Abstract
Classic psychedelics (serotonin 2A receptor agonists) and dissociative hallucinogens (NMDA receptor antagonists), though differing in pharmacology, may share neuropsychological effects. These drugs, however, have undergone limited direct comparison. This report presents data from a double-blind, placebo controlled within- subjects study comparing the neuropsychological effects of multiple doses of the classic psychedelic psilocybin (10, 20, and 30 mg/70 kg) with the effects of a single high dose of the dissociative hallucinogen dextromethorphan (DXM; 400 mg/70 kg). Twenty hallucinogen users (11 females) completed neurocognitive assessments during five blinded drug administration sessions in which participants and study staff were informed that a large range of possible drug conditions may have been administered. Previously reported subjective effects data from this study show that DXM produces a drug effect strength time-course similar to 20 and 30 mg/70 kg of psilocybin (Carbonaro et al., 2018). Global cognitive impairment, assessed using the Mini-Mental State Examination, was not observed with psilocybin or DXM. Orderly and dose-dependent effects of psilocybin were observed on psychomotor performance, working memory, episodic memory, associative learning, and visual perception. Effects of DXM on psychomotor performance, visual perception, and associative learning were in the range of effects of a moderate to high dose (20 to 30 mg/70 kg) of psilocybin. Psilocybin had greater effects than DXM on working memory. DXM had greater effects than all psilocybin doses on balance, episodic memory, response inhibition, and executive control.
Introduction
Classic psychedelic drugs (serotonin 2A, or 5HT2a, receptor agonists) such as lysergic acid diethylamide (LSD), psilocybin, and N, N-dimethyltryptamine (DMT), and dissociative hallucinogens (NMDA receptor antagonists) such as dextromethorphan (DXM) and ketamine, are used nonmedically and abused as psychedelic drugs (Bern and Peck 1992; Banken and Foster 2008; Wilson et al. 2011; Tylš et al. 2014; NIDA 2015; SAMHSA 2015) and are of concern to the Food and Drug Administration, Drug Enforcement Agency, and the National Institute on Drug Abuse. Classic and dissociative hallucinogens differ in primary receptor mechanism of action, but may share a profile of subjective effects (Reissig et al. 2012; Carbonaro et al. 2018), and underlying interactions between serotonergic and glutamatergic systems may mediate the effects of both classic and dissociative hallucinogens (Aghajanian and Marek 1999; Vollenweider and Kometer 2010; Nichols 2016). A recent dose-effects study of DXM demonstrated that, under blinded conditions, experienced hallucinogen users responded on a pharmacological class questionnaire that the subjective effects of a high dose of DXM (400 mg/70 kg) were most similar to those produced by classic hallucinogens (but not 9 other drug classes, including dissociative hallucinogens), although no classic hallucinogens were administered in that study (Reissig et al. 2012). Ratings of subjective effects of 400 mg/70 kg DXM on a series of subjective effects questionnaires that are sensitive to the effects of hallucinogens (Reissig et al. 2012) were similar to previously reported ratings for classic hallucinogens (Griffiths et al. 2006, 2011).
Similar subjective effects between 400 mg/70 kg dose of DXM and both 20 and 30 mg/70 kg psilocybin were recently shown using a within-subject design (Carbonaro et al. 2018). In that study, 400 mg/70 kg dose of DXM produced subjective ratings similar to both 20 and 30 mg/70 kg psilocybin when volunteers rated the overall strength of drug effect, distance from normal reality, ineffability, somatic effects (e.g. numbness/tingling, temperature change), impaired cognition, and ratings of challenging experience. DXM also produced lower scores than 20 and 30 mg/70 kg doses of psilocybin on subjective ratings of personal insight, visual effects and imagery, absorption in music, spiritual or mystical experience, and affect, but higher scores on subjective ratings of dizziness, nausea, and disembodiment. Doses of psilocybin in the range of 20–30 mg/70 kg are being investigated for therapeutic efficacy in treating both mood disorders (Griffiths et al. 2016; Ross et al. 2016; Carhart-Harris et al. 2016a) and substance use disorders (Johnson et al. 2014, 2017; Bogenschutz et al. 2015; Johnson and Griffiths 2017). While subjective effects of classic and dissociative hallucinogens have both directly and indirectly compared, little attention has been paid to the comparative effects of these drugs on cognition.
Classic hallucinogens have been shown to acutely disrupt visual perception (Carter et al. 2004; Kometer et al. 2011, 2013), attention (Gouzoulis-Mayfrank et al. 2006; Heekeren et al. 2007, 2008; Daumann et al. 2008, 2010), and spatial working memory (Vollenweider et al. 1998). Recent neuroimaging studies have confirmed that psychedelic drugs modulate the activity and connectivity of brain regions involved in 7/24/2018 1:28:00 AMmemory (Carhart-Harris et al. 2012, 2014; Kaelen et al. 2016), inhibitory processing (Quednow et al. 2012; Schmidt et al. 2018), and visual processing (Kometer et al. 2011, 2013; Kraehenmann et al. 2015; Kaelen et al. 2016; Carhart-Harris et al. 2016b).
Dissociative hallucinogens (NMDA antagonists) including dextromethorphan (DXM), ketamine, and phencyclidine have been shown to alter performance on episodic memory, psychomotor function, attention, vigilance, continuous performance, executive function, meta cognition, and visual perception tasks (Curran and Morgan 2000; Carter et al. 2013; Giorgetti et al. 2015). Ketamine has been shown to shift brain functional connectivity from hubs primarily centered in cortical regions to those primarily centered in subcortical regions (Joules et al. 2015), and alter human brain activity in regions involved in vision (Musso et al. 2011), verbal fluency (Nagels et al. 2011), memory (Honey et al. 2005; Kraguljac et al. 2017), and executive function (Vollenweider et al. 1997; Deakin et al. 2008).
Few studies have directly compared the acute effects of classic and dissociative hallucinogens on cognitive performance. In a series of studies comparing DMT and S- ketamine, DMT was shown to impair orienting of attention (Gouzoulis-Mayfrank et al. 2006; Daumann et al. 2008), alertness (Daumann et al. 2010), and accuracy in a continuous performance task (Heekeren et al. 2008) more strongly than S-ketamine, and S-ketamine but not DMT was shown to decreased startle response and enhance sensorimotor gating (Heekeren et al. 2007). However, studies of LSD (Schmid et al. 2015) and psilocybin (Gouzoulis-Mayfrank et al. 1998; Vollenweider et al. 2007) demonstrated impaired sensorimotor gating, suggesting that there may not be consistency in effects of different serotonergic hallucinogens, at least in the domain of sensorimotor gating. No other cognitive domains have been directly compared between classic and dissociative hallucinogens.
The current study used a computerized battery of validated neurocognitive tasks in a double-blind, placebo controlled, complete-crossover design to compare the effects of a low (10 mg/70 kg), moderate (20 mg/70 kg), and high (30 mg/70 kg) oral dose of the classic psychedelic psilocybin to the effects of a high (400 mg/70 kg) oral dose of the dissociative hallucinogen DXM that has been shown to produce effects similar to classic hallucinogens (Reissig et al. 2012; Carbonaro et al. 2018). Psilocybin and DXM were compared on measures of psychomotor functioning, working and episodic memory, executive function, and visual perception. These are domains that are impacted by classic and/or dissociative hallucinogens, but that have not been directly compared during the acute effects of these drugs.
A single dose of DXM (400 mg/70 kg) was chosen as the active comparator condition to three doses of psilocybin. This dose of DXM was selected based on previous studies (Reissig et al. 2012) demonstrating that, under blinded conditions, this dose of DXM produced subjective effects most similar to that of a classic hallucinogen and lower doses of DXM produced subjectively similar but less intense effects. 400 mg/70 kg was also the highest dose tolerated by all volunteers in that study, and therefore it is the highest dose that we believed that we could reliably administer in this study. Given the potential interaction of serotonergic and glutamatergic systems in mediating hallucinogen effects of both classic and dissociative hallucinogens (Vollenweider and Kometer 2010), a dose of DXM (400 mg/70 kg) that yields classic hallucinogen effects is appropriate to compare the cognitive effects of these two mechanistically different hallucinogens.
We tested the hypothesis that, based on previous behavioral and neuroimaging findings with DXM (Carter et al. 2013; Braun et al. 2016), DXM would have greater effects on performance of psychomotor, memory, and executive function tasks than psilocybin. We also tested the hypothesis that, given the visual effects of psilocybin (Kometer and Vollenweider 2018) and effects of psilocybin on activity and connectivity of visual brain networks (Kaelen et al. 2016; Roseman et al. 2016), psilocybin would have greater effects on performance of a visual perception task than DXM.
Methods and Materials
The cognitive assessment data reported here have not been published elsewhere, but were collected as part of a larger study. Subjective effects measures assessed within this same study are reported elsewhere (Carbonaro et al. 2018). However, we present the time course of volunteer-rated strength of drug effects that was initially reported by Carbonaro et al. (2018) in Figure 1 of the current report to document the subjective strength of drug effects when each cognitive assessment was conducted.
Figure 1.
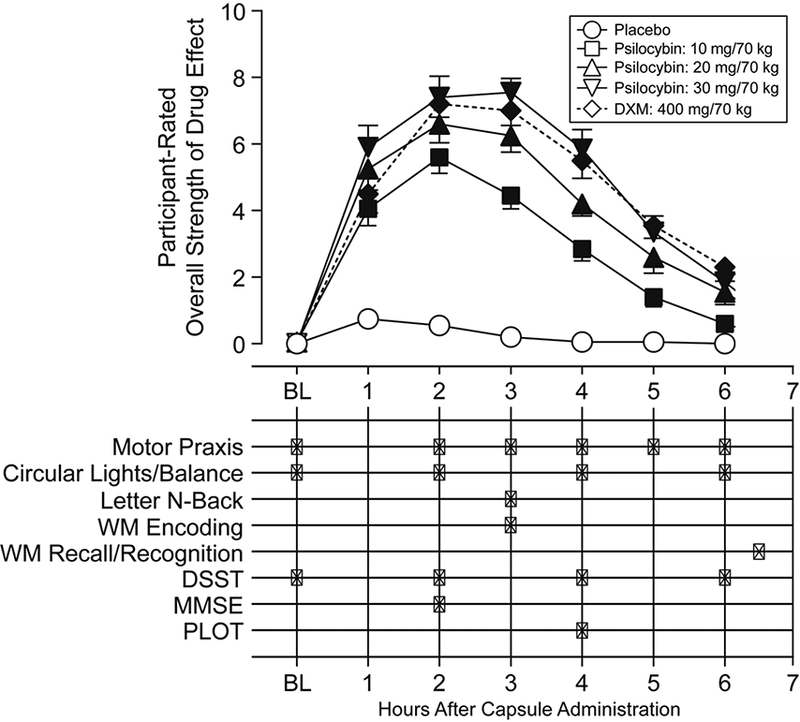
Timeline of drug effects and neurocognitive assessments. The abscissa indicates time relative to capsule administration. The top half of the figure displays the average time course of participant-rated strength of drug effects, with error bars denoting SEM, and filled symbols indicating a significant difference from placebo (p < 0.05) at the respective time point. Each “X” in the bottom half of the figure designates the time, relative to capsule administration, at which each neurocognitive assessment was administered in each drug administration session. BL: Baseline measurements, before capsule administration; DSST: Digit Symbol Substitution Task; MMSE: Mini-Mental Status Examination; PLOT: Penn Line Orientation Test.
Participants
20 medically and psychiatrically healthy participants (11 females; 19 Caucasian, 1 Asian American; mean age = 28.5 years, range 22–43) with a history of both classic hallucinogen use (mean = 60.9 uses, range 16–183) and dissociative hallucinogen use (mean = 19.0 uses, range = 1–154) gave written informed consent before participating in any study procedures. Individuals with a history of substance dependence according to DSM-IV-TR criteria (excluding nicotine and caffeine), those with a current significant medical or psychiatric condition, those with a personal or immediate family history of psychosis or bipolar affective disorder, and women who were pregnant or nursing were excluded from participation. Procedures were approved by the Johns Hopkins Medicine Institutional Review Boards. This study was registered with ClinicalTrials.gov (NCT02033707).
Procedures
After enrollment, participants completed two preparation visits during which they met and built rapport with study monitors and received training and practice on the computerized neurocognitive tasks (described in Supplementary Materials and Methods). Each participant completed a total of five drug administration sessions under blinded conditions, one each with inactive placebo, high dose DXM (400 mg/70 kg) and a low (10 mg/70 kg), medium (20 mg/70 kg), and high (30 mg/70 kg) dose of psilocybin. Procedures for blinding conditions included instructing participants 38 possible drug conditions could be administered but obscuring the specific drug conditions to be administered (see Carbonaro et al., 2018, and Supplementary Materials and Methods). Participants were instructed to eat a light, low-fat breakfast before each session, and were allowed to consume a light snack during the afternoon. Each drug administration session began with administration of two identically-appearing opaque capsules. Negative urine screening for recent use of cocaine, benzodiazepines, and opioids was required before each drug administration. While volunteers were not tested for presence of cannabinoids or amphetamines before each drug administration, prospective volunteers meeting criteria for current or recent substance use disorders (including these drug classes) were excluded at screening. Dose order was counterbalanced across participants using a 5-order Williams design matrix. The mean days between drug administration sessions was 10 days (range: 3–28 days). Given that elimination half-life of psilocin (the active metabolite of psilocybin) is roughly 3 hours (Passie et al. 2002; Brown et al. 2017), and the elimination half-life of dextromethorphan is roughly 2 hours (Schadel et al. 1995), session schedules ensured washout of drug between sessions.
Drug administration followed previously reported procedures (Griffiths et al. 2011; Reissig et al. 2012) and safety guidelines applicable to the study of high doses of classic hallucinogens (Johnson et al. 2008). Briefly, each drug administration was conducted in a comfortable living room-like setting. Participants were continuously monitored by at least one staff member at all times during acute drug effects. Cardiovascular measures (heart rate and blood pressure) were recorded at regular intervals, and are reported elsewhere (Carbonaro et al. 2018). If a volunteer reported significant fear or anxiety, the volunteer would be provided verbal reassurance or physical reassurance in the form of supportive hand-holding. After drug administration, participants were instructed to lie on a couch with eyeshades and headphones and turn their attention inward while listening to a standard playlist of music that has been provided in previous studies (Griffiths et al. 2011; Reissig et al. 2012).
At regular intervals (Figure 1), participants moved from the couch to an upright chair at a desk and completed a series of neurocognitive assessments using a laptop computer and a mouse. Every hour after capsule administration, monitors rated the observed strength of drug effects on a 5 point scale (0: none, 4: extreme). Every two hours after capsule administration, participants completed gross motor performance tasks: the circular lights task (Mumford et al. 1995) and the balance task (Carter et al. 2006). The circular lights task is a hand-eye coordination task, with the outcome measure being the number of correct presses in 60 seconds. The balance task involves balancing on one foot with both eyes closed, and the outcome is the number of seconds that a person can maintain balance, summed across both feet (60 seconds maximum). Gross motor performance tasks are expected to be sensitive to the overall strength of drug effect, while being independent of potential specific cognitive effects of the drugs in question. Other assessments, including subjective effects assessments, were administered during and after the study and are reported elsewhere (Carbonaro et al. 2018). Although peak effects of each drug condition on circular lights and balance tasks were reported by Carbonaro and colleagues (2018), we report time courses for those measures in the current report.
Neurocognitive Assessments
Neurocognitive assessments included measures of psychomotor performance, working memory, episodic memory, executive functioning and overall cognitive impairment, and visual perception. Four tasks were administered from the Penn Computerized Neurocognitive Battery (CNB) (Gur et al. 2010). One task (the MMSE) was administered verbally. The remaining tasks were programmed and presented using Presentation software (http://www.neurobs.com). The order of forms for tasks with multiple forms (the MMSE, letter N-back, and word encoding/recognition tasks) was counter-balanced across participants, and orthogonalized in relation to dose order. This orthogonalization is described in the Supplemental Materials and Methods. A more detailed description of each neurocognitive task is contained in the Supplemental Materials and Methods. An additional task (the emotional conflict Stroop task) was administered 4 hours after capsule administration in each experimental session. This task is described and reported in Supplemental Materials and Methods, but is not further described in the main text as it yielded no drug by task interactions.
Psychomotor Performance.
The Motor Praxis (mpraxis) task (Gur et al. 2001) from the CNB was administered at every assessment period as a test of psychomotor ability. In this task, participants are instructed to click on a series of progressively smaller green squares on a computer screen. Outcome measures for this task are average response time and number of squares clicked (accuracy) for the timed response block for this task.
Memory.
A word-encoding, recall, and recognition task including 36 target and 36 lure words was administered as a measure of short-term memory performance, as in previous studies with DXM in our laboratory (Carter et al. 2013). Participants were instructed to categorize the concrete nouns (target words) presented at encoding as “artificial” (i.e., man-made) or “natural” to encourage deep (rather than shallow) encoding (Rose and Craik 2012). Participants completed a free recall task for 5 minutes, beginning 195 minutes after encoding, followed immediately by a recognition task in which participants were instructed to indicate whether randomly presented targets and lures were old or new, using a 6-point confidence scale (definitely old, probably old, maybe old, maybe new, probably new, definitely new). The dependent measure for recall was the number of correctly recalled words. Dependent measures for recognition were derived from a dual-process signal detection model (Yonelinas and Parks 2007) applied to receiver operating characteristic (ROC) curve analysis conducted on confidence rating data pooled across subjects using the ROC Toolbox in Matlab (Koen et al. 2017). Dependent measures were area under the ROC curve (AUC), the non-parametric index of sensitivity in distinguishing between old and new words (A’), and dual-process model parameters for recollection and familiarity (Yonelinas and Parks 2007).
The letter N-back task from the CNB was administered as a test of working memory and vigilance (Gur et al. 2010). This is a continuous performance task that is sensitive to working memory load (Kearney-Ramos et al. 2014), and also requires attention, rapid response, and executive function (Yoran-Hegesh et al. 2009). Outcome measures were average response time for correct responses for each task condition, as well as discriminability (defined as hit rate [HR] minus the false alarm rate [FAR]) and response bias (defined as the FAR/[1-discriminability]) (Snodgrass and Corwin 1988).
Executive Function and Overall Cognitive Impairment.
The Digit Symbol Substitution Task (McLeod et al. 1982) was administered to measure executive function, mental flexibility, and associative learning (described further in Supplemental Materials and Methods). Outcome measures are total number of trials attempted within 90 seconds, proportion of attempted trials that were correct, and the number of correctly identified symbols in the final associative learning test. The Mini-Mental Status Examination (MMSE) (Folstein et al. 1975), which is used as a clinical test of delirium and provides a global measure of cognition, was also administered, with the MMSE total score as the outcome measure.
Visual Perception.
The Penn Line Orientation Test (PLOT) (Moore et al. 2015) from the CNB was administered as a measure of spatial orientation ability. Outcome measures are total number of correct trials, median response time for correct and incorrect trials, and mean excess clicks for correct and incorrect trials.
Analysis
Mpraxis scores, MMSE scores, and peak monitor-rated strength of drug effect were analyzed using a mixed effects repeated measures ANOVA, assessing a main effect of drug condition. All other outcome measures were submitted to a mixed effects repeated measures ANCOVA, with responses nested within participant, testing a main effect of drug condition and using average mpraxis response times for the given assessment period as a covariate to control for general effects of psychomotor slowing. Mixed effects models allow for assessment of effects of drug condition while controlling for individual differences in overall task performance in the presence of missing data. For the DSST and letter n-back analyses, responses were also nested within drug condition and a main effect of time point (for the DSST) or task condition (for the letter n-back) was assessed.
Statistical models were estimated using the Imer function of the lme4 library (Bates et al. 2015) and the ImerTest library (Kuznetsova et al. 2016) within the R statistical environment (R Core Team 2015) (version 3.2.2). Tukey’s method was used to correct for multiple comparisons in all analyses. Missing data are outlined in the Supplemental Material and Methods.
Results
Missing Data
Baseline assessments were missing for the motor praxis task (1 volunteer) and the DSST (3 volunteers) for all drug conditions. Four volunteers were unable to complete cognitive assessments in the 400 mg/70 kg DXM condition due to impairing drug effects: two were unable to complete any tasks, one was unable to complete the motor praxis or DSST at the 2-hour time point, and one was unable to complete the Letter N-Back task. Three volunteers were unable to complete cognitive assessments in the 30 mg/70 kg psilocybin condition due to impairing drug effects: one volunteer was unable to complete any tasks during the first three hours of drug effects, one volunteer was unable to complete the DSST during the 2-hour time point, and one volunteer was unable to complete the Letter N-Back task. One volunteer was unable to complete the DSST 4 hours after drug administration in the 20 mg/70 kg psilocybin condition. An additional volunteer was unable to complete the Letter N-Back task due to technical difficulties during both the 20 mg/70 kg and the 30 mg/70 kg psilocybin conditions. All other data were collected for all participants in all conditions. Partial data from remaining tasks and drug conditions was retained for analysis.
Gross Motor Effects, Strength of Drug Effect, and Psychomotor Slowing
Both the circular lights and balance tasks yielded orderly time- and dose- dependent effects of drug condition (Figure 2). DXM exerted a greater effect than the 10 and 20 mg/70 kg conditions on the circular lights task (Figure 2A), and DXM also exerted a greater effect than any psilocybin condition on balance task performance (Figure 2B).
Figure 2.
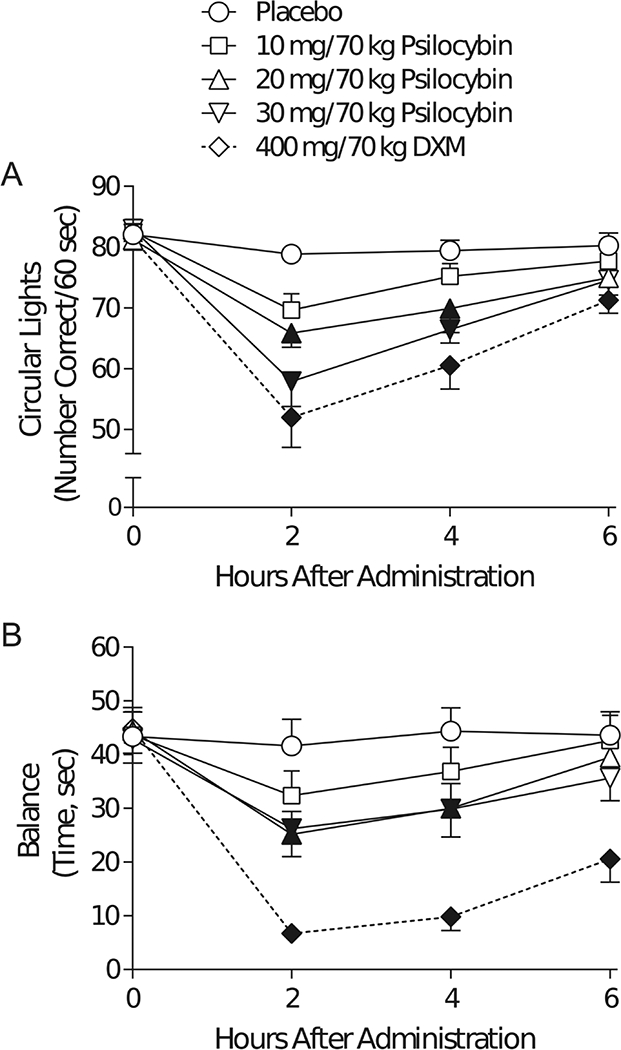
Time Course of Effects of Drug Condition on Measures of Gross Motor Function. The outcome measure (on the ordinate) for (A) the circular lights task, and (B) the balance task are plotted against the time point during the experimental drug sessions at which assessments of gross motor function were made (on the abscissa): 0 (before capsule administration) and 2, 4, and 6 hours after capsule administration. Brackets indicate ±1 standard error. Filled markers indicate significant difference from placebo at that time point (p < 0.05, using Tukey’s correction for multiple comparisons).
Peak monitor-rated strength of observed drug effects were significantly different from placebo in each drug condition, and were significantly lower for 10 mg/70 kg psilocybin than for 20 and 30 mg/70 kg psilocybin and DXM (Figure 3A). There was a main effect of drug condition on response time (F[4] = 6.52, p < 0.001) but not on accuracy in the timed portion of the motor praxis (mpraxis) task, which assessed psychomotor slowing. Post-hoc tests revealed that responses were slower during the 20 and 30 mg/70 kg psilocybin conditions and the 400 mg/70 kg DXM condition than during placebo (Figure 3B).
Figure 3.
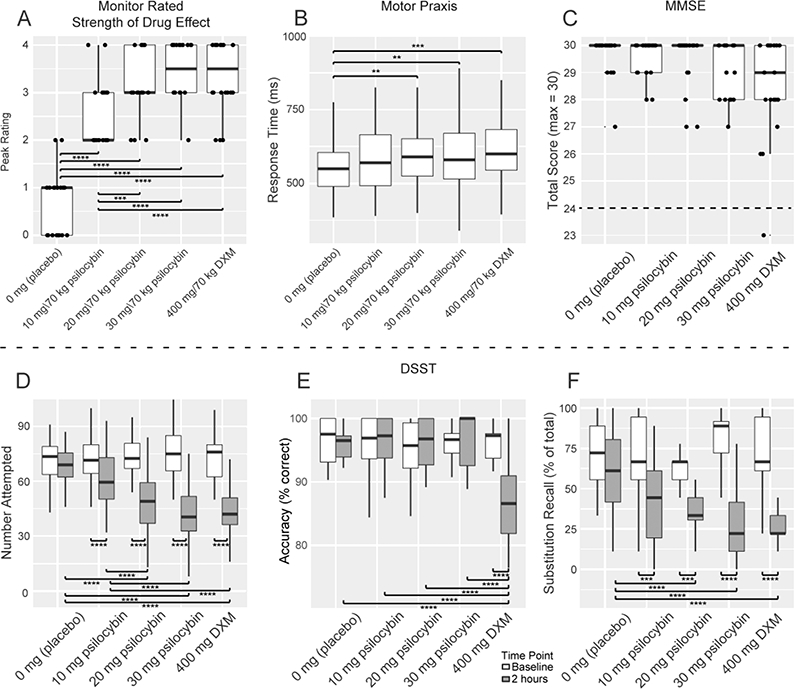
Effects of Drug Condition on Rated Strength of Drug Effects, Psychomotor Performance, and Executive Function. Outcome measures in each panel (on the ordinate) are plotted against the drug conditions of this experiment (on the abscissa): 0 mg (placebo), 10 mg/70 kg psilocybin, 20 mg/70 kg psilocybin, and 30 mg/70 kg psilocybin, and 400 mg/70 kg dextromethorphan (DXM). The thick horizontal lines in each panel indicate the medians, the outer boxes indicate the 1st and 3rd quartiles (25% and 75%), and vertical lines (or “whiskers”) indicate 1.5 times the inter-quartile range. (A) Observed strength of drug effects was rated by study monitors at regular intervals during each drug administration session. The average of the peak of observed strength of drug effects for each session are plotted. (B) Effects of drug condition on psychomotor performance were assessed using average response time during the timed portion of the motor praxis task. (C) Effects of drug condition on cognitive impairment were assessed using the total score from the Mini-Mental Status Examination (MMSE). A dashed line at 24 on the ordinate indicates the cutoff for a “cognitively normal” score on the MMSE, where scores at or above this score are considered to not be impaired. (D-F) Effects of drug condition on executive function as assessed using the Digit Symbol Substitution Task (DSST) were analyzed using (D) number of trials attempted, (E) accuracy (percent correct) of responses in trials attempted, and (F) accuracy (percent correct) of substitution recall trials at the end of the DSST. DSST responses from baseline as well as 2, 4, and 6 hours post-drug-administration were included in the analysis. Because peak effects generally occurred at 2 hours, data at that time point are displayed graphically. A more detailed figure including data from 4 and 6 hours post-drug-administration is included in the online Supplemental Materials and Methods (Figure S1-S3). * = p < 0.05, ** = p < 0.01, *** = p < 0.001, **** = p < 0.0001, using Tukey’s correction for multiple comparisons.
Global Cognitive Impairment and Executive Function
Mini-mental status examination (MMSE).
MMSE scores were not significantly associated with drug condition (Figure 3C). One participant tested outside of the “normal” range of 24–30 (Folstein et al., 1976) in their session with 400 mg DXM, but all other observed scores were greater than or equal to 26.
Digit Symbol Substitution Task.
A main effect of drug condition (F[4] = 23.52, p < 0.0001) and time point (F[3] = 123.28, p < 0.0001), and an interaction between drug condition and time point (F[12] = 10.28, p < 0.0001), was observed on the number of attempted trials (Figure 3D). Main effects of drug condition (F[4] = 10.56, p < 0.0001) and time point (F[3] = 6.05, p < 0.0005), and an interaction between drug condition and time point (F[12] = 4.10, p < 0.0001), on accuracy were observed (Figure 3E). Main effects of drug condition (F[4] = 11.59, p < 0.0001) and time point (F[3] = 40.92, p < 0.0001), and an interaction between drug condition and time point (F[12] = 2.07, p < 0.05), were also observed for substitution recall accuracy (Figure 3F).
Working Memory (the Letter N-Back Task).
Main effects of n-back condition (F[2] = 38.53, p < 0.0001) and drug condition (F[4] = 3.47, p < 0.05), as well as an interaction between drug and n-back condition (F[8] = 3.51,p<0.001), were observed on discriminability (Figure 4A). A main effect of n- back condition (F[2] = 62.30, p < 0.0001) and drug condition (F[4] = 6.75, p < 0.001), as well as an interaction between drug and n-back condition (F[8] = 3.36, p < 0.005), was observed on response bias (Figure 4B). Main effects of drug condition (F[4] = 8.50, p < 0.0005) and n-back condition (F[2] = 86.38, p < 0.0001), as well as an interaction of drug and n-back conditions (F[8] = 6.02, p < 0.0001), were also observed on response time (Figure 4C).
Figure 4.
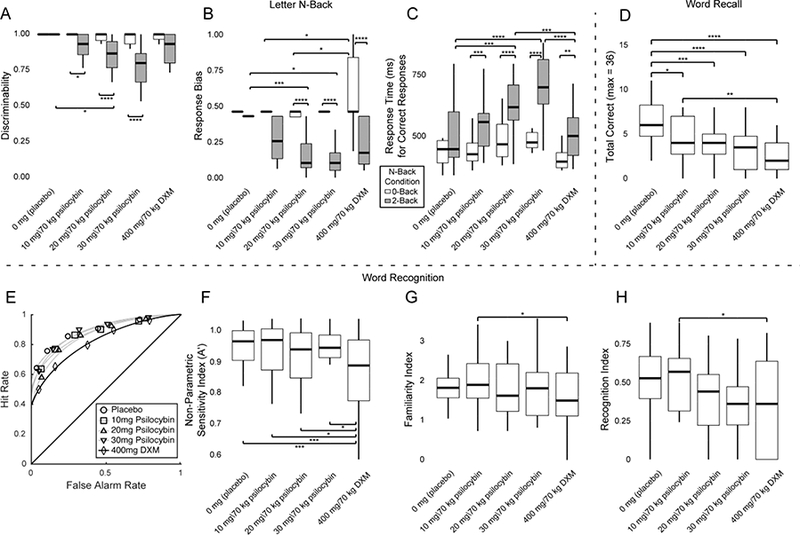
Effects of Drug Condition on Memory Outcome Measures. Outcome measures in each panel (on the ordinate) are plotted against the drug conditions of this experiment (on the abscissa): 0 mg (placebo), 10 mg/70 kg psilocybin, 20 mg/70 kg psilocybin, and 30 mg/70 kg psilocybin, and 400 mg/70 kg dextromethorphan (DXM). (A-C) Effects of drug condition on working memory performance in the letter n-back task were assessed using (A) discriminability, (B) response bias, and (C) median response time (in ms) to correct responses. 0-back, 1-back, and 2-back conditions were contained within the analysis, but no significant differences were observed between 0-back and 1-back conditions. Therefore, plotting is restricted to 0-back (red bars) and 2-back (green bars) conditions for brevity. Full plots including the 1-back condition are available in Figure S4-S6, in the Supplemental Materials and Methods. (D) Word recall performance is expressed as the total number of encoded words that were freely recalled. (E-H) Effects of drug condition on outcome measures for word recognition were assessed using (E) Receiver Operating Characteristic (ROC) curve analysis and ROC area under the curve, (F) the non-parametric sensitivity index A’, and the (G) familiarity index and (H) recollection index derived from the dual-process ROC curve analysis model. The horizontal lines of each panel indicate the median, the outer boxes indicate the 1st and 3rd quartiles (25% and 75%), and vertical lines (or “whiskers”) indicate 1.5 times the interquartile range. * = p < 0.05, ** = p < 0.01, *** = p < 0.001, **** = p < 0.0001, using Tukey’s correction for multiple comparisons.
Episodic Memory
Main effects of drug condition were observed on word recall accuracy (F[4] = 11.22, p < 0.0001; Figure 4D). Main effects in the word recognition task of drug condition were observed on the area under the curve (AUC) of the Receiver Operator Characteristic (ROC) curves (F[4] = 6.42, p < 0.0005; Figure 4E) and sensitivity (A’; F[4] = 5.94, p < 0.0005; Figure 4F). Planned comparisons revealed a significant difference between the DXM and 10 mg/70 kg psilocybin conditions for the familiarity index (z = −2.08, p < 0.05; Figure 4G) and recollection index (z = −2.15, p < 0.05; Figure 4H).
Penn Line Orientation Task
No significant effects of drug condition were observed on accuracy, median response time for correct trials, or mean excess clicks for incorrect trials. A main effect of drug condition was observed on mean excess clicks for correct trials (F[4] = 4.80, p < 0.005; Figure 5A) and median response time for incorrect trials (F[4] = 4.48, p < 0.005; Figure 5B).
Figure 5.
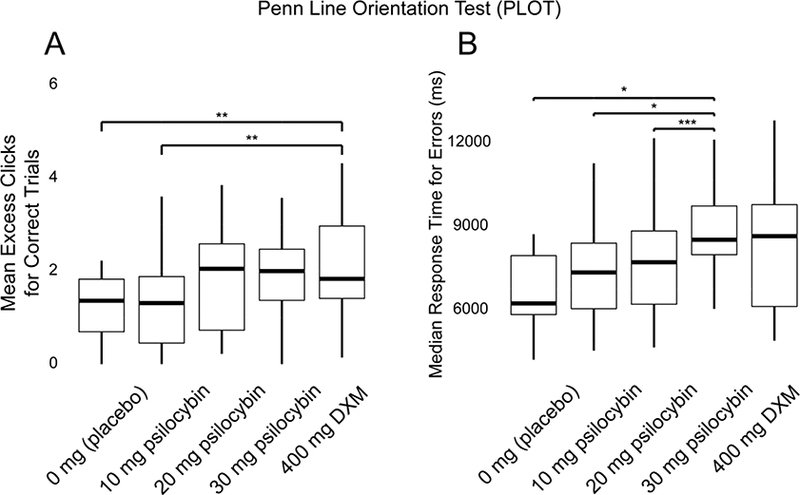
Effects of Drug Condition on Visual Perception. Outcome measures in each panel (on the ordinate) are plotted against the drug conditions of this experiment (on the abscissa): 0 mg (placebo), 10 mg/70 kg psilocybin, 20 mg/70 kg psilocybin, and 30 mg/70 kg psilocybin, and 400 mg/70 kg dextromethorphan (DXM). The horizontal lines of each panel indicate the median, the outer boxes indicate the 1st and 3rd quartiles (25% and 75%), and vertical lines (or “whiskers”) indicate 1.5 times the inter-quartile range. Effects of drug condition on visual perceptual abilities as assessed using the Penn Line Orientation Test (PLOT) were demonstrated on (A) mean excess clicks for correct trials, and (B) median response time (in ms) for errors. * = p < 0.05, ** = p < 0.01, *** = p < 0.001, **** = p < 0.0001, using Tukey’s correction for multiple comparisons.
Discussion
Orderly, dose-dependent effects of psilocybin were demonstrated on outcome measures related to associative learning (Figure 3F), working memory (Figure 4A–4C), episodic memory (Figure 4D), and visual perception (Figure 5B). In most cases, the 400 mg/70 kg dose of dextromethorphan had effects on cognition in the range of those produced by 20 to 30 mg/70 kg psilocybin. Both drugs produced psychomotor slowing (Figure 3B), and all cognitive effects were controlled for psychomotor slowing. General cognitive impairment and delirium, as assessed with the Mini-Mental State Examination (MMSE), were not observed with DXM (with the exception of one individual) or at any dose of psilocybin (Figure 3C). Notably, effects of drug on MMSE scores, and effects of psilocybin on accuracy on the DSST, were absent at 2 hours post-capsule administration despite the substantial effects of these drugs on gross motor functioning at this time point (Figure 2). Thus, while global cognitive impairment and delirium (e.g. drug-induced impairment of all cognitive domains) was not observed, “local” cognitive impairments (e.g. more subtle impariments within individual cognitive domains) were observed, and were both dose and drug dependent. Importantly, strong changes in perception and affect typical of classical hallucinogens were observed at the time that MMSE was administered (Carbonaro et al. 2018), strongly suggesting that subjective and cognitive effects of hallucinogens are not accurately described as being similar to clinical delirium states.
Effects of psilocybin and DXM on learning and memory
The dual-process signal detection model of episodic memory assumes separate psychological processes for “remember” and “know” memory decisions, and yields separate model parameters for “recollection” and “familiarity” judgments (related to “remember” and “know” processes, respectively) (Yonelinas and Parks 2007). DXM selectively decreased recognition sensitivity (Figure 4F) and decreased the engagement of both familiarity (Figure 4G) and recognition (Figure 4H) processes compared to the 10 mg/70 kg dose of psilocybin. This is consistent with previous literature indicating a decrease in accuracy and discrimination, and an increase in response time for episodic recognition during the effects of a high dose of DXM (Carter et al. 2013).
Both psilocybin and DXM decreased the free recall of words, but DXM decreased free recall of words to a greater extent than psilocybin (Figure 4D). Both psilocybin and DXM caused impairments in associative learning, as measured using the substitution recall portion of the DSST (Figure 3F). Overall, this suggests an impact of both drugs on incidental associative learning, and a selective effect of DXM, but not psilocybin, on episodic memory.
Effects of psilocybin and DXM on executive function
Psilocybin exerted a dose-dependent impairing effect on discriminability (Figures 4A, 4C) and both DXM and psilocybin increased response time for correct responses (Figure 4C) during the 2-back condition compared to the 0-back condition in the N-back task, and psilocybin did not affect any outcome measures associated with 0-back task performance (Figure 4A–4C). A dose-dependent decrease in response bias was observed during the 2-back condition with psilocybin, while a liberal response bias was observed with DXM during the 0-back condition of the N-Back (Figure 4B). The 0-back condition of the N-back task may be thought of in terms of a response inhibition task (such as a go/no-go task), during which participants must provide a response only when presented with the target stimulus (in this case, the letter “X”), and during which participants must inhibit responses to all other stimuli. Liberal response bias during the 0-back task under the effects of DXM suggests that response inhibition is impaired by DXM. Both 0-back and 2-back conditions require vigilance, while only the 2-back condition requires working memory. Thus, the specific effects of psilocybin and DXM on performance of the N-back task suggest that psilocybin selectively impairs working memory, while DXM selectively impairs response inhibition, which is a key component of executive control.
Speeded responding, working memory, and executive function are all required for successful performance of the DSST (Yoran-Hegesh et al. 2009). Sacrificing the speed of a response to maintain the accuracy of a response (the speed-accuracy tradeoff) is a well- established performance strategy in cognitive literature (Heitz 2014). When two groups or experimental conditions yield differences in response time but not accuracy on a given task, this may reflect a difference in response strategy rather than impairment of a specific cognitive process (Luck and Gold 2008). However, when responses are both slower and less accurate, this is more likely to indicate impairment of a given cognitive process.
Psilocybin did not exert an effect on the accuracy of attempted responses (Figure 3E), but psilocybin did cause a dose-dependent decrease in attempted responses at 2 hours after drug administration on the number of trials attempted in 90 seconds in the Digit Symbol Substitution Task (DSST) (Figure 3D). This suggests a successful speed- accuracy trade-off during the effects of psilocybin. Consistent with previous literature (L. P. Carter et al., 2013), participants were impaired in both number attempted and accuracy of attempted trials in the DSST during the effects of DXM (Figure 3D-E). Given that other measures involving working memory (Figure 4A) did not show impairment during DXM compared to placebo, impairment of accuracy in the DSST suggests that DXM, but not psilocybin, selectively impaired executive function.
Effects of psilocybin and DXM on visual processing
While previous literature noted that psilocybin impaired visual motion coherence detection (Carter et al. 2004) and modal visual object completion (Kometer et al. 2011), the current study did not find an effect of psilocybin on accuracy in the perception of line orientation. Given that the perception of line orientation may be considered a function of lower-level perceptual processing, this is consistent with previous literature that showed selective impairment of higher-level rather than lower-level aspects of visual perception (Carter et al. 2004). A dose-dependent effect of psilocybin was observed on response time during the commission of errors in the PLOT (Figure 5B). In contrast, mean excess clicks for correct trials were greater during DXM than during placebo and the low dose of psilocybin (Figure 5A). This suggests that while increased effort may be exerted for difficult trials during the effects of psilocybin, increased effort may be exerted for all trials during the effects of DXM.
Strengths and Limitations
Dose-effects of psilocybin on cognition were investigated, and clear dose- dependent effects of psilocybin were observed. This yielded a range of doses of psilocybin against which to compare the effects of a high dose of DXM. Dose effects of DXM on measures of working and episodic memory, executive function, and attention were previously demonstrated (Carter et al. 2013), and the dose of DXM chosen for the current study (400 mg/70 kg) had the greatest effects on cognition in the previous study, while also being the highest dose tolerated by all participants in that previous study. Previous work has demonstrated a similar profile of rated drug strength and most subjective effects of a 400 mg/70 kg dose of DXM with a 20–30 mg/70 kg dose of psilocybin (Reissig et al. 2012; Carbonaro et al. 2018), and this dose of DXM was consistently rated as producing subjective effects similar to those of classic hallucinogens. While a single dose of DXM in the current study allows for a limited direct comparison, it does not provide for the full comparison that would have been allowed with multiple doses of both drugs. Also, without measurement of plasma drug concentration, we are unable to correlate pharmacokinetic measures with performance measures, or assess the role of drug metabolism, for example, on task performance, even given a single dose condition for DXM. However, given that lower doses of DXM were previously shown to yield subjective (Reissig et al. 2012) and cognitive (Carter et al. 2013) effects qualitatively similar but quantitatively less intense as a high (400 mg/70 kg) dose of DXM, observed differences between DXM and psilocybin on working memory in our sample can be generalized to untested lower doses of DXM. Though it is unclear if observed differences between DXM and psilocybin on balance, episodic memory, response inhibition, and executive control can likewise be generalized to lower doses of DXM, differences between psilocybin and this high dose of DXM on these measures are most informative when comparing the effects of these two drugs at strongly, typically ‘psychedelic’ doses.
Given the length of each task, the entire neurocognitive battery was not administered at every time point, and the MMSE, Letter N-Back, and PLOT were only administered once within the session. This leads to an inevitable confound between timing of task performance and the strength of subjective effects at that given time point (Figure 1). However, almost all tasks were administered at times of peak subjective effects, which were maintained across the 2 and 3 hour time points for all drug conditions except 10 mg/70 kg psilocybin (Figure 1). Thus, the tasks administered at these timepoints are likely not compromised by differences in task timing relative to drug strength ratings. While fine motor (Motor Praxis task), gross motor (Balance and Circular Lights tasks), and executive function measures (DSST) were acquired at multiple time- points before and after drug administration (see Supplemental Information for time course of Motor Praxis and DSST performance), future studies may benefit from a more complete sampling of the interaction of task performance and strength of subjective effects for other cognitive domains.
Though no effects of drug condition were found in the PLOT, this task was administered fairly late in each drug session (4 hours after capsule administration, when drug effects were at approximately quarter-maximum to half-maximum levels; Figure 1). Thus, null effects reported for the PLOT may be a function of the timing of the task administration. However, substantial drug effects were still detected on balance and circular lights (Figure 2) as well as the DSST (Figure S1, S3) tasks at 4 hours post-capsule. While drug strength ratings were still rather high at this time point, it may be the case that effects of drug condition on visual perception may have abated by this time.
Volunteers in this study had extensive drug use histories, including substantial prior exposure to both classic and dissociative hallucinogens. Thus, we cannot rule out the possibility that the reported findings are limited to those with extensive drug use history. In particular, it is possible that null effects on the MMSE may be a function of the hallucinogen experience of this sample of volunteers. However, our observations are consistent with responses to high doses of psilocybin that we have observed in those with little or no prior exposure to hallucinogens (Carter et al. 2006; Griffiths et al. 2011, 2018).
We employed a fully counterbalanced experimental design that was completed with the planned 20 volunteers in order to maintain drug blinding and control for both potential learning and drug order effects, but missing data, although rare, might have resulted in incomplete balancing. Further, while participants completed two practice sessions for the cognitive battery before any drug administration, subtle learning or practice effects may still present in the data.
Conclusion
The current report describes the dose-dependent effects of psilocybin on cognition and the comparative neuropsychopharmacology of psilocybin and DXM. Despite similarities in subjective effects (Reissig et al. 2012; Carbonaro et al. 2018), and similarly strong effects on psychomotor performance, visual perception, and associative learning in the current report, DXM and psilocybin were found to differ in their effects in important ways. Psilocybin exerted greater effects than DXM on measures of working memory, and DXM exerted selective effects on episodic memory, response inhibition, and executive control. Impairments observed in executive function and episodic memory during DXM compared to psilocybin may account for less psychological insight reported after DXM compared to psilocybin (Carbonaro et al. 2018), and also may underlie lower ratings of personal meaningfulness and spiritual significance during DXM compared to psilocybin (unpublished data). Impairment of balance and a greater number of cognitive domains during the effects of DXM compared to psilocybin may also indicate greater risk of DXM if abused or consumed in uncontrolled settings.
Supplementary Material
Acknowledgements
We thank Mary Cosimano, M.S.W., Taylor Marcus, Darrick May, MD, Albert Garcia-Romeu, Ph.D., Mary Sweeney, Ph.D., William Richards, Ph.D., Brennan Kersgaard, and Eileen Rosello for their roles as session monitors, Dr. Annie Umbricht, MD and the medical staff at the Behavioral Pharmacology Research Unit for medical screening and medical coverage, and Lisa Schade for technical assistance. We also thank David Nichols, Ph.D. for synthesizing the psilocybin. The study was conducted in compliance with United States laws.
Funding and Disclosure
This research was supported by NIH grant R01DA03889 to RRG. FSB and TC were supported in part by NIH grant 5T32 DA007209. FSB was supported in part by NIH grant R03DA042336. MWJ was supported in part by R01DA035277. TC is an employee of the U.S. Food and Drug Administration (FDA), however, the views presented in this article do not necessarily reflect those of the FDA and no official support or endorsement of this article by the FDA is intended or should be inferred. RRG is a board member of the Heffter Research Institute. Other authors have no conflicts of interest to disclose.
Footnotes
Supplementary information is available at the Psychopharmacology website.
References
- Aghajanian GK, Marek GJ (1999) Serotonin and hallucinogens. Neuropsychopharmacology 21:16S–23S. doi: 10.1016/S0893-133X(98)00135-3 [DOI] [PubMed] [Google Scholar]
- Banken JA, Foster H (2008) Dextromethorphan. Ann N Y Acad Sci 1139:402–411. doi: 10.1196/annals.1432.003 [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S (2015) Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software 67:. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Bem JL, Peck R (1992) Dextromethorphan. An overview of safety issues. Drug Saf 7:190–199 [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, Forcehimes AA, Pommy JA, et al. (2015) Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol (Oxford) 29:289–299. doi: 10.1177/0269881114565144 [DOI] [PubMed] [Google Scholar]
- Braun U, Schäfer A, Bassett DS, et al. (2016) Dynamic brain network reconfiguration as a potential schizophrenia genetic risk mechanism modulated by NMDA receptor function. Proc Natl Acad Sci USA 113:12568–12573. doi: 10.1073/pnas.1608819113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RT, Nicholas CR, Cozzi NV, et al. (2017) Pharmacokinetics of Escalating Doses of Oral Psilocybin in Healthy Adults. Clin Pharmacokinet 56:1543–1554. doi: 10.1007/s40262-017-0540-6 [DOI] [PubMed] [Google Scholar]
- Carbonaro TM, Johnson MW, Hurwitz E, Griffiths RR (2018) Double-blind comparison of the two hallucinogens psilocybin and dextromethorphan: similarities and differences in subjective experiences. Psychopharmacology (Berl) 235:521–534. doi: 10.1007/s00213-017-4769-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Bolstridge M, Rucker J, et al. (2016a) Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry 3:619–627. doi: 10.1016/S2215-0366(16)30065-7 [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Erritzoe D, Williams T, et al. (2012) Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci USA 109:2138–2143. doi: 10.1073/pnas.1119598109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Leech R, Hellyer PJ, et al. (2014) The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci 8:20. doi: 10.3389/fnhum.2014.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Muthukumaraswamy S, Roseman L, et al. (2016b) Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci USA 113:4853–4858. doi: 10.1073/pnas.l518377113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Reissig CJ, Johnson MW, et al. (2013) Acute cognitive effects of high doses of dextromethorphan relative to triazolam in humans. Drug Alcohol Depend 128:206–213. doi: 10.1016/j.drugalcdep.2012.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Richards BD, Mintzer MZ, Griffiths RR (2006) Relative abuse liability of GHB in humans: A comparison of psychomotor, subjective, and cognitive effects of supratherapeutic doses of triazolam, pentobarbital, and GHB. Neuropsychopharmacology 31:2537–2551. doi: 10.1038/sj.npp.l301146 [DOI] [PubMed] [Google Scholar]
- Carter OL, Pettigrew JD, Burr DC, et al. (2004) Psilocybin impairs high-level but not low-level motion perception. Neuroreport 15:1947–1951 [DOI] [PubMed] [Google Scholar]
- Curran HV, Morgan C (2000) Cognitive, dissociative and psychotogenic effects of ketamine in recreational users on the night of drug use and 3 days later. Addiction 95:575–590 [DOI] [PubMed] [Google Scholar]
- Daumann J, Heekeren K, Neukirch A, et al. (2008) Pharmacological modulation of the neural basis underlying inhibition of return (IOR) in the human 5-HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology (Berl) 200:573–583. doi: 10.1007/s00213-008-1237-l [DOI] [PubMed] [Google Scholar]
- Daumann J, Wagner D, Heekeren K, et al. (2010) Neuronal correlates of visual and auditory alertness in the DMT and ketamine model of psychosis. J Psychopharmacol (Oxford) 24:1515–1524. doi: 10.1177/0269881109103227 [DOI] [PubMed] [Google Scholar]
- Deakin JFW, Lees J, McKie S, et al. (2008) Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry 65:154–164. doi: 10.1001/archgenpsychiatry.2007.37 [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198 [DOI] [PubMed] [Google Scholar]
- Giorgetti R, Marcotulli D, Tagliabracci A, Schifano F (2015) Effects of ketamine on psychomotor, sensory and cognitive functions relevant for driving ability. Forensic Sci Int 252:127–142. doi: 10.1016/j.forsciint.2015.04.024 [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Heekeren K, Neukirch A, et al. (2006) Inhibition of return in the human 5HT2A agonist and NMDA antagonist model of psychosis. Neuropsychopharmacology 31:431–441. doi: 10.1038/sj.npp.1300882 [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Heekeren K, Thelen B, et al. (1998) Effects of the hallucinogen psilocybin on habituation and prepulse inhibition of the startle reflex in humans. Behav Pharmacol 9:561–566 [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Carducci MA, et al. (2016) Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharmacol (Oxford) 30:1181–1197. doi: 10.1177/0269881116675513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, et al. (2011) Psilocybin occasioned mystical- type experiences: immediate and persisting dose-related effects. Psychopharmacology (Berl) 218:649–665. doi: 10.1007/s00213-011-2358-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, et al. (2018) Psilocybin-occasioned mystical- type experience in combination with meditation and other spiritual practices produces enduring positive changes in psychological functioning and in trait measures of prosocial attitudes and behaviors. J Psychopharmacol (Oxford) 32:49–69. doi: 10.1177/0269881117731279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, Jesse R (2006) Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl) 187:268–283; discussion 284– 292. doi: 10.1007/s00213-006-0457-5 [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, et al. (2001) Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology 25:766–776. doi: 10.1016/S0893-133X(01)00278-0 [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, et al. (2010) A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. JNeurosci Methods 187:254–262. doi: 10.1016/j.jneumeth.2009.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heekeren K, Daumann J, Neukirch A, et al. (2008) Mismatch negativity generation in the human 5HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology (Berl) 199:77–88. doi: 10.1007/s00213-008-l129-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heekeren K, Neukirch A, Daumann J, et al. (2007) Prepulse inhibition of the startle reflex and its attentional modulation in the human S-ketamine and N,N- dimethyltryptamine (DMT) models of psychosis. J Psychopharmacol (Oxford) 21:312–320. doi: 10.1177/0269881107077734 [DOI] [PubMed] [Google Scholar]
- Heitz RP (2014) The speed-accuracy tradeoff: history, physiology, methodology, and behavior. Front Neurosci 8:150. doi: 10.3389/fnins.2014.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey GD, Honey R a. E, O’Loughlin C, et al. (2005) Ketamine disrupts frontal and hippocampal contribution to encoding and retrieval of episodic memory: an fMRI study. Cereb Cortex 15:749–759. doi: 10.1093/cercor/bhhl76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Richards W, Griffiths R (2008) Human hallucinogen research: guidelines for safety. J Psychopharmacol (Oxford) 22:603–620. doi: 10.1177/0269881108093587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Garcia-Romeu A, Cosimano MP, Griffiths RR (2014) Pilot study of the 5- HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol (Oxford) 28:983–992. doi: 10.1177/0269881114548296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Garcia-Romeu A, Griffiths RR (2017) Long-term follow-up of psilocybin- facilitated smoking cessation. Am J Drug Alcohol Abuse 43:55–60. doi: 10.3109/00952990.2016.1170135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Griffiths RR (2017) Potential Therapeutic Effects of Psilocybin. Neurotherapeutics 14:734–740. doi: 10.1007/sl3311-017-0542-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joules R, Doyle OM, Schwarz AJ, et al. (2015) Ketamine induces a robust whole-brain connectivity pattern that can be differentially modulated by drugs of different mechanism and clinical profile. Psychopharmacology (Berl) 232:4205–4218. doi: 10.1007/s00213-015-3951-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelen M, Roseman L, Kahan J, et al. (2016) LSD modulates music-induced imagery via changes in parahippocampal connectivity. Eur Neuropsychopharmacol 26:1099–1109. doi: 10.1016/j.euroneuro.2016.03.018 [DOI] [PubMed] [Google Scholar]
- Kearney-Ramos TE, Fausett JS, Gess JL, et al. (2014) Merging clinical neuropsychology and functional neuroimaging to evaluate the construct validity and neural network engagement of the n-back task. J Int Neuropsychol Soc 20:736–750. doi: 10.1017/S135561771400054X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koen JD, Barrett FS, Harlow IΜ, Yonelinas AP (2017) The ROC Toolbox: A toolbox for analyzing receiver-operating characteristics derived from confidence ratings. Behav Res Methods 49:1399–1406. doi: 10.3758/sl3428-016-0796-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kometer M, Cahn BR, Andel D, et al. (2011) The 5-HT2A/1A agonist psilocybin disrupts modal object completion associated with visual hallucinations. Biol Psychiatry 69:399–406. doi: 10.1016/j.biopsych.2010.10.002 [DOI] [PubMed] [Google Scholar]
- Kometer M, Schmidt A, Jancke L, Vollenweider FX (2013) Activation of serotonin 2A receptors underlies the psilocybin-induced effects on a oscillations, N170 visual- evoked potentials, and visual hallucinations. J Neurosci 33:10544–10551. doi: 10.1523/JNEUROSCI.3007-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kometer M, Vollenweider FX (2018) Serotonergic Hallucinogen-Induced Visual Perceptual Alterations. Curr Top Behav Neurosci 36:257–282. doi: 10.1007/7854_2016_461 [DOI] [PubMed] [Google Scholar]
- Kraehenmann R, Schmidt A, Friston K, et al. (2015) The mixed serotonin receptor agonist psilocybin reduces threat-induced modulation of amygdala connectivity. Neuroimage Clin 11:53–60. doi: 10.1016/j.nicl.2015.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac NV, Frolich MA, Tran S, et al. (2017) Ketamine modulates hippocampal neurochemistry and functional connectivity: a combined magnetic resonance spectroscopy and resting-state fMRI study in healthy volunteers. Mol Psychiatry 22:562–569. doi: 10.1038/mp.2016.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB (2016) lmerTest: Tests in linear mixed effects models
- Luck SJ, Gold JM (2008) The translation of cognitive paradigms for patient research. Schizophr Bull 34:629–644. doi: 10.1093/schbul/sbn036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J (1982) An automated version of the digit symbol substitution test (DSST). Behavior Research Methods & Instrumentation 14:463–466. doi: 10.3758/BF03203313 [DOI] [Google Scholar]
- Moore TM, Reise SP, Gur RE, et al. (2015) Psychometric properties of the Penn Computerized Neurocognitive Battery. Neuropsychology 29:235–246. doi: 10.1037/neu0000093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford GK, Rush CR, Griffiths RR (1995) Alprazolam and DN-2327 (Pazinaclone) in Humans: Psychomotor, Memory, Subjective, and Reinforcing Effects. Experimental and clinical psychopharmacology 3:39–48 [Google Scholar]
- Musso F, Brinkmeyer J, Ecker D, et al. (2011) Ketamine effects on brain function- simultaneous fMRI/EEG during a visual oddball task. Neuroimage 58:508–525. doi: 10.1016/j.neuroimage.2011.06.045 [DOI] [PubMed] [Google Scholar]
- Nagels A, Kirner-Veselinovic A, Krach S, Kircher T (2011) Neural correlates of S- ketamine induced psychosis during overt continuous verbal fluency. Neuroimage 54:1307–1314. doi: 10.1016/j.neuroimage.2010.08.021 [DOI] [PubMed] [Google Scholar]
- Nichols DE (2016) Psychedelics. Pharmacol Rev 68:264–355. doi: 10.1124/pr.115.011478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIDA (2015) Hallucinogens and dissociative drugs, including LSD, psilocybin, peyote, DMT, ayahuasca, PCP, ketamine, dextromethorphan, and salvia. NIH [Google Scholar]
- Passie T, Seifert J, Schneider U, Emrich HM (2002) The pharmacology of psilocybin. Addict Biol 7:357–364. doi: 10.1080/1355621021000005937 [DOI] [PubMed] [Google Scholar]
- Quednow BB, Kometer M, Geyer MA, Vollenweider FX (2012) Psilocybin-Induced Deficits in Automatic and Controlled Inhibition are Attenuated by Ketanserin in Healthy Human Volunteers. Neuropsychopharmacology 37:630–640. doi: 10.1038/npp.2011.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- Reissig CJ, Carter LP, Johnson MW, et al. (2012) High doses of dextromethorphan, an NMDA antagonist, produce effects similar to classic hallucinogens. Psychopharmacology (Berl) 223:1–15. doi: 10.1007/s00213-012-2680-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose NS, Craik FIM (2012) A processing approach to the working memory/long-term memory distinction: evidence from the levels-of-processing span task. J Exp Psychol Learn Mem Cogn 38:1019–1029. doi: 10.1037/a0026976 [DOI] [PubMed] [Google Scholar]
- Roseman L, Sereno MI, Leech R, et al. (2016) LSD alters eyes-closed functional connectivity within the early visual cortex in a retinotopic fashion. Hum Brain Mapp 37:3031–3040. doi: 10.1002/hbm.23224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S, Bossis A, Guss J, et al. (2016) Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol (Oxford) 30:1165–1180. doi: 10.1177/0269881116675512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA (2015) 2014 national survey on drug use and health: Detailed tables.
- Schadel M, Wu D, Otton SV, et al. (1995) Pharmacokinetics of dextromethorphan and metabolites in humans: influence of the CYP2D6 phenotype and quinidine inhibition. J Clin Psychopharmacol 15:263–269 [DOI] [PubMed] [Google Scholar]
- Schmid Y, Enzler F, Gasser P, et al. (2015) Acute Effects of Lysergic Acid Diethylamide in Healthy Subjects. Biol Psychiatry 78:544–553. doi: 10.1016/j.biopsych.2014.11.015 [DOI] [PubMed] [Google Scholar]
- Schmidt A, Muller F, Lenz C, et al. (2018) Acute LSD effects on response inhibition neural networks. Psychol Med 48:1464–1473. doi: 10.1017/S0033291717002914 [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J (1988) Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen 117:34–50 [DOI] [PubMed] [Google Scholar]
- Tylš F, Páleníč ek T, Horáček J (2014) Psilocybin—summary of knowledge and new perspectives. Eur Neuropsychopharmacol 24:342–356. doi: 10.1016/j.euroneuro.2013.12.006 [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Csomor PA, Knappe B, et al. (2007) The effects of the preferential 5- HT2A agonist psilocybin on prepulse inhibition of startle in healthy human volunteers depend on interstimulus interval. Neuropsychopharmacology 32:1876–1887. doi: 10.1038/sj.npp.l301324 [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Kometer M (2010) The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci 11:642–651. doi: 10.1038/nrn2884 [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Oye I, et al. (1997) Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET). Eur Neuropsychopharmacol 7:25–38 [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, et al. (1998) Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9:3897–3902 [DOI] [PubMed] [Google Scholar]
- Wilson MD, Ferguson RW, Mazer ME, Litovitz TL (2011) Monitoring trends in dextromethorphan abuse using the National Poison Data System: 2000–2010. Clin Toxicol (Phila) 49:409–415. doi: 10.3109/15563650.2011.585429 [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Parks CM (2007) Receiver operating characteristics (ROCs) in recognition memory: a review. Psychol Bull 133:800–832. doi: 10.1037/0033-2909.133.5.800 [DOI] [PubMed] [Google Scholar]
- Yoran-Hegesh R, Kertzman S, Vishne T, et al. (2009) Neuropsychological mechanisms of Digit Symbol Substitution Test impairment in Asperger Disorder. Psychiatry Res 166:35–45. doi: 10.1016/j.psychres.2007.11.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


