaD had smoked significantly longer than normal patients at the first visit. VaD patients had the most frequent diagnoses of recent/active diabetes and hypertension, and one of the most frequent hypercholesterolemia diagnoses, which were expected by definition of the VaD subtype. MMSE and CDR-SUM values at baseline were similar for the four dementia subtypes evaluated, although as expected, the average MMSE score at baseline was significantly higher for the normal group and the average CDR-SUM score at baseline was significantly lower for the normal group.
3.2. Relationship of Cardiovascular Risk Factors to Cognitive Score Across Dementia Subtypes
A summary of the results from each of the linear mixed models, one for each diagnosis of interest, are displayed in Tables 2 and 3 for MMSE and CDR-SUM, respectively. The VaD group had a small sample size (n=13), thus the results should be interpreted carefully as the findings may not be applicable to larger populations. Significant cardiovascular risk factors are listed in bold. The estimate displayed in the table is the predicted change in cognitive score per month for a change in the cardiovascular risk factor status. For MMSE, a positive estimate indicates that an increase in the continuous variables (BMI and years smoking) or a recent/active status for the categorical variables (atrial fibrillation, diabetes, hypertension, and hypercholesterolemia) is associated with an increase in MMSE and thus an increase in cognitive functioning. For CDR-SUM, negative estimates indicate associations with increased cognitive functioning.
Table 2.
Model results for the impact of cardiovascular risk factors (CRF) on the change in Mini-Mental State Examination (MMSE) per month for each of the diagnoses of interest.
| AD n=1899, nt=7519 |
DLB n=65, nt=236 |
FTD n=168, nt=626 |
VaD** n=13, nt=45 |
Normal n=3583, nt=18468 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRF | Est. | SE | p-value | Est. | SE | p-value | Est. | SE | p-value | Est. | SE | p-value | Est. | SE | p-value |
| BMI | 0.0006 | 0.0005 | 0.2294 | −0.0027 | 0.0045 | 0.5514 | 0.0092 | 0.0023 | <0.0001 | −0.0016 | 0.0042 | 0.6975 | 0.0001 | 0.0001 | 0.3265 |
| Years Smoking | 0.0002 | 0.0001 | 0.2048 | 0.0029 | 0.0012 | 0.0144 | 0.0023 | 0.0006 | 0.0003 | 0.0015 | 0.0013 | 0.2496 | 0.0000* | 0.0000* | 0.4473 |
| Atrial Fibrillation | 0.0069 | 0.0093 | 0.4569 | −0.0643 | 0.0632 | 0.3096 | 0.0954 | 0.0400 | 0.0174 | −0.0675 | 0.1624 | 0.6811 | −0.0010 | 0.0011 | 0.3980 |
| Diabetes | 0.0081 | 0.0070 | 0.2492 | 0.0740 | 0.0892 | 0.4074 | 0.0463 | 0.0348 | 0.1836 | −0.0068 | 0.0661 | 0.9194 | 0.0014 | 0.0009 | 0.1297 |
| Hypertension | 0.0177 | 0.0044 | <0.0001 | 0.0235 | 0.0318 | 0.4618 | −0.0384 | 0.0253 | 0.1300 | 0.0094 | 0.1383 | 0.9465 | −0.0027 | 0.0006 | <0.0001 |
| Hyperchol. | 0.0100 | 0.0044 | 0.0241 | −0.0447 | 0.0301 | 0.1393 | 0.0050 | 0.0230 | 0.8271 | −0.0313 | 0.0958 | 0.7467 | 0.0001 | 0.0005 | 0.8885 |
n=number of subjects, nt=total number of visits/observations
: due to rounding, estimate (Est.) or standard error (SE) is 0.00
: due to a small sample size, caution should be used in interpreting the results.
Table 3.
Model results for the impact of cardiovascular risk factors (CRF) on the change in Clinical Dementia Rating Sum of Boxes (CDR-SUM) per month for each of the diagnoses of interest.
| AD n=1899, nt=7519 |
DLB n=65, nt=236 |
FTD n=168, nt=626 |
VaD** n=13, nt=45 |
Normal n=3583, nt=18468 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRF | Est. | SE | p-value | Est. | SE | p-value | Est. | SE | p-value | Est. | SE | p-value | Est. | SE | p-value |
| BMI | −0.0005 | 0.0003 | 0.0816 | −0.0024 | 0.0026 | 0.3575 | −0.0034 | 0.0013 | 0.0126 | −0.0025 | 0.0025 | 0.3227 | 0.0000* | 0.0000* | 0.8975 |
| Years Smoking | −0.0001 | 0.0001 | 0.1295 | −0.0005 | 0.0007 | 0.4486 | −0.0008 | 0.0004 | 0.0288 | 0.0009 | 0.0008 | 0.2404 | 0.0000* | 0.0000* | 0.3073 |
| Atrial Fibrillation | −0.0016 | 0.0059 | 0.7848 | −0.0070 | 0.0368 | 0.8495 | −0.0406 | 0.0234 | 0.0838 | −0.0032 | 0.0965 | 0.9739 | 0.0001 | 0.0002 | 0.5026 |
| Diabetes | 0.0019 | 0.0044 | 0.6647 | −0.0648 | 0.0521 | 0.2154 | −0.0250 | 0.0204 | 0.2210 | 0.0932 | 0.0399 | 0.0279 | −0.0001 | 0.0002 | 0.6964 |
| Hypertension | −0.0109 | 0.0028 | 0.0001 | −0.0036 | 0.0184 | 0.8436 | 0.0019 | 0.0149 | 0.8967 | 0.0205 | 0.0789 | 0.7972 | 0.0002 | 0.0001 | 0.0661 |
| Hyperchol. | −0.0061 | 0.0028 | 0.0294 | 0.0521 | 0.0175 | 0.0032 | −0.0155 | 0.0136 | 0.2546 | 0.0240 | 0.0527 | 0.6534 | −0.0001 | 0.0001 | 0.2466 |
n=number of subjects, nt=total number of visits/observations
: due to rounding, estimate (Est.) or standard error (SE) is 0.00
: due to a small sample size, caution should be used in interpreting the results.
3.2.1. Mini-Mental State Examination Analysis Results
For this sample of patients, select cardiovascular risk factors were found to be significantly associated with a change in MMSE for individuals with a diagnosis of AD, DLB, FTD, or normal. In the group with a diagnosis of AD, having recent or active hypertension resulted in a significant increase in cognitive score per month (p-value < 0.0001). In addition, recent or active hypercholesterolemia was associated with a significant monthly increase in MMSE score (p-value = 0.0241). For patients with a DLB diagnosis, the data suggests that years smoking is associated with an increase in MMSE score for each additional year the patient smoked (p-value = 0.0144). For patients with FTD, BMI, years smoking, and recent/active atrial fibrillation were all significantly associated with an increase in MMSE score each month. In the normal group, recent/active hypertension was associated with a decline in MMSE score. Baseline MMSE score was significantly positively associated with the patient’s MMSE scores over the course of the study for all diagnoses except VaD. The demographic variables (age, sex, race, and education) were also found to be significant in some of the generalized linear mixed models. Age was a significant predictor in the AD mixed model and was positively associated with the overall MMSE score. All four demographic variables were statistically significant in the model for the normal diagnosis group. For the normal diagnosis, age was negatively associated, education was positively associated, and females had higher MMSE scores relative to males. Caucasians had higher MMSE scores compared to other races (African American, American Indian, Hawaiian/Pacific Islanders, Asian, and multiracial). The random intercept at the subject, or patient, level was statistically significant in four models (AD, DLB, FTD, and the normal diagnosis). This indicates significant correlation between the measurements collected from the same patient. The random effect for ADC, accounting for similarities between MMSE measurements collected at the same clinic, was statistically significant in the models for patients with a diagnosis of AD and the normal group.
Figure 2 demonstrates the differing impact of hypertension on MMSE score over time for the four dementia subtypes and the normal group (denoted by black lines). Each score is evaluated using the average demographic characteristics of patients from the longitudinal study at baseline. As seen from the plot, a diagnosis of recent/active hypertension (solid line) results in a more rapid decline in MMSE over time for the FTD (Fig 2c) and normal subgroups (Fig 2e), although the association is only significant for the normal group. However, recent/active hypertension was positively associated with cognition for the AD (Fig 2a) and DLB (Fig 2b) subgroups, as can be seen by the slower cognitive decline over time (the effect is only significant for AD patients). This plot reveals the significantly different cognitive functioning patients with varying dementia diagnoses may have over five years given the presence of cardiovascular risk factors.
Figure 2.
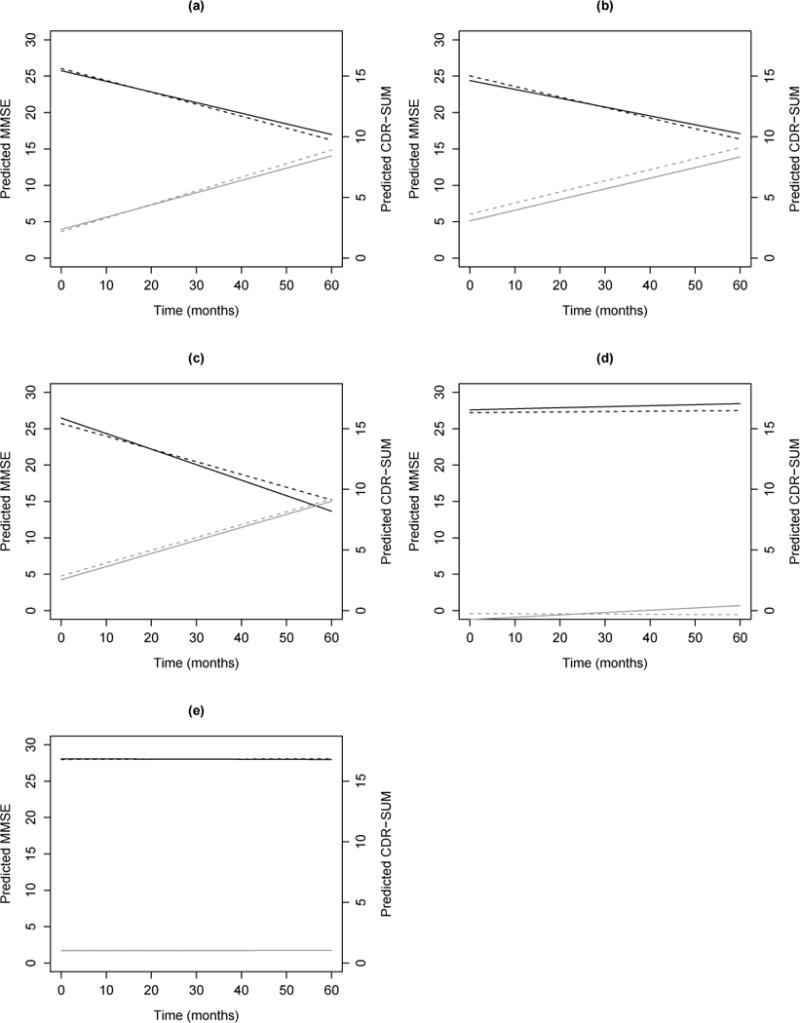
Plots of Predicted Mini-Mental State Examination (MMSE, black) and Clinical Dementia Rating Sum of Boxes (CDR-SUM, gray) scores vs time in months since baseline for patients with recent/active hypertension (solid lines) vs. remote/inactive/absent hypertension (dashed lines) for (a) Alzheimer’s disease (AD), (b) dementia with Lewy bodies (DLB), (c) frontotemporal dementia (FTD), (d) vascular dementia (VaD), and (e) normal patients. Predictions for MMSE and CDR-SUM scores based on average demographic characteristics of patients from the longitudinal sample at baseline: white female, age 71.7, 15.4 years of education, BMI of 27.0, smoked for 9.7 years, with remote/inactive/absent atrial fibrillation, diabetes, and hypercholesterolemia.
3.2.2. Clinical Dementia Rating Sum of Boxes Analysis Results
For the CDR-SUM outcome, cardiovascular risk factors were found to be significantly associated with a change in cognitive score over time for patients with a diagnosis of AD, DLB, FTD, or VaD. For AD patients, recent/active hypertension and recent/active hypercholesterolemia were associated with a decrease in CDR-SUM score per month (p-values = 0.0001 and 0.0294, respectively), which was an increase in cognition. Patients diagnosed with DLB showed a decrease in cognition per month which was significantly associated with recent or active hypercholesterolemia (p-value = 0.0032). For FTD patients, BMI and years smoking were significantly associated with a decrease in CDR-SUM each month. In the VaD group, recent/active diabetes was associated with a significant increase in CDR-SUM per month, which resulted in a decrease in cognition. The baseline CDR-SUM score was positively associated with future CDR-SUM scores for all diagnoses. The demographic variables (age, sex, race, and education) were also found to be significant in some of the generalized linear mixed models. In the AD mixed model, age was negatively associated with CDR-SUM score. None of the demographic variables were significantly associated with CDR-SUM for DLB and FTD patients. For VaD patients, race was significantly associated to CDR-SUM. African Americans and multiracial individuals had higher CDR-SUM scores compared to Caucasians. All four demographic variables were statistically significant in the model for the normal diagnosis group. Age was positively associated to CDR-SUM while years of education was negatively associated. Females had lower CDR-SUM scores, and thus higher cognitive functioning, compared to males. In the normal group, Caucasians had significantly lower CDR-SUM scores than American Indians/Alaska Natives. The patient level random intercept was statistically significant in the AD, DLB, FTD, and normal diagnosis models. The ADC random effect was statistically significant in the AD and normal group models.
Figure 2 also displays the impact of hypertension on CDR-SUM scores over time (denoted by gray lines), given their dementia diagnosis (including a normal diagnosis). The predicted cognitive scores are evaluated using the average demographic characteristics at baseline. Although the predicted CDR-SUM scores are close, it can be seen that a diagnosis of recent/active hypertension has a slower rate of cognitive decline for AD (Fig 2a) and DLB patients (Fig 2b). This association is significant for AD patients. On the other hand, for patients diagnosed with FTD (Fig 2c), VaD (Fig 2d), or as normal (Fig 2e), recent/active hypertension appears to be associated with faster cognitive decline, although none of these associations are statistically significant.
3.2.3. Co-occurrences of Cardiovascular Risk Factors
At baseline, the most common co-occurrences of two cardiovascular risk factors in the longitudinal dataset included recent/active hypertension and hypercholesterolemia (28.0 percent), hypercholesterolemia and smoking more than 10 years (16.1 percent), hypertension and smoking more than 10 years (15.9 percent), hypertension and BMI greater than 30 (14.2 percent), and hypercholesterolemia and BMI greater than 30 (12.1 percent). Table 4 displays the most frequent cardiovascular factors that co-occurred by disease diagnosis. For all diagnoses, including the four dementia types and the normal group, recent or active hypertension and recent or active hypercholesterolemia were the two cardiovascular risk factors that most frequently co-occurred at baseline.
Table 4.
Most frequent co-occurrences of cardiovascular risk factors at baseline by diagnosis.
| AD (n=1899) | ||||||
|---|---|---|---|---|---|---|
| BMI > 30 | Years Smoking > 10 | Atrial Fibrillation | Diabetes | Hypertension | Hyperchol. | Frequency, % |
| X | X | 30.8 | ||||
| X | X | 17.8 | ||||
| X | X | 16.5 | ||||
| X | X | X | 11.7 | |||
| X | X | 10.2 | ||||
| DLB (n=65) | ||||||
| BMI > 30 | Years Smoking > 10 | Atrial Fibrillation | Diabetes | Hypertension | Hyperchol. | Frequency, % |
| X | X | 29.2 | ||||
| X | X | 27.7 | ||||
| X | X | 20.0 | ||||
| X | X | 16.9 | ||||
| X | X | X | 15.4 | |||
| FTD (n=168) | ||||||
| BMI > 30 | Years Smoking > 10 | Atrial Fibrillation | Diabetes | Hypertension | Hyperchol. | Frequency, % |
| X | X | 24.4 | ||||
| X | X | 17.3 | ||||
| X | X | 14.3 | ||||
| X | X | 13.7 | ||||
| X | X | 13.1 | ||||
| VaD (n=13) | ||||||
| BMI > 30 | Years Smoking > 10 | Atrial Fibrillation | Diabetes | Hypertension | Hyperchol. | Frequency, % |
| X | X | 53.9 | ||||
| X | X | 46.2 | ||||
| X | X | 30.8 | ||||
| X | X | 23.1 | ||||
| X | X | 23.1 | ||||
| X | X | X | 23.1 | |||
| X | X | X | 23.1 | |||
| X | X | X | 23.1 | |||
| Normal (n=3583) | ||||||
| BMI > 30 | Years Smoking > 10 | Atrial Fibrillation | Diabetes | Hypertension | Hyperchol. | Frequency, % |
| X | X | 26.6 | ||||
| X | X | 16.4 | ||||
| X | X | 15.6 | ||||
| X | X | 14.9 | ||||
| X | X | 13.4 | ||||
4. DISCUSSION
In a sample of patients from the NACC UDS with diagnoses of AD, DLB, FTD, VaD, and normal, we evaluated the differential associations of cardiovascular risk factors and cognitive score, measured using MMSE and CDR-SUM. The analyses indicate select cardiovascular factors are significantly associated with cognitive decline for select dementia subtypes. Four associations between changes in cognition and cardiovascular risk factors were identified that were in agreement between both the MMSE and CDR-SUM measures. In the AD group, recent/active hypertension and recent/active hypercholesterolemia were both found to be associated with higher cognitive functioning. For FTD patients, an increase in BMI or an increase in the number of years smoking were both associated with higher cognition. Additional associations were also identified for each cognitive measure as mentioned in the results.
Our findings are in agreement with existing studies that have found a significant association between hypertension and hypercholesterolemia and increased cognitive scores, using MMSE, for patients with AD [8, 40]. While Bergland’s study [8] shows mild significance of these two cardiovascular risk factors for AD patients, our study indicates a highly statistically significant association with the rate of change in cognition. Although a previous study found increased systolic blood pressure to be associated with a higher rate of cognitive decline in patients with AD [41], we found that the presence of hypertension was associated with a smaller decrease in cognitive scores for this subtype. These differences may be due to differences in study cohorts as well as methodologies used. Furthermore, many individuals with hypertension may not be optimally treated [42] potentially creating distinct subgroups (treated vs. untreated). This is imperative as treatment for high blood pressure has been noted to decrease cognitive decline in patients with cerebrovascular disease [43]; more research is needed to understand these effects across dementia subtypes.
Our study also identifies associations between cardiovascular risk factors and changes in cognition for the FTD subtype (BMI, years smoked, and atrial fibrillation). While previous studies on the FTD dementia subtype are limited, studies have demonstrated an association between increased BMI and a lower risk of cognitive decline [44]. We similarly found a higher BMI in late life was associated with an increase in MMSE scores or a decrease in CDR-SUM, with statistically significant associations using both measures. From a mechanistic standpoint, the association of increased BMI with less cognitive impairment may be related to glucose metabolism, which is disrupted in mild cognitive impairment and dementia [48, 49] or may be related to changes in body composition which in turn alters many physiological processes [44]. More research is needed to develop a causal link.
For the DLB subtype, we found that number of years smoked was associated with a significant increase in MMSE score per month although we did not identify an association between years smoked and CDR-SUM. This result agrees with a previous study which also investigated both cognitive measures and only found a statistically significant association between smoking and change in MMSE [8]. Many previous studies have indicated that smoking may have a protective effect from Parkinson’s disease [50–54], which is consistent with the association between smoking and the cognitive rate of change measured using MMSE. This association may be explained by select underlying physiological processes as mentioned in previous interpretations of similar findings including reduction of the enzyme monoamine B oxidase, interaction with glutathione S-transferases, or induction of cytochrome P-450 [8].
For the VaD subgroup, diabetes was identified to be significantly associated to an increase in the change of CDR-SUM over time, although other associations between cardiovascular risk factors and cognitive decline were not identified. While the VaD group had a small sample size (n=13) and should be assessed carefully, there are several factors that could contribute to a lack of associations between cardiovascular risk factors and cognitive decline for VaD patients. By definition, VaD patients have been previously diagnosed with cardiovascular risk factors and over time the factors may fall within the remote/inactive category. Moreover, VaD is not a progressive disease, unlike AD, DLB, and FTD and studies have noted less of a cognitive decline in VaD when compared to AD [55]. Recent studies have also shown that VaD patients often have mixed dementia [56], which the current study excluded. In addition, individual cardiovascular risk factors may not contribute to cognitive decline in VaD patients as these factors define a VaD diagnosis. Since cardiovascular disease is very common in VaD patients, it is difficult to identify the impact of individual cardiovascular risk factors. In this study, 85.7 percent of patients had recent/active hypertension and 64.3 percent of patients had recent/active hypercholesterolemia (Table 1). Since patients with VaD often have these cardiovascular risk factors, we do not suspect changes should have a significant impact on cognitive decline but due to limitations in our sample size, this should be further investigated.
Although MMSE and CDR-SUM are both measures of cognition, the differing associations between cardiovascular risk factors and each measure can be expected. A study evaluating 19 different cognitive tests to assess a range of cognitive abilities was performed and it was found that the rates of change in cognitive score varied depending on the test [57]. MMSE is considered more of a cognitive assessment, while CDR-SUM is more of a global measure as it assesses skills other than memory and cognition. The scales for both measures vary greatly as well. Previous comparisons between the two measures found that while MMSE and CDR-SUM can generally capture similar levels of dementia severity, CDR-SUM is more precise in measuring cognitive decline [58, 59].
In this study, we identify several associations between cardiovascular risk factors and the rate of cognitive decline. While we examine each factor independently to identify cardiovascular risk factors that may have the most influence, co-occurrences of risk factors can have varying impacts on the rate of decline. For patients in our study, recent or active hypertension and hypercholesterolemia most frequently co-occurred at baseline for all dementia diagnoses and the normal subgroup, as shown in Table 4. In the NACC data, hypertension and hypercholesterolemia were both associated with a smaller decline in cognition for AD patients, while the two cardiovascular risk factors had opposing effects for other disease diagnoses. For patients with both hypertension and hypercholesterolemia, the predicted monthly rate of change would be a net decline for diagnoses of DLB, VaD and normal. In our study, the change in cognition for FTD patients with both of these cardiovascular risk factors had conflicting results between MMSE and CDR-SUM. As co-occurrences of cardiovascular risk factors are common and other studies have determined that the aggregation of vascular risk factors can be associated with a higher risk of disease [60], we recommend future studies investigate the impact of aggregated risk factors should be further investigated on cognitive change.
As clinical diagnosis accuracy rates can vary, one might evaluate post-mortem data to understand the underlying pathology and the corresponding associations. Autopsy data was available for 692 patients in the longitudinal sample (approximately 12.1 percent of patients). Of these subjects, a primary diagnosis was determined for 416 patients. Using the post mortem diagnosis, nearly 67 percent of the 416 patients corresponded directly to the clinical diagnosis. Of the patients with conflicting primary diagnoses, most had been classified as AD or normal in the NACC data but during the autopsy had been diagnosed with a disease other than those evaluated in this study, including mixed diagnoses. On average, the patients with post mortem diagnoses (n=416) died 19.2 months after their last visit to the ADC (min = 0 months, max = 71 months). It is possible that patients who were considered part of the normal group during the study later developed dementia due to the length of time between the last visit and death.
Using the NACC UDS as the source of data provides multiple strengths for this study, including standardized protocols for data collection across the ADCs as well as a large number of patients, which results in reliable statistical models and estimates. In addition, the study design is longitudinal, across 34 ADCs in the United States, which includes many different patients with mean follow-up times of 36 months (for the DLB group) or longer. The evaluation of cardiovascular factors across multiple dementia subtypes allows for flexibility as vascular risk factors may be associated with only select neurodegenerative diseases.
The present study does have some limitations. We had very stringent inclusion criteria, including having only one consistent dementia diagnosis over the course of all visits to reduce confounding variables. The authors are aware that most dementias are that of a mixed etiology which is revealed at the time of a post-mortem neuropathological evaluation [61, 62]. Furthermore, while the NACC database overall contains many patients, most patients in the subset of data selected for the study had a diagnosis of AD or normal. The DLB, FTD, and VaD subtypes had much smaller sample sizes and should be further analyzed in subsequent analyses. In addition, some visits were missing the MMSE value or contained invalid values and were excluded. These missing datapoints may obscure important changes in the MMSE value or associations with the cognitive score and can create bias. Categorical cardiovascular risk factors evaluated, including atrial fibrillation, diabetes, hypertension, and hypercholesterolemia, varied for some of the patients as cardiovascular diseases developed or became inactive during the study. Of the sample evaluated, 5.8 percent of patients had a change in atrial fibrillation status, 4.1 percent had a change in diabetes status, 16.2 percent had a change in hypertension status, and 21.7 percent had a change in hypercholesterolemia status during the study. Some patients had multiple changes in status for these variables. Across the four categorical cardiovascular risk factors, 42.2 percent of the statuses switched from absent to recent/active and 17.4 percent switched from remote/inactive to recent/active, which is expected as the population ages over the duration of the study and develops cardiovascular diseases. In 5.3 percent of status changes, the cardiovascular risk factor status switched from absent to remote/inactive. In 35.0 percent of cases, patients had a cardiovascular risk factor change from recent/active to remote/inactive. There was one case of a patient changing from remote/inactive atrial fibrillation to the status absent, although this is likely an error in the data. Although we accounted for changing statuses rather than assigning each patient one cardiovascular risk factor status at the beginning of study, follow up studies should be performed to understand the impact on cognition when a cardiovascular risk factor becomes present after the onset of dementia. Moreover, cardiovascular risk factors may affect cognition differently based on the length of time and the age the patient acquired the condition [10, 63–66]. In addition, this study focuses on a select subset of cardiovascular risk factors. A recent study has suggested that autonomic dysfunction, measured using heart rate variability, may also be associated with dementia and cognitive impairment [67]. Follow up investigations should further evaluate this condition as well as other cardiovascular risk factors.
This study investigated multiple dementia subtypes, as well as a non-demented group, and enhanced previous studies of this type. We identified clear associations between cardiovascular risk factors and the rate of cognitive decline for patients with diagnoses of AD, DLB, FTD and normal. This study indicates the necessity of evaluating cardiovascular risk factors to understand dementia patient outcomes and identified other potential risk factors including demographic characteristics such as age, sex, race, and education that should be further investigated in future studies.
Supplementary Material
Acknowledgments
Source of Funding:
KI has received funding support from the National Science Foundation, Grant No. DGE-1311230.
BD has received funding support from grants AG002132 (Core C) (PI: Dugger) from the National Institutes of Health, as well as the CurePSP foundation (PI: Dugger), the Alzheimer’s Association (PI: Dugger), the Henry M. Jackson Foundation (HU0001-15-2-0020, PI: Prusiner), and Daiichi Sankyo Co., Ltd. (PI: Prusiner).
JW has received funding support from the National Institute of Health Alzheimer’s Consortium Fellowship Grant, Grant No. NHS0007.
The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest pertaining to this study.
References
- 1.Beeri M, Ravona-Springer R, Silverman J, Haroutunian V. The effects of cardiovascular risk factors on cognitive compromise. Dialogues Clin Neurosci. 2009;11:201–12. doi: 10.31887/DCNS.2009.11.2/msbeeri. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monsell S, Liu D, Weintraub S, Kukull W. Comparing measures of decline to dementia in amnestic MCI subjects in the National Alzheimer’s Coordinating Center (NACC) Uniform Data Set. Int Psychogeriatr. 2012;24:1553–60. doi: 10.1017/S1041610212000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dregan A, Stewart R, Gulliford M. Cardiovascular risk factors and cognitive decline in adults aged 50 and over: a population-based cohort study. Age Ageing. 2013;42:338–45. doi: 10.1093/ageing/afs166. [DOI] [PubMed] [Google Scholar]
- 4.Gill D, Hubbard R, Koepsell T, Borrie M, Petrella R, Knopman D, et al. Differences in rate of functional decline across three dementia types. Alzheimers Dement. 2013;9(5 Suppl):S63–71. doi: 10.1016/j.jalz.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pillai J, Butler R, Bonner-Jackson A, Leverenz J. Impact of Alzheimer’s Disease, Lewy Body and Vascular Co-Pathologies on Clinical Transition to Dementia in a National Autopsy Cohort. Dement Geriatr Cogn Disord. 2016;42:106–16. doi: 10.1159/000448243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenowitz W, Hubbard R, Keene C, Hawes S, Longstreth WJ, Woltjer R, et al. Mixed neuropathies and estimated rates of clinical progression in a large autopsy sample. Alzheimers Dement. 2016 doi: 10.1016/j.jalz.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dugger B, Malek-Ahmadi M, Monsell S, Kukull W, Woodruff B, Reiman E, et al. A Cross-Sectional Analysis of Late-Life Cardiovascular Factors and Their Relation to Clinical Defined Neurodegenerative Diseases. Alzheimer Dis Assoc Disord. 2016;30:223–9. doi: 10.1097/WAD.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergland A, Dalen I, Larsen A, Aarsland D, Soennesyn H. Effect of Vascular Risk Factors on the Progression of Mild Alzheimer’s Disease and Lewy Body Dementia. J Alzheimers Dis. 2017;56:575–84. doi: 10.3233/JAD-160847. [DOI] [PubMed] [Google Scholar]
- 9.White H, Pieper C, Schmader K. The association of weight change in Alzheimer’s disease with severity of disease and mortality: a longitudinal analysis. J Am Geriatr Soc. 1998;46:1223–7. doi: 10.1111/j.1532-5415.1998.tb04537.x. [DOI] [PubMed] [Google Scholar]
- 10.Posner H, Tang M, Luchsinger J, Lantigua R, Stern Y, Mayeux R. The relationship of hypertension in the elderly to AD, vascular dementia, and cognitive function. Neurology. 2002;58:1175–81. doi: 10.1212/wnl.58.8.1175. [DOI] [PubMed] [Google Scholar]
- 11.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163:1524–8. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 12.Buchman A, Wilson R, Bienias J, Shah R, Evans D, Bennett D. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65:892–7. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 13.Mielke M, Zandi P, Sjogren M, Gustafson D, Ostling S, Steen B, et al. High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology. 2005;64:1689–95. doi: 10.1212/01.WNL.0000161870.78572.A5. [DOI] [PubMed] [Google Scholar]
- 14.Stewart R, Masaki K, Xue Q, Peila R, Petrovitch H, White L, et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005;62:55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 15.Johnson D, Wilkins C, Morris J. Accelerated weight loss may precede diagnosis in Alzheimer disease. Arch Neurol. 2006;63:1312–7. doi: 10.1001/archneur.63.9.1312. [DOI] [PubMed] [Google Scholar]
- 16.Rosano C, Newman A. Cardiovascular disease and risk of Alzheimer’s disease. Neurol Res. 2006;28:612–20. doi: 10.1179/016164106X130407. [DOI] [PubMed] [Google Scholar]
- 17.Rosendorff C, Beeri M, Silverman J. Cardiovascular risk factors for Alzheimer’s disease. Am J Geriatr Cardiol. 2007;16:143–9. doi: 10.1111/j.1076-7460.2007.06696.x. [DOI] [PubMed] [Google Scholar]
- 18.Ettorre E, Cicerchia M, De Benedetto G, Fossati C, Guglielmi S, Manzon L, et al. A possible role of atrial fibrillation as a risk factor for dementia. Arch Gerontol Geriatr. 2009;49(Suppl 1):71–6. doi: 10.1016/j.archger.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Fitzpatrick A, Kuller L, Lopez O, Diehr P, O’Meara E, Longstreth WJ, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336–42. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naderali E, Ratcliffe S, Dale M. Obesity and Alzheimer’s disease: a link between body weight and cognitive function in old age. Am J Alzheimers Dis Other Demen. 2009;24:445–9. doi: 10.1177/1533317509348208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anstey K, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev. 2011;12:e426–37. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 22.Gorelick P, Scuteri A, Black S, Decarli C, Greenberg S, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thacker E, McKnight B, Psaty B, Longstreth WJ, Dublin S, Jensen P, et al. Association of body mass index, diabetes, hypertension, and blood pressure levels with risk of permanent atrial fibrillation. J Gen Intern Med. 2013;28:247–53. doi: 10.1007/s11606-012-2220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Besser L, Gill D, Monsell S, Brenowitz W, Meranus D, Kukull W, et al. Body mass index, weight change, and clinical progression in mild cognitive impairment and Alzheimer disease. Alzheimer Dis Assoc Disord. 2014;28:36–43. doi: 10.1097/WAD.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu Y, Scarmeas N, Cosentino S, Brandt J, Albert M, Blacker D, et al. Change in body mass index before and after Alzheimer’s disease onset. Curr Alzheimer Res. 2014;11:349–56. doi: 10.2174/1567205010666131120110930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tolppanen A, Ngandu T, Kareholt I, Laatikainen T, Rusanen M, Soininen H, et al. Midlife and late-life body mass index and late-life dementia: results from a prospective population-based cohort. J Alzheimers Dis. 2014;38:201–9. doi: 10.3233/JAD-130698. [DOI] [PubMed] [Google Scholar]
- 27.Emmerzaal T, Kiliaan A, Gustafson D. 2003-2013: a decade of body mass index, Alzheimer’s disease, and dementia. J Alzheimers Dis. 2015;43:739–55. doi: 10.3233/JAD-141086. [DOI] [PubMed] [Google Scholar]
- 28.Sposato L, Vargas E, Riccio P, Toledo J, Trojanowski J, Kukull W, et al. Milder Alzheimer’s disease pathology in heart failure and atrial fibrillation. Alzheimers Dement. 2017:S1552–5260. 33122–3. doi: 10.1016/j.jalz.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedrinolla A, Schena F, Venturelli M. Resilience to Alzheimer’s disease: the role of physical activity. Curr Alzheimer Res. 2017 doi: 10.2174/1567205014666170111145817. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Casado A, Toledano-Diaz A, Toledano A. Defective Insulin Signalling, Mediated by Inflammation, Connects Obesity to Alzheimer Disease; Relevant Pharmacological Therapies and Preventive Dietary Interventions. Curr Alzheimer Res. 2017;14:894–911. doi: 10.2174/1567205014666170316161848. [DOI] [PubMed] [Google Scholar]
- 31.Folstein M, Folstein S, McHugh P. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 32.Hughes C, Berg L, Danziger W, Coben L, Martin R. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–72. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 33.O’Bryant S, Waring S, Cullum C, Hall J, Lacritz L, Massman P, et al. Staging Dementia Using Clincial Dementia Rating Scale Sum of Boxes Scores. Arch Neurol. 2008;65:1091–5. doi: 10.1001/archneur.65.8.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris J, Weintraub S, Chui H, Cummings J, DeCarli C, Ferris S, et al. The Uniform Data Set (UDS): Clinical and Cognitive Variables and Descriptive Data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–6. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 35.Beekly D, Ramos E, Lee W, Deitrich W, Jacka M, Wu J, et al. The National Alzheimer’s Coordinating Center (NACC) Database: The Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21:249–58. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 36.Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford N, Chui H, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): The Neuropsychologic Test Battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKhann G, Knopman D, Chertkow H, Hyman B, Jack C, Kawas C, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations form the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson P, Schmitt F, Jicha G, Kryscio R, Abner E, Smith C, et al. Association between male gender and cortical Lewy body pathology in large autopsy series. J Neurol. 2010;257:1875–81. doi: 10.1007/s00415-010-5630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dugger B, Boeve B, Murray M, Parisi J, Fujishiro H, Dickson D, et al. Rapid eye movement sleep behavior disorder and subtypes in autopsy-confirmed dementia with Lewy bodies. Mov Disord. 2012;27:72–8. doi: 10.1002/mds.24003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldstein F, Ashley A, Endeshaw Y, Hanfelt J, Lah J, Levey A. Effects of Hypertension and Hypercholesterolemia on Cognitive Functioning in Patients with Alzheimer’s Disease. Alzheimer Dis Assoc Disord. 2008;22:336–42. doi: 10.1097/wad.0b013e318188e80d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blom K, Emmelot-Vonk M, Koek H. The influence of vascular risk factors on cognitive decline in patients with dementia: a systematic review. Maturitas. 2013;76:113–7. doi: 10.1016/j.maturitas.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Rodriguez CJ, Wang K. Prevalence and trends of isolated systolic hypertension among untreated adults in the United States. Journal of the American Society of Hypertension. 2015;9:197–205. doi: 10.1016/j.jash.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tzourio C, Anderson C, Chapman N, Woodward M, Neal B, MacMahon S, et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003;163:1069–75. doi: 10.1001/archinte.163.9.1069. [DOI] [PubMed] [Google Scholar]
- 44.Kim S, Kim Y, Park SM. Body Mass Index and Decline of Cognitive function. PLOS One. 2016 doi: 10.1371/journal.pone.0148908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawamura T, Umemura T, Hotta N. Cognitive impairment in diabetic patients: Can diabetic control prevent cognitive decline? J Diabetes Investig. 2012;3:413–23. doi: 10.1111/j.2040-1124.2012.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gasecki D, Kwarciany M, Nyka W, Narkiewicz K. Hypertension, Brain Damage and Cognitive Decline. Curr Hypertens Rep. 2013;15:547–58. doi: 10.1007/s11906-013-0398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalantarian S, Stern T, Mansour M, Ruskin J. Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann Intern Med. 2013;158:338–46. doi: 10.7326/0003-4819-158-5-201303050-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kantarci K, Senjem M, Lowe V, Wiste H, Weigand S, Kemp B, et al. Effects of age on the glucose metabolic changes in mild cognitive impairment. AJNR Am J Neuroradiol. 2010;31:1247–53. doi: 10.3174/ajnr.A2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging. 2005;32:486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 50.Jimenez-Jimenez F, Mateo D, Gimenez-Roldan S. Premorbid smoking, alcohol consumption, and coffee drinking habits in Parkinson’s disease: a case-control study. Mov Disord. 1992;7:339–44. doi: 10.1002/mds.870070407. [DOI] [PubMed] [Google Scholar]
- 51.Butterfield P, Valanis B, Spencer P, Lindeman C, Nutt J. Environmental antecedents of young-onset Parkinson’s disease. Neurology. 1993;43:1150–8. doi: 10.1212/wnl.43.6.1150. [DOI] [PubMed] [Google Scholar]
- 52.Mayeux R, Ting M-X, Marder K, Cote L, Stern Y. Smoking and Parkinson’s disease. Mov Disord. 1994;9:207–12. doi: 10.1002/mds.870090215. [DOI] [PubMed] [Google Scholar]
- 53.Morens D, Grandinetti A, Reed D, White L, Ross G. Cigarette smoking and protection from Parkinson’s disease: false association or etiologic clue? Neurology. 1995;45:1041–51. doi: 10.1212/wnl.45.6.1041. [DOI] [PubMed] [Google Scholar]
- 54.Gorell J, Rybicki B, Johnson C, Peterson E. Smoking and Parkinson’s disease: a dose-response relationship. Neurology. 1999;52:115–9. doi: 10.1212/wnl.52.1.115. [DOI] [PubMed] [Google Scholar]
- 55.Pilon M, Poulin S, Fortin M, Houde M, Verrt L, Bouchard R, et al. Differences in Rate of Cognitive Decline and Caregiver Burden between Alzheimer’s Disease and Vascular Dementia: a Retrospective Study. Neurology. 2016;2:278–86. [PMC free article] [PubMed] [Google Scholar]
- 56.Kapasi A, DeCarli C, Schneider J. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017 doi: 10.1007/s00401-017-1717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arvanitakis Z, Wilson R, Bienias J, Evans D, Bennett D. Diabetes Mellitus and Risk of Alzheimer Disease and Decline in Cognitive Function. Arch Neurol. 2004;61:661–6. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 58.Perneczky R, Wagenpfeil S, Komossa K, Grimmer T, Diehl J, Kurz A. Mapping scores onto stages: mini-mental state examination and clinical dementia rating. Am J Geriatr Psychiatry. 2006;14:139–44. doi: 10.1097/01.JGP.0000192478.82189.a8. [DOI] [PubMed] [Google Scholar]
- 59.Balsis S, Benge J, Lowe D, Geraci L, Doody R. How Do Scores on the ADAS-Cog, MMSE, and CDR-SOB Correspond? Clin Neruopsychol. 2015;29:1002–9. doi: 10.1080/13854046.2015.1119312. [DOI] [PubMed] [Google Scholar]
- 60.Luchsinger J, Reitz C, Honig L, Tang M, Shea S, R M. Aggregation of vascular risk fctors and risk of incident Alzheimer disease. Neurology. 2005;65:545–51. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schneider J, Arvanitakis Z, Bang W, Bennett D. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 62.Kawas C, Kim R, Sonnen J, Bullain S, Trieu T, Corrada M. Multiple pathologies are common and related to dementia in the oldest-old: The 90+ Study. Neurology. 2015;85:535–42. doi: 10.1212/WNL.0000000000001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Debette S, Seshadri S, Beiser A, Au R, Himali J, Palumbo C, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–8. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Launer L, Ross G, Petrovitch H, Masaki K, Foley D, White L, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 65.Elias M, Elias P, Sullivan L, Wolf P, D’Agostino R. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes Relat Metab Disord. 2003;27:260–8. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 66.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–99. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 67.Pereira da Silva V, Ribeiro Ramalho Oliveira B, Gomes Tavares Mello R, Moraes H, Camaz Deslandes A, Laks J. Heart Rate Variability Indexes in Dementia: A Systematic Review with a Quantitative Analysis. Curr Alzheimer Res. 2018;15:80–8. doi: 10.2174/1567205014666170531082352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


