Abstract
BACKGROUND
The ability to effectively cope with stress is a critical determinant of disease susceptibility. The lateral habenula (LHb) and the endocannabinoid (ECB) system have independently been shown to be involved in the selection of stress coping strategies, yet the role of ECB signaling in the LHb remains unknown.
METHODS
Using a battery of complementary techniques in rats, we examined the localization of type-1 cannabinoid receptors (CB1R) and assessed the behavioral and neuroendocrine effects of intra-LHb CB1R manipulations. We further tested the extent to which the ECB system in the LHb is impacted following chronic unpredictable stress (CUS) or social defeat stress (SDS), and whether manipulation of LHb CB1Rs can bias coping strategies in rats with a history of chronic stress.
RESULTS
Electron microscopy studies revealed CB1R expression on presynaptic axon terminals, postsynaptic membranes, mitochondria, and glial processes in the rat LHb. In vivo microdialysis experiments indicated that acute stress increased the amount of 2-arachidonoylglycerol (2-AG) in the LHb, while intra-LHb CB1R blockade increased basal corticosterone, augmented proactive coping strategies, and reduced anxiety-like behavior. Basal LHb 2-AG content was similarly elevated in rats subjected to CUS or SDS and positively correlated with adrenal weight. Finally, intra-LHb CB1R blockade increased proactive behaviors in response to a novel conspecific, increasing approach behaviors irrespective of stress history and decreasing the latency to be attacked during an agonistic encounter.
CONCLUSIONS
Alterations in LHb ECB signaling may be relevant for development of stress-related pathologies in which LHb dysfunction and stress coping impairments are hallmark symptoms.
Keywords: endocannabinoid, CB1 receptor, lateral habenula, stress coping, anxiety, rat
INTRODUCTION
In navigating stressful environments, all organisms must evaluate the potential risks of different response options and ultimately select the coping strategy that befits goal-directed actions and survival in the face of adversity (1). However, not all organisms cope with stress in the same manner. A significant body of literature indicates that coping styles can be dichotomized into proactive and reactive subtypes (2). Organisms that utilize proactive coping styles are more aggressive, less behaviorally flexible, show high sympathetic and emotional reactivity, and are more likely to engage in novelty seeking, risk taking, defensive postures, and/or escape-related behaviors (3–5). In contrast, organisms that utilize reactive coping styles are less aggressive, more risk averse, and favor avoidance rather than escape in situations of distress (3–5). These distinct coping styles may represent stable endophenotypes that underlie individual vulnerability to neuropsychiatric illnesses (6–7). Thus, a more complete understanding of the neural mechanisms that confer divergent stress coping strategies may help to identify populations that may be at risk for developing stress-related disorders.
The endocannabinoid (ECB) system fundamentally participates in the behavioral and neuroendocrine response to stress (8–11). Generally speaking, low-dose CB1R activation dampens the physiological stress response (12–13), engages proactive stress coping strategies (14–20), and increases the exploration of novel, potentially threatening environments (21–24), whereas CB1R blockade often does the opposite (12,24–26). Conversely, chronic stress causes widespread ECB disturbances that contribute to manifestation of depression-related symptoms in rodents (27). Accordingly, the ECB system has become an important focus for understanding the pathology and treatment of stress-related disorders (28).
The lateral habenula (LHb) has also emerged as an appealing therapeutic target, in part due to its unique position at the center of the mesocorticolimbic stress/reward circuit (29,30). The LHb integrates forebrain inputs from the basal ganglia and limbic system and sends coordinated output to midbrain dopamine (DA)-and serotonin (5-HT)-containing neurons in the ventral tegmental area (VTA) and dorsal raphe nucleus (DRN), respectively (31–35). Behaviorally, LHb activation facilitates avoidance (36), whereas LHb lesions reduce avoidance behaviors (37), augment proactive coping strategies (38,39), and promote exploration of anxiogenic environments (40). In the context of major depression, human studies have demonstrated pathological hyperactivity of the LHb (41,42), and deep brain stimulation of the LHb has emerged as a viable therapeutic approach in treatment-resistant patients (43). Importantly, similar observations have been reported following chronic stress exposure in rodents and in congenital learned helpless rats (44–52).
Given the therapeutic potential of targeting the ECB system and the LHb independently, it is surprising that few studies have examined the role of the ECB system in the LHb. Slice electrophysiology experiments have recently shown that the ECB system participates in long-term depression at excitatory LHb synapses (53). Moreover, acute stress impairs ECB-dependent plasticity in the LHb (54). These alterations in LHb ECB signaling could be important for the manifestation of depressive-like symptoms; however, the role of the ECB system in the LHb in vivo has yet to be studied and the effects of chronic stress on this system remain unknown. To this end, we employed a battery of complementary approaches to determine the anatomical localization and functional role of CB1Rs in the LHb under conditions of stress. Additionally, we examined the extent to which the LHb ECB system is affected following chronic unpredictable stress (CUS) or social defeat stress (SDS) exposure, and whether manipulation of LHb CB1Rs can bias coping strategies in rats with a history of chronic stress.
METHODS AND MATERIALS
A full description of all experimental procedures is provided in the Supplemental Materials.
Animals and Housing
Male and female Sprague Dawley rats (Simonsen Laboratories, Santa Clara, CA) arriving at 70 days of age were used for all experiments, except for microdialysis and SDS experiments where only male rats were used. Retired breeder male Long Evans rats were used as resident aggressors in SDS experiments. CB1R knockout (CB1R-KO) mice on a C57BL/6 N background were used to confirm CB1R immunolabeling (kindly provided by Dr. Giovanni Marsicano to Dr. Pedro Grandes). Animals were isolate-housed in a temperature-controlled (21 ± 2 °C) vivarium maintained on a 12-hr reverse light cycle with ad libitum food and water access. Estrous stage was determined in female rats daily via vaginal lavage and cytological identification (55). Animal care adhered to the National Research Council’s Guide for the Care and Use of Laboratory Animals (1996) and Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (2003), with all procedures approved by the Washington State University Institutional Animal Care and Use Committee, The Scripps Research Institute Institutional Animal Care and Use Committee, or the Committee of Ethics for Animal Welfare of the University of the Basque Country, where appropriate.
Drugs
Drugs were obtained from Tocris Biosciences Ltd. (Bristol, UK). The CB1R inverse agonist rimonabant (RIM) and the CB1R agonist WIN 55,212-2 (WIN) were dissolved in a 1:1:18 solution of dimethyl sulfoxide:Tween 80:0.9% saline (0.15 μg/0.1 μl and 0.5 μg/0.1 μl, respectively) (VEH).
Electron Microscopy
Coronal sections encompassing the LHb were obtained from CB1R-WT and CB1R-KO mice and a pre-embedding silver-intensified immunogold method was used for localization of the CB1R protein, as described (56). Details in Supplement.
In Vivo Microdialysis
A unilateral guide cannula was implanted above the right LHb as described in the Supplement (n=10). One week later, 16 hr prior to sample collection, a microdialysis probe with 1 mm active length polyethyl sulfone dialysis membrane and 15 kDa MW cutoff (MAB 6.14.2) was inserted and secured to the guide cannula (57). Rats were then subjected to 30 min of acute immobilization stress, dialysis samples were collected every 10 min for 180 min, and concentrations of AEA and 2-AG were analyzed using liquid chromatography tandem mass spectrometry (LC/MS/MS) as described previously (57).
Intracranial Surgery and Microinfusion Procedure
Rats were implanted with bilateral cannulae directly above the LHb as described (58) (see Supplemental Methods). On test days, internal cannulae (Plastics One, Roanoke, VA) extending 2 mm below the cannulae guides were inserted. Microinfusions occurred at a rate of 0.1 μl/min over 2 min. To maximize diffusion, the internal cannulae were kept in place for an additional 2 min after the infusion.
Behavioral Phenotyping
Separate cohorts of male and female rats (n=12-19/group) received intra-LHb microinfusions of WIN, RIM, or VEH. Fifteen min later, rats were subjected to one of four behavioral tests (acute restraint stress, forced swim test [FST], elevated plus maze [EPM], or novelty-suppressed feeding test [NSFT]). Details are provided in the Supplement. For rats subjected to acute restraint stress, blood samples (~100 μl) were collected via tail nick at 0, 30, 60, and 90 min following stress onset and serum corticosterone (CORT) concentration was analyzed in duplicate using radioimmunoassay kits (MP Biomedicals, Solon, OH; CV=2.56%) (59).
Chronic Unpredictable Stress Paradigm
Male and female rats (n=20-26/sex/group) were subjected to six weeks of chronic unpredictable stress (CUS) as described previously (58). Twenty-four hr after the final stressor, rats were decapitated and LHb tissue was harvested for analysis of ECB content, monoacylglycerol lipase (MAGL) activity, and CB1R binding. Weight gain and the number of days in estrus/proestrus were measured over the course of the experiment (Fig. S3). Adrenal and thymus glands were harvested and trunk blood was collected at the time of decapitation to assess physiological indices of CUS exposure.
Endocannabinoid Quantification
Lipids were extracted from LHb tissue samples using previously described methods (60) and AEA and 2-AG content were determined using LC-MS/MS (n=4-6/sex/group) (Details in Supplement).
MAGL Activity Assay
MAGL enzyme binding affinity (KM) and maximal hydrolytic activity (Vmax) were assessed using radiolabeled 2-oleoylglycerol and membranes that were prepared from LHb tissue as described (61) (n=5-6/sex/group) (Details in Supplement).
CB1R Binding Assay
CB1R binding assays were performed using a Multiscreen Filtration System with Durapore 1.2-μM filters as described previously (58) (n=5-6/sex/group) (Details in Supplement).
Social Defeat Stress Paradigm
Male rats were subjected to the resident-intruder social defeat stress (SDS) paradigm for seven days as described (62) (see Supplemental Material). Control rats received daily interactions with a novel, non-aggressive conspecific. Twenty-four hr after the last interaction, rats were decapitated, LHb AEA and 2-AG content was quantified, and LHb MAGL activity was assessed. Another group of rats (n=6-10/group) was implanted with cannulae above the LHb and then subjected to SDS or control conditions upon recovery. These rats received microinfusions of WIN, RIM, or VEH in their home cage 15 min prior to the final SDS (or control) interaction and behaviors during the interaction were scored (see Supplemental Methods).
In Vivo Electrophysiology
An additional cohort of rats was subjected to CUS, SDS or non-stress conditions (n=5/group) and single unit extracellular recordings of spontaneously active LHb neurons were performed 24 hr after the final stressor as described in the Supplemental Methods.
Statistical Analysis
Effects of treatment or stress exposure were analyzed using two-way analysis of variance (ANOVA), with sex and treatment or stress as between-group factors. Tukey’s post-hoc tests were used to probe significant interactions. Behavioral and neuroendocrine data in females were further analyzed with stage of estrous (proestrus, estrus, or diestrus) as a covariate. Since biochemical assays of MAGL activity and CB1R binding in males and females were run independently, these data were analyzed using independent samples t-tests. ECB microdialysis data were analyzed using repeated measures ANOVA, while CORT time course data, body weight gain, and estrous cycling were analyzed using a two-way between-within design with sex (or estrous stage) as the between factor and time as the within factor. Significance for all tests was set as p<0.05 and estimated effect sizes are reported as partial eta squared (ηp2) or Cohen’s d (d) for ANOVAs and t-tests, respectively.
RESULTS
The CB1R is Diversely Expressed in the Lateral Habenula
A high-resolution pre-embedding immunogold method for electron microscopy revealed that CB1Rs were localized on presynaptic axon boutons making asymmetric and symmetric synapses with dendrites in the LHb (Figure 1). Semi-quantification analysis showed that 18.58±1.25% of the total CB1R immunoparticles were in presynaptic terminal membranes; 8.82±0.60% of those particles were on synaptic profiles exhibiting characteristics of excitatory terminals with round and clear synaptic vesicles making asymmetric synapses; the rest of CB1R particles in presynaptic terminals (9.76±0.66%) were present in inhibitory terminals containing more pleomorphic vesicles and forming asymmetric synapses. Low levels of CB1R immunolabeling were also observed postsynaptically on dendritic membranes receiving synaptic terminals, as shown previously (63). 14.37±1.41% of the total CB1R particles were distributed on postsynaptic dendrites; 5.51±0.54% and 8.86±0.87% of those immunoparticles were on dendritic membranes that received excitatory or inhibitory synaptic terminals, respectively. CB1R immunoparticles on presynaptic terminals and postsynaptic dendrites were always outside the active zone and postsynaptic specialization of synapses (Figure 1). Furthermore, CB1R immunolabeling was also on glial cell membranes filling up the neuropil between neuronal elements. Indeed, the highest proportion of total CB1R labeling detected in the analyzed area of the LHb (65 μm2) was in glial processes (31.68±1.76%). There were also CB1R-positive immunoparticles located on mitochondrial membranes (26.46±1.69%). Importantly, CB1R immunolabeling was not observed in the LHb of CB1R-KO mice (Figure 1E), indicating that the CB1R antibody used was specific.
Figure 1. Type-1 cannabinoid receptors (CB1R) are present on presynaptic terminals, postsynaptic dendrites, and glial cells in the lateral habenula (LHb).
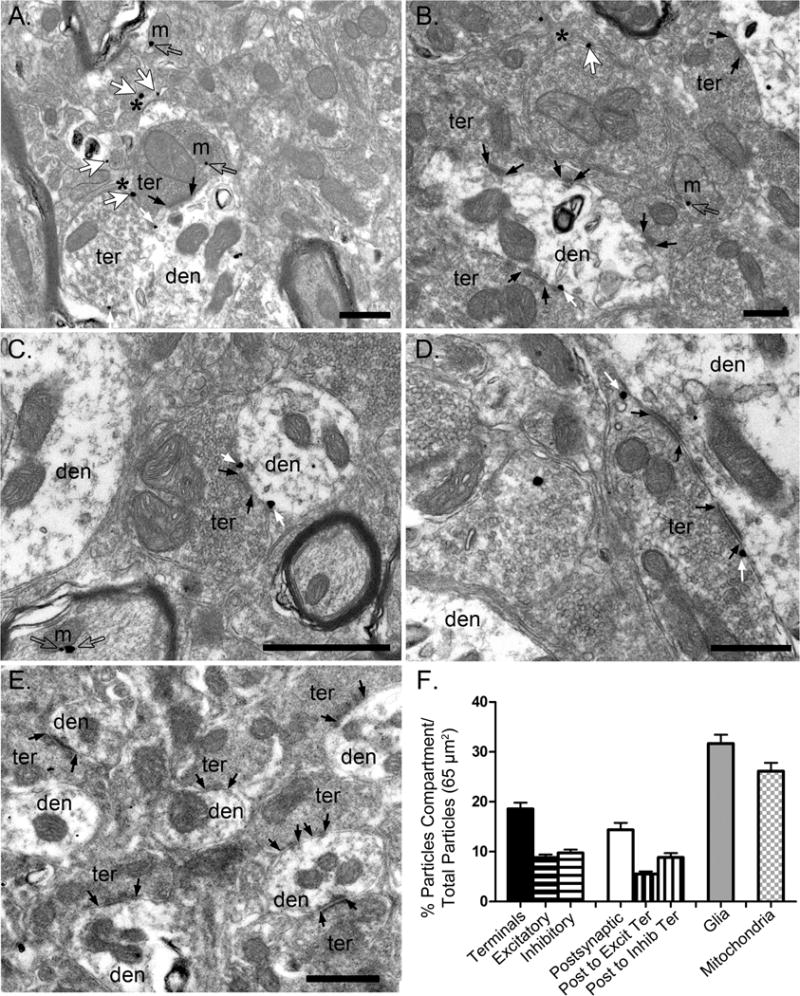
(A-D) Representative electron microscopy images illustrating the presence of CB1R-positive immunogold labeling in the rat LHb. (E) No CB1R labeling was observed in CB1R-KO LHb. Black arrows indicate synapses, small white arrows indicate CB1R-positive immunoparticles on excitatory and inhibitory presynaptic terminals (ter) impinging on postsynaptic dendrites (den), and large white arrows indicate CB1R-positive immunoparticles on glial cells which are indicated with a black asterisk. Scale bars = 500 nm. (F) Bar graph illustrating the percentage of CB1R-positive immunoparticles on excitatory and inhibitory presynaptic terminals (black - horizontal lines), postsynaptic membranes forming synapses with excitatory or inhibitory terminals (white - vertical lines), and glial cells (gray) in the LHb.
Acute Stress Increases in 2-Arachidonoylglycerol in the Lateral Habenula
Acute restraint stress increased 2-AG (Figure 2A), but not AEA (Figure 2C), in male rats. When data were analyzed according to area under the curve (AUC) for pre-stress vs. post-stress time points, stress significantly increased 2-AG (Figure 2B) but not AEA (Figure 2D).
Figure 2. Acute restraint stress mobilizes 2-arachidonoylglycerol (2-AG) in the lateral habenula (LHb).
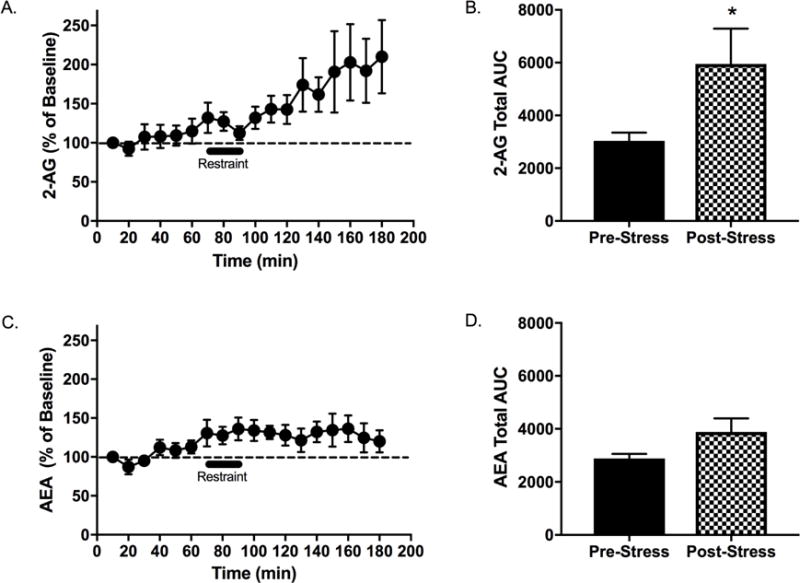
In vivo microdialysis recordings revealed a significant effect of time on the concentration of (A) 2-AG (F17,136 =3.50, p<0.001, ηp2 =0.30) and (B) anandamide (AEA) (F17,136 =3.30, p<0.001, ηp2 =0.29) in the LHb. Area under the curve (AUC) analysis comparing the 30-min pre-stress epoch to the final 30-min post-stress epoch revealed a significant effect of immobilization stress to increase the concentration of (C) 2-AG (t36=2.79, p=0.01, d=3.0), but not (D) AEA (t36=0.96, p=0.69, d=0.82) in the LHb. n=10, * denotes significance at p<0.05.
Site-Specific CB1R Activation in the Lateral Habenula Dampens Basal Corticosterone Release
Acute restraint stress increased CORT in all groups, and to a greater extent in females (Figure 3). Within-sex analyses revealed that intra-LHb RIM administration increased plasma CORT compared to VEH at baseline in male but not female rats, while intra-LHb WIN administration decreased CORT at baseline in both sexes (Figures 3A and 3C). Intra-LHb CB1R manipulations had no effect on plasma CORT concentrations at 30, 60, or 90 min post-stress onset. WIN (but not RIM) administration significantly reduced the total AUC compared to VEH in males (Figure 3B) but not females (Figure 3D).
Figure 3. Local manipulation of type-1 cannabinoid receptors (CB1R) in the lateral habenula (LHb) alters basal corticosterone (CORT) secretion.
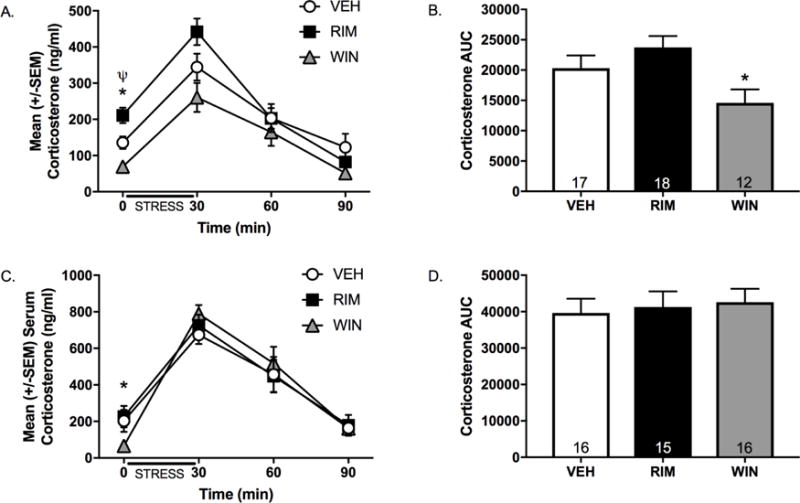
Animals received an intra-LHb infusion of the CB1R agonist WIN 55212-2 (WIN), the CB1R antagonist rimonabant (RIM), or vehicle (VEH) 15 min prior to a 30-min restraint stress episode and blood samples were collected to assay circulating CORT prior to stress onset (i.e., time 0) and at 30, 60, and 90 min post-stress onset. Acute stress exposure produced a significant elevation in CORT in both (A) males and (C) females (Time: F3,267=83.87, p<0.001, ηp2=0.15), with females exhibiting higher corticosterone levels at all time points (Time × Sex interaction: F3,267=16.98, p<0.001, ηp2 =0.16). Subsequent within-sex analyses revealed that intra-LHb WIN administration significantly reduced CORT concentrations at time 0 in males (t27=2.84, p=0.01, d=1.12) and females (t30=2.94, p=0.01, d=1.04), whereas intra-LHb RIM administration significantly increased CORT in males t33=−2.74, p=0.01, d=0.95) but not females (t29=0.34, p=0.74, d=0.49). Area under the curve (AUC) analyses indicated that intra-LHb WIN administration decreased CORT in (B) males (t27=1.82, p=0.04, d=0.70) but not (D) females (t30=0.33, p=0.37, d=0.12). n= 12-19/group/sex; * denotes significance at p<0.05.
Intra-Habenular CB1R Manipulations Bidirectionally Alter Stress Coping Strategies
RIM-treated rats spent significantly less time immobile (Figure 4A), and spent more time struggling/climbing (Figure 4C) compared to VEH-treated rats, with no differences in swimming time in the FST (Figure 4B). In contrast, WIN-treated rats spent more time immobile (Figure 4A), and spent less time swimming (Figure 4B) compared to VEH-treated rats, with no differences in struggling/climbing time (Figure 4C).
Figure 4. Local manipulation of type-1 cannabinoid receptors (CB1R) in the lateral habenula (LHb) dictates coping strategies in the forced swim test (FST).
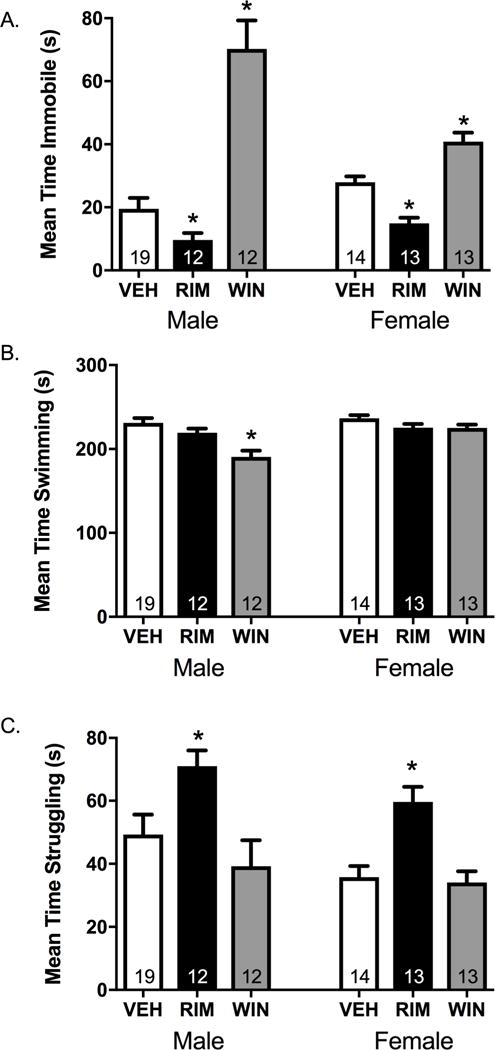
Rats received an intra-LHb infusion of the CB1R agonist WIN 55212-2 (WIN), the CB1R antagonist rimonabant (RIM), or vehicle (VEH) 15 min prior to a 5-min forced swim session where time spent immobile, swimming, and struggling were quantified. There was a main effect of treatment on (A) immobility time (F3,92=32.51, p<0.001, ηp2 =0.54), (B) swimming time (F3,92=6.49, p<0.001, ηp2=0.19), and (C) struggling time (F3,92=7.91, p<0.001, ηp2=0.22), and a sex × treatment interaction but no consistent effect of sex for immobility (F2,58=31.03, p =<0.001, ηp2 =0.52 and F1,58=2.44, p=0.12, ηp2 =0.04), for struggling (F2,58=7.32, p = 0.001, ηp2 =0.20 and F1,58=1.13, p=0.31, ηp2 =0.02), and swimming (F2,58=2.18, p =0.12, ηp2 =0.07 and F1,58=5.05, p=0.03, ηp2 =0.08). Post-hoc analyses revealed that intra-LHb RIM treatment (A) decreased total immobility time (male: t29=2.08, p=0.05, d=0.84, female: t25=5.01, p<0.001, d=1.93), (B) had no significant effect on swimming time (male: t29=1.44, p=0.16, d=0.56, female: t25=1.43, p=0.18, d=0.41) and (C) significantly increased struggling time (male: t29=2.44, p=0.02, d=0.96; female: t25=−4.07, p<0.001, d=1.56). Conversely, intra-LHb WIN treatment (A) significantly increased total immobility time in both sexes (male: t29=6.04, p<0.001, d=2.07; female: t26=−3.73, p<0.001, d=1.41), and (B) decreased swimming time in males (t29=4.33, p<0.001, d=1.61) but not females (t26=1.40, p=0.19, d=0.74), with (C) no differences in struggling time in either sex (male: t29=0.98, p=0.34, d=0.36, female: t26=0.44, p=0.67, d=0.16). n=12-19/group/sex; * denotes significance at p<0.05.
Site-Specific CB1R Blockade in the Lateral Habenula Reduces Anxiety-Like Behaviors
In the EPM, female rats spent more time exploring the open arms (Figure 5A), entered the open arms more frequently (Figure 5B), and traveled significantly farther in the open arms (Figure 5C) compared to male rats. Intra-LHb RIM administration increased the percentage of time spent in the open arms of the EPM in both male and female rats, whereas intra-LHb WIN treatment was without effect (Figure 5A). Total locomotor activity was not altered in RIM-treated rats, while WIN-treated rats unexpectedly showed enhanced locomotor activity (Figure S3).
Figure 5. Local blockade of type-1 cannabinoid receptors (CB1R) in the lateral habenula (LHb) decreases anxiety-like behavior.
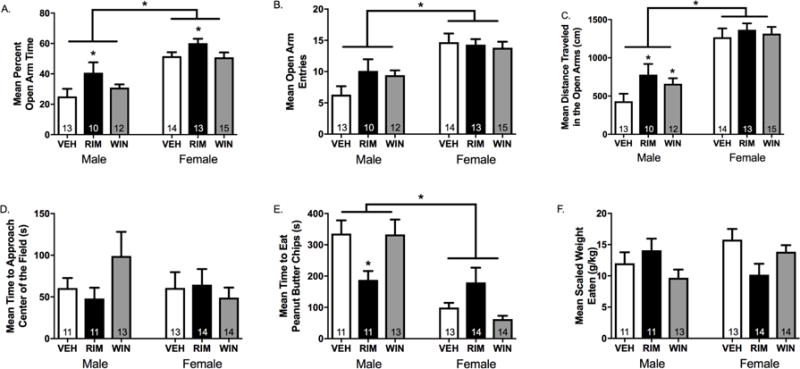
Rats received an intra-LHb infusion of the CB1R agonist WIN 55212-2 (WIN), the CB1R antagonist rimonabant (RIM), or vehicle (VEH) 15 min prior testing in either the elevated plus maze (EPM) or novelty-suppressed feeding test (NSFT). (A) With respect to the percentage of time spent exploring the open arms of the EPM, there was a main effect of treatment (F2,86=3.98, p=0.03, ηp2=0.14), a main effect of sex (F1,86=41.89, p<0.001, ηp2=0.45), but no treatment × sex interaction (F2,86=0.53, p=0.59, ηp2=0.02). Post-hoc analyses revealed that intra-LHb RIM administration significantly increased the percentage of time spent exploring the open arms in male (t20=−2.26, p=0.04, d=0.96) and female (t24=−2.24, p=0.04, d=1.0) rats compared to VEH. (B) There was no effect of treatment on the total number of open arm entries (F2,86=2.69, p=0.08, ηp2=0.10), a main effect of sex (F1,86=19.80, p<0.001, ηp2=0.28), and no significant interaction treatment × sex interaction (F2,86=0.24, p=0.79, ηp2=0.01). (C) There was a main effect of treatment on the total distance travelled in the open arms of the EPM (F2,86=4.17, p=0.02, ηp2=0.14), a main effect of sex (F1,86=33.17, p<0.001, ηp2 =0.39), and no significant treatment × sex interaction (F2,86=0.31, p=0.74, ηp2=0.01). Post-hoc analyses revealed that, in male rats, the total distance travelled in the open arms was significantly increased following intra-LHb RIM (t20=−2.45, p=0.02, d=1.03) or WIN (t22=−2.55, p=0.02, d=1.04) treatment compared to VEH. In female rats, there was no effect of RIM (t25=−1.73, p=0.11, d=0.93) or WIN (t13=−1.89, p=0.08, d=0.99) on distance travelled in the open arms. (D) In the NSFT, there was no effect of treatment (F2,86=0.15, p=0.86, ηp2=0.01), sex (F1, 86=0.20, p=0.66, ηp2<0.01), or treatment × sex interaction (F2,86=1.39, p=0.26, ηp2=0.05) on the latency to approach the peanut butter chips. (E) However, with respect to the latency to consume the peanut butter chips, there was a main effect of treatment (F2,86=0.02, p=0.98, ηp2<0.01), sex (F1,86=28.35, p<0.001, ηp2=0.26), and a significant treatment × sex interaction (F3,86=5.06, p=0.003, ηp2=0.16). Post-hoc analyses revealed that intra-LHb RIM administration significantly decreased the latency to consume the peanut butter chips compared to VEH in male (t20=3.25, p=0.004, d=1.39), but not female (t25=−1.46, p=0.16, d=0.58) rats. (F) There was no effect of treatment (F2,86=0.64, p=0.53, ηp2=0.05), sex (F2,86=0.79, p=0.38, ηp2=0.02), or treatment × sex interaction (F2,86=1.80, p=0.18, ηp2=0.07) with respect to the weight of peanut butter chips consumed in the home cage after the test, thus indicating that the effects observed were not due to treatment-induced hyperphagia. n=10-15/group/sex; * denotes significance at p<0.05.
In the NSFT, there were no sex differences in latency to approach (Figure 5D); however, female rats began eating the peanut butter chips in the novel open field faster than males (Figure 5E). Intra-LHb RIM administration decreased the latency to consume the peanut butter chips in male, but not female rats (Figure 5E). This was not due to a general hyperphagic effect of intra-LHb RIM administration, because the weight of peanut butter chips eaten in the home cage after the test was similar across treatment groups (Figure 5F).
Chronic Unpredictable Stress Augments Bursting of Lateral Habenula Neurons In Vivo
LHb neurons become hyperactive in various animal models of depressive-like behavior (see (64) for review). We tested whether 6 weeks of CUS or 7 days of SDS induces hyperexcitability of LHb neurons in vivo. The firing rate of LHb neurons was increased in CUS-exposed male and female rats, but no differences were found between SDS-exposed rats and male non-stressed rats (Figure 6A). CUS also increased 1) the percentage of spikes occurring in burst (in male rats only), 2) the number of spikes/burst, 3) burst interspike interval (ISI) (in females only), and 4) burst length (Figure 6C–F). This is in line with enhanced LHb burst activity observed in congenital learned helpless rats (65). Apart from a decrease in the number of active cells/track (Figure 6B), there were no significant effects of SDS exposure on LHb firing. Notably, non-stressed female rats exhibited lower LHb firing rates, fewer spikes/burst, and shorter burst ISIs relative to non-stressed male rats.
Figure 6. Chronic unpredictable stress (CUS), but not social defeat stress (SDS), increases bursting activity of lateral habenula (LHb) cells in vivo.
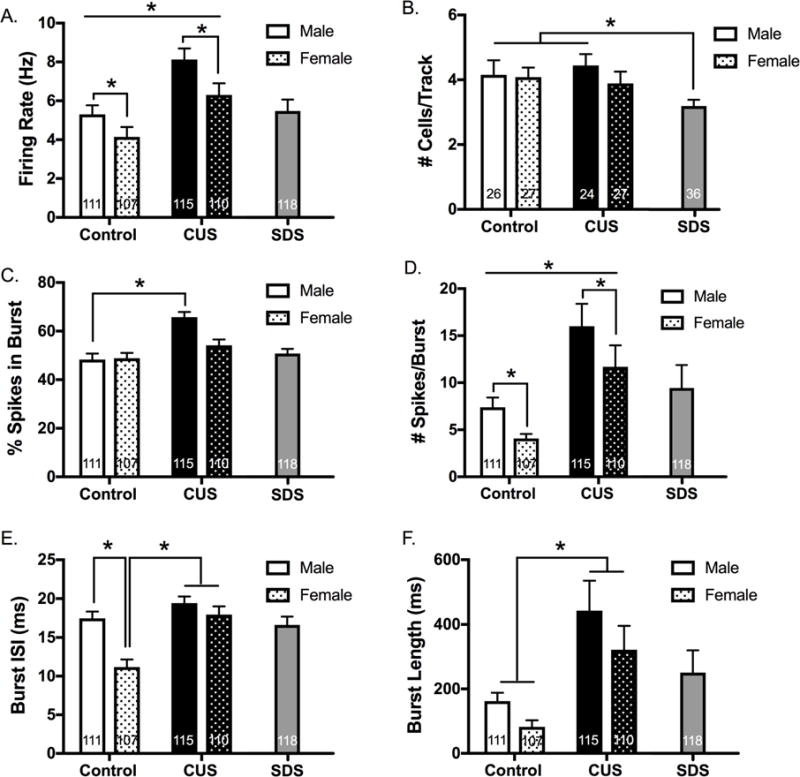
(A) There was a main effect of CUS to increase the firing rate of LHb neurons compared to non-stressed rats (F1,439=21.30, p<0.0001, ηp2=0.046). There was also a main effect of sex, with females exhibiting reduced LHb firing rates compared to males (F1,439=7.65, p=0.006, ηp2=0.017), but no significant interaction (F1,439=0.39, p=0.53, ηp2=0.001). When SDS-exposed rats were compared to non-stressed male rats, there was no significant difference in LHb firing rate (t227=−0.30, p=0.76, d=0.04). (B) There was no effect of CUS on the number of spontaneously active LHb cells encountered per track (F1,100=0.02, p=0.90, ηp2=0.0002), no main effect of sex (F1,100=0.72, p=0.40, ηp2=0.007), and no significant interaction (F1,100=0.43, p=0.51, ηp2=0.004). However, SDS-exposed rats exhibited fewer spontaneously active LHb cells compared to non-stressed male rats (t60=2.104, p=0.04, d=0.51) and non-stressed female rats (t58=2.539, p=0.014, d= 0.11). (C) There was a main effect of CUS on the percentage of spikes occurring in burst (F1,439=24.51, p<0.0001, ηp2=0.052), a main effect of sex (F1,439=5.83, p=0.016, ηp2=0.013), and a significant interaction (F1,439=7.01, p=0.0084, ηp2=0.015). Tukey’s post-hoc tests revealed that male (but not female) rats experienced an increase in the percentage of spikes occurring in burst (male: t224=5.43, p<0.05, d=0.72; female: t215=1.61, p>0.05, d=0.22). There was no difference between SDS-exposed males and non-stressed males (t229=0.97, p=0.33, d=0.13). (D) There was a main effect of CUS to increase the mean number of spikes per burst (F1,439=20.29, p<0.0001, ηp2=0.043), a main effect of sex (F1,439=4.49, p=0.035, ηp2=0.010), but no significant interaction (F1,439=0.08, p=0.78, ηp2<0.0001). There was no difference between SDS and non-stressed male rats (t229=0.79, p=0.43, d=0.0014). (E) With respect to the mean burst interspike interval (ISI), there was a main effect of CUS (F1,439=21.10, p<0.0001, ηp2=0.045), a main effect of sex (F1,439=16.82, p<0.0001, ηp2=0.036), and a significant interaction (F1,439=6.41, p=0.012, ηp2=0.014). Post-hoc tests revealed that non-stressed female rats had a significantly shorter burst ISI compared to male non-stressed rats (t224=4.62, p<0.05, d=0.62). Additionally, CUS-exposed female rats had a significantly longer burst ISI compared to non-stressed females (t215=4.99, p<0.05, d=0.68). SDS-exposed male rats did not differ significantly from non-stressed male rats (t226=0.45, p=0.65, d=0.0059). (F) There was a significant main effect of CUS to increase the mean burst length, (F1,439=16.97, p<0.0001, ηp2=0.036), no effect of sex (F1,439=2.54, p=0.11, ηp2=0.006), and no significant interaction (F1,439=0.11, p=0.74, ηp2<0.0001). SDS-exposed male rats did not differ significantly from non-stressed male rats (t229=1.20, p=0.23, d=0.16). n=5/group/sex; * denotes significance at p<0.05.
Chronic Unpredictable Stress Selectively Increases 2-AG/CB1R Signaling and Disrupts Monoacylglycerol Lipase Activity in the Lateral Habenula
CUS decreased weight gain, produced adrenal hypertrophy, and increased basal CORT in both sexes, and disturbed estrous cyclicity in females (Figure S4). CUS augmented 2-AG content in the LHb in both sexes when examined 24 hr after the final stressor (Figure 7A), with no significant differences in AEA content (Figure 7C). Interestingly, both AEA and 2-AG contents were positively correlated with adrenal weights (Figures 7B and 7D). Also, females had higher AEA content in the LHb, irrespective of stress history (Figure 7C).
Figure 7. Six weeks of chronic unpredictable stress (CUS) exposure increases 2-arachidonoylglycerol (2-AG) content, alters monoacylglycerol lipase (MAGL) hydrolytic activity, and decreases type-1 cannabinoid receptor (CB1R) binding in the lateral habenula (LHb).
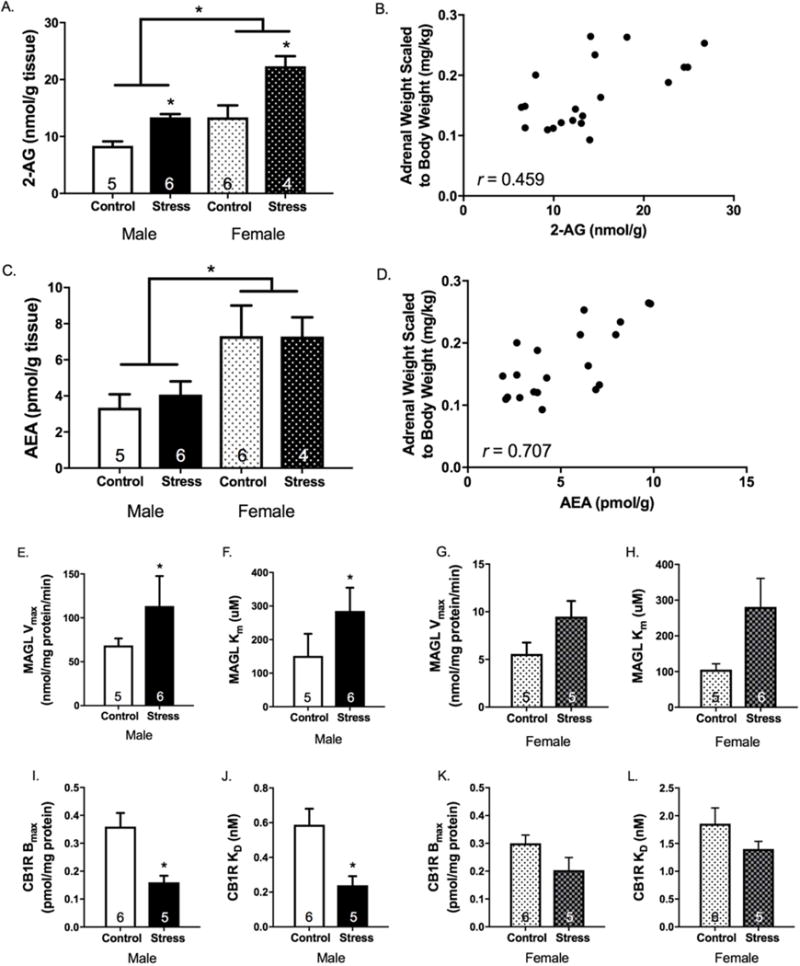
(A) There was a main effect of CUS to increase 2-AG content in the LHb (F1,17=24.85, p<0.001, ηp2=0.59), a main effect of sex (F1,17=24.84, p<0.001, ηp2 =0.59), but no stress × sex interaction (F1,17=2.0, p=0.18, ηp2=0.11). (B) With respect to anandamide (AEA) content, there was no effect of stress (F1,17=0.11, p=0.74, ηp2=0.01), however, females had significantly higher AEA levels compared to males (F1,17=11.75, p=0.003, ηp2=0.41) and there was no stress × sex interaction (F1,17=0.13, p=0.72, ηp2=0.01). Both (C) 2-AG and (D) AEA were positively correlated with scaled adrenal weights (2-AG: r=0.459, p=0.005; AEA: r=0.707, p<0.001). (E) In males, the hydrolytic activity (Vmax) of MAGL in the LHb was significantly increased in CUS-exposed rats compared to non-stressed rats (t9=−2.71, p=0.02, d=1.56). (F) In females, there was a trend for CUS to increase the Vmax of MAGL in the LHb (t8=−1.94, p=0.09, d=1.56). (G) The KM of MAGL was also significantly increased in CUS-exposed male rats (t9=−2.29, p=0.05, d=1.34). (H) In females, there was once again a trend for CUS to increase the MAGL KM in the LHb (t8=−2.17, p=0.06, d=1.38). (I and J) In males, the Bmax and KD of CB1Rs in the LHb were both significantly decreased following CUS exposure (Bmax: t9=3.38, p=0.004, d=2.17; KD: t9=3.06, p=0.007, d=1.96). (K and L) In females, the Bmax and KD for CB1Rs in the LHb was similarly reduced following CUS exposure, however, this did not reach statistical significance (Bmax: t9=1.84, p=0.099, d=1.16; KD: t9=1.36, p=0.21, d=0.85). n= 4-6/group/sex; * denotes significance at p<0.05.
CUS exposure increased both the Vmax (Figures 7E and 7F) and KM (Figures 7G and 7H) of MAGL in the LHb, which is indicative of enhanced hydrolytic activity but reduced binding affinity. CUS exposure also significantly decreased the Bmax (Figures 7I and 6K) and KD (Figures 7J and 7L) for the CB1R in the LHb, particularly in males, which indicates a reduction in the number of CB1R binding sites but an increase in agonist binding affinity.
Social Defeat Stress Augments Basal Endocannabinoid Content in the Lateral Habenula
SDS produced physiological indices of chronic stress-related pathology (Figure S5). Rats subjected to SDS displayed increased 2-AG and AEA content in the LHb compared to non-stressed rats (Figures 8A and 8B). Both AEA and 2-AG content in the LHb were positively correlated with adrenal weights (Figures 8C and 8D). SDS exposure did not significantly alter MAGL activity relative to non-stressed conditions (Figure S6).
Figure 8. Local manipulation of type-1 cannabinoid receptors (CB1R) in the lateral habenula (LHb) dictates coping strategies in chronically stressed male rats.
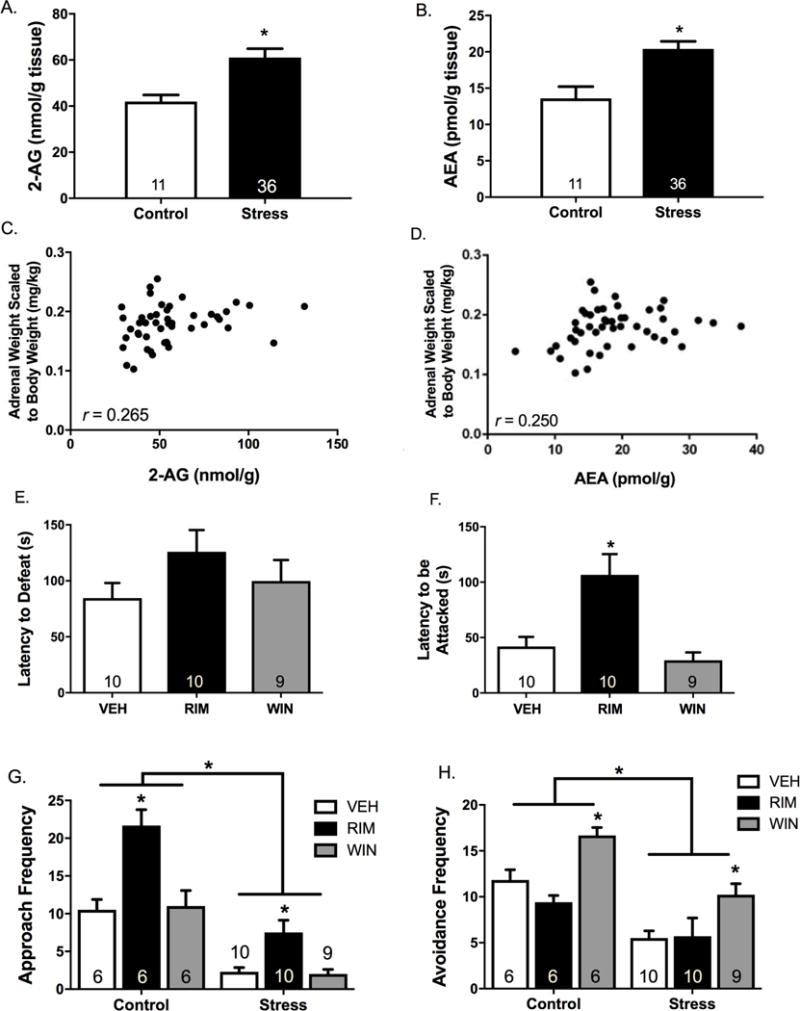
Male rats were exposed to seven days of social defeat stress (SDS), and LHb tissue was collected 24 hr after the final defeat episode (under stress free conditions). SDS exposure significantly increased (A) 2-arachidonoylglycerol (2-AG) (F1,45=10.74, p=0.002, ηp2 =0.19) and (B) anandamide (AEA) (F1,45=6.57, p=0.01, ηp2 =0.13) content compared to non-stressed rats. Both (C) 2-AG and (D) AEA content were positively correlated with scaled adrenal weights (2-AG: r=0.27, p=0.04; AEA: r=0.25, p=0.05). A separate cohort of rats was implanted with bilateral cannula aimed at the LHb and the CB1R agonist WIN 55212-2 (WIN), the CB1R antagonist rimonabant (RIM), or vehicle (VEH) were administered 15 min prior to the final SDS episode. There was no effect of treatment on (E) the latency to defeat (F2,26=1.50, p=0.24, ηp2=0.10), however, there was a significant effect of treatment on (F) the latency to be attacked (F2,26=10.20, p=0.001, ηp2=0.44). Post-hoc analyses confirmed that intra-LHb RIM administration significantly increased the latency to be attacked compared to VEH (t18=−3.11, p=0.01, d=1.42), while intra-LHb WIN administration failed to significantly affect the latency to be attacked (t17=1.10, p=0.29, d=0.50). (G) Additionally, RIM-treated animals engaged in significantly more approach behaviors during the SDS episode compared to VEH-treated animals (t18=−3.65, p=0.002, d=1.63), while WIN-treated animals were not significantly different from VEH (t17=0.36, p=0.72, d=0.17). The same effect was also observed in non-stressed rats interacting with a non-aggressive conspecific (t10=−4.69, p=0.001, d=2.64), whereas WIN-treated rats did not differ significantly from VEH-treated rats with respect to the frequency of approach behaviors (t10=−0.09, p=0.93, d=0.05). (H) Intra-LHb WIN treatment significantly increased the frequency of avoidance behaviors in both SDS-exposed (t17=−3.33, p=0.004, d=1.45) and non-stressed (t10=−3.41, p=0.01, d=1.99) rats. Conversely, intra-LHb RIM treatment did not significantly affect the frequency of avoidance behaviors in SDS-exposed (t18=−1.11, p=0.29, d=0.49) or non-stressed (t10=1.11, p=0.29, d=0.63) rats. For biochemical assays, control n=11 and SDS n=36. For behavioral assays, n=6/group for control experiments and n=9-10/group for SDS experiments. * denotes significance at p<0.05.
Intra-LHb CB1R Manipulations Bias Action Selection Independent of Stress History
We examined whether the LHb ECB system can be targeted to alter coping strategies in rats exposed to SDS. Although the effect of intra-LHb RIM administration to increase the latency to defeat did not reach statistical significance (Figure 8E), RIM significantly increased latency to the first attack episode (Figure 8F). When behaviors during the session were scored, RIM administration was found to increase the frequency of approach behaviors (Figure 8G), whereas WIN administration increased the frequency of avoidance behaviors (Figure 8H). These effects of RIM and WIN were also observed in rats with no previous history of stress exposure.
DISCUSSION
We found that CB1Rs are expressed on presynaptic, postsynaptic, astrocytic, and mitochondrial membranes in the LHb, and that exposure to either acute or chronic stress increases the concentration of 2-AG in the LHb. In the case of CUS, this is associated with increased LHb burst firing and deficits in MAGL-mediated 2-AG hydrolysis, coupled to a reduction in the number of available CB1R binding sites. We further show that site-specific CB1R blockade engages proactive coping strategies in the FST, reduces anxiety-like behavior in the EPM and NSFT, and augments basal CORT secretion. Moreover, local CB1R blockade significantly increased the latency to be attacked by a territorial conspecific and increased approach towards either an aggressive or non-aggressive conspecific, while local CB1R activation had the opposite effect. Thus, stress enhances ECB signaling in the LHb, while disruption of stress-induced LHb ECB/CB1R signaling induces a state of arousal that facilitates a “switch” in behavioral coping styles to a more proactive strategy that favors exploration over avoidance.
These results are surprising given that CB1R activation typically recruits proactive stress coping strategies (14–20) and reduces anxiety-like behavior (21–24). Moreover, systemic CB1R blockade usually increases anxiety-like behaviors (24–26), particularly under stressful conditions (21). In contrast, our data indicate that intra-LHb CB1R activation increased immobility in the FST, whereas blocking these receptors had the opposite effect. Accordingly, intra-LHb CB1R blockade increased exploration in the EPM and NSFT. Behavioral engagement was also evident in the SDS paradigm, where intra-LHb CB1R blockade facilitated defensive aggression during an agonistic encounter and increased social approach behaviors. Although unexpected, the ability of CB1R blockade to increase proactive coping responses and decrease anxiety-like behavior has been documented previously (66–71). Moreover, site-specific CB1R activation strongly potentiates fear-related freezing responses, which supports these data (72–76). Thus, the LHb may contribute to the anxiolytic and antidepressant-like effects that are sometimes observed after systemic CB1R antagonism.
CUS exposure caused profound alterations in ECB content, MAGL activity, and CB1R binding that collectively result in augmented LHb ECB signaling under basal conditions. In the case of CUS, we found an increase in 2-AG content that was likely driven by stress-induced alterations in the affinity of MAGL for 2-AG, thereby leading to impaired 2-AG hydrolysis. CUS also produced alterations in LHb CB1R binding that are presumably secondary to CUS-induced alterations in 2-AG. This is supported by studies showing that sustained inactivation of MAGL increases 2-AG and causes CB1R desensitization (77), whereas CB1R deletion fails to alter activity of ECB hydrolases (78). Thus, elevated 2-AG tone in the LHb likely decreases the number of available CB1R binding sites, with the remaining binding sites being more sensitive to stimulation. The magnitude of stress-induced 2-AG signaling could be a relevant signal in determining the nature of the stress coping response. Under acute stress, there is considerable variation in coping responses, whereas chronic stress conditions confer greater reliance on passive coping strategies (58,79). As such, the progressive increase in 2-AG signaling may be a factor favoring the development of passive coping responses after chronic stress.
The ability of chronic stress to augment basal LHb 2-AG content was robust and replicated in two preclinical models, the CUS and SDS paradigms, each of which have been extensively used to model symptoms of major depression (63,80). However, increased LHb neuronal firing (and changes in MAGL activity; see Figure S6) was only observed following 6 weeks of CUS. This could be due to differences in the duration of the stress regimen, as 7 days of SDS may not be sufficient to produce changes in MAGL that support LHb hyperactivity, at least when examined 24 hr following the final stress exposure. It may also be that LHb hyperactivity only emerges in a unique subset of SDS-exposed rats (i.e., stress susceptible rats).
Since males and females exhibit sexual dimorphism in the ECB system (81) and respond differently to stress (82), we also assessed sex differences in LHb ECB signaling. Although effects of RIM were similar in both sexes, effects of WIN were often only evident in males (see Table 1). This could be due to elevated ECB tone in the female LHb (see Figures 7A and 7C), which may occlude the effects of WIN. Sex differences in LHb ECB signaling may confer distinct alterations in LHb function and could help to explain why females engage in differential coping styles than males (83) and exhibit greater susceptibility for developing stress-related illnesses (82).
Table 1.
Observed Sex Differences Following Lateral Habenula CB1R Manipulation or Chronic Unpredictable Stress Exposure.
| Endpoint Measured | ♂ | ♀ |
|---|---|---|
| CB1R Manipulation Studies | ||
| Basal CORT | ||
| RIM Effect | ↑ | –– |
| Acute Stress CORT (AUC) | ||
| WIN Effect | ↓ | –– |
| FST Swimming Time | ||
| WIN Effect | ↓ | –– |
| EPM Open Arm Distance | ||
| RIM Effect | ↑ | –– |
| WIN Effect | ↑ | –– |
| NSF Latency To Eat | ||
| RIM Effect | ↓ | –– |
| Chronic Unpredictable Stress Studies | ||
| Percent LHb Spikes in Burst | ||
| CUS Effect | ↑ | –– |
| LHb Burst Interspike Interval | ||
| CUS Effect | –– | ↑ |
| MAGL Vmax | ||
| CUS Effect | ↑ | ↑ |
| MAGL Km | ||
| CUS Effect | ↑ | ↑ |
| CB1R Bmax | ||
| CUS Effect | ↓ | ↓ |
| CB1R Kd | ||
| CUS Effect | ↓ | –– |
↓↑ = Significant Increase/Decrease, ↓↑ = Trend (p=0.051-0.10), –– = No Change
Exposure to stress increases LHb activation (84–91), which suppresses exploratory behavior and facilitates avoidance under aversive conditions (92). Accordingly, silencing the LHb engages proactive coping strategies in the FST and in models of learned helplessness (48–49, 95–97). Our behavioral data, together with the studies described above, support the notion that local CB1R activation causes a net increase in the activity of LHb neurons. However, this is contrary to what was expected and appears to be at odds with the recently established role for CB1Rs in regulating long-term depression at excitatory LHb synapses (53,54). The most parsimonious explanation is that activation of CB1Rs in the LHb preferentially suppresses GABA release onto LHb projection neurons. There are prominent GABAergic inputs to the LHb from the basal forebrain, and our data indicate that CB1Rs are equally distributed on excitatory vs. inhibitory terminals in the LHb, supporting this possibility. Moreover, CB1R populations on GABAergic terminals have been shown to be necessary for the anxiogenic effects of CB1R agonism (98). An alternative explanation is that ECB signaling increases LHb activity via activation of CB1Rs on glial cells. In the hippocampus, ECBs have been shown to activate astrocytes via a Gq/11-mediated mechanism, which indirectly potentiates synaptic transmission at more distal synapses by stimulating the release of excitatory gliotransmitters (reviewed in (99)). This could explain how CB1R-mediated synaptic depression at local excitatory synapses coincides with an increase in LHb activation. In support of this hypothesis, our electron microscopy data revealed that the majority of CB1Rs are localized to glial cells in the LHb. Moreover, chronic stress has deleterious effects on glial cells in the LHb, which have been shown to contribute to LHb hyperactivity, anxiety-like behavior, and the emergence of a depressive-like phenotype (100,101). Future studies exploring the relative roles for ECBs in gating excitatory and inhibitory transmission from both neuronal and glial cells in the LHb could help to reconcile these findings.
There are multiple downstream systems that ECB signaling in the LHb may influence to produce behavioral effects observed herein. In particular, DA and 5-HT activity are strongly modulated by the LHb, and each has been implicated in the behavioral response to stress. For instance, DA is required for the initiation of voluntary motor movements (102), is recruited under conditions of stress (103), and photostimulation of VTA DA neurons produces enhances escape-directed struggling responses in the FST in rats subjected to CUS (104). Our results are consistent with the idea that intra-LHb CB1R inactivation may be promoting behavioral activation via indirect enhancement of DA transmission. Moreover, a vast body of literature has described a fundamental role for 5-HT in regulating the behavioral response to stress (35), and LHb-DRN projections modulate 5-HT release into downstream circuits that mediate anxiety-like behavior (89). Additional studies will be needed to understand the precise mechanisms by which ECB signaling in the LHb influences monoaminergic transmission, and how this modulates stress coping strategies. This could offer insight into the pathophysiology of stress-related disorders given that ECB alterations and LHb hyperactivity have been independently associated with affective disorders in the clinical population.
Supplementary Material
Acknowledgments
This work was supported by an intramural grant from the College of Veterinary Medicine at Washington State University awarded to RJM and a Foundation grant from the Canadian Institutes of Health Research (CIHR) to MNH. MAS and MM are recipients of postdoctoral scholarships from CIHR and MM is also supported by postdoctoral funding from Alberta Innovates Health Solutions. Pedro Grandes’ laboratory is supported by The Basque Government [grant number BCG IT764-13]; MINECO/FEDER, UE [grant number SAF2015-65034-R]; University of the Basque Country [UPV/EHU UFI11/41]; and Red de Trastornos Adictivos UE/ERDF [grant number RD16/0017/0012]. Work in the Parsons laboratory was supported by grants from the US National Institutes of Health (NIH): AA020404, AA006420, AA022249 and AA017447 to LHP, and T32 AA 7456-33 and F32 AA025257-01 to SAL. Work in the Hillard laboratory was supported by the Research and Education Component of the Advancing a Healthier Wisconsin Endowment at the Medical College of Wisconsin and by the Department of Pharmacology Mass Spectrometry Facility. The authors would also like to thank the Southern Alberta Mass Spectrometry Centre, located in and supported by the Cumming School of Medicine, University of Calgary, for their services in targeted liquid chromatography tandem mass spectrometry. This paper is dedicated to the memory of our dear friend and colleague Larry Parsons.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Hermans EJ, Henckens MJ, Joëls M, Fernández G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 1990;37:304–314. doi: 10.1016/j.tins.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Koolhaas JM, De Boer SF, Buwalda B, Van Reenen K. Individual variation in coping with stress: A multidimensional approach of ultimate and proximate mechanisms. Brain Behav Evol. 2007;70:218–226. doi: 10.1159/000105485. [DOI] [PubMed] [Google Scholar]
- 3.De Boer S, Buwalda B, Koolhaas J. Untangling the neurobiology of coping styles in rodents: towards neural mechanisms underlying individual differences in disease susceptibility. Neurosci Biobehav Rev. 2016;16:30222–30226. doi: 10.1016/j.neubiorev.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Veenema AH, Koolhaas JM, De Kloet ER. Basal and stress-induced differences in HPA axis, 5-HT responsiveness, and hippocampal cell proliferation in two mouse lines. Ann NY Acad Sci. 2004;1018:255–265. doi: 10.1196/annals.1296.030. [DOI] [PubMed] [Google Scholar]
- 5.Veenema AH, Cremers TI, Jongsma ME, Steenbergen PJ, De Boer SF, Koolhaas JM. Differences in the effects of 5-HT1A receptor agonists on forced swimming behavior and brain 5-HT metabolism between low and high aggressive mice. Psychopharmacology (Berl) 2005;178:151–160. doi: 10.1007/s00213-004-2005-5. [DOI] [PubMed] [Google Scholar]
- 6.Koolhaas JM, de Boer SF, Coppens CM, Buwalda B. Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front Neuroendocrinol. 2010;31:307–321. doi: 10.1016/j.yfrne.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Yen YC, Anderzhanova E, Bunck M, Schuller J, Landgraf R, Wotjak CT. Co-segregation of hyperactivity, active coping styles, and cognitive dysfunction in mice selectively bred for low levels of anxiety. Front Behav Neurosci. 2013;7:103. doi: 10.3389/fnbeh.2013.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaughlin RJ, Gobbi G. Cannabinoids and emotionality: a neuroanatomical perspective. Neuroscience. 2012;204:134–144. doi: 10.1016/j.neuroscience.2011.07.052. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin RJ, Hill MN, Gorzalka BB. A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emotional behavior. Neurosci Biobehav Rev. 2014;42:116–131. doi: 10.1016/j.neubiorev.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Hillard CJ, Beatka M, Sarvaideo J. Endocannabinoid signaling and the hypothalamic-pituitary-adrenal axis. Compr Physiol. 2016;7:1–15. doi: 10.1002/cphy.c160005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balsevich G, Petrie GN, Hill MN. Endocannabinoids: Effectors of glucocorticoid signaling. Front Neuroendocrinol. 2017 doi: 10.1016/j.yfrne.2017.07.005. S0091-3022(17)30039-0. [DOI] [PubMed] [Google Scholar]
- 12.Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–5438. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- 13.Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ, Gorzalka BB. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2009;34:2733–2745. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill MN, Gorzalka BB. Pharmacological enhancement of cannabinoid CB1 receptor activity elicits an antidepressant-like response in the rat forced swim test. Eur Neuropsychopharmacol. 2005;15:593–599. doi: 10.1016/j.euroneuro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Bambico FR, Katz N, Debonnel G, Gobbi G. Cannabinoids elicit antidepressant-like behavior and activate serotonergic neurons through the medial prefrontal cortex. J Neurosci. 2007;27:11700–11711. doi: 10.1523/JNEUROSCI.1636-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLaughlin RJ, Hill MN, Morrish AC, Gorzalka BB. Local enhancement of cannabinoid CB1 receptor signalling in the dorsal hippocampus elicits an antidepressant-like effect. Behav Pharmacol. 2007;18:431–438. doi: 10.1097/FBP.0b013e3282ee7b44. [DOI] [PubMed] [Google Scholar]
- 17.Adamczyk P, Gołda A, Mccreary AC, Filip M, Przegaliński E. Activation of endocannabinoid transmission induces antidepressant-like effects in rats. J Physiol Pharmacol. 2008;59:217–228. [PubMed] [Google Scholar]
- 18.El-Alfy AT, Ivey K, Robinson K, Ahmed S, Radwan M, Slade D, et al. Antidepressant-like effect of delta9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol Biochem Behav. 2010;95:434–442. doi: 10.1016/j.pbb.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLaughlin RJ, Hill MN, Bambico FR, Stuhr KL, Gobbi G, Hillard CJ, Gorzalka BB. Prefrontal cortical anandamide signaling coordinates coping responses to stress through a serotonergic pathway. Eur Neuropsychopharmacol. 2012;22:664–671. doi: 10.1016/j.euroneuro.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kruk-Slomka M, Michalak A, Biala G. Antidepressant-like effects of the cannabinoid receptor ligands in the forced swimming test in mice: mechanism of action and possible interactions with cholinergic system. Behav Brain Res. 2015;284:24–36. doi: 10.1016/j.bbr.2015.01.051. [DOI] [PubMed] [Google Scholar]
- 21.Haller J, Varga B, Ledent C, Freund TF. CB1 cannabinoid receptors mediate anxiolytic effects: convergent genetic and pharmacological evidence with CB1-specific agents. Behav Pharmacol. 2004;15:299–304. doi: 10.1097/01.fbp.0000135704.56422.40. [DOI] [PubMed] [Google Scholar]
- 22.Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- 23.Braida D, Limonta V, Malabarba L, Zani A, Sala M. 5-HT1A receptors are involved in the anxiolytic effect of Δ9-tetrahydrocannabinol and AM 404, the anandamide transport inhibitor, in Sprague-Dawley rats. Eur J Pharmacol. 2007;555:156–163. doi: 10.1016/j.ejphar.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 24.Gamble-George JC, Conger JR, Hartley ND, Gupta P, Sumislawski JJ, Patel S. Dissociable effects of CB1 receptor blockade on anxiety-like and consummatory behaviors in the novelty-induced hypophagia test in mice. Psychopharmacology (Berl) 2013;228:401–409. doi: 10.1007/s00213-013-3042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navarro M, Hernandez E, Munoz RM, del Arco I, Villanua MA, Carrera MR, Rodriguez de Fonseca F. Acute administration of the CB1 cannabinoid receptor antagonist SR 141716A induces anxiety-like responses in the rat. Neuroreport. 1997;8:491–496. doi: 10.1097/00001756-199701200-00023. [DOI] [PubMed] [Google Scholar]
- 26.Rodgers RJ, Evans PM, Murphy A. Anxiogenic profile of AM-251, a selective cannabinoid CB1 receptor antagonist, in plus-maze-naïve and plus-maze-experienced mice. Behav Pharmacol. 2005;16:405–413. doi: 10.1097/00008877-200509000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Morena M, Patel S, Bains JS, Hill MN. Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology. 2016;41:80–102. doi: 10.1038/npp.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wyrofsky R, McGonigle P, Van Bockstaele EJ. Drug discovery strategies that focus on the endocannabinoid signaling system in psychiatric disease. Expert Opin Drug Discov. 2015;10:17–36. doi: 10.1517/17460441.2014.966680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boulos LJ, Darcq E, Kieffer BL. Translating the habenula - from rodents to humans. Biol Psychiatry. 2017;81:296–305. doi: 10.1016/j.biopsych.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fakhoury M. The habenula in psychiatric disorders: More than three decades of translational investigation. Neurosci Biobehav Rev. 2017;7634 doi: 10.1016/j.neubiorev.2017.02.010. S0149-7634(16)30654-6. [DOI] [PubMed] [Google Scholar]
- 31.Omelchenko N, Bell R, Sesack SR. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur J Neurosci. 2009;30:1239–1250. doi: 10.1111/j.1460-9568.2009.06924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR. The inhibitory influence of the lateral habenula on midbrain dopamine cells: ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. J Comp Neurol. 2011;519:1143–1164. doi: 10.1002/cne.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao H, Zhang BL, Yang SJ, Rusak B. The role of lateral habenula-dorsal raphe nucleus circuits in higher brain functions and psychiatric illness. Behav Brain Res. 2015;15:89–98. doi: 10.1016/j.bbr.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 35.Metzger M, Bueno D, Lima LB. The lateral habenula and the serotonergic system. Pharmacol Biochem Behav. 2017;3057:30330–30336. doi: 10.1016/j.pbb.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15:1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thornton EW, Bradbury GE. Effort and stress influence the effect of lesion of the habenula complex in one-way active avoidance learning. Physiol Behav. 1989;45:929–935. doi: 10.1016/0031-9384(89)90217-5. [DOI] [PubMed] [Google Scholar]
- 38.Luo XF, Zhang BL, Li JC, Yang YY, Sun YF, Zhao H. Lateral habenula as a link between dopaminergic and serotonergic systems contributes to depressive symptoms in Parkinson’s disease. Brain Res Bull. 2015;110:40–46. doi: 10.1016/j.brainresbull.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Wang Y, Xuan C, Li Y, Piao L, Li J, Zhao H. Role of the lateral habenula in pain-associated depression. Front Behav Neurosci. 2017;11:31. doi: 10.3389/fnbeh.2017.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gill MJ, Ghee SM, Harper SM, See RE. Inactivation of the lateral habenula reduces anxiogenic behavior and cocaine seeking under conditions of heightened stress. Pharmacol Biochem Behav. 2013;111:24–29. doi: 10.1016/j.pbb.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ely BA, Xu J, Goodman WK, Lapidus KA, Gabbay V, Stern ER. Resting-state functional connectivity of the human habenula in healthy individuals: Associations with subclinical depression. Hum Brain Mapp. 2016;37:2369–2384. doi: 10.1002/hbm.23179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt FM, Schindler S, Adamidis M, Strauß M, Tränkner A, Trampel R, et al. Habenula volume increases with disease severity in unmedicated major depressive disorder as revealed by 7T MRI. Eur Arch Psychiatry Clin Neurosci. 2017;267:107–115. doi: 10.1007/s00406-016-0675-8. [DOI] [PubMed] [Google Scholar]
- 43.Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 44.Lim LW, Prickaerts J, Huguet G, Kadar E, Hartung H, Sharp T, Temel Y. Electrical stimulation alleviates depressive-like behaviors of rats: investigation of brain targets and potential mechanisms. Transl Psychiatry. 2015;5:e535. doi: 10.1038/tp.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim Y, Morath B, Hu C, Byrne LK, Sutor SL, Frye MA, Tye SJ. Antidepressant actions of lateral habenula deep brain stimulation differentially correlate with CaMKII/GSK3/AMPK signaling locally and in the infralimbic cortex. Behav Brain Res. 2016;306:170–177. doi: 10.1016/j.bbr.2016.02.039. [DOI] [PubMed] [Google Scholar]
- 46.Shumake J, Edwards E, Gonzalez-Lima F. Opposite metabolic changes in the habenula and ventral tegmental area of a genetic model of helpless behavior. Brain Res. 2003;963:275–281. doi: 10.1016/s0006-8993(02)04048-9. [DOI] [PubMed] [Google Scholar]
- 47.Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meng H, Wang Y, Huang M, Lin W, Wang S, Zhang B. Chronic deep brain stimulation of the lateral habenula nucleus in a rat model of depression. Brain Res. 2011;1422:32–38. doi: 10.1016/j.brainres.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 49.Winter C, Vollmayr B, Djodari-Irani A, Klein J, Sartorius A. Pharmacological inhibition of the lateral habenula improves depressive-like behavior in an animal model of treatment resistant depression. Behav Brain Res. 2011;216:463–465. doi: 10.1016/j.bbr.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 50.Li K, Zhou T, Liao L, Yang Z, Wong C, Henn F, et al. βCaMKII in lateral habenula mediates core symptoms of depression. Science. 2013;341:1016–1020. doi: 10.1126/science.1240729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shabel SJ, Proulx CD, Piriz J, Malinow R. Mood regulation. GABA/glutamate co-release controls habenula output and is modified by antidepressant treatment. Science. 2014;345:1494–1498. doi: 10.1126/science.1250469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cui W, Mizukami H, Yanagisawa M, Aida T, Nomura M, Isomura Y, et al. Glial dysfunction in the mouse habenula causes depressive-like behaviors and sleep disturbance. J Neurosci. 2014;34:16273–16285. doi: 10.1523/JNEUROSCI.1465-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valentinova K, Mameli M. mGluR-LTD at excitatory and inhibitory synapses in the lateral habenula tunes neuronal output. Cell Rep. 2016;16:2298–2307. doi: 10.1016/j.celrep.2016.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park H, Rhee J, Lee S, Chung C. Selectively impaired endocannabinoid-dependent long-term depression in the lateral habenula in an animal model of depression. Cell Rep. 2017;20:289–296. doi: 10.1016/j.celrep.2017.06.049. [DOI] [PubMed] [Google Scholar]
- 55.Henricks AM, Berger AL, Lugo JM, Baxter-Potter LN, Bieniasz KV, Petrie G, et al. Sex-and hormone-dependent alterations in alcohol withdrawal-induced anxiety and corticolimbic endocannabinoid signaling. Neuropharmacology. 2017;124:121–133. doi: 10.1016/j.neuropharm.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 56.Gutiérrez-Rodríguez A, Puente N, Elezgarai I, Ruehle S, Lutz B, Reguero L, et al. Anatomical characterization of the cannabinoid CB1 receptor in cell-type-specific mutant mouse rescue models. J Comp Neurol. 2017;525:302–318. doi: 10.1002/cne.24066. [DOI] [PubMed] [Google Scholar]
- 57.Caillé S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McLaughlin RJ, Hill MN, Dang SS, Wainwright SR, Galea LA, Hillard CJ, Gorzalka BB. Upregulation of CB₁ receptor binding in the ventromedial prefrontal cortex promotes proactive stress-coping strategies following chronic stress exposure. Behav Brain Res. 2013;237:333–337. doi: 10.1016/j.bbr.2012.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hill MN, McLaughlin RJ, Pan B, Fitzgerald ML, Roberts CJ, Lee TT, et al. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci. 2011;31:10506–10515. doi: 10.1523/JNEUROSCI.0496-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qin Z, Zhou X, Pandey NR, Vecchiarelli HA, Stewart CA, Zhang X, et al. Chronic stress induces anxiety via an amygdalar intracellular cascade that impairs endocannabinoid signaling. Neuron. 2015;85:1319–1331. doi: 10.1016/j.neuron.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 61.Morena M, De Castro V, Gray JM, Palmery M, Trezza V, Roozendaal B, et al. Training-associated emotional arousal shapes endocannabinoid modulation of spatial memory retrieval in rats. J Neurosci. 2015;35:13962–113974. doi: 10.1523/JNEUROSCI.1983-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koolhaas JM, Coppens CM, de Boer SF, Buwalda B, Meerlo P, Timmermans PJ. The resident-intruder paradigm: a standardized test for aggression, violence and social stress. J Vis Exp. 2013;77:e4367. doi: 10.3791/4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maroso M, Szabo GG, Kim HK, Alexander A, Bui AD, Lee SH, et al. Cannabinoid control of learning and memory through HCN channels. Neuron. 2016;89:1059–1073. doi: 10.1016/j.neuron.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang Y, Wang H, Hu J, Hu H. Lateral habenula in the pathophysiology of depression. Curr Opin Neurobiol. 2018;48:90–96. doi: 10.1016/j.conb.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 65.Yang Y, Cui Y, Sang K, Dong Y, Zheyi N, Ma S, Hu H. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018;554:317–322. doi: 10.1038/nature25509. [DOI] [PubMed] [Google Scholar]
- 66.Shearman LP, Rosko KM, Fleischer R, Wang J, Xu S, Tong XS, Rocha BA. Antidepressant-like and anorectic effects of the cannabinoid CB1 receptor inverse agonist AM251 in mice. Behav Pharmacol. 2003;14:573–582. doi: 10.1097/00008877-200312000-00001. [DOI] [PubMed] [Google Scholar]
- 67.Tzavara ET, Davis RJ, Perry KW, Li X, Salhoff C, Bymaster FP, et al. The CB1 receptor antagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: implications for therapeutic actions. Br J Pharmacol. 2003;138:544–553. doi: 10.1038/sj.bjp.0705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Griebel G, Stemmelin J, Scatton B. Effects of the cannabinoid CB1 receptor antagonist rimonabant in models of emotional reactivity in rodents. Biol Psychiatry. 2005;57:261–267. doi: 10.1016/j.biopsych.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 69.Steiner MA, Marsicano G, Nestler EJ, Holsboer F, Lutz B, Wotjak CT. Antidepressant-like behavioral effects of impaired cannabinoid receptor type 1 signaling coincide with exaggerated corticosterone secretion in mice. Psychoneuroendocrinology. 2008;33:54–67. doi: 10.1016/j.psyneuen.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee S, Kim DH, Yoon SH, Ryu JH. Sub-chronic administration of rimonabant causes loss of antidepressive activity and decreases doublecortin immunoreactivity in the mouse hippocampus. Neurosci Lett. 2009;467:111–116. doi: 10.1016/j.neulet.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 71.Häring M, Grieb M, Monory K, Lutz B, Moreira FA. Cannabinoid CB₁ receptor in the modulation of stress coping behavior in mice: the role of serotonin and different forebrain neuronal subpopulations. Neuropharmacology. 2013;65:83–89. doi: 10.1016/j.neuropharm.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 72.Laviolette SR, Grace AA. Cannabinoids potentiate emotional learning plasticity in neurons of the medial prefrontal cortex through basolateral amygdala inputs. J Neurosci. 2006;26:6458–6468. doi: 10.1523/JNEUROSCI.0707-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tan H, Lauzon NM, Bishop SF, Chi N, Bechard M, Laviolette SR. Cannabinoid transmission in the basolateral amygdala modulates fear memory formation via functional inputs to the prelimbic cortex. J Neurosci. 2011;31:5300–5312. doi: 10.1523/JNEUROSCI.4718-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ahmad T, Lauzon NM, de Jaeger X, Laviolette SR. Cannabinoid transmission in the prelimbic cortex bidirectionally controls opiate reward and aversion signaling through dissociable kappa versus μ-opiate receptor dependent mechanisms. J Neurosci. 2013;33:15642–15651. doi: 10.1523/JNEUROSCI.1686-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Draycott B, Loureiro M, Ahmad T, Tan H, Zunder J, Laviolette SR. Cannabinoid transmission in the prefrontal cortex bi-phasically controls emotional memory formation via functional interactions with the ventral tegmental area. J Neurosci. 2014;34:13096–13109. doi: 10.1523/JNEUROSCI.1297-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahmad T, Sun N, Lyons D, Laviolette SR. Bi-directional cannabinoid signalling in the basolateral amygdala controls rewarding and aversive emotional processing via functional regulation of the nucleus accumbens. Addict Biol. 2017;22:1218–1231. doi: 10.1111/adb.12406. [DOI] [PubMed] [Google Scholar]
- 77.Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 2010;13:1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baggelaar MP, van Esbroeck AC, van Rooden EJ, Florea BI, Overkleeft HS, Marsicano G, et al. Chemical proteomics maps brain region specific activity of endocannabinoid hydrolases. ACS Chem Biol. 2017;12:852–861. doi: 10.1021/acschembio.6b01052. [DOI] [PubMed] [Google Scholar]
- 79.Roth MK, Bingham B, Shah A, Joshi A, Frazer A, Strong R, Morilak DA. Effects of chronic plus acute prolonged stress on measures of coping style, anxiety, and evoked HPA-axis reactivity. Neuropharmacology. 2012;63:1118–1126. doi: 10.1016/j.neuropharm.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hill MN, Hellemans KG, Verma P, Gorzalka BB, Weinberg J. Neurobiology of chronic mild stress: parallels to major depression. Neurosci Biobehav Rev. 2012;36:2085–2117. doi: 10.1016/j.neubiorev.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cooper ZD, Craft RM. Sex-dependent effects of cannabis and cannabinoids: A translational perspective. Neuropsychopharmacology. 2018;43:34–51. doi: 10.1038/npp.2017.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol. 2014;35:303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hanninen V, Aro H. Sex differences in coping and depression among young adults. Soc Sci Med. 1996;43:1453–1460. doi: 10.1016/0277-9536(96)00045-7. [DOI] [PubMed] [Google Scholar]
- 84.Wirtshafter D, Asin KE, Pitzer MR. Dopamine agonists and stress produce different patterns of Fos-like immunoreactivity in the lateral habenula. Brain Res. 1994;633:21–26. doi: 10.1016/0006-8993(94)91517-2. [DOI] [PubMed] [Google Scholar]
- 85.Shumake J, Edwards E, Gonzalez-Lima F. Opposite metabolic changes in the habenula and ventral tegmental area of a genetic model of helpless behavior. Brain Res. 2003;963:274–281. doi: 10.1016/s0006-8993(02)04048-9. [DOI] [PubMed] [Google Scholar]
- 86.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 87.Mirrione MM, Schulz D, Lapidus KA, Zhang S, Goodman W, Henn FA. Increased metabolic activity in the septum and habenula during stress is linked to subsequent expression of learned helplessness behavior. Front Hum Neurosci. 2014;8:29. doi: 10.3389/fnhum.2014.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ootsuka Y, Mohammed M. Activation of the habenula complex evokes autonomic physiological responses similar to those associated with emotional stress. Physiol Rep. 2015;3(2) doi: 10.14814/phy2.12297. pii: e12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dolzani SD, Baratta MV, Amat J, Agster KL, Saddoris MP, Watkins LR, Maier SF. Activation of a habenulo-raphe circuit Is critical for the behavioral and neurochemical consequences of uncontrollable stress in the male rat. eNeuro. 2016;3 doi: 10.1523/ENEURO.0229-16.2016. pii ENEURO.0229-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park H, Rhee J, Park K, Han JS, Malinow R, Chung C. Exposure to stressors facilitates long-term synaptic potentiation in the lateral habenula. J Neurosci. 2017;37:6021–6030. doi: 10.1523/JNEUROSCI.2281-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Seo JS, Zhong P, Liu A, Yan Z, Greengard P. Elevation of p11 in lateral habenula mediates depression-like behavior. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.96. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lecca S, Pelosi A, Tchenio A, Moutkine I, Lujan R, Hervé D, Mameli M. Rescue of GABAB and GIRK function in the lateral habenula by protein phosphatase 2A inhibition ameliorates depression-like phenotypes in mice. Nat Med. 2016;22:254–261. doi: 10.1038/nm.4037. [DOI] [PubMed] [Google Scholar]
- 94.Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Henn F, Malinow R. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Amat J, Sparks PD, Matus-Amat P, Griggs J, Watkins LR, Maier SF. The role of the habenular complex in the elevation of dorsal raphe nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Res. 2001;917:118–126. doi: 10.1016/s0006-8993(01)02934-1. [DOI] [PubMed] [Google Scholar]
- 96.Luo XF, Zhang BL, Li JC, Yang YY, Sun YF, Zhao H. Lateral habenula as a link between dopaminergic and serotonergic systems contributes to depressive symptoms in Parkinson’s disease. Brain Res Bull. 2015;110:40–46. doi: 10.1016/j.brainresbull.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 97.Yang LM, Hu B, Xia YH, Zhang BL, Zhao H. Lateral habenula lesions improve the behavioral response in depressed rats via increasing the serotonin level in dorsal raphe nucleus. Behav Brain Res. 2008;188:84–90. doi: 10.1016/j.bbr.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 98.Rey AA, Purrio M, Viveros MP, Lutz B. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABAB receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology. 2012;37:2624–2634. doi: 10.1038/npp.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Navarrete M, Diez A, Araque A. Astrocytes in endocannabinoid signalling. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130599. doi: 10.1098/rstb.2013.0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cui W, Mizukami H, Yanagisawa M, Aida T, Nomura M, Isomura Y, et al. Glial dysfunction in the mouse habenula causes depressive-like behaviors and sleep disturbance. J Neurosci. 2014;34:16273–16285. doi: 10.1523/JNEUROSCI.1465-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jacinto LR, Mata R, Novais A, Marques F, Sousa N. The habenula as a critical node in chronic stress-related anxiety. Exp Neurol. 2017;289:46–54. doi: 10.1016/j.expneurol.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 102.Selby G. Cerebral atrophy in parkinsonism. Proc Aust Assoc Neurol. 1968;5:299–303. [PubMed] [Google Scholar]
- 103.Holly EN, Miczek KA. Ventral tegmental area dopamine revisited: effects of acute and repeated stress. Psychopharmacology (Berl) 2016;233:163–186. doi: 10.1007/s00213-015-4151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


