Abstract
Gait speed is an indicator of overall functional health and is correlated with survival in older adults. We prospectively evaluated the long-term association between cerebral vasoreactivity and gait speed during normal walking (NW) and dual-task walking (DTW) in older adults with and without type 2 diabetes mellitus (T2DM). Forty participants (aged 67.3±8.8 years, 20 with T2DM) completed a two-year prospective study consisting of MRI, blood sampling, and gait assessments. The whole brain vasoreactivity was quantified using continuous arterial spin labeling MRI. Gait speed during DTW was assessed by subtracting serial sevens. Dual-task cost was calculated as the percent change in gait speed from NW to DTW. In the entire cohort, higher glycemic profiles were associated with a slower gait speed. In the diabetic group, lower vasoreactivity was associated with a slower gait speed during NW (r2adj=0.30, p=0.019) and DTW (r2adj=0.35, p=0.01) and a higher dual-task cost (r2adj=0.69, p=0.009) at two-year follow-up. The participants with T2DM and lower cerebral vasoreactivity had a greater decrease in gait speed during NW and DTW after the two-year follow-up (r2adj=0.17, p=0.04 and r2adj=0.28, p=0.03, respectively). Longer diabetes duration was associated with a higher dual-task cost (r2adj=0.19, p=0.04) and a greater decrease in gait speed during NW (r2adj=0.17, p=0.02). These findings indicate that in older adults with type 2 diabetes, gait performance is highly dependent on the integrity of cerebrovascular regulation.
Keywords: vasoreactivity, vasoregulation, perfusion, type 2 diabetes, cognition, gait, walking
INTRODUCTION
Gait speed is an important indicator of overall functional health and is correlated with survival in older adults [1]. Type 2 diabetes mellitus (T2DM) has been separately linked to reduced gait speed and increased risk of falls [2–4]. Impaired cerebral vasoreactivity has been cross-sectionally associated with slower walking speed in older adults with and without T2DM [5, 6]. However, the long-term effect of diabetes on gait speed and the relationship between cerebral vasoreactivity and mobility remains unknown. Gait performance while performing a cognitive task and its association with cerebral vasoregulation in T2DM has not been previously reported. The aim of the current study is to determine prospectively 1) the effects of T2DM on gait during normal walking (NW) and walking while performing a serial subtraction cognitive task (dual-task walking, DTW) and 2) the relationship between cerebral vasoreactivity and gait performance in older adults with and without T2DM over a two-year period. We hypothesized that: 1) participants with T2DM would demonstrate a worse gait performance as compared to controls, 2) diabetic participants with lower cerebral vasoreactivity would demonstrate a greater decline in gait speed after two-years of follow-up.
MATERIAL AND METHODS
Participants
The study was conducted between August 2009 and July 2013 at the Syncope and Falls in the Elderly Laboratory in the Clinical Research Center and at the MRI Center at Beth Israel Deaconess Medical Center (BIDMC). Potential participants were recruited consecutively through advertisements in the local community. Inclusion criteria for the diabetes group included: age 50–85 years and diagnosed with T2DM for more than 5 years. Inclusion criteria for the nondiabetic group included age- and sex-matched controls with normal fasting glucose and hemoglobin A1c (HbA1c). All participants were able to walk independently without assistance or walking aids. Exclusion criteria included type 1 diabetes, history of stroke or other neurological disorders that may cause gait disturbances, dementia or subthreshold Mini-Mental Status Examination (MMSE) ≤ 24 [7], myocardial infarction, congestive heart failure, severe hypertension (systolic blood pressure [BP] > 200, diastolic BP > 110 mmHg or taking three or more antihypertensive medications), and MRI incompatibilities (i.e., claustrophobia, metal implants, pacemakers, and arterial stents).
This study was carried out in accordance with the recommendations of ethical standards of the BIDMC and approved by the Institutional Review Board. All participants signed the written informed consent in accordance with the Declaration of Helsinki.
Protocol
Participants completed the same protocol during two overnight study visits separated by a two-year interval (baseline and two-year follow-up). Prior to each study visit, BP was continuously measured for 3 days by using a wearable 24-h ambulatory BP monitoring device (Dynapulse, Inc., Vista, CA, USA). Upon admission, participants completed medical history and physical activity questionnaires. A study physician performed physical and neurological examinations. The clinical signs and symptoms of diabetic polyneuropathy were assessed using the Toronto Clinical Neuropathy Score [8]. Fasting laboratory chemistries were obtained the next morning, followed by the completion of the gait tests and brain MRI protocols. Antihypertensive medications were withdrawn during the morning of the study. Glycemic control medications were administered as prescribed.
Walking test
Two 6-min walking trials were performed along a 75-m course within an 80 × 4 m indoor hallway. The start and end points were marked on the floor with tape. For the NW trial, participants were instructed to walk at a self-preferred pace. For the DTW trial, participants were instructed to walk while verbally subtracting serial 7s from a random 3-digit number. The time taken to complete each 75-m length and total distance were recorded. For the final analysis, only the data of the first 75 m walk were examined to minimize potential confounders of turning and fatigue. The change in gait speed during NW and DTW was calculated as the difference between the gait speed at baseline visit and that at the two-year follow-up visit. Dual-task cost to gait speed was calculated as the percent change in gait speed from NW to DTW conditions, i.e., [(NW – DTW)/NW] × 100 [9].
MRI
Three-dimensional continuous arterial spin labeling (CASL) brain imaging was performed using a 3-T GE HDx MRI scanner (GE Medical Systems, Milwaukee, WI, USA). This noninvasive mapping of cerebral blood flow was quantified during normal breathing (normocapnia), CO2 rebreathing with 95% air and 5% CO2 (hypercapnia), and hyperventilation (hypocapnia). Two-minute scans were acquired during each condition. Respiratory rate, tidal volume, and end-tidal CO2 values were recorded during each scan, and vital signs were measured at 1-minute intervals using an upper arm automatic pressure cuff and finger photoplethysmogram.
Image processing
Images were analyzed using tools developed in interactive data language (Research Systems, Boulder, CO, USA) and MATLAB (MathWorks, Natick, MA, USA). Quantitative perfusion and vasoreactivity maps were co-registered with anatomical images, and calculations were performed globally and within each brain region of interest (frontal, temporal, parietal, and occipital lobes). Cerebral vasoreactivity was calculated as the slope of the regression between perfusion and CO2 (mL/100 g/min/mmHg) during normocapnia, hypercapnia, and hypocapnia [10, 11]. The change in vasoreactivity was calculated as the difference between the vasoreactivity at baseline and at the two-year follow-up visit.
The volume and distribution of white matter hyperintensities (WMH) were quantified on high resolution T2-weighted fluid-attenuation inversion recovery (FLAIR). WMH seeds were identified via thresholding of hyperintense pixels using a region growing method applied on WMH seeds. Periventricular WMH are described as ill-defined and moderately hypodense (>30% signal intensity change) areas of ≥ 5 mm on FLAIR.
The brain volume was computed from the T1-weighted magnetization-prepared rapid gradient-echo (MP-RAGE) image using an inherently circular model in Statistical Parametric Mapping software package (SPM, Wellcome Department of Imaging Neuroscience, University College London, UK) involving spatial normalization and tissue classification.
Statistical analysis
All variables were summarized using descriptive statistics. Demographic data, laboratory variables, and gait-related outcomes were compared between the groups by using one-way analysis of variance (ANOVA). Matched-pair t-tests were used to compare the differences in gait speed between NW and DTW within each group and across the entire cohort at each visit as well as between the baseline and two-year follow-up visits. A correlation matrix was used to determine the associations between the following variables: age, sex, body mass index (BMI), glucose, HbA1c, duration of diabetes, hematocrit (Hct), BP, hypertension, gait speed, and cerebral vasoreactivity. The variables with r2 > 0.1 or p < 0.05 were included in the models. Least square models were used to assess the relationships between cerebral vasoreactivity, gait speed, dual-task cost, and changes in gait speed over two years. Modeling was conducted globally and regionally for the frontal, parietal, temporal, and occipital regions and the models were adjusted for age, sex, BMI, Hct, and the Toronto Clinical Neuropathy Score. Hct was included in the model because this variable is inversely correlated with cerebral perfusion [10, 12]. Tertiles of cerebral vasoreactivity were calculated and their associations with gait speed were analyzed. All analyses were performed using JMP software (SAS Institute, Cary, NC, USA).
RESULTS
Cohort Characteristics
Of the 131 participants that provided written informed consent, 75 participants were enrolled in the study and of those, 40 participants (20 with T2DM), who completed both the baseline and two-year follow-up visits, were included in the final analyses. Participants were excluded from baseline analyses for the following reasons: withdrawal of consent (n = 11), lost to follow-up (n = 10), poor glycemic control (n = 4), diabetes<5 years (n = 3), smoking (n = 1), stroke or TIA (n =2), unidentified neurologic disorders (n = 2), subthreshold MMSE score (n = 3), arrhythmia (n = 4), heart failure (n = 1), uncontrolled hypertension (n = 3), MRI-incompatible implants (n = 1), renal insufficiency (n = 1), cancer (n = 2), adverse event (n = 1), and incomplete datasets (n = 7). Participants were excluded from the 2-year follow-up analyses for the following reasons: withdrawal of consent (n = 5), lost to follow-up (n = 25), subthreshold MMSE score (n = 1), and incomplete datasets (n = 4).
At baseline, participants with T2DM were treated with insulin (n= 6), oral glucose-control agents (n = 15), and diet only (n = 3) for hyperglycemia and for hypertension (n = 17) and hypercholesterolemia (n = 11). Control participants were treated for hypertension (n = 4) and hypercholesterolemia (n = 4). At the two-year follow-up, 7 participants with T2DM were treated with insulin, 15 were taking oral glucose-control agents, and 4 participants controlled hyperglycemia with diet only.
At baseline, the diabetic and control groups had similar age and sex profiles. The diabetic group had higher BMI, fasting glucose, HbA1c levels, and Toronto Clinical Neuropathy scores (Table 1). Although the prevalence of hypertension was higher in the diabetic group, the 24-h systolic and diastolic BP was similar between the groups. At baseline, groups did not differ based on educational level, MMSE scores, geriatric depression scale (GDS), instrumental activities of daily living scales (IADL), global gray matter, white matter, or WMH volumes. In the entire cohort, higher HbA1c was associated with an increase in the WMH volume (p = 0.017). At the two-year follow-up, global gray matter volume remained similar between groups (p = 0.11). The diabetic group had lower MMSE scores as compared to the control group (26.9 ± 2.3 versus 28.9 ± 1.4, p = 0.002, respectively).
Table 1.
Demographic characteristics and laboratory results
| Control | T2DM | p | |
|---|---|---|---|
| N | 20 | 20 | |
| Age (years) | 67.6 ± 9.83 | 67.05 ± 7.97 | NS |
| Sex (men/women) | 9/11 | 7/13 | NS |
| Diabetic duration | 12.15 ± 6.0 | ||
| Hypertension (with/without) | 4/16 | 16/4 | <0.0001 |
| Body mass index (kg/m2) | 24.91 ± 4.15 | 29.28 ± 5.65 | 0.0086 |
| Education level (years) | 16.45 ± 2.81 | 15.18 ± 4.90 | NS |
| MMSE | 28.7 ± 1.76 | 28.9 ± 1.2 | NS |
| Geriatric Depression Scale (GDS) | 5.82 ± 7.11 | 8.94 ± 8.14 | NS |
| Instrumental Activities of Daily Living (IADL) | 26.43 ± 1.60 | 26.00 ± 1.21 | NS |
| Toronto Clinical Neuropathy score | 3.00 ± 2.34 | 6.05 ± 4.57 | 0.015 |
| Global gray matter volume (mL) | 632.67 ± 88.11 | 589.56 ± 65.77 | NS |
| Global white matter volume (mL) | 446.63 ± 63.70 | 413.13 ± 70.73 | NS |
| Global white matter hyperintensities (mL) | 15.83 ± 5.59 | 14.93 ± 5.64 | NS |
| Systolic blood pressure (mmHg) | 131.35 ± 7.11 | 132.43 ± 2.19 | NS |
| Diastolic blood pressure (mmHg) | 68.51 ± 8.76 | 69.34 ± 6.41 | NS |
| Fasting glucose (mg/dL) | 88.50 ± 10.93 | 114.05 ± 27.64 | 0.001 |
| HbA1c (%) | 5.71 ± 0.29 | 7.11 ± 1.26 | <0.0001 |
| HbA1c (mmol/mol) | 38.9 ± 3.1 | 54.3 ± 13.9 | <0.0001 |
| Cholesterol (mg/dL) | 179.79 ± 35.7 | 154.80 ± 37.30 | 0.039 |
| Hematocrit (%) | 39.26 ± 4.54 | 38.56 ± 4.06 | NS |
Continuous variables are presented as mean ± SD. p = between-group comparisons. One-way ANOVA was used for continuous variables and Fisher’s exact test was used for categorical variables. HbA1c = hemoglobin A1c; MMSE = Mini-mental State Exam; NS = comparison not significantly different if p > 0.05.
Vasoreactivity and the Effect of Diabetes
At baseline, the diabetic and the control groups did not differ in global or regional cerebral vasoreactivity (Table 2). At two-year follow-up, the diabetic group exhibited decreases in global (p = 0.046) and regional vasoreactivity (frontal lobes, p = 0.047; temporal lobes, p = 0.025; Table 2) as compared to that at baseline. In the control group, no significant decrease in cerebral vasoreactivity was observed.
Table 2.
Comparison of functional outcomes between baseline and two-year follow-up visits
| Control (N = 20) | T2DM (N = 20) | |||
|---|---|---|---|---|
| Visit | Baseline | 2 years | Baseline | 2 years |
| Vasoreactivity (mL/100 g/min/mmHg) | ||||
| Global | 0.95 ± 0.57 | 0.69 ± 0.45 | 1.33 ± 1.07 | 0.72 ± 0.42‡ |
| Frontal | 0.83 ± 0.58 | 0.62 ± 0.46 | 1.17 ± 0.86 | 0.69 ± 0.50‡ |
| Parietal | 1.06 ± 0.75 | 0.82 ± 0.52 | 1.33 ± 0.83 | 0.88 ± 0.57 |
| Temporal | 0.89 ± 0.53 | 0.62 ± 0.39 | 1.17 ± 0.72 | 0.68 ± 0.35‡ |
| Occipital | 1.31 ± 0.91 | 1.02 ± 0.56 | 1.52 ± 0.84 | 1.02 ± 0.71 |
| Gait measures | ||||
| Normal walking (m/s) | 1.16 ± 0.18 | 1.16 ± 0.13 | 1.05 ± 0.14§, | 1.16 ± 0.30 |
| Dual-task walking (m/s) | 1.04 ± 0.52† | 1.05 ± 0.15† | 0.97± 0.19 | 1.05 ± 0.33† |
| Dual-task cost (%) | 8.93 ± 10.32 | 9.41 ± 7.01 | 6.44 ± 17.35 | 10.09 ± 11.50 |
Comparison between normal walking and dual-task walking (two-tailed matched pairs), if p < 0.05;
if p < 0.01
Comparison between baseline and two-year follow-up visits (one-tailed matched pairs), if p < 0.05
Between-group comparison (ANOVA), if p < 0.05
Gait Performance and the Effect of Diabetes
Baseline visit
Compared with controls, the diabetic group walked more slowly during NW (p = 0.017; Table 2). In controls, gait speed was slower during DTW compared with that during NW (p = 0.004).
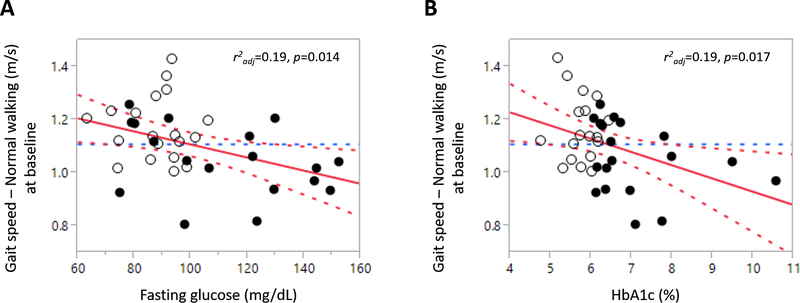
In the entire cohort, higher fasting glucose and HbA1c levels were associated with a slower gait speed during NW (r2adj = 0.19, p = 0.014, Fig. 1a; r2adj = 0.19, p = 0.017, Fig. 1b), independent of age and sex. In the diabetic group, longer diabetes duration was associated with a higher dual-task cost during walking (r2adj = 0.19, p = 0.04), independent of age, sex, BMI, and neuropathy score.
Fig. 1.
Associations between the glycemic profiles and gait measures.
Higher fasting glucose (a) and HbA1c (b) are associated with a slower gait speed during NW. White circles = controls; black circles = participants with T2DM; best fit = red solid line; confidence interval = red dotted lines; mean = blue dotted line.
Two-year follow-up visit
Both the diabetic (p = 0.0006) and control (p < 0.0001) groups had a slower gait speed during DTW compared to NW. The diabetic and the control groups did not differ in their gait speed during NW or DTW. When compared to the baseline, there was no difference between the gait speed during NW or DTW in both diabetic and control groups (Table 2).
In the entire cohort, higher fasting glucose was associated with higher dual-task cost during walking (r2adj = 0.17, p = 0.017), independent of age, sex, BMI, and neuropathy score.
In the entire cohort, higher WMH volume at baseline was associated with a greater decrease in gait speed during DTW after the two-year follow-up period (r2adj = 0.04, p = 0.038), independent of age, BMI, neuropathy score, and HbA1c.
In the diabetic group, those with better glycemic control (i.e., HbA1c less than 7%) exhibited a decrease in their dual-task cost in the follow up visit, while those with less favorable glycemic control (i.e., HbA1c greater than 7%) exhibited an increase in their dual-task cost at the two-year follow-up, independent of age, sex, BMI, and WMH volume (p = 0.016).
Relationship between Vasoreactivity and Gait Performance
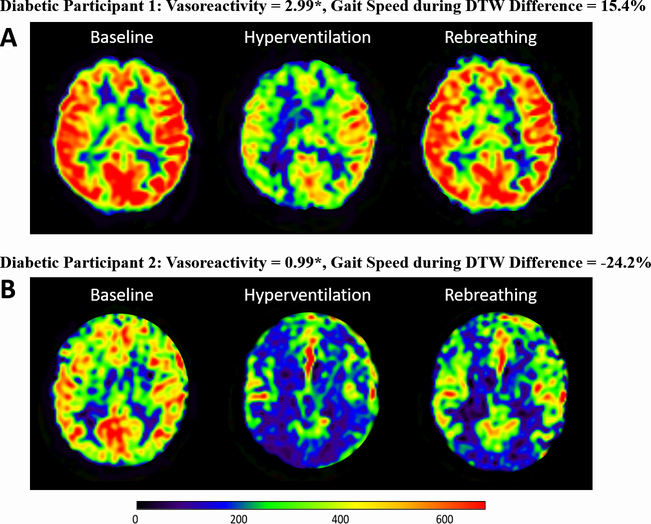
Fig. 2 presents an example of perfusion registration for two representative diabetic participants: one with higher global vasoreactivity at baseline and faster gait speed during DTW at the two-year follow-up (a), and the other with lower global vasoreactivity at baseline and slower gait speed during DTW at follow-up (b).
Fig. 2.
Perfusion maps, vasoreactivity, and gait speed for two participants with T2DM.
Perfusion maps for two representative participants with T2DM using a 3T, 3-dimensional CASL MRI show (a) a participant with higher global vasoreactivity at baseline and has faster gait speed during DTW at the two-year follow-up, and (B) a participant with lower global vasoreactivity and has slower gait speed during DTW at the two-year follow-up. The gait speed difference shows the percent changes in gait speed between the two-year follow-up and baseline. *unit–mL/100g/min/mmHg.
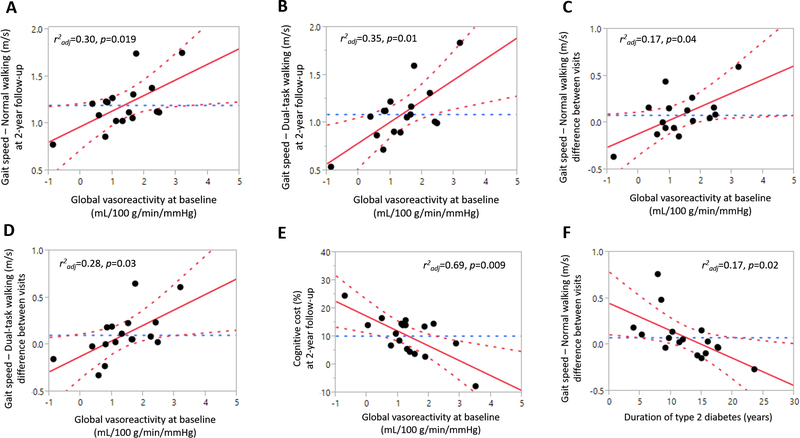
In the diabetic group, a lower global vasoreactivity at baseline was associated with a slower gait speed during both NW (r2adj = 0.30, p = 0.019, Fig. 3a) and DTW (r2adj = 0.35, p = 0.01, Fig. 3b) at the two-year follow-up, independent of age, BMI, neuropathy score, Hct, and WMH volume. In this group, a lower global vasoreactivity at baseline was also associated with a greater decrease in gait speed during both NW (r2adj = 0.17, p = 0.04, Fig. 3c) and DTW (r2adj = 0.28, p = 0.03, Fig. 3d) at the two-year follow-up, independent of age, BMI, neuropathy score, Hct, and WMH volume. In the control group, there were no similar relationships between vasoreactivity and gait speed.
Fig 3.
Associations between vasoreactivity and gait measures in participants with T2DM.
Lower cerebral vasoreactivity is associated with slower gait speed during NW (a) and DTW (b) and greater decreases in gait speed during NW (c) and DTW (d) in the diabetic group. Lower cerebral vasoreactivity is associated with higher dual-task costs during walking (e). Duration of diabetes is associated with greater decrease in gait speed during NW (f). In the control group, no similar association was observed. Best fit = red solid line; confidence interval = red dotted lines; mean = blue dotted line.
In the diabetic group, a lower global vasoreactivity at baseline was associated with a higher dual-task cost at the two-year follow-up visit, (r2adj = 0.69, p = 0.009, Fig. 3e), independent of age, sex, BMI, neuropathy score, Hct, and educational level. In the diabetic group, a longer duration of diabetes was also associated with a greater decrease in gait speed during NW (r2adj = 0.17, p = 0.02, Fig. 3f), independent of age, sex, BMI, neuropathy score, and fasting glucose level.
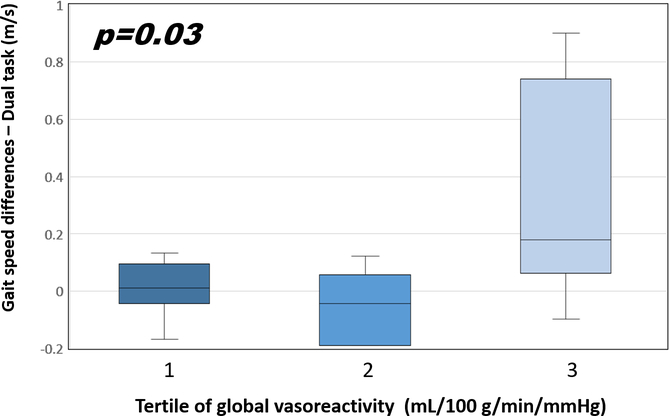
In order to explore nonlinear associations, we examined the associations between gait speed and tertiles of global vasoreactivity, with tertile 1 representing the lowest and tertile 3 representing the highest tertile of vasoreactivity. In the diabetic group, the differences in gait speed during DTW between the two-year follow-up and baseline visits were 0.01±0.1, −0.05±0.1, and 0.33±0.1 (m/s) in tertile 1, 2, and 3 respectively (p=0.03, Fig. 4). Post-hoc testing revealed that tertile 2 demonstrated a greater decrease in gait speed during DTW as compared to tertile 3 (p=0.03).
Fig 4.
Changes of gait speed and vasoreactivity.
The gait speed differences show the percent changes in gait speed during DTW between the two-year follow-up and baseline and are stratified by global cerebral vasoreactivity in the diabetic group. Tertile 3 is the highest and the referent tertile. The error bars represent the standard deviation of the vasoreactivity.
DISCUSSION
This study demonstrated that alteration of cerebral vasoreactivity was independently associated with a greater decrease in gait speed during NW and DTW in the diabetic group over the two-year follow-up period. In the diabetic group, the greater decline in cerebral vasoreactivity was the predictor of the greater decrease in gait speed and greater increase in dual-task cost to gait speed. The duration of diabetes was correlated with the severity of decline in cerebral vasoreactivity. These findings were independent of the presence and severity of peripheral neuropathy or WMH, and were not observed in the age-matched control group. This study further demonstrated that glycemic control was correlated with gait speed. In the entire cohort, higher glycemic indices, measured by fasting glucose and HbA1c, were associated with a slower gait speed during NW and higher dual-task cost to gait speed.
Vasoreactivity is a measure of vasoregulation and cerebral endothelial function [13, 14] and is directly implicated in the distribution of adequate blood perfusion by vasodilation and vasoconstriction to areas of increased or decreased neuronal activity [5, 15]. Impaired cerebral vasoreactivity has been linked to slow gait speed and falls in elderly people [6]. T2DM is characterized by altered endothelial dysfunction and blood–brain barrier permeability, which can ultimately lead to a decline in vasoreactivity [11, 16, 17]. Previous studies have reported the association between cerebral vasoreactivity and gait speed in T2DM in a cross-sectional manner [5, 6]. Our study further investigated the long-term effects of lower cerebral vasoreactivity on normal and dual task walking speed in T2DM, demonstrating that the lower global vasoreactivity is the predictor of greater deterioration of gait performance in the diabetic group.
Gait speed reflects overall health and functional status and is recommended as a potentially useful clinical indicator of well-being among older adults [1]. Numerous reports have revealed that gait abnormalities and the risk of falls in T2DM [3, 4] are associated with reduced peripheral nerve function [18, 19], ankle muscle strength [20], and balance [21]. Moreover, T2DM is associated with altered gait pattern and speed despite the absence of peripheral neuropathy [2]. Previous studies on older adults have reported an association between gray matter volume and gait speed in T2DM, thus emphasizing the importance of cerebral control to walking in this population [11, 21].
WMH has been linked to slower gait disturbance and balance dysfunction in older adults [22, 23]. In our study, we demonstrated the association between HbA1c, WMH volume and slower gait speed, suggesting the importance of the integrity of the central nervous system pathway in gait performance, and their links to glycemic control.
Walking while performing a secondary task (i.e., dual-task walking) has been used to assess the interaction between cognition, gait, and risk of falls [24] and has been suggested as a sensitive indicator of gait performance [9, 25]. The percent change in an individual’s gait speed from a single- to a dual-task is known as the dual-task cost. The underlying hypothesis suggests that two simultaneously performed tasks interfere with each other and compete for brain cortical resources [26]. The magnitude of these dual-task costs reveals the insufficiency of cortical control for regulating walking and has been associated with a slower gait speed in individuals with cognitive dysfunctions such as mild cognitive impairment and Alzheimer’s disease [27].
Previous studies have reported that in individuals with T2DM, gait is affected by reduced cognitive function irrespective of the presence of neuropathy [25, 28]. We are the first to report gait performance including the dual-task cost to gait speed in T2DM, and its association with cerebral vasoregulation. Our study investigated gait speed during NW and DTW in participants with and without T2DM and found that in the diabetic group, adequate cerebral vasoregulation was a predictor of a faster gait speed. At the two-year follow-up, participants with T2DM and a lower cerebral vasoreactivity at baseline had a greater decline in gait speed during both NW and DTW. It appears that those in the lower vasoreactivity tertile at baseline had a slower gait speed and greater decrease in gait speed during DTW as compared to those in the highest tertile who did not have significant decrease of gait speed. Therefore, in participants with diabetes, the higher cerebral vasoreactivity is an indicator of better overall functionality as indicted by faster walking.
Walking has been proposed to be not only an autonomous activity, but also one involved in the integration of multiple systems, including the central nervous systems [21, 29]. In individuals with T2DM, walking regulation greatly depends on the cerebral locomotor control system and is affected by cognitive function [4]. When the dual-task paradigm is used to evaluate the cognitive cost of walking, the relationship between cerebral vasoreactivity and gait performance indicates the importance of cerebral vascular regulation in gait control in individuals with T2DM.
Intact cerebral vasoreactivity allows for regional brain blood perfusion to meet metabolic demands while performing specific tasks [30, 31]. T2DM-related hyperglycemia, insulin resistance, and inflammatory processes may facilitate nitric oxide-dependent endothelial dysfunction [11], subsequently causing impaired cerebral vasoreactivity and a related decline in walking performance [5, 6]. During DTW, performance in the subtraction task depends on cognitive functions related to working memory and attention [32]. The association between cerebral vasoreactivity, slower DTW gait speed, and higher dual-task cost represents the insufficiency of the cerebral capacity to perform a concurrent cognitive load during walking. The deficiency is evidenced by the rapid deterioration in cognitive function and cerebral vasoregulation in T2DM [25, 28] and is readily observed during dual-task walking. In our study, the participants with T2DM exhibited a large increase of dual-task cost when the two visits were compared (6.44 at baseline versus 10.09 at two-year follow-up) and also exhibited a large standard deviation of dual-test cost at baseline and at two-year follow-up. In this group, those with a lower vasoreactivity developed a greater increase in dual-task cost. This vasoreactivity-dependent difference might have caused the wide-ranged presentation of gait speed and dual-task cost in the T2DM group. Moreover, T2DM with poor glycemic control typically presents a significant glycemic swing between hyper- and hypoglycemia. Increased glycemic variability may independently contribute to the progression of cerebral microvascular disease and is associated with greater cognitive decline in diabetes [33]. The association between the decline in vasoreactivity and the duration of diabetes in our study support the notion of the chronic hyperglycemic damaging effect on cerebral vasoregulation, subsequently causing deterioration in gait performance in T2DM. The relationship between global cerebral vasoreactivity and gait speed also suggests the complexity of the central nervous system mechanism that controls walking. The integration and integrity of various cerebral regions responsible for different functions, including cognition, are essential and critical to maintain normal gait performance in individuals with T2DM [34, 35].
Although there are now more complex methods to evaluate gait performance, a simple gait speed measure remains highly clinically relevant in the geriatric population. In a study conducted by Studenski et al. (2011), a pooled analysis of 34,485 community-dwelling older from 9 cohort studies revealed that gait speed was associated with survival in older adults [1]. Though complex technology and devices have led to a more comprehensive understanding in mechanism of human gait, a simple method which is clinically relevant, may provide a generalized rule that could be easily applied in the clinical settings. In our study, we measured gait speed during normal walking and dual-task walking, finding that gait performance in T2DM is highly dependent on the integrity of cerebral vasoregulation. In the future, more complex methods for evaluating gait performance may be used based on current results.
CONCLUSION
Our study demonstrated that in the older adults with T2DM, a lower cerebral vasoreactivity was prospectively associated with a greater decrease in gait speed and a higher dual-task cost over the two-year follow-up period. These findings highlight the importance of the long-term effect of cerebral vasoregulation on gait speed in diabetes. Our study had a relatively moderate sample size and follow-up period. Further research involving more complex gait measures (e.g., temporo-spatial parameters) to yield more comprehensive understandings of gait performance in this population is warranted. The strength of our study is the prospective association between cerebral vasoreactivity, gait speed decline, and dual-task cost to gait speed in older adults with T2DM. The results have the clinical implications that cerebral vasoreactivity is an indicator for detecting and predicting the deterioration of gait performance in older adults with T2DM. Furthermore, the development of novel treatment strategies to improve cerebral vasoregulation and cognitive functions during dual-task performance may effectively improve gait performance, prevent falls, and increase survival in older adults with T2DM.
Acknowledgments
The work was supported by the National Institutes of Health (NIH-NIA 1R01-AG0287601A2, NIH-NIDDK 5R21 DK084463), American Diabetes Association (Clinical 1–03-CR-23 and 1–06-CR-25 to Dr. Vera Novak). The work was also in part supported NIH-NIDDK 1R01 1R01DK103902–01A1 to Dr. Novak. The project described was supported by Harvard Clinical and Translational Science Center (Grant Number UL1 RR025758) and National Center for Research Resources (M01-RR-01032).
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R01DK103902. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- NW
normal walking
- DTW
dual-task walking
- T2DM
type 2 diabetes mellitus
- CASL
3-D continuous arterial spin labeling
- WMH
white matter hyperintensities
Footnotes
Compliance with ethical standards
Conflicts of interest Chen-Chih Chung, Daniela A. Pimentel, Freddy J. Alfaro, Vasileios-Arsenios Lioutas, Maria Zunilda Núñez report no disclosures. Vera Novak was funded by the National Institutes of Health (NIH-NIDDK 1R01DK103902 A1, NIH-NIA 1R01- AG0287601A2, NIH-NIDDK 5R21 DK084463), American Diabetes Association (Clinical 1–03-CR-23 and 1–06-CR-25). Azizah J. Jor’dan was funded by NIH T32 Fellowship (5T32AG023480 and HL007374–36), a Diversity Supplement Award (3R01AG041785–02S1) and a Career Development Award (K99AG051766).
Ethical standards All participants that met inclusion/exclusion criteria signed an Informed consent form (ICF) as approved by the Institutional review board (IRB) at Beth Israel Deaconess Medical Center.
REFERENCES
- 1.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J (2011) Gait speed and survival in older adults. Jama 305:50–58. https://10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ko SU, Stenholm S, Chia CW, Simonsick EM, Ferrucci L (2011) Gait pattern alterations in older adults associated with type 2 diabetes in the absence of peripheral neuropathy--results from the Baltimore Longitudinal Study of Aging. Gait & posture 34:548–552. https://10.1016/j.gaitpost.2011.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz AV, Hillier TA, Sellmeyer DE, Resnick HE, Gregg E, Ensrud KE, Schreiner PJ, Margolis KL, Cauley JA, Nevitt MC, Black DM, Cummings SR (2002) Older women with diabetes have a higher risk of falls: a prospective study. Diabetes care 25:1749–1754. [DOI] [PubMed] [Google Scholar]
- 4.Roman de Mettelinge T, Cambier D, Calders P, Van Den Noortgate N, Delbaere K (2013) Understanding the relationship between type 2 diabetes mellitus and falls in older adults: a prospective cohort study. PloS one 8:e67055 https://10.1371/journal.pone.0067055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jor’dan AJ, Manor B, Novak V (2014) Slow gait speed - an indicator of lower cerebral vasoreactivity in type 2 diabetes mellitus. Frontiers in aging neuroscience 6:135 https://10.3389/fnagi.2014.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorond FA, Galica A, Serrador JM, Kiely DK, Iloputaife I, Cupples LA, Lipsitz LA (2010) Cerebrovascular hemodynamics, gait, and falls in an elderly population: MOBILIZE Boston Study. Neurology 74:1627–1633. https://10.1212/WNL.0b013e3181df0982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alencar RC, Cobas RA, Gomes MB (2010) Assessment of cognitive status in patients with type 2 diabetes through the Mini-Mental Status Examination: a cross-sectional study. Diabetology & metabolic syndrome 2:10 https://10.1186/1758-5996-2-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bril V, Tomioka S, Buchanan RA, Perkins BA (2009) Reliability and validity of the modified Toronto Clinical Neuropathy Score in diabetic sensorimotor polyneuropathy. Diabetic medicine : a journal of the British Diabetic Association 26:240–246. https://10.1111/j.1464-5491.2009.02667.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindenberger U, Marsiske M, Baltes PB (2000) Memorizing while walking: increase in dual-task costs from young adulthood to old age. Psychology and aging 15:417–436. [DOI] [PubMed] [Google Scholar]
- 10.Last D, Alsop DC, Abduljalil AM, Marquis RP, de Bazelaire C, Hu K, Cavallerano J, Novak V (2007) Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes care 30:1193–1199. https://10.2337/dc06-2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novak V, Zhao P, Manor B, Sejdic E, Alsop D, Abduljalil A, Roberson PK, Munshi M, Novak P (2011) Adhesion molecules, altered vasoreactivity, and brain atrophy in type 2 diabetes. Diabetes care 34:2438–2441. https://10.2337/dc11-0969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells RE, Jr., Merrill EW (1962) Influence of flow properties of blood upon viscosity-hematocrit relationships. The Journal of clinical investigation 41:1591–1598. https://10.1172/jci104617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavi S, Egbarya R, Lavi R, Jacob G (2003) Role of nitric oxide in the regulation of cerebral blood flow in humans: chemoregulation versus mechanoregulation. Circulation 107:1901–1905. https://10.1161/01.cir.0000057973.99140.5a [DOI] [PubMed] [Google Scholar]
- 14.Lavi S, Gaitini D, Milloul V, Jacob G (2006) Impaired cerebral CO2 vasoreactivity: association with endothelial dysfunction. American journal of physiology Heart and circulatory physiology 291:H1856–1861. https://10.1152/ajpheart.00014.2006 [DOI] [PubMed] [Google Scholar]
- 15.Fujishima M, Scheinberg P, Busto R, Reinmuth OM (1971) The relation between cerebral oxygen consumption and cerebral vascular reactivity to carbon dioxide. Stroke 2:251–257. [DOI] [PubMed] [Google Scholar]
- 16.Chung CC, Pimentel D, Jor’dan AJ, Hao Y, Milberg W, Novak V (2015) Inflammation-associated declines in cerebral vasoreactivity and cognition in type 2 diabetes. Neurology 85:450–458. https://10.1212/wnl.0000000000001820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, Marshall I (2003) Increased blood-brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. Journal of neurology, neurosurgery, and psychiatry 74:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allet L, Armand S, Golay A, Monnin D, de Bie RA, de Bruin ED (2008) Gait characteristics of diabetic patients: a systematic review. Diabetes/metabolism research and reviews 24:173–191. https://10.1002/dmrr.809 [DOI] [PubMed] [Google Scholar]
- 19.Fernando M, Crowther R, Lazzarini P, Sangla K, Cunningham M, Buttner P, Golledge J (2013) Biomechanical characteristics of peripheral diabetic neuropathy: A systematic review and meta-analysis of findings from the gait cycle, muscle activity and dynamic barefoot plantar pressure. Clinical biomechanics (Bristol, Avon) 28:831–845. https://10.1016/j.clinbiomech.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 20.Camargo MR, Barela JA, Nozabieli AJ, Mantovani AM, Martinelli AR, Fregonesi CE (2015) Balance and ankle muscle strength predict spatiotemporal gait parameters in individuals with diabetic peripheral neuropathy. Diabetes & metabolic syndrome 9:79–84. https://10.1016/j.dsx.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 21.Manor B, Newton E, Abduljalil A, Novak V (2012) The relationship between brain volume and walking outcomes in older adults with and without diabetic peripheral neuropathy. Diabetes care 35:1907–1912. https://10.2337/dc11-2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novak V, Last D, Alsop DC, Abduljalil AM, Hu K, Lepicovsky L, Cavallerano J, Lipsitz LA (2006) Cerebral blood flow velocity and periventricular white matter hyperintensities in type 2 diabetes. Diabetes care 29:1529–1534. https://10.2337/dc06-0261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novak V, Haertle M, Zhao P, Hu K, Munshi M, Novak P, Abduljalil A, Alsop D (2009) White matter hyperintensities and dynamics of postural control. Magnetic resonance imaging 27:752–759. https://10.1016/j.mri.2009.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lundin-Olsson L, Nyberg L, Gustafson Y (1997) “Stops walking when talking” as a predictor of falls in elderly people. Lancet (London, England) 349:617 https://10.1016/s0140-6736(97)24009-2 [DOI] [PubMed] [Google Scholar]
- 25.Paul L, Ellis BM, Leese GP, McFadyen AK, McMurray B (2009) The effect of a cognitive or motor task on gait parameters of diabetic patients, with and without neuropathy. Diabetic medicine : a journal of the British Diabetic Association 26:234–239. https://10.1111/j.1464-5491.2008.02655.x [DOI] [PubMed] [Google Scholar]
- 26.Montero-Odasso M, Muir SW, Speechley M (2012) Dual-task complexity affects gait in people with mild cognitive impairment: the interplay between gait variability, dual tasking, and risk of falls. Archives of physical medicine and rehabilitation 93:293–299. https://10.1016/j.apmr.2011.08.026 [DOI] [PubMed] [Google Scholar]
- 27.Camicioli R, Howieson D, Lehman S, Kaye J (1997) Talking while walking: the effect of a dual task in aging and Alzheimer’s disease. Neurology 48:955–958. [DOI] [PubMed] [Google Scholar]
- 28.Roman de Mettelinge T, Delbaere K, Calders P, Gysel T, Van Den Noortgate N, Cambier D (2013) The impact of peripheral neuropathy and cognitive decrements on gait in older adults with type 2 diabetes mellitus. Archives of physical medicine and rehabilitation 94:1074–1079. https://10.1016/j.apmr.2013.01.018 [DOI] [PubMed] [Google Scholar]
- 29.Kloter E, Wirz M, Dietz V (2011) Locomotion in stroke subjects: interactions between unaffected and affected sides. Brain : a journal of neurology 134:721–731. https://10.1093/brain/awq370 [DOI] [PubMed] [Google Scholar]
- 30.Perrey S (2013) Promoting motor function by exercising the brain. Brain sciences 3:101–122. https://10.3390/brainsci3010101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorond FA, Kiely DK, Galica A, Moscufo N, Serrador JM, Iloputaife I, Egorova S, Dell’Oglio E, Meier DS, Newton E, Milberg WP, Guttmann CR, Lipsitz LA (2011) Neurovascular coupling is impaired in slow walkers: the MOBILIZE Boston Study. Annals of neurology 70:213–220. https://10.1002/ana.22433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hittmair-Delazer M, Semenza C, Denes G (1994) Concepts and facts in calculation. Brain : a journal of neurology 117 ( Pt 4):715–728. [DOI] [PubMed] [Google Scholar]
- 33.Cui X, Abduljalil A, Manor BD, Peng CK, Novak V (2014) Multi-scale glycemic variability: a link to gray matter atrophy and cognitive decline in type 2 diabetes. PloS one 9:e86284 https://10.1371/journal.pone.0086284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atkinson HH, Rosano C, Simonsick EM, Williamson JD, Davis C, Ambrosius WT, Rapp SR, Cesari M, Newman AB, Harris TB, Rubin SM, Yaffe K, Satterfield S, Kritchevsky SB (2007) Cognitive function, gait speed decline, and comorbidities: the health, aging and body composition study. The journals of gerontology Series A, Biological sciences and medical sciences 62:844–850. [DOI] [PubMed] [Google Scholar]
- 35.Zhou J, Hao Y, Wang Y, Jor’dan A, Pascual-Leone A, Zhang J, Fang J, Manor B (2014) Transcranial direct current stimulation reduces the cost of performing a cognitive task on gait and postural control. The European journal of neuroscience 39:1343–1348. https://10.1111/ejn.12492 [DOI] [PMC free article] [PubMed] [Google Scholar]