Abstract
An enteric virus surrogate and reliable domestic wastewater tracer is needed to manage microbial quality of food and water as (waste)water reuse becomes more prevalent in response to population growth, urbanization, and climate change. Pepper mild mottle virus (PMMoV), a plant pathogen found at high concentrations in domestic wastewater, is a promising surrogate for enteric viruses that has been incorporated into over 29 investigations of water- and food-related microbial quality and technology around the world. This review consolidates the available literature from across disciplines to provide guidance on the utility of PMMoV as either an enteric virus surrogate and/or domestic wastewater marker in various situations. Synthesis of the available research supports PMMoV as a useful enteric virus process indicator since its high concentrations in source water allow for identifying the extent of virus log-reductions in field, pilot, and full-scale (waste)water treatment systems. PMMoV reduction levels during many forms of wastewater treatment were less than or equal to the reduction of other viruses, suggesting this virus can serve as an enteric virus surrogate when evaluating new treatment technologies. PMMoV excels as an index virus for enteric viruses in environmental waters exposed to untreated domestic wastewater because it was detected more frequently and in higher concentrations than other human viruses in groundwater (72.2%) and surface waters (freshwater, 94.5% and coastal, 72.2%), with pathogen co-detection rates as high as 72.3%. Additionally, PMMoV is an important microbial source tracking marker, most appropriately associated with untreated domestic wastewater, where its pooled-specificity is 90% and pooled-sensitivity is 100%, as opposed to human feces where its pooled-sensitivity is only 11.3%. A limited number of studies have also suggested that PMMoV may be a useful index virus for enteric viruses in monitoring the microbial quality of fresh produce and shellfish, but further research is needed on these topics. Finally, future work is needed to fill in knowledge gaps regarding PMMoV’s global specificity and sensitivity.
Keywords: Domestic wastewater, water reuse, fecal pollution, water quality, indicator, enteric pathogens
1. Introduction
Domestic wastewater pollution is a major, global public health concern due to the high concentrations of diverse pathogens in feces, the prevalence of wastewater pollution worldwide, and the important role water plays in everyday activities (UNICEF and World Health Organization, 2015). Specifically, enteric viruses are one the most problematic pathogen types and among the most important emerging waterborne pathogens because of their low infectious doses, high concentrations in human feces, long persistence in the environment, and resistance to (waste)water treatment (summarized in Fong and Lipp, 2005, Gerba et al., 2017, Lin and Ganesh, 2013, Nwachcuku and Gerba, 2004, Sinclair et al., 2009, Symonds and Breitbart, 2015, US National Research Council, 2012). Therefore, to manage the health risks associated with wastewater pollution and achieve the United Nations’ Sustainable Development Goals (United Nations, 2015), it is important to measure enteric viruses in environmental waters and (waste)water treatment and reuse systems.
Directly measuring enteric viruses in water and food can be difficult and expensive; consequently, surrogates are often used to indirectly measure the presence of enteric viruses (Ashbolt et al., 2001, Sinclair et al., 2012). In public health-related microbiology, a surrogate is a substance (e.g., caffeine) or microbe (e.g., human norovirus (HNoV), Escherichia coli) whose presence reflects pathogen persistence and can therefore be used to explore the risk of contamination in food and in the environment, as well as the efficacy of various (waste)water treatment technologies. With respect to human enteric pathogens, microbial surrogates are divided into the following categories: process indicators, index or model surrogates (also termed organisms), reference pathogens, and fecal indicators. Surrogates that characterize the efficacy of a specific treatment are classified as process indicators. Index and model surrogates are most frequently used to represent a specific pathogen type (e.g., E. coli for bacterial enteric pathogens), while reference pathogens are human pathogens that are used to infer the occurrence of similar pathogens (e.g., HNoV is a reference pathogen for enteric viruses). Finally, fecal indicators and markers refer to surrogates that identify the presence of fecal material and not necessarily the presence of a particular pathogen or group of pathogens. The World Health Organization (WHO) recommends monitoring the microbial quality of (waste)water and food using indicator organisms (e.g., fecal indicator bacteria (FIB), bacteriophages) and reference pathogens (e.g., HNoV) in lieu of measuring all of the human pathogens that are possibly present (World Health Organization, 2006;2009;2010;2011).
Over the last four decades, numerous studies have demonstrated that FIB, one of the most frequently used and easily measured water quality parameters (Ashbolt et al., 2001), do not correlate with or predict the presence of enteric viruses in treated (waste)water, shellfish, environmental waters, and fresh produce (Carducci et al., 2009, Eregno et al., 2018, Fout et al., 2017, Gerba et al., 2017, Griffin et al., 2001, Harwood et al., 2005, LaBelle et al., 1980, Li et al., 2015, Savichtcheva and Okabe, 2006, Schets et al., 2008, Sinclair et al., 2012, Stewart et al., 2008, Symonds et al., 2014, Verbyla et al., 2016, Wu et al., 2011, Yates, 2007). This is not surprising given the vastly different physical characteristics of FIB and enteric viruses, which results in disparate responses to treatment and environmental conditions. An alternate surrogate for enteric viruses is therefore needed in order to better predict risk and protect human health.
While a variety of bacteriophages can be measured with culture-based assays and are therefore affordable enteric virus surrogates to monitor fecal pollution and (waste)water treatment efficiency, these bacteriophages do not consistently correlate with the presence of enteric viruses (Eregno et al., 2018, Fout et al., 2017, Lin and Ganesh, 2013, Santiago-Rodriguez et al., 2013, Savichtcheva and Okabe, 2006). Additionally, a recently identified and abundant crAssphage has been proposed as a viral fecal pollution indicator (Dutilh et al., 2014, Stachler and Bibby, 2014); however, more research is needed to understand its correlation with infectious human enteric viruses in contaminated environments and throughout wastewater treatment before crAssphage can be used as an index or process enteric virus indicator (García-Aljaro et al., 2017, Stachler et al., 2017). The concentrations of some commonly used reference enteric viruses, such as HNoV or rotavirus group A (ARV), vary widely based on disease epidemiology, ranging from undetected to nearly 107 virus targets l−1 wastewater (Gerba et al., 2017, Kitajima et al., 2014). Other reference enteric viruses include human adenoviruses (HAdV), aichiviruses (HAiV), and polyomaviruses (HPyV), which are consistently measured at 104 to 107 virus targets l−1 domestic wastewater (Kitajima et al., 2014, Schmitz et al., 2016). However, since the abundance of reference enteric viruses found in wastewater is dependent upon the extent of infection and shedding in the population, their use as enteric virus surrogates to test (waste)water treatment technologies (Gerba et al., 2017, Kato et al., 2018, Li et al., 2015, Sano et al., 2016) and manage microbial water and food quality may be limited (Lin and Ganesh, 2013, Sinclair et al., 2012, Symonds and Breitbart, 2015, U.S. EPA, 2006, Verbyla et al., 2016).
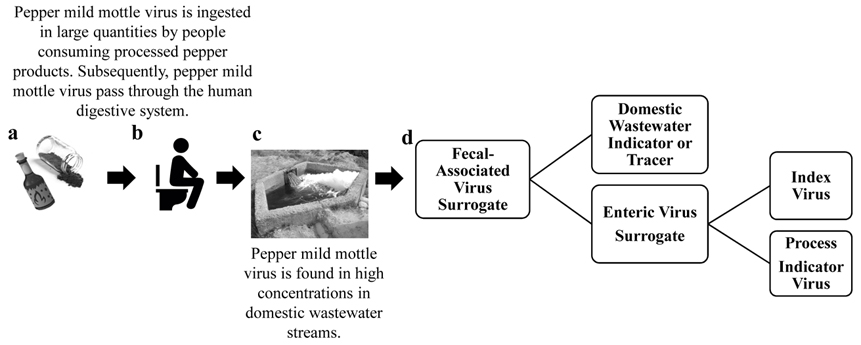
Pepper mild mottle virus (PMMoV) was first proposed as an indicator of fecal pollution for coastal, sub-tropical waters in 2009 (Rosario et al., 2009b) after it was identified by metagenomics as the dominant RNA virus in human feces and shown by qPCR to be present at concentrations as great as 109 viral targets g−1 dry weight (Zhang et al., 2006). Since Zhang et al. published their study in 2006, other metagenomic studies have verified the abundance of PMMoV in human feces (Moore et al., 2015, Nakamura et al., 2009, Victoria et al., 2009), untreated domestic wastewater (Wang et al., 2018), tertiary-treated domestic wastewater (Rosario et al., 2009a), and even indoor air filters (Rosario et al., 2018). Additionally, PMMoV has been incorporated in over 29 peer-reviewed and published investigations related to microbial water and food quality as well as (waste)water treatment technologies throughout the world (Ahmed et al., 2018, Asami et al., 2016, Betancourt et al., 2014, Fout et al., 2017, Gu et al., 2018, Hamza et al., 2011, Han et al., 2014, Haramoto et al., 2013, Hruby et al., 2013, Hughes et al., 2017, Kato et al., 2018, Kitajima et al., 2014, Kuroda et al., 2015, Lee et al., 2017, Rachmadi et al., 2015, Rosiles-González et al., 2017, Saeidi et al., 2018, Sangsanont et al., 2016, Sassi et al., 2018, Schmitz et al., 2016, Shirasaki et al., 2017, Shirasaki et al., 2018, Shrestha et al., 2018, Symonds et al., 2015, Symonds et al., 2016, Symonds et al., 2014, Symonds et al., 2017, Tandukar et al., 2018, Verbyla et al., 2016, Wang et al., 2018). The main purpose of this review is to consolidate the available information from a variety of different disciplines to understand how and when PMMoV can best be used as an enteric virus surrogate and/or domestic wastewater marker (Figure 1).
Figure 1:
The origin and use of pepper mild mottle virus (PMMoV) to assess the microbial quality of water and fresh food: a) large quantities of PMMoV are ingested by people consuming processed pepper products; b) which pass through the human digestive system; and c) enter domestic wastewater streams in high concentrations; which consequently, d) can be used to determine microbial quality with respect to fecal pollution as a domestic wastewater indicator or a surrogate for enteric viruses.
2. Methods
2.1. Literature search
All published, peer-reviewed wastewater treatment and water quality studies using PMMoV were identified via Google Scholar searches executed from May 2017 to May 2018. To identify these studies, Google Scholar searches were executed using the following keywords in May 2017: fecal pollution, pepper mild mottle virus, and wastewater pollution. At that time, Google Scholar alerts were created for studies citing previously published PMMoV-related articles (specifically Rosario et al., 2009b, Symonds et al., 2016, Symonds et al., 2014, Verbyla et al., 2016), as well as the aforementioned keywords. Lastly, an additional Google Scholar search using the same keywords was executed prior to manuscript publication June 2018 to ensure that all peer-reviewed, published, water quality studies using PMMoV had been identified and considered in this review.
2.2. Pooled data calculations
The data from a total of 22 peer-reviewed, published studies were reviewed and consolidated with respect to PMMoV detection, concentration, and pathogen co-occurrence. The pooled human-associated sensitivity (e.g., the percentage of PMMoV positive human fecal and domestic wastewater samples) and specificity (e.g., the percentage of PMMoV negative non-human fecal samples) were determined by consolidating the data from 16 and three investigations, respectively. Additionally, environmental water samples from 17 different published studies were classified by water type (groundwater, coastal seawater and brackish water, and inland freshwater) and subsequently by the type of domestic wastewater contamination, which was characterized as point or non-point sources of untreated or treated domestic wastewater. For each combination of environmental water sample type and type of domestic wastewater contamination, the pooled positive PMMoV detection rate, PMMoV minimum and maximum concentration reported, and the PMMoV pooled pathogen co-occurrence rate was determined. While the majority of studies specifically compared PMMoV presence to those of other enteric viruses, several studies also included enteric parasites and bacteria; thus, the pooled pathogen co-occurrence rate refers to the co-occurrence of PMMoV and any human enteric pathogen.
2.3. Review of the (waste)water treatment and water quality studies
This review summarizes all the previously published, and some unpublished, investigations that have incorporated the use of PMMoV as a fecal-associated virus surrogate. This manuscript begins by discussing PMMoV ecology and origin in human feces. Next, it summarizes how PMMoV is identified and quantified in a variety of sample types, followed by a review of PMMoV occurrence and persistence in natural environments and engineered-systems. A more detailed discussion is included on the use of PMMoV as a domestic wastewater microbial source tracking (MST) tool. Finally, this review discusses the compiled and pooled data, as well as identifies directions for future research.
3. Pepper mild mottle virus ecology and its origin in human feces
PMMoV is a rod-shaped, non-enveloped, positive-sense, single-stranded RNA virus approximately 18 nm in diameter and 300-310 nm in length, with an isoelectric point (the pH when the virus has a neutral charge) ranging from 3.7 – 3.8 (King et al., 2011, Michen and Graule, 2010, Wetter et al., 1984). Belonging to the Virgaviridae family and Tobamovirus genus, this pepper plant pathogen is transmitted via mechanical contact with contaminated soil and/or seed and causes fruit malformation and leaf mosaic in Capsicum species throughout Asia, Australia, Europe, Middle East, and North and South America (Adkins et al., 2001, Ahmad et al., 2015, Alonso et al., 1991, Beczner et al., 1997, Çağlar et al., 2013, Garcia-Luque et al., 1993, Genda et al., 2007, Han et al., 2017, Ichiki et al., 2009, Kaur et al., 2014, Milošević et al., 2015, Oliveira et al., 2010, Wang et al., 2006, Wetter et al., 1984, Yu et al., 2018). PMMoV has a 6,356 bp genome that encodes for a capsid, two replication-associated proteins (126 kDa and 183 kDa), and a cell-to-cell movement-associated protein (Banović Đeri et al., 2018, Oliveira et al., 2010). Given the limited genetic variability of PMMoV, it has been suggested that the PMMoV genome is highly stable (Rodriguez-Cerezo et al., 1989) and limited sequence variation has been observed among geographic regions (Yu et al., 2018).
Like many other plant pathogens belonging to the Virgaviradae family that are found in domestic wastewater (Cantalupo et al., 2011), PMMoV has a dietary origin in human feces (Colson et al., 2010, Zhang et al., 2006). In 2016, approximately 39 million tonnes of peppers were produced throughout the world (Food and Agriculture Organization of the United Nations, 2018). Processed pepper products, such as dry spices and sauces, from the United States of America, France, Mexico, China and elsewhere have tested positive for PMMoV and sauces can contain as many as 108.8 PMMoV targets ml−1 (Colson et al., 2010, Peng et al., 2015, Zhang et al., 2006). Remarkably, infectious PMMoV are present in food products as well as feces (Colson et al., 2010, Peng et al., 2015, Zhang et al., 2006). While PMMoV has not been reported to replicate in the human gut, scientists have hypothesized and investigated its replication in humans due to its high concentration in feces (Colson et al., 2010, Mandal and Jain, 2010, Zhang et al., 2006) and association with fever and other immune responses (Colson et al., 2010). Taken together, PMMoV’s genetic stability and its persistence in globally distributed processed pepper products and human feces make it an attractive domestic wastewater indicator, which is defined in this review as a microbe or chemical that identifies the presence of fecal material and not necessarily the presence of a particular pathogen or group of pathogens (Sinclair et al., 2012).
4. Identifying and quantifying pepper mild mottle virus
4.1. Virus isolation and/or concentration methods
Nucleic acid isolation is the first step in identifying PMMoV, and the methods to do so vary by sample type. For pepper produce in an agricultural context, PMMoV RNA is usually isolated from either the seeds or leaves of the infected Capsicum plant via mechanical disruption (Çağlar et al., 2013, Ichiki et al., 2009, Ikegashira et al., 2004, Kaur et al., 2014, Wang et al., 2006). In processed food products, domestic wastewater, and feces (supernatant), PMMoV RNA is often in high enough concentrations to be directly detected (Colson et al., 2010, Peng et al., 2015, Symonds et al., 2016). If PMMoV is suspected to be in lower concentrations, which is often the case in settings where fecal pollution is being measured, then virus concentration methods are necessary prior to detection assays.
Standard methods (ISO/TS15216-1, 2013) for viruses have been used to isolate and concentrate PMMoV from lettuce (Verbyla et al., 2016) and shellfish (Ingeborg Boxman; personal communication). For water matrices, the virus isolation and concentration methods vary by water type. PMMoV, as well as enteric viruses, in small-volume samples (< 2 l) are typically isolated using adsorption-elution methods with electronegative membrane filters (Betancourt et al., 2014, Han et al., 2014, Kato et al., 2018, Kitajima et al., 2014, Sangsanont et al., 2016, Sassi et al., 2018, Schmitz et al., 2016, Shrestha et al., 2018, Tandukar et al., 2018), which is sometimes followed by the concentration of the eluent using PEG precipitation (Hamza et al., 2011) or centrifugal filter concentrators (Asami et al., 2016, Kitajima et al., 2014, Kuroda et al., 2015, Lee et al., 2017, Rachmadi et al., 2015, Sangsanont et al., 2016, Sassi et al., 2018, Schmitz et al., 2016, Tandukar et al., 2018).
Alternatively, it is possible to directly purify virus nucleic acids from the electronegative membrane filter used in the adsorption-elution methods (Ahmed et al., 2018, Hughes et al., 2017, Symonds et al., 2014, Symonds et al., 2017, Verbyla et al., 2016). Centrifugal filter concentrators have also been used to directly concentrate smaller volumes (< 20 ml) of (waste)water when high virus concentrations were anticipated (Rosario et al., 2009b, Symonds et al., 2015). Viruses, including PMMoV, in higher-volume samples (> 30 l), typically used to test groundwater and potable water, have been concentrated with Nanoceram filters (Betancourt et al., 2014), glass-wool filters (Hruby et al., 2013), cartridge mixed cellulose ester filters (Kuroda et al., 2015), and hollow-fiber ultrafilters (Gu et al., 2018, McGinnis et al., 2018, Rosiles-González et al., 2017). Since all of the aforementioned virus concentration methods have been developed for enteric viruses that have a different capsid structure compared to PMMoV, future investigations should ensure that PMMoV is similarly concentrated (Haramoto et al., 2018). The theoretical process limit of detection, which assumes 100% recovery of PMMoV cDNA from the original volume samples, is often not reported; however, it can be as low as 4.28 targets l−1 when 1000-l volumes are concentrated (Hruby et al., 2013) and as high as 1000 targets l−1 when 50-ml volumes are concentrated (Shrestha et al., 2018). The use of process controls, which is usually a virus similar to the enteric reference virus analyzed, is necessary to ensure effective virus concentration and detection (Haramoto et al., 2018). Recently, one study incorporated cucumber green mottle mosaic virus, also belonging to the Tobamovirus genus, as a virus concentration process control for PMMoV (Kato et al., 2018).
4.2. Detection and quantification methods
While PMMoV-like particles can be visualized using electron microscopy (Colson et al., 2010, Peng et al., 2015), PMMoV is most frequently detected using either molecular- or immunological-based methods in agriculture (Çağlar et al., 2013, Ikegashira et al., 2004, Kaur et al., 2014, Wang et al., 2006). Rapid, lab-free immunological-based tests designed for agricultural contexts are available to test leaf tissue or seeds for PMMoV (ImmunoStrip®, Agdia, Elkhart, IN, USA). Reverse transcriptase-polymerase chain reaction (RT-PCR; Beczner et al., 1997, Çağlar et al., 2013, Kaur et al., 2014, Milošević et al., 2015, Wang et al., 2006) is frequently used to identify PMMoV and the complete genomes of several strains have been sequenced (Alonso et al., 1991, Avila-Rincon et al., 1989, Garcia-Luque et al., 1990, Hagiwara et al., 2002, Oliveira et al., 2010, Velasco et al., 2002, Wang et al., 2006). In contrast to agriculture, molecular-based methods are primarily used for detecting PMMoV in microbial water and food quality contexts (Hamza et al., 2011, Haramoto et al., 2013, Rosario et al., 2009b, Zhang et al., 2006) and methods have even been created to discriminate between infectious and non-infectious PMMoV particles (Lee et al., 2018).
In fecal pollution studies, RT-PCR and RT-quantitative PCR (RT-qPCR) are the most common forms of molecular detection and quantification of PMMoV. A variety of RNA purification kits have been used successfully, with the most appropriate kit dependent on the sample type (Asami et al., 2016, Betancourt et al., 2014, Colson et al., 2010, Haramoto et al., 2013, Symonds et al., 2015, Symonds et al., 2016, Symonds et al., 2014, Symonds et al., 2017, Verbyla et al., 2016). Reverse transcription is generally executed as a two-step process, in which purified RNA is reversed transcribed with random hexamer primers using the First-Strand Synthesis Superscript III reverse transcription kit (Invitrogen, Carlsbad, CA, USA; Ahmed et al., 2018, Hughes et al., 2017, Rosario et al., 2009b, Rosiles-González et al., 2017, Symonds et al., 2015, Symonds et al., 2016, Symonds et al., 2014, Symonds et al., 2017, Verbyla et al., 2016) or the High Capacity cDNA reverse transcription kit (Life Technologies, Carlsbad, CA, USA; (Asami et al., 2016, Betancourt et al., 2014, Haramoto et al., 2013, Kato et al., 2018, Kitajima et al., 2014, Kuroda et al., 2015, Lee et al., 2017, Rachmadi et al., 2015, Sassi et al., 2018, Schmitz et al., 2016, Shirasaki et al., 2017, Shirasaki et al., 2018, Shrestha et al., 2018, Tandukar et al., 2018). Less frequently, a one-step RT approach has been taken using the Quantitect probe one-step RT-PCR kit (Qiagen, Hilden, Germany; Hamza et al., 2011) or the Superscript III Platinum One-Step Quantitative RT-PCR System (Life Technologies, Carlsbad, CA, USA; Colson et al., 2010).
Several endpoint RT-PCR and RT-qPCR assays have been developed, which target either the PMMoV capsid or replication-associated proteins (Table 1). Although some of the PCR assays were developed for infected Capsicum specimens, Haramoto et al. (2013) and Rosiles-Gonzalez et al. (2017) have used these assays for water matrices (Çağlar et al., 2013) and processed food products (Peng et al., 2015), respectively. Additionally, the PCR assay developed by Zhang et al. (2006) has also been used to test feces and (waste)water matrices (Symonds et al., 2017). With respect to quantitative methods, Hamza et al. (2009) created a SYBR-based RT-qPCR assay with a reported detection limit of 5 targets. The PMMoV hydrolysis qPCR assay, which is preceded by a separate RT reaction, was originally designed by Zhang et al. (2006) and then the forward primer was modified by the addition of a thymine nucleotide to better match PMMoV isolates (Haramoto et al., 2013). Five years ago, Haramoto et al. (2013) replaced the TAMRA quencher with a minor groove binding nonfluorescent quencher (MBGNFQ); this modification lowered the limit of quantification from 100 targets to 10 targets. While many papers have utilized the Zhang et al. primers and probes (2006; Ahmed et al., 2018, Colson et al., 2010, Hruby et al., 2013, Hughes et al., 2017, Peng et al., 2015, Rosario et al., 2009b, Sangsanont et al., 2016, Symonds et al., 2014), most recent papers have used the PMMV-FP1-rev and PMMV-RP1 primers, with the PMMV-Taqman MGB probe or equivalent type of probe (Asami et al., 2016, Betancourt et al., 2014, Gu et al., 2018, Haramoto et al., 2013, Kato et al., 2018, Kitajima et al., 2014, Kuroda et al., 2015, Lee et al., 2018, Lee et al., 2017, Rachmadi et al., 2015, Rosiles-González et al., 2017, Saeidi et al., 2018, Sassi et al., 2018, Schmitz et al., 2016, Shirasaki et al., 2017, Shirasaki et al., 2018, Shrestha et al., 2018, Symonds et al., 2015, Symonds et al., 2016, Symonds et al., 2017, Tandukar et al., 2018, Verbyla et al., 2016).
Table 1.
The reverse transcription-PCR and qPCR assays most commonly used to detect PMMoV in (waste)water and food matrices.
| Assay type | Oligonucleotide name | Sequence (5’ – 3’) | Target gene | Amplicon size (bp) | Thermocycler conditions |
Citation |
|---|---|---|---|---|---|---|
| PCR | PMMV-F | AACCTTTCCAGCACTGCG | Replication- associated protein |
201 | 94°C 5 min, 40x (94°C 1 min, 50°C 45 s, 72°C 1 min), 72°C 5 min |
Zhang et al. 2006 |
| PMMV-R | GCGCCTATGTCGTCAAGACT | |||||
| PCR | PMMoV-CP forward | ATGGCTTACACAGTTTCCAGT | Capsid protein |
474 | 94°C 3 min, 40x (94°C 30 s, 55°C 30 s, 72°C 30 s) |
Peng et al. 2015 |
| PMMoV-CP reverse | CTAAGGAGTTGTAGCCCAGGTG | |||||
| PCR | CP/s | ATGGCATACACAGTTACCAGT | Capsid protein |
470 | 94°C 2 min, 40x (94°C 1 min, 49°C 45 s, 72°C 1 min), 72°C 7 min |
Çağlar et al. 2013 |
| CP/a | TTAAGGAGTTGTAGCCCACGTA | |||||
| qPCR (SYBR green) |
Ha-PMMV1 | GTGGCAGCAAAGGTAATGGT | Replication- associated protein |
80 | 95°C 15 min, 45x (58°C 45 s, 72°C 15 s) |
Hamza et al. 2011 |
| Ha-PMMV2 | ATTTGCTTCGGTAGGCCTCT | |||||
| PM1602 | TGTTTCGGAAAAGGCTCTTG | |||||
| qPCR (hydrolysis probe) |
PMMV-FP1 | GAGTGGTTTGACCTTAACGTTGA | Replication- associated protein |
68 | 95°C 10 min, 40x (95°C 30 s, 60°C 1 min) |
Zhang et al. 2006 |
| PMMV-RP1 | TTGTCGGTTGCAATGCAAGT | |||||
| PMMV-probe1 | 6FAM-CCTACCGAAGCAAATG-TAMRA | |||||
| PMMV-FP1-rev | GAGTGGTTTGACCTTAACGTTTGA | Haramoto et al. 2013 | ||||
| PMMV- Taqman MGB probe | 6FAM-CCTACCGAAGCAAATG-MGB-NFQ |
5. Occurrence and persistence in natural environments and engineered-systems
5.1. Domestic wastewater and treatment
Since people consume food products cultivated and produced from around the world, it is reasonable to believe that PMMoV will be found in domestic wastewater worldwide; however, studies are lacking to prove this assertion. To date, PMMoV targets have been detected in domestic wastewater from Australia (Hughes et al., 2017), Bolivia (Symonds et al., 2014), Brasil (Verbyla & Symonds, unpublished data), Costa Rica (Symonds et al., 2017), Germany (Hamza et al., 2011), Guatemala (Symonds & Breitbart, unpublished data), Japan (Lee et al., 2017), Kenya (Nicolette Zhou & Scott Meschke, personal communication), Nepal (Shrestha et al., 2018), South Korea (Han et al., 2014), the United States of America (Kitajima et al., 2014, Rosario et al., 2009b, Schmitz et al., 2016), and Vietnam (Kuroda et al., 2015); PMMoV is typically found at concentrations ranging from 106 to 1010 targets l−1 in untreated domestic wastewater (Table 2). PMMoV abundance in domestic wastewater did not significantly vary when measured daily for two weeks (Rosario et al., 2009b) nor monthly for one year (Kitajima et al., 2014). Additionally, PMMoV is consistently more abundant than other human enteric viruses and proposed virus surrogates in domestic wastewater (Bofill-Mas et al., 2006, Carducci et al., 2009, Jothikumar et al., 2005, Kitajima et al., 2014, Laverick et al., 2004, Schmitz et al., 2016, Symonds et al., 2014, Symonds et al., 2017). The consistently high concentrations of PMMoV in untreated domestic wastewater make it a good candidate as an index virus for enteric viruses in polluted environmental waters, produce, and shellfish, as well as a process indicator for measuring enteric virus removal during (waste)water treatment and water reclamation.
Table 2.
Sensitivity of pepper mild mottle virus (PMMoV) to domestic wastewater in target (human-associated) samples pooled from multiple studies (n = number of samples; sensitivity is the percentage of PMMoV positive human fecal and domestic wastewater samples). PMMoV concentrations are reported where available.
| Fecal source | Human- associated sensitivity (n) |
Units | Minimum concentration |
Maximum concentration |
Median concentration |
Countryb | Citation(s) |
|---|---|---|---|---|---|---|---|
| Adult human feces | 10.6% (321) | targets g−1 dry |
1.00 × 106 | 1.00 × 109 | n/a | FR, SG, US | Colson et al. 2010, Zhang et al. 2006 |
| Adult human feces | 95.0% (20) | targets g−1 wet |
3.80 × 105 | 9.80 × 109 | 1.93 × 108 | DE | Hamza et al. 2011 |
| Child human feces | 0.7% (137) | targets g−1 dry |
n/a | n/a | n/a | FR | Colson et al. 2010 |
| Wastewater treatment plant effluenta |
97.7% (133) | targets l−1 | < 6.60 × 101 | 6.31 × 109 | n/a | AU, BO, DE, JP, NP, US, VN |
Betancourt et al. 2014, Hamza et al. 2011, Hughes et al. 2017, Kitajima et al. 2014, Kuroda et al. 2015 Lee et al. 2017, Rosario et al. 2009b, Schmitz et al. 2016, Symonds et al. 2014, Sassi et al. 2018 |
| Untreated domestic wastewater |
100.0% (126) | targets l−1 | 1.00 × 106 | 2.00 × 1010 | n/a | AU, BO, CR, DE, NP, US, VN |
Hamza et al. 2011, Hughes et al. 2017, Kitajima et al. 2014, Kuroda et al. 2015, Rosario et al. 2009b, Schmitz et al. 2016, Shrestha et al. 2018, Symonds et al. 2014, Symonds et al. 2016, Symonds et al. 2017, Verbyla et al. 2016 |
All types of domestic wastewater treatment effluent samples (e.g. activated sludge, modified bardenpho, etc.) were pooled for simplicity.
Australia (AU), Bolivia (BO), Costa Rica (CR), France (FR), Germany (DE), Japan (JP), Nepal (NP), Singapore (SG), United States of America (US), Vietnam (VN)
In full-scale domestic wastewater effluent, PMMoV has been consistently found in concentrations ranging from 101 – 109 targets l−1 (Table 2), with reduction levels less than or equal to the reduction of other viruses (Hamza et al., 2011, Hughes et al., 2017, Kitajima et al., 2014, Kuroda et al., 2015, Rosario et al., 2009b, Schmitz et al., 2016, Shrestha et al., 2018, Symonds et al., 2014). To date, a benchtop electrocoagulation unit is the only wastewater treatment technology that has demonstrated PMMoV reductions to concentrations below the limit of detection, corresponding to reductions as great as 5-log10, (Symonds et al., 2015).The greatest PMMoV target reductions on full-scale systems were observed at two US wastewater treatment plants (WWTPs) using advanced Bardenpho technologies, in which PMMoV targets were reduced as great as 4-log10 and coincided with greater human virus reductions (Schmitz et al., 2016). For a conventional activated sludge system in Germany, approximately 3-log10 PMMoV target reduction was observed, which was similar to reductions of HPyV, torque teno virus, and slightly greater than culturable somatic coliphage (Hamza et al., 2011). Rosario et al. (2009b) determined similar log10-reductions for some conventional activated sludge systems with disinfection in the United States of America; however, one third of the systems analyzed had as ≤ 1-log10 PMMoV target reductions.
Several other studies, analyzing a variety of full-scale domestic WWTPs, also demonstrated approximately ≤ 1-log10 PMMoV reductions. At an Australian activated sludge WWTP with disinfection, a 1.1-log10 PMMoV target reduction was observed, which was approximately 2-log10 less than the reduction of HAdV and HPyV; HNoV and HEV reductions were slightly greater (≤2-log10) than those observed for PMMoV (Hughes et al., 2017). Similarly, PMMoV and HAdV had ≤ 1-log10 target reductions at a sequential batch reactor WWTP in Vietnam (Kuroda et al., 2015). In two wastewater treatment pond systems in Bolivia, Symonds et al. (2014) also observed ≤ 1-log10 PMMoV, HNoV, and ARV reductions; however, culturable human enteroviruses (HEV) were reduced by ≤ 1-log10. In two other studies on virus reduction during activated sludge and biological trickling filter, PMMoV had < 1-log10 reductions, which was similar to HAiV and coincided with minimum reductions in HNoV, sapovirus (HSaV), HEV, HAdV, HPyV, and ARV (Kitajima et al., 2014, Schmitz et al., 2016).
5.2. Drinking water treatment and reclamation systems
The consistently high PMMoV concentrations present in domestic wastewater make PMMoV a potential process indicator of enteric virus removal in drinking water treatment and reclamation systems. Currently, PMMoV has been incorporated into seven investigations researching the viral log-reduction in point-of-use (Sangsanont et al., 2016) as well as drinking water treatment plant technologies (e.g., coagulation, rapid sand filtration, membrane filtration; (Asami et al., 2016, Kato et al., 2018, Lee et al., 2017, Shirasaki et al., 2017, Shirasaki et al., 2018). Unlike HAdV, HAiV, HEV, PMMoV, and F-specific coliphage, PMMoV was the only waterborne virus detected in drinking water sources (e.g., pond and tapwater) prior to point-of-use treatment in a study executed in Vietnam; thus, PMMoV was an essential enteric virus process indicator during point-of-use field testing (Sangsanont et al., 2016). In addition, laboratory testing determined that its log-removal was comparable to that of HAdV and HAiV and less than HEV (Sangsanont et al., 2016). PMMoV was also a useful process indicator of enteric viruses in a pilot-scale treatment plant as well as full-scale drinking water treatment plants due to its consistently high concentrations in source water in Japan (Kato et al., 2018, Lee et al., 2017) and Thailand (Asami et al., 2016).
In addition to its high concentrations in source water, PMMoV removal was similar to that of human viral pathogens of interest and rarely overestimated removal of these viruses by the drinking water treatment technologies tested to date (Asami et al., 2016, Kato et al., 2018, Lee et al., 2017, Shirasaki et al., 2017, Shirasaki et al., 2018). No correlation was identified between human viral pathogens and FIB in source water and post-treatment waters; FIB had greater log-removal than that of PMMoV and other viruses (Asami et al., 2016, Kato et al., 2018, Sangsanont et al., 2016). PMMoV removal was most significantly, positively correlated with the removal of HAdV, HAV, murine NoV (MNV), HAiV, HNoVGII, and human coxsackieviruses (HCV) via coagulation-rapid sand filtration as well as via membrane filtration procesesses; thus, it is a more appropriate process indicator of viral removal in comparison to bacteriophages MS2 and φX174, which did not correlate as well with human viral pathogens (Kato et al., 2018, Shirasaki et al., 2017, Shirasaki et al., 2018). In another study on ultrafiltration with coagulation-sedimentation virus removal, PMMoV removal rates were also similar to human enteric viruses (HNoV and HAiV); however, only culturable bacteriophage demonstrated additional virus removal when coagulation-sedimentation was combined with ultrafiltration (Lee et al., 2017). Despite PMMoV’s utility as an enteric virus removal process indicator, it is possible that PMMoV may need to be combined with a culturable assay or other type of selective treatment for infectious particles (e.g. (Lee et al., 2018) in order to effectively measure virus inactivation during treatment.
5.3. Contaminated surface waters
Since PMMoV was first proposed as an indicator of fecal pollution, this virus has been incorporated into 16 published studies analyzing microbial quality of freshwater, brackish waters, and seawater with and without known sources of domestic wastewater pollution in Australia (Hughes et al., 2017), Bolivia (Verbyla et al., 2016), Costa Rica (Symonds et al., 2017), Germany (Hamza et al., 2011), Japan (Haramoto et al., 2013), Mexico (Rosiles-González et al., 2017), Nepal (Shrestha et al., 2018, Tandukar et al., 2018), Singapore (Gu et al., 2018, Saeidi et al., 2018), United States of America (Betancourt et al., 2014, McGinnis et al., 2018, Rosario et al., 2009b, Sassi et al., 2018, Symonds et al., 2016), and Vietnam (Kuroda et al., 2015). It is important to note that PMMoV has not been detected in environmental waters in the absence of human fecal pollution and that PMMoV RNA persisted in seawater for approximately one week at temperatures ranging from 31 to 33°C, and in river water for approximately 10 days at 25°C and as long as 21 days at 4°C (Hamza et al., 2011, Rosario et al., 2009b). The persistence of PMMoV in surface freshwater was comparable to caffeine in surface freshwater contaminated with known, point sources of domestic wastewater (Kuroda et al., 2015). Additionally, PMMoV detection exceeded the detection of other enteric viruses in rivers dominated by domestic wastewater treatment effluent, and was detected as far as 32 km downstream from the discharge pipe (Sassi et al., 2018). PMMoV did not significantly correlate with FIB in coastal waters (Hughes et al., 2017, Symonds et al., 2016, Symonds et al., 2017) or inland freshwater (Hamza et al., 2011, McGinnis et al., 2018, Symonds et al., 2016). However, one study in Singapore identified significant positive correlations between PMMoV and the FIB Enterococci and E. coli in fresh surface waters (Saeidi et al., 2018). PMMoV concentrations were also correlated with residential and green areas in Singapore (Saeidi et al., 2018).
In coastal waters, PMMoV was detected in 72.2%, 52.9%, and 11.4% of surface water samples exposed to ocean outfalls discharging secondary-treated, chlorinated domestic waster; known non-point domestic wastewater sources; and possible but unconfirmed domestic wastewater pollution, respectively (Table 3; Hughes et al., 2017, Rosario et al., 2009b, Symonds et al., 2016, Symonds et al., 2017). Sixty-one percent of coastal water samples exposed to ocean outfalls discharging secondary-treated and chlorinated domestic wastewater were positive for both PMMoV and at least one human pathogen (e.g., HPyV, HNoV, HAdV, HEV) and this percentage decreased to 0% as the extent of known domestic wastewater pollution decreased (Table 3). In coastal waters without known domestic wastewater pollution, it is possible that PMMoV may persist for longer periods of time in comparison to other enteric viruses because PMMoV concentrations were quantified at 104 targets l−1 with a 0% pathogen co-occurrence; however, care is needed when making this observation since the sample size was limited to just five positive samples. In coastal waters exposed to point and non-point domestic wastewater pollution, PMMoV exhibited a significant positive correlation with the human-associated MST markers, Bacteroides HF183 and HPyV (Symonds et al., 2016); however, in coastal waters exposed to stormwater runoff, PMMoV did not co-occur with Bacteroides HF183, which was present more often and in greater concentrations (Hughes et al., 2017).
Table 3.
Pooled co-occurrence rates of pepper mild mottle virus and human pathogens in environmental waters (n = number of samples) from the studies cited. Minimum and maximum PMMoV concentrations reported are those measured above the limit of quantification by RT-qPCR, as available in the literature, for all the studies cited.
| Water type | Extent of domestic wastewater contamination |
Pooled PMMOV detection rate |
Minimum concentration reported (targets l−1) |
Maximum concentration reported (targets l−1) |
Pooled pathogen co-occurrence rate |
Citation(s) |
|---|---|---|---|---|---|---|
| Groundwater | Managed aquifer recharge, treated |
72.2% (n = 18) |
1.35 × 101 | 1.44 × 106 | 23.1% | Betancourt et al. 2014 |
| Known non-point sources |
35.3% (n = 102) |
4.00 × 10−1 | ~1.00 × 107 | 5.3%a |
Hruby et al. 2013, Kuroda et al. 2015, Rosiles-González et al. 2017, Shrestha et al. 2018 |
|
| Coastal seawater and brackish water | Outfall, secondary- treated and chlorinated |
72.2% (n = 18) |
1.99 × 102 | 6.00 × 107 | 61.1% |
Rosario et al. 2009b, Symonds et al. 2016 |
| Known non-point sources | 52.9% (n = 17) |
1.90 × 102 | 1.92 × 103 | 11.8% | Symonds et al. 2016 | |
| Unknown sources |
11.4% (n = 44) |
3.60 × 104 | 8.60 × 104 | 0% |
Hughes et al. 2017, Symonds et al. 2017 |
|
| Inland freshwater |
Point sources, untreated | 94.2% (n = 69) |
2.23 × 101 | 5.01 × 108 | 72.3% |
Kuroda et al. 2015, Verbyla et al. 2016, Shrestha et al. 2018, McGinnis et al. 2018 |
| Point sources, secondary-treated & disinfected | 86.3% (n = 335) |
2.49 × 102 | 2.90 × 106 | 67.5% |
Hamza et al. 2011, Haramoto et al. 2013, Betancourt et al. 2014, Sassi et al. 2018 |
|
| Unknown sources |
67.5% (n = 209) |
9.58 × 102 | 7.63 × 103 | n/ab | Gu et al. 2018, Saeidi et al. 2018 |
Seventeen of the PMMoV positive samples were not analyzed for human pathogens; thus, the pooled pathogen co-occurrence rate excludes these samples.
One hundred and eighteen of the PMMoV positive samples were not analyzed for human pathogens. While Gu et al. 2018 analyzed all samples for human pathogens, it was impossible based upon their manuscript to determine the pooled pathogen co-occurrence rate for PMMoV.
Initially it was thought that PMMoV may be an ultraconservative fecal pollution indicator due to its persistence throughout wastewater treatment (Hamza et al., 2011, Kuroda et al., 2015, Rosario et al., 2009b, Sassi et al., 2018); however, recent coastal fecal pollution studies have shown that PMMoV detection appropriately relates to risk of illness (Ahmed et al., 2018, Hughes et al., 2017, Symonds et al., 2016). For example, two of these investigations on the PMMoV process limits of detection and quantification demonstrated that the mere detection of PMMoV in coastal water samples seeded with untreated and secondary treated domestic wastewater corresponded to health risks of HNoV and HAdV infection exceeding the United States of America Environmental Protection Agency (U.S. EPA) health benchmark (36/1000 people) for safe recreation (Ahmed et al., 2018, Symonds et al., 2016). Specifically, 5.44×103 PMMoV targets l−1 corresponded to the health benchmark for HNoV and HAdV illness when coastal water was contaminated with untreated domestic wastewater (Ahmed et al., 2018). For coastal water contaminated with secondary treated domestic wastewater, 2.00 × 104 PMMoV targets l−1 and 1.78 × 103 PMMoV targets l−1 corresponded to the health benchmark for HNoV and HAdV, respectively. The health risks of HNoV and HAdV infection associated with detectable and quantifiable PMMoV concentrations, per untreated and secondary treated domestic wastewater pollution, were similar to those previously identified for other human-associated fecal markers; however, HF183 was more sensitive and was able to identify contamination below the health benchmark (Ahmed et al., 2018, Boehm et al., 2015, Staley et al., 2012, Symonds et al., 2016). In comparison to HPyV, PMMoV may be a more sensitive index virus for enteric viruses because HPyV limit of detection and quantification corresponded to health risks exceeding the U.S. EPA health benchmark (Ahmed et al., 2018, Staley et al., 2012).
Moving inland to freshwater systems, PMMoV was detected in 90.8%, 86.3%, and 67.5% of river, creek, canal, and pond surface waters exposed to point sources of untreated domestic wastewater, secondary treated domestic wastewater, and unknown sources, respectively (Table 3). PMMoV co-detection with human pathogens in inland freshwater systems exposed to point source pollution was 72.3% when domestic wastewater was not treated and 67.5% when it was treated; these rates are greater than the rates observed for coastal seawater and groundwater. Additionally, PMMoV significantly correlated with enteric viruses and HPyV even though it was more prevalent in microbial water quality investigations in Germany (Hamza et al., 2011) and Nepal (Tandukar et al., 2018). Despite similar co-detection rates of PMMoV and human pathogens in systems receiving treated versus untreated wastewater, PMMoV concentrations were greatest in surface freshwater exposed to untreated domestic wastewater, which includes studies executed in regions lacking sanitation (Kuroda et al., 2015, Shrestha et al., 2018, Verbyla et al., 2016) as well as those exposed to combined-sewer overflows (CSOs; (McGinnis et al., 2018). Unlike the studies executed in areas lacking sanitation, CSO-creek water had the lowest PMMoV concentrations and only 57% of CSO-creek water samples positive for PMMoV were also positive for at least one of the following pathogens: HAdV, HEV, HNoVGI, HNoVGII, Campylobacter jejuni, Enterohemorragic E. coli, and Salmonella (McGinnis et al., 2018). Interestingly, PMMoV and human-marker Bacteroides HF183 were detected in all creek water samples collected for this study despite problems with RT-qPCR inhibition.
5.4. Groundwater
PMMoV has been integrated into five groundwater studies in North America and Asia. PMMoV was detected in 72.2% and 35.3% of groundwater samples collected from areas with known point source and non-point source domestic wastewater pollution, respectively, in concentrations ranging from 0.4 to 1.00 × 107 targets l−1 (Table 3). The first groundwater fecal pollution study to include PMMoV was executed in Iowa, USA and only 22% of the wells sampled (n = 66) tested positive for at least one fecal-associated microorganism (Hruby et al., 2013). PMMoV was the most frequently detected microbial target (found in 17% of samples); however, no correlation was identified between PMMoV and any of the microbial indicators (E.coli, enterococci, total coliforms, male-specific and somatic coliphage) or pathogens (HNoV, HAdV, HPyV, bovine polyomavirus, swine hepatitis E, Salmonella, Campylobacter, and Enterohemorrhagic E.coli). In another study, groundwater collected near managed aquifer recharge sites in the USA more frequently contained PMMoV, which often co-occurred with the presence of pathogens (Betancourt et al., 2014).
Outside of the USA, PMMoV was found in only 38% of samples (n =8) while total coliforms and E. coli were found in 78% and 11%, respectively, in Vietnam (Kuroda et al., 2015). In an area with extensive domestic wastewater pollution in Nepal, PMMoV and tobacco mosaic virus were detected in 春83% of groundwater samples (n = 6), while HNoV was only found in one sample and HSaV, HEV, HAiV, HAdV, HPyV, and ARV were not detected (Shrestha et al., 2018). In the northeastern Yucatan Peninsula aquifer system in Mexico, PMMoV was detected in 85% of sink hole samples (mixture of fresh and brackish water; n = 20), while coliphages were found in 80% of samples and total coliforms exceeded concentrations of 770 MPN/100 ml in 100% of samples (Rosiles-González et al., 2017). No correlation was identified between PMMoV and coliphages, total coliforms, or E. coli in this study; unfortunately, no pathogens were analyzed. Given the limited sample size and number of investigations and the difficulties measuring enteric viruses in groundwater, the correlation and co-detection of PMMoV and various pathogens in groundwater remains to be determined (Table 3). Nevertheless, PMMoV appears to be a conservative surrogate for enteric viruses in groundwater given its presence in greater concentrations compared to human viruses (Betancourt et al., 2014, Hruby et al., 2013, Kuroda et al., 2015, Shrestha et al., 2018).
5.5. Bottled water and food safety
The use of PMMoV as an indicator of microbial quality in food and bottled water has yet to be fully explored. In a study comparing PMMoV and pharmaceuticals and personal care products (PPCPs) as tracers of fecal pollution in a watershed, PMMoV was not identified in bottled water (n = 3) despite the occurrence of PPCPs (n = 2), FIB (n = 1), and/or HAdV (n = 1) (Kuroda et al., 2015). Based upon this study, it appears that PMMoV could underestimate and PPCPs could overestimate the extent of fecal pollution in bottled water. PMMoV has also been used in two studies investigating microbial quality of irrigation water, in which is most detected more frequently than human viral pathogens (Shrestha et al., 2018, Verbyla et al., 2016). Only Verbyla et al. (2016) measured crop microbial quality and demonstrated that PMMoV may be a conservative enteric virus surrogate to manage the microbial quality of crops irrigated with wastewater-contaminated water sources. Future studies are needed to ascertain the role of PMMoV as an enteric virus surrogate in these contexts.
To date, PMMoV has only been incorporated into one investigation on shellfish microbial quality, which has not been published previously (Ingeborg Boxman; personal communication). In a Dutch program (2008-2009) monitoring shellfish on the Dutch market and directly collected from European harvesting areas, viruses were isolated from shellfish samples using standard methods (ISO/TS15216-1, 2013). PMMoV was detected in a higher percentage of oyster and mussel samples than NoV, with both viruses assayed by RT-qPCR. PMMoV and NoV were detected in 65% (75/115) and 18% (21/115) of the oyster samples, respectively, whereas PMMoV and NoV were detected in 74% (64/86) and 42% (36/86) of the mussels sampled. Out of the NoV-positive shellfish samples, PMMoV co-occurred with NoV in 86% (18/21) of oysters and 92% (33/36) of mussels. Out of the PMMoV-positive shellfish samples tested, NoV co-occurred in 24% (18/75) of oysters and 52% (33/64) of mussels. Thus, PMMoV shows promise as a conservative viral indicator of microbial quality in shellfish; future studies are needed to understand how PMMoV depuration and retention rates compare with pathogens of interest.
6. Pepper mild mottle virus as a domestic wastewater microbial source tracking marker
MST was developed to discriminate between human and wildlife sources of fecal pollution in water bodies. MST markers aim to target genes of microbes that are specific to the host species (specificity) and prevalent within the host species (sensitivity; as summarized in (Harwood et al., 2013b)). Identifying genes of human-associated bacteria and viruses are of particular interest because sewage pollution poses a greater threat to human health than wildlife sources in most cases (Harwood et al., 2013b, Soller et al., 2010, Stoeckel and Harwood, 2007). The most routinely used human-associated markers include HF183, HPyV, and HumM2 and HumM3 (summarized in (Harwood et al., 2013b). These and other human-associated markers are often used in conjunction with wildlife markers to characterize fecal contamination in impaired water bodies. MST methodologies ultimately help identify dominant sources of fecal contamination, leading to more informed water quality assessment and targeted remediation practices.
PMMoV has been utilized in many fecal pollution studies, but only three studies to date have specifically assessed its potential as a domestic wastewater marker by testing for specificity (i.e., the ratio of PMMoV positive non-human feces samples to total non-human feces tested) in fecal samples from chickens, cows, dogs, horses, pigs, seabirds, and other organisms (Table 4; Hamza et al., 2011, Rosario et al., 2009b, Symonds et al., 2017). While specificity testing across non-target organisms remains geographically limited to Costa Rica, Germany, and the United States of America, sensitivity testing has occurred with numerous types of domestic wastewater samples across ten countries. PMMoV sensitivity across numerous studies was 97.9% (n = 446) in domestic wastewater and/or effluent undergoing different treatments, river water exposed to WWTP effluent, and surface water exposed to untreated wastewater (Tables 2 and 3). The specificity and sensitivity of PMMoV as a domestic wastewater associated marker are similar to those of HF183, HPyVs, HumM2, HumM3, and other human-associated MST markers (summarized in Harwood et al., 2013b).
Table 4.
Human-associated specificity of pepper mild mottle virus (PMMoV) in non-target (animal) fecal samples from multiple studies (n = number of samples; specificity is the percentage of PMMoV negative, non-human fecal samples). PMMoV concentrations are reported where available in the literature.
| Fecal source |
Human- associated specificity (n) |
Minimum concentration (targets g−1) |
Maximum concentration (targets g−1) |
Median concentration (targets g−1) |
Countrya | Citation(s) |
|---|---|---|---|---|---|---|
| Chicken | 50.0% (16) | 2.40 × 105 | 2.20 × 107 | 7.30 × 105 | DE, US | Hamza et al. 2011, Rosario et al. 2009b |
| Seagull | 70.6% (17) | 5.84 × 105 | 9.55 × 105 | n/a | US | Rosario et al. 2009b |
| Seabirds | 100.0% (8) | n/a | n/a | n/a | CR | Symonds et al. 2017 |
| Geese | 90.0% (10) | n/a | n/a | 9.0 × 104 | DE | Hamza et al. 2011 |
| Cow | 94.4% (18) | n/a | n/a | 2.1 × 105 | CR, DE, US |
Hamza et al. 2011, Rosario et al. 2009b, Symonds et al. 2017 |
| Coyote | 100.0% (3) | n/a | n/a | n/a | US | Rosario et al. 2009b |
| Dog | 100.0% (17) | n/a | n/a | n/a | CR, US | Rosario et al. 2009b, Symonds et al. 2017 |
| Duck | 100.0% (6) | n/a | n/a | n/a | DE | Hamza et al. 2011 |
| Hog (wild) |
100.0% (1) | n/a | n/a | n/a | US | Rosario et al. 2009b |
| Horse | 100.0% (18) | n/a | n/a | n/a | CR, DE, US |
Hamza et al. 2011, Rosario et al. 2009b, Symonds et al. 2017 |
| Pig | 100.0% (24) | n/a | n/a | n/a | CR, US & CA |
Rosario et al. 2009b, Symonds et al. 2017 |
| Raccoon | 100.0% (1) | n/a | n/a | n/a | US | Rosario et al. 2009b |
| Sheep | 100.0% (11) | n/a | n/a | n/a | DE, US | Hamza et al. 2011, Rosario et al. 2009b |
Canada (CA), Costa Rica (CR), Germany (DE), United States of America (US)
Similar to other markers, one potential limitation of PMMoV for MST is its lower specificity due to cross-reactivity with chicken, seagull, geese, and cow feces, where PMMoV can be detected at the same concentration range as human feces; thus, PMMoV may not be as suitable marker in areas where these animals are prevalent (Table 4). Additionally, PMMoV was not always present at detectable levels in individual fecal samples from children and adults, which were mostly collected in France (Table 2). PMMoV’s sensitivity in human feces is only slightly lower than those reported for HPyV and some Bacteroidales assays (Harwood et al., 2013b) and it is possible that PMMoV may be more frequently encountered in regions that consume more processed pepper products. To obtain a clearer global specificity value, we recommend specificity testing with non-target organisms across a wider geographic range. Despite these limitations, our meta-analysis suggests that PMMoV currently has a specificity of 90.0% (n = 150), and sensitivity ranging from 11.3% (n = 478, human feces only) to 100% (n = 126, untreated domestic wastewater only). Thus, it is more appropriate to consider PMMoV a domestic wastewater associated marker than a human fecal marker.
Overall, the concomitant presence of PMMoV in domestic wastewater effluent and water sources exposed to different effluent sources suggests that PMMoV is mostly human-specific and should be incorporated into the MST toolbox for use as a domestic wastewater marker. The utility of PMMoV as a tool for MST was exemplified in an investigation of the Reedy Creek watershed (Kissimmee, FL, USA), in which the absence of PMMoV confirmed that the high FIB concentrations were from natural sources (e.g., wildlife, vegetation) and not from the water reclamation facility (Young et al., 2014). While HF183, typically a domestic wastewater marker, was measured in this study, it could not serve as a domestic wastewater marker since it was identified in natural sources as well as deer feces (Nguyen et al., in review). This study highlights the need to have multiple domestic wastewater markers available for MST. Finally, caution needs to be used when applying PMMoV as a pollution marker in areas where pepper processing occurring as well as in areas irrigating with reclaimed water (e.g., tertiary-treated and disinfected wastewater).
7. Discussion
This comprehensive review indicates that PMMoV can be used to inform microbial water quality and shows potential to characterize the microbial quality of fresh produce and shellfish cultivated in or with environmental waters in the Americas, Australia, Asia, and Europe. Our synthesis of the available literature supports PMMoV as a useful enteric virus process indicator to evaluate (waste)water treatment technologies and a unique and important process indicator to measure virus removal in full-scale systems. Even though PMMoV removal and reduction did not always coincide with all enteric viruses or culturable virus indicators, PMMoV frequently co-occurred with pathogens of interest. Furthermore, PMMoV’s naturally high concentrations in wastewater were essential for determining the degree and mechanisms of viral reduction during full-scale treatment; a requisite as we work towards reaching the United Nations’ Sustainable Development Goals, particularly goal 6 (clean water and sanitation) that aims to increase (waste)water reuse (Gerba et al., 2017, Sano et al., 2016).
While PMMoV can be considered an index virus for enteric viruses in areas with known, untreated wastewater pollution sources, it may not correlate with infectious enteric viruses in areas with better sanitation, leading to an overestimation of the consequent risk of illness; thus, more research is necessary to understand the fate of infectious enteric viruses under various treatment scenarios and their correlation to PMMoV. However, it is possible that PMMoV could be an appropriate index virus for enteric viruses in environmental waters exposed to (mixed) treated domestic water if a PMMoV threshold could be identified to distinguish likely enteric virus presence and/or risk of enteric virus infection exceeding established health targets. Additional studies that link PMMoV detection and quantification with risk of enteric virus infection in a variety of fecal pollution contexts are needed to determine the suitability of PMMoV as an index virus for enteric viruses in environmental waters exposed to mixed-treated domestic wastewater pollution.
Even though it is likely that PMMoV will be equally applicable throughout Africa and the Middle East due to the globalization of food products and the prevalence of PMMoV in agriculture, the sensitivity and specificity of PMMoV as a domestic wastewater tracer has yet to be investigated in these regions. Before PMMoV is described as a universal domestic wastewater tracer in environmental waters, studies are needed to confirm the absence of non-fecal sources (e.g., food processing plant effluent, agricultural fields). The elevated concentrations of PMMoV in (treated) domestic wastewater provide an advantage as a domestic wastewater tracer by overcoming the methodological difficulties of false-negative results, which commonly occur as a result of low virus concentration and detection efficiencies (Gentry-Shields and Stewart, 2013, Harwood et al., 2013a). For example, PMMoV was used to justify modeling the risk of HAdV, ARV, and HNoV infection with virus concentrations between 0 and the limit of detection for samples with high concentrations of inhibitors, an advantage that allowed for the use of QMRA and informed conclusions for managing indirect wastewater reuse (Verbyla et al., 2016).
During the last decade, public health-related water microbiology has witnessed the rapid development of molecular-based technologies, which have enabled researchers to measure virus concentrations in (waste)water and food. Concomitantly, these studies identified the need to replace and/or complement routine FIB analyses with an indicator specific to enteric viruses (Gerba et al., 2017, Li et al., 2015, Sano et al., 2016, Symonds and Breitbart, 2015). PMMoV is a proven domestic wastewater tracer, as well as promising process indicator and index virus for enteric viruses; thus, this virus is a worthwhile target for rapid, lab-free, molecular-based analyses to facilitate rapid microbial quality management. Future research on the applications of PMMoV as a domestic wastewater tracer and surrogate for enteric viruses in the management of food and water quality, as well as wastewater reuse, will help engineers, environmental scientists, policy makers, and other stakeholders achieve the post-2015 United Nations’ Sustainable Development Goals (United Nations, 2015, United Nations Water, 2015).
8. Conclusion
PMMoV is a useful process indicator for enteric virus removal because its high concentrations allow for measuring removal at all treatment technology scales.
PMMoV is an appropriate index virus for enteric viruses in environmental waters exposed to untreated domestic wastewater.
Even with known secondary-treated wastewater, PMMoV concentrations greater than 1.78 × 103 targets l-1 correspond to health risks exceeding the US EPA health benchmark for recreational waters.
Further research is needed to determine if PMMoV correlates with enteric viruses and the concentrations of PMMoV that correspond to high human health risks in environmental waters contaminated by tertiary-treated wastewater.
PMMoV is a promising index virus to determine the microbial quality of shellfish as well as agricultural products irrigated with poor or vulnerable water sources.
Highlights.
PMMoV is a useful process indicator for field and full-scale treatment evaluation
PMMoV is 100% sensitive to domestic wastewater and 90% specific, per published studies
PMMoV and pathogen co-detection rate is as high as 72% for waters exposed to raw sewage
PMMoV and pathogen co-detection rate is as high as 67% for waters exposed to treated sewage
PMMoV is an effective, conservative index virus for enteric viruses to assess water quality
Acknowledgements
E.M.S., V.J.H., and M.B. were supported by U.S. National Science Foundation (US NSF) grant OCE-1566562. K.H. N. was supported by U.S. NSF grant EF-1241889, U.S. National Institutes of Health grant R01TW010286, and U.S. Department of Agriculture grant 2009-35102-0543. Special thanks to Dr. Ingeborg Boxman, from the Netherlands Food and Consumer Product Safety Authority, for sharing previously unpublished data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins S, Lamb EM, Roberts PD, Gooch MD, Breman L and Shuler KD (2001) Identification of pepper mild mottle virus in commercial bell pepper in Florida. Plant Dis 85(6), 679–679. [DOI] [PubMed] [Google Scholar]
- Ahmad A, Tiberini A, Ashfaq M and Tomassoli L (2015) First report of pepper mild mottle virus infecting chilli pepper in Pakistan. New Dis Rep 32, 31. [Google Scholar]
- Ahmed W, Hamilton KA, Lobos A, Hughes B, Staley C, Sadowsky MJ and Harwood VJ (2018) Quantitative microbial risk assessment of microbial source tracking markers in recreational water contaminated with fresh untreated and secondary treated sewage. Environ Int 117, 243–249. [DOI] [PubMed] [Google Scholar]
- Alonso E, García-Luque I, de la Cruz A, Wicke B, Avila-Rincón MJ, Serra MT, Castresana C and Diaz-Ruíz JR (1991) Nucleotide sequence of the genomic RNA of pepper mild mottle virus, a resistance-breaking tobamovirus in pepper. J Gen Virol 72(12), 2875–2884. [DOI] [PubMed] [Google Scholar]
- Asami T, Katayama H, Torrey JR, Visvanathan C and Furumai H (2016) Evaluation of virus removal efficiency of coagulation-sedimentation and rapid sand filtration processes in a drinking water treatment plant in Bangkok, Thailand. Water Res 101, 84–94. [DOI] [PubMed] [Google Scholar]
- Ashbolt N, Grawbow WOK and Snozzi M (2001) Water quality guidelines, standards and health: Assessment of risk and risk assessment for water-related infectious disease. World Health Organization (ed), pp. 289–316, IWA Publishing, London, UK. [Google Scholar]
- Avila-Rincon MJ, Ferrero ML, Alonso E, García-Luque I and Díaz-Ruíz JR (1989) Nucleotide Sequences of 5′ and 3′ Non-coding Regions of Pepper Mild Mottle Virus Strain S RNA. J Gen Virol 70(11), 3025–3031. [DOI] [PubMed] [Google Scholar]
- Banović Đeri B, Pajić V and Dudic D (2018) Revealing new information from existing genomic data for pepper mild mottle virus pathotype determination. Crop Prot 107, 93–103. [Google Scholar]
- Beczner L, Rochon D and Hamilton RI (1997) Characterization of an isolate of pepper mild mottle tobamovirus occurring in Canada. Can J Plant Pathol 19(1), 83–88. [Google Scholar]
- Betancourt WQ, Kitajima M, Wing AD, Regnery J, Drewes JE, Pepper IL and Gerba CP (2014) Assessment of virus removal by managed aquifer recharge at three full-scale operations. J Environ Sci Health Part A Toxic-Hazard Subst Environ Eng 49(14), 1685–1692. [DOI] [PubMed] [Google Scholar]
- Boehm AB, Soller JA and Shanks OC (2015) Human-associated fecal quantitative polymerase chain reaction measurements and simulated risk of gastrointestinal illness in recreational waters contaminated with raw sewage. Environ Sci Technol Lett 2, 270–275. [Google Scholar]
- Bofill-Mas S, Albinana-Gimenez N, Clemente-Casares P, Hundesa A, Rodriguez-Manzano J, Allard A, Calvo M and Girones R (2006) Quantification and stability of human adenoviruses and polyomavirus JCPyV in wastewater matrices. Appl Environ Microbiol 72(12), 7894–7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çağlar BK, Fidan H and Elbeaino T (2013) Detection and molecular characterization of pepper mild mottle virus from Turkey. J Phytopath 161(6), 434–438. [Google Scholar]
- Cantalupo PG, Calgua B, Zhao G, Hundesa A, Wier AD, Katz JP, Grabe M, Hendrix RW, Girones R, Wang D and Pipas JM (2011) Raw sewage harbors diverse viral populations. mBio 2(5), e00180–00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carducci A, Battistini R, Rovini E and Verani M (2009) Viral removal by wastewater treatment: Monitoring of indicators and pathogens. Food Environ Virol 1(2), 85–91. [Google Scholar]
- Colson P, Richet H, Desnues C, Balique F, Moal V, Grob J-J, Berbis P, Lecoq H, Harlé J-R, Berland Y and Raoult D (2010) Pepper mild mottle virus, a plant virus associated with specific immune responses, fever, abdominal pains, and pruritus in humans. PLoS ONE 5(4), e10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutilh BE, Cassman N, McNair K, Sanchez SE, Silva GGZ, Boling L, Barr JJ, Speth DR, Seguritan V, Aziz RK, Felts B, Dinsdale EA, Mokili JL and Edwards RA (2014) A highly abundant bacteriophage discovered in the unknown sequences of human faecal metagenomes. Nat Commun 5, 4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eregno FE, Tryland I, Myrmel M, Wennberg A, Oliinyk A, Khatri M and Heistad A (2018) Decay rate of virus and faecal indicator bacteria (FIB) in seawater and the concentration of FIBs in different wastewater systems. Microb Risk Anal 8, 14–21. [Google Scholar]
- Fong TT and Lipp EK (2005) Enteric viruses of humans and animals in aquatic environments: Health risks, detection, and potential water quality assessment tools. Microbiol Mol Biol Rev 69(2), 357–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations (2018) FAOSTAT Crops. http://www.fao.org/faostat/en/#data/QC/visualize, (accessed) May 8.
- Fout GS, Borchardt MA, Kieke BA and Karim MR (2017) Human virus and microbial indicator occurrence in public-supply groundwater systems: Meta-analysis of 12 international studies. Hydrogeol J, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Luque I, Ferrero M, Rodríguez J, Alonso E, De La Cruz A, Sanz A, Vaquero C, Serra M and Díaz-Ruíz J (1993) The nucleotide sequence of the coat protein genes and 3′ non-coding regions of two resistance-breaking tobamoviruses in pepper shows that they are different viruses. Arch Virol 131(1-2), 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Luque I, Serra MT, Alonso E, Wicke B, Ferrero ML and Diaz-Ruiz JR (1990) Characterization of a Spanish strain of pepper mild mottle virus (PMMV-S) and its relationship to other tobamoviruses. J Phytopath 129(1), 1–8. [Google Scholar]
- García-Aljaro C, Balleste E, Muniesa M and Jofre J (2017) Determination of crAssphage in water samples and applicability for tracking human faecal pollution. Microb Biotechnol 10(6), 1775–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genda Y, Kanda A, Hamada H, Sato K, Ohnishi J and Tsuda S (2007) Two amino acid substitutions in the coat protein of pepper mild mottle virus are responsible for overcoming the L4 gene-mediated resistance in Capsicum spp. Phytopath 97(7), 787–793. [DOI] [PubMed] [Google Scholar]
- Gentry-Shields J and Stewart JR (2013) Similar concentration and extraction recoveries allow for use of turnip crinkle virus as a process control for enteroviruses in water. J Virol Methods 189, 250–257. [DOI] [PubMed] [Google Scholar]
- Gerba CP, Betancourt WQ and Kitajima M (2017) How much reduction of virus is needed for recycled water: A continuous changing need for assessment? Water Res 108, 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DW, Lipp EK, McLaughlin MR and Rose JB (2001) Marine recreation and public health microbiology: Quest for the ideal indicator. Biosci 51(10), 817–825. [Google Scholar]
- Gu X, Tay QXM, Te SH, Saeidi N, Goh SG, Kushmaro A, Thompson JR and Gin KY-H (2018) Geospatial distribution of viromes in tropical freshwater ecosystems. Water Res 137, 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara K, Ichiki TU, Ogawa Y, Omura T and Tsuda S (2002) A single amino acid substitution in 126-kDa protein of Pepper mild mottle virus associates with symptom attenuation in pepper; the complete nucleotide sequence of an attenuated strain, C-1421. Arch Virol 147(4), 833–840. [DOI] [PubMed] [Google Scholar]
- Hamza IA, Jurzik L, Überla K and Wilhelm M (2011) Evaluation of pepper mild mottle virus, human picobirnavirus and torque teno virus as indicators of fecal contamination in river water. Water Res 45(3), 1358–1368. [DOI] [PubMed] [Google Scholar]
- Han S-H, Park J-S, Han J-Y, Gong J-S, Park C-H, Kim J-K, Seo E-Y, Domier LL, Hammond J and Lim H-S (2017) New Korean isolates of Pepper mild mottle virus (PMMoV) differ in symptom severity and subcellular localization of the 126 kDa protein. Virus Genes, 1–12. [DOI] [PubMed] [Google Scholar]
- Han TH, Kim SC, Kim ST, Chung CH and Chung JY (2014) Detection of norovirus genogroup IV, klassevirus, and pepper mild mottle virus in sewage samples in South Korea. Arch Virol 159(3), 457–463. [DOI] [PubMed] [Google Scholar]
- Haramoto E, Kitajima M, Hata A, Torrey JR, Masago Y, Sano D and Katayama H (2018) A review on recent progress in the detection methods and prevalence of human enteric viruses in water. Water Res 135, 168–186. [DOI] [PubMed] [Google Scholar]
- Haramoto E, Kitajima M, Kishida N, Konno Y, Katayama H, Asami M and Akiba M (2013) Occurrence of pepper mild mottle virus in drinking water sources in Japan. Appl Environ Microbiol 79(23), 7413–7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood VJ, Boehm AB, Sassoubre LM, Vijayavel K, Stewart JR, Fong T-T, Caprais M-P, Converse RR, Diston D, Ebdon J, Fuhrman JA, Gourmelon M, Gentry-Shields J, Griffith JF, Kashian DR, Noble RT, Taylor H and Wicki M (2013a) Performance of viruses and bacteriophages for fecal source determination in a multi-laboratory, comparative study. Water Res 47(18), 6929–6943. [DOI] [PubMed] [Google Scholar]
- Harwood VJ, Levine AD, Scott TM, Chivukula V, Lukasik J, Farrah SR and Rose JB (2005) Validity of the indicator organism paradigm for pathogen reduction in reclaimed water and public health protection. Appl Environ Microbiol 71(6), 3163–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood VJ, Staley C, Badgley BD, Borges K and Korajkic A (2013b) Microbial source tracking markers for detection of fecal contamination in environmental waters: Relationships to pathogens and human health outcomes. FEMS Microbiol Rev 38(1), 1–40. [DOI] [PubMed] [Google Scholar]
- Hruby CE, Libra RD, Fields CL, Kolpin DW, Hubbard LE, Borchardt MR, Spencer SK, Wichmand MD, Hall N, Schueller MD, Furlong ET and Weyer PJ (2013) 2013 Survey of Iowa groundwater and Evaluation of Public Well Vulnerability Classifications for Contaminants of Emerging Concern. Resources, I.D.o.N. (ed), Iowa Geological and Water Survey, Technical Information Series 57. [Google Scholar]
- Hughes B, Beale DJ, Dennis PG, Cook S and Ahmed W (2017) Cross-comparison of human wastewater-associated molecular markers in relation to fecal indicator bacteria and enteric viruses in recreational beach waters. Appl Environ Microbiol 83(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiki T, Nagaoka E, Hagiwara K, Sasaya T and Omura T (2009) A single residue in the 126-kDa protein of pepper mild mottle virus controls the severity of symptoms on infected green bell pepper plants. Arch Virol 154(3), 489–493. [DOI] [PubMed] [Google Scholar]
- Ikegashira Y, Ohki T, Ichiki UT, Higashi T, Hagiwara K, Omura T, Honda Y and Tsuda S (2004) An Immunological System for the Detection of Pepper mild mottle virus in Soil from Green Pepper Fields. Plant Dis 88(6), 650–656. [DOI] [PubMed] [Google Scholar]
- ISO/TS15216-1 (2013) Microbiology of food and animal feed – Horizontal method for determination of hepatitis A virus and norovirus in food using real-time RT-PCR Part 1: Method for quantification, International Standards Organization. [Google Scholar]
- Jothikumar N, Cromeans TL, Hill VR, Lu X, Sobsey MD and Erdman DD (2005) Quantitative real-time PCR assays for detection of human adenoviruses and identification of serotypes 40 and 41. Appl Environ Microbiol 71(6), 3131–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato R, Asami T, Utagawa E, Furumai H and Katayama H (2018) Pepper mild mottle virus as a process indicator at drinking water treatment plants employing coagulation-sedimentation, rapid sand filtration, ozonation, and biological activated carbon treatments in Japan. Water Res 132, 61–70. [DOI] [PubMed] [Google Scholar]
- Kaur S, Kang S, Sharma A and Sharma S (2014) First report of pepper mottle virus infecting chilli pepper in India. New Dis Rep 30, 14. [Google Scholar]
- King AM, Adams MJ and Lefkowitz EJ (2011) Virus taxonomy: Classification and nomenclature of viruses: Ninth report of the International Committee on Taxonomy of Viruses, Elsevier, Philadelphia, PA, U.S. [Google Scholar]
- Kitajima M, Iker BC, Pepper IL and Gerba CP (2014) Relative abundance and treatment reduction of viruses during wastewater treatment processes — Identification of potential viral indicators. Sci Total Environ 488–489, 290–296. [DOI] [PubMed] [Google Scholar]
- Kuroda K, Nakada N, Hanamoto S, Inaba M, Katayama H, Do AT, Nga TTV, Oguma K, Hayashi T and Takizawa S (2015) Pepper mild mottle virus as an indicator and a tracer of fecal pollution in water environments: Comparative evaluation with wastewater-tracer pharmaceuticals in Hanoi, Vietnam. Sci Total Environ 506–507, 287–298. [DOI] [PubMed] [Google Scholar]
- LaBelle RL, Gerba CP, Goyal SM, Melnick JL, Cech I and Bogdan GF (1980) Relationships between environmental factors, bacterial Indicators, and the occurrence of enteric viruses in estuarine sediments. Appl Environ Microbiol 39(3), 588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverick MA, Wyn-Jones AP and Carter MJ (2004) Quantitative RT-PCR for the enumeration of noroviruses (Norwalk-like viruses) in water and sewage. Lett Appl Microbiol 39(2), 127–136. [DOI] [PubMed] [Google Scholar]
- Lee H-W, Lee H-M, Yoon S-R, Kim SH and Ha J-H (2018) Pretreatment with propidium monoazide/sodium lauroyl sarcosinate improves discrimination of infectious waterborne virus by RT-qPCR combined with magnetic separation. Environ Pollut 233(Supplement C), 306–314. [DOI] [PubMed] [Google Scholar]
- Lee S, Hata A, Yamashita N and Tanaka H (2017) Evaluation of virus reduction by ultrafiltration with coagulation–sedimentation in water reclamation. Food Environ Virol, 1–11. [DOI] [PubMed] [Google Scholar]
- Li D, De Keuckelaere A and Uyttendaele M (2015) Fate of foodborne viruses in the “Farm to Fork” chain of fresh produce. Compr Rev Food Sci Food Saf 14(6), 755–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J and Ganesh A (2013) Water quality indicators: bacteria, coliphages, enteric viruses. Int J Environ Health Res 23(6), 484–506. [DOI] [PubMed] [Google Scholar]
- Mandal B and Jain R (2010) Can plant virus infect human being? Indian J Virol 21(1), 92–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis S, Spencer S, Firnstahl A, Stokdyk J, Borchardt M, McCarthy DT and Murphy HM (2018) Human Bacteroides and total coliforms as indicators of recent combined sewer overflows and rain events in urban creeks. Sci Total Environ 630, 967–976. [DOI] [PubMed] [Google Scholar]
- Michen B and Graule T (2010) Isoelectric points of viruses. J Appl Microbiol 109(2), 388–397. [DOI] [PubMed] [Google Scholar]
- Milošević D, Stanković I, Bulajić A, Ignjatov M, Nikolić Z, Petrović G and Krstić B (2015) Detection and molecular characterization of pepper mild mottle virus in Serbia. Genetika 47(2), 651–663. [Google Scholar]
- Moore NE, Wang J, Hewitt J, Croucher D, Williamson DA, Paine S, Yen S, Greening GE and Hall RJ (2015) Metagenomic analysis of vruses in feces from unsolved outbreaks of gastroenteritis in Humans. J Clin Microbiol 53(1), 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Yang C-S, Sakon N, Ueda M, Tougan T, Yamashita A, Goto N, Takahashi K, Yasunaga T, Ikuta K, Mizutani T, Okamoto Y, Tagami M, Morita R, Maeda N, Kawai J, Hayashizaki Y, Nagai Y, Horii T, Iida T and Nakaya T (2009) Direct Metagenomic Detection of Viral Pathogens in Nasal and Fecal Specimens Using an Unbiased High-Throughput Sequencing Approach. PLoS ONE 4(1), e4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen K, Senay C, Young S, Nayak B, Lobos A, Conrad J and Harwood VJ (in review) Determination of Wild Animal Sources of Fecal Indicator Bacteria by Microbial Source Tracking (MST) Influences Regulatory Decisions. Water Res. [DOI] [PubMed] [Google Scholar]
- Nwachcuku N and Gerba CP (2004) Emerging waterborne pathogens: Can we kill them all? Curr Opin Biotechnol 15(3), 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira L.M.d., Inoue-Nagata AK and Nagata T (2010) Complete genome nucleotide sequence of pepper mild mottle virus isolated in the Federal District, Brazil. Trop Plant Pathol 35, 373–376. [Google Scholar]
- Peng J, Shi B, Zheng H, Lu Y, Lin L, Jiang T, Chen J and Yan F (2015) Detection of pepper mild mottle virus in pepper sauce in China. Arch Virol 160(8), 2079–2082. [DOI] [PubMed] [Google Scholar]
- Rachmadi A, Kitajima M, Pepper I and Gerba C (2015) Enteric and indicator virus removal by surface flow wetlands. Sci Total Environ 542, 976–982. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Cerezo E, Moya A and Garcia-Arenal F (1989) Variability and evolution of the plant RNA virus pepper mild mottle virus. J Virol 63(5), 2198–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario K, Fierer N, Miller S, Luongo J and Breitbart M (2018) Diversity of DNA and RNA Viruses in Indoor Air As Assessed via Metagenomic Sequencing. Environ Sci Technol 52(3), 1014–1027. [DOI] [PubMed] [Google Scholar]
- Rosario K, Nilsson C, Lim YW, Ruan Y and Breitbart M (2009a) Metagenomic analysis of viruses in reclaimed water. Environ. Microbiol 11(11), 2806–2820. [DOI] [PubMed] [Google Scholar]
- Rosario K, Symonds EM, Sinigalliano C, Stewart J and Breitbart M (2009b) Pepper mild mottle virus as an indicator of fecal pollution. Appl Environ Microbiol 75(22), 7261–7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosiles-González G, Ávila-Torres G, Moreno-Valenzuela OA, Acosta-González G, Leal-Bautista RM, Grimaldo-Hernández CD, Brown JK, Chaidez-Quiroz C, Betancourt WQ, Gerba CP and Hernández-Zepeda C (2017) Occurrence of Pepper Mild Mottle Virus (PMMoV) in Groundwater from a Karst Aquifer System in the Yucatan Peninsula, Mexico. Food Environ Virol 9(4), 487–497. [DOI] [PubMed] [Google Scholar]
- Saeidi N, Gu X, Han TN, Goh SG, Kitajima M, Kushmaro A, Schmitz BW and Gin KY-H (2018) Occurrence of traditional and alternative fecal indicators in tropical urban environments under different land use patterns. Appl Environ Microbiol doi: 10.1128/AEM.00287-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangsanont J, The Dan D, Thi Viet Nga T, Katayama H and Furumai H (2016) Detection of pepper mild mottle virus as an indicator for drinking water quality in Hanoi, Vietnam, in large volume of water after household treatment. J Environ Sci Health [A] 51(13), 1100–1106. [DOI] [PubMed] [Google Scholar]
- Sano D, Amarasiri M, Hata A, Watanabe T and Katayama H (2016) Risk management of viral infectious diseases in wastewater reclamation and reuse: Review. Environ Int 91, 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Rodriguez TM, Marcos P, Monteiro S, Urdaneta M, Santos R and Toranzos GA (2013) Evaluation of Enterococcus-infecting phages as indices of fecal pollution. J Water Health 11(1), 51–63. [DOI] [PubMed] [Google Scholar]
- Sassi HP, Tuttle KD, Betancourt WQ, Kitajima M and Gerba CP (2018) Persistence of viruses by qPCR downstream of three effluent-dominated rivers in the western United States. Food Environ Virol. doi: 10.1007/s12560-018-9343-7. [DOI] [PubMed] [Google Scholar]
- Savichtcheva O and Okabe S (2006) Alternative indicators of fecal pollution: Relations with pathogens and conventional indicators, current methodologies for direct pathogen monitoring and future application perspectives. Water Res 40(13), 2463–2476. [DOI] [PubMed] [Google Scholar]
- Schets FM, van Wijnen JH, Schijven JF, Schoon H and de Roda Husman AM (2008) Monitoring of waterborne pathogens in surface waters in Amsterdam, The Netherlands, and the potential health risk associated with exposure to Cryptosporidium and Giardia in these waters. Appl Environ Microbiol 74(7), 2069–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz BW, Kitajima M, Campillo ME, Gerba C and Pepper IL (2016) Virus reduction during advanced bardenpho and conventional wastewater treatment process. Environ Sci Technol 50(17), 9524–9532. [DOI] [PubMed] [Google Scholar]
- Shirasaki N, Matsushita T, Matsui Y and Murai K (2017) Assessment of the efficacy of membrane filtration processes to remove human enteric viruses and the suitability of bacteriophages and a plant virus as surrogates for those viruses. Water Res 115, 29–39. [DOI] [PubMed] [Google Scholar]
- Shirasaki N, Matsushita T, Matsui Y and Yamashita R (2018) Evaluation of the suitability of a plant virus, pepper mild mottle virus, as a surrogate of human enteric viruses for assessment of the efficacy of coagulation–rapid sand filtration to remove those viruses. Water Res 129, 460–469. [DOI] [PubMed] [Google Scholar]
- Shrestha S, Shrestha S, Shindo J, Sherchand JB and Haramoto E (2018) Virological quality of irrigation water sources and pepper mild mottle virus and tobacco mosaic virus as index of pathogenic virus contamination level. Food Environ Virol 10(1), 107–120. [DOI] [PubMed] [Google Scholar]
- Sinclair RG, Jones EL and Gerba CP (2009) Viruses in recreational water-borne disease outbreaks: a review. J Appl Microbiol 107(6), 1769–1780. [DOI] [PubMed] [Google Scholar]
- Sinclair RG, Rose JB, Hashsham SA, Gerba CP and Haas CN (2012) A criteria for selection of surrogates used to study the fate and control of pathogens in the environment. Appl Environ Microbiol 78(6), 1969–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soller JA, Schoen ME, Bartrand T, Ravenscroft JE and Ashbolt NJ (2010) Estimated human health risks from exposure to recreational waters impacted by human and non-human sources of faecal contamination. Water Res 44(16), 4674–4691. [DOI] [PubMed] [Google Scholar]
- Stachler E and Bibby K (2014) Metagenomic evaluation of the highly abundant human gut bacteriophage crAssphage for source tracking of human fecal pollution. Environ Sci Technol Lett 1(10), 405–409. [Google Scholar]
- Stachler E, Kelty C, Sivaganesan M, Li X, Bibby K and Shanks OC (2017) Quantitative CrAssphage PCR assays for human fecal pollution measurement. Environ Sci Technol 51(16), 9146–9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley C, Gordon KV, Schoen ME and Harwood VJ (2012) Performance of two quantitative PCR methods for microbial source tracking of human sewage and implications for microbial risk assessment in recreational waters. Appl Environ Microbiol 78(20), 7317–7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JR, Gast RJ, Fujioka RS, Solo-Gabriele HM, Meschke JS, Amaral-Zettler LA, del Castillo E, Polz MF, Collier TK and Strom MS (2008) The coastal environment and human health: microbial indicators, pathogens, sentinels and reservoirs. Environ Health 7(Suppl 2), S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel DM and Harwood VJ (2007) Performance, design, and analysis in microbial source tracking studies. Appl Environ Microbiol 73(8), 2405–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds EM and Breitbart M (2015) Affordable enteric virus detection techniques are needed to support changing paradigms in water quality management. CLEAN 43(1), 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds EM, Cook MM, McQuaig SM, Ulrich RM, Schenck RO, Lukasik JO, Van Vleet ES and Breitbart M (2015) Reduction of nutrients, microbes, and personal care products in domestic wastewater by a benchtop electrocoagulation unit. Sci Rep 5(9380). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds EM, Sinigalliano C, Gidley ML, Ahmed W, McQuaig S and Breitbart M (2016) Faecal pollution along the southeastern coast of Florida and insight into the use of pepper mild mottle virus as an indicator. J Appl Microbiol 121(5), 1469–1481. [DOI] [PubMed] [Google Scholar]
- Symonds EM, Verbyla ME, Lukasik JO, Kafle RC, Breitbart M and Mihelcic JR (2014) A case study of enteric virus removal and insights into the associated risk of water reuse for two wastewater treatment pond systems in Bolivia. Water Res 65, 257–270. [DOI] [PubMed] [Google Scholar]
- Symonds EM, Young S, Verbyla ME, McQuaig-Ulrich SM, Ross E, Jimenez JA, Harwood VJ and Breitbart M (2017) Microbial source tracking in shellfish harvesting waters in the Gulf of Nicoya, Costa Rica. Water Res 111, 177–184. [DOI] [PubMed] [Google Scholar]
- Tandukar S, Sherchand J, Bhandari D, Sherchan S, Malla B, Ghaju Shrestha R and Haramoto E (2018) Presence of human enteric viruses, protozoa, and indicators of pathogens in the Bagmati River, Nepal. Pathog 7(2), 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency (2006) Implementing the BEACH Act of 2000 Report to Congress, EPA-823-R-06-001, Office of Water, Washington, DC, USA. [Google Scholar]
- UNICEF and World Health Organization (2015) Progress on sanitation and drinking water: 2015 update and MDG assessment, UNICEF and World Health Organization, New York, NY, USA. [Google Scholar]
- United Nations (2015) Sustainable development goals. General Assembly. United Nations General Assembly, New York, NY, USA: http://www.un.org/sustainabledevelopment/sustainable-development-goals/, (accessed) October 6, 2016. [Google Scholar]
- United Nations Water (2015) Wastewater management: A UN-Water Analytical Brief, United Nations, New York, NY, USA. [Google Scholar]
- U.S. National Research Council (2012) Water Reuse: Potential for Expanding the Nation's Water Supply Through Reuse of Municipal Wastewater, National Academies Press, Washington, DC, USA. [Google Scholar]
- Velasco L, Janssen D, Ruiz-Garcia L, Segundo E and Cuadrado IM (2002) The complete nucleotide sequence and development of a diferential detection assay for a pepper mild mottle virus (PMMoV) isolate that overcomes L3 resistance in pepper. J Virol Methods 106(1), 135–140. [DOI] [PubMed] [Google Scholar]
- Verbyla ME, Symonds EM, Kafle RC, Cairns MR, Iriarte M, Mercado A, Coronado O, Breitbart M, Ledo C and Mihelcic JR (2016) Managing microbial risks from indirect wastewater reuse for irrigation in urbanizing watersheds. Environ Sci Technol 50(13), 6803–6813. [DOI] [PubMed] [Google Scholar]
- Victoria JG, Kapoor A, Li L, Blinkova O, Slikas B, Wang C, Naeem A, Zaidi S and Delwart E (2009) Metagenomic analyses of viruses in stool samples from children with acute flaccid paralysis. J Virol 83(9), 4642–4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Sikora P, Rutgersson C, Lindh M, Brodin T, Bjorlenius B, Larsson DGJ and Norder H (2018) Differential removal of human pathogenic viruses from sewage by conventional and ozone treatments. Int J Hyg Environ Health 221(3), 479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu F, Zhou G, Li XH and Li Z (2006) Detection and molecular characterization of pepper mild mottle virus in China. J Phytopath 154(11–12), 755–757. [Google Scholar]
- Wetter C, Conti M, Altschuh D, Tabillion R and Van Regenmortel MHV (1984) Pepper mild mottle virus, a tobamovirus infecting pepper cultivars in Sicily. Phytopath 74, 405–410. [Google Scholar]
- World Health Organization (2006) WHO guidelines for the safe use of wastewater, excreta, and greywater 4th edition, WHO Press, Geneva, Switzerland. [Google Scholar]
- World Health Organization (2009) Addendum to the WHO guideliens for safe recreational water environments, volume 1, coastal and fresh waters, Geneva, Switzerland. [Google Scholar]
- World Health Organization (2010) Safe management of shellfish and harvest waters, International Water Association, London, UK. [Google Scholar]
- World Health Organization (2011) Guidelines for drinking water quality 4th edition, Geneva, Switzerland. [Google Scholar]
- Wu J, Long S, Das D and Dorner S (2011) Are microbial indicators and pathogens correlated? A statistical analysis of 40 years of research. J Water Health 9(2), 265–278. [DOI] [PubMed] [Google Scholar]
- Yates MV (2007) Classical indicators in the 21st century - Far and beyond the coliform. Water Environ Res 79(3), 279–286. [DOI] [PubMed] [Google Scholar]
- Young S, Nayak B and Harwood VJ (2014) Assessing Animal and Human Fecal Contamination in a Watershed Managed for Wildlife Conservation, Boston, MA, USA. [Google Scholar]
- Yu M, Zhou T, Wu Y and An M (2018) Complete Genome Sequence of a Pepper mild mottle virus Isolate from Northeast China. Genome Announcements 6(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Breitbart M, Lee WH, Run JQ, Wei CL, Soh SWL, Hibberd ML, Liu ET, Rohwer F and Ruan YJ (2006) RNA viral community in human feces: Prevalence of plant pathogenic viruses. PLoS Biol 4(1), 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]



