Supplemental digital content is available in the text.
Key words: analgesia, meditation, naloxone, opioid antagonist, pain relief
ABSTRACT
Objective
Studies have consistently shown that long-term meditation practice is associated with reduced pain, but the neural mechanisms by which long-term meditation practice reduces pain remain unclear. This study tested endogenous opioid involvement in meditation analgesia associated with long-term meditation practice.
Methods
Electrical pain was induced with randomized, double-blind, cross-over administration of the opioid antagonist naloxone (0.15-mg/kg bolus dose, then 0.2-mg/kg per hour infusion dose) with 32 healthy, experienced meditation practitioners and a standardized open monitoring meditation.
Results
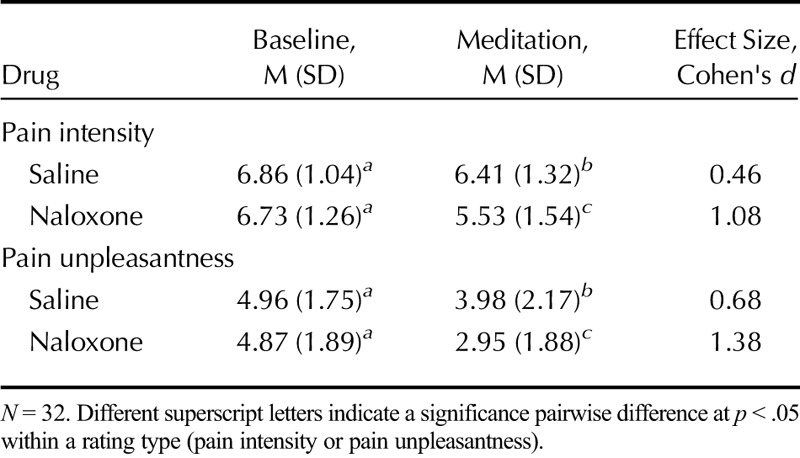
Under saline, pain ratings were significantly lower during meditation (pain intensity: 6.41 ± 1.32; pain unpleasantness: 3.98 ± 2.17) than at baseline (pain intensity: 6.86 ±1.04, t(31) = 2.476, p = .019, Cohen's d = 0.46; pain unpleasantness: 4.96 ±1.75, t(31) = 3.746, p = .001, Cohen's d = 0.68), confirming the presence of meditation analgesia. Comparing saline and naloxone revealed significantly lower pain intensity (t(31) = 3.12, p = .004, d = 0.56), and pain unpleasantness (t(31) = 3.47, p = .002, d = 0.62), during meditation under naloxone (pain intensity: 5.53 ± 1.54; pain unpleasantness: 2.95 ± 1.88) than under saline (pain intensity: 6.41 ± 1.32; pain unpleasantness: 3.98 ± 2.17). Naloxone not only failed to eliminate meditation analgesia but also made meditation analgesia stronger.
Conclusions
Long-term meditation practice does not rely on endogenous opioids to reduce pain. Naloxone's blockade of opioid receptors enhanced meditation analgesia; pain ratings during meditation were significantly lower under naloxone than under saline. Possible biological mechanisms by which naloxone-induced opioid receptor blockade enhances meditation analgesia are discussed.
INTRODUCTION
Meditation's potential to reduce pain is well established (1–5). The pathways by which meditation reduces pain are beginning to receive empirical attention, although important questions remain unanswered. Given the prevalence and costs of chronic pain (6–8), concomitant opioid medication abuse/addiction (9–12), and meditation's potential for drug-free analgesia, developing a more complete picture of the pathway or pathways by which meditation can cause analgesia is an important public health priority.
Understanding the neural mechanisms of meditation analgesia could contribute to improved treatment efficacy by elucidating for whom meditation is effective. It could also inform treatment of other health states, because meditation likely influences diverse outcomes through shared neurobiological pathways. In addition, in the case of a nonopioid pathway, meditation could represent a promising pain-reducing intervention for people with disrupted endogenous opioid function associated with opioid use/abuse or other disorders.
The endogenous opioid system is a viable candidate neural mechanism by which long-term meditation practice could reduce pain. Several other cognitive and affective factors reduce pain via endogenous opioids, including placebo (13–15), conditioned pain modulation (16), and attentional control (17). In addition to the theoretical rationale, testing this candidate neural mechanism was also attractive from a methodological perspective. Decades of work has established that pharmacological manipulation of opioid receptors is safe and effective for investigating the psychological and biological factors that contribute to pain relief (18). This method allows for greater causal inference than imaging methods typically used for other neural candidates such as dopamine (19–21) without the logistic and safety challenges presented by less well-established antagonists such as those targeting the endocannabinoid system (22,23).
We set out to test whether, and to what extent, endogenous opioids contribute to meditation analgesia in experienced meditators and whether additional mechanisms might be at play. The goal of this study was to test endogenous opioid involvement in meditation analgesia using healthy, experienced meditation practitioners, experimentally induced pain, and the opioid antagonist naloxone. We used a style of meditation called open monitoring, characterized by “nonreactively monitoring the content of experience from moment to moment, primarily as a means to recognize the nature of emotional and cognitive patterns (24).” Open monitoring is a foundational practice of many Buddhist contemplative traditions and secular derivatives and is thought to reduce pain by reducing emotionally reactive responses to pain by training nonjudgment and acceptance (5).
We recently demonstrated that meditation analgesia in novice meditators does not rely on the endogenous opioid system (25), but given that experienced meditators typically experience a greater analgesic effect from meditation than do novices (1,3,26), we hypothesized that endogenous opioids are one neurochemical pathway by which long-term meditation reduces pain. This hypothesis entailed the following specific predictions: first, on the basis of previous studies that examined the use of high-dose naloxone (~0.1-mg/kg or greater) (27–29), we predicted that naloxone would not have a significant impact on baseline pain measurements. Second, we reasoned that there are two possible outcomes of naloxone's temporary blockade of opioid receptors during meditation. On one hand, if endogenous opioids are involved, naloxone would increase pain during meditation. As such, we would expect higher pain ratings in the naloxone meditation condition than in the saline meditation condition and equivalent pain ratings in the naloxone meditation and naloxone baseline conditions (Figure 2, predicted results A). On the other hand, if endogenous opioids are not involved, naloxone would have no impact on pain during meditation. In this case, we would expect similar pain ratings in the naloxone meditation condition and in the saline meditation condition and lower pain ratings in both meditation conditions versus the baseline conditions (Figure 2, predicted results B).
FIGURE 2.
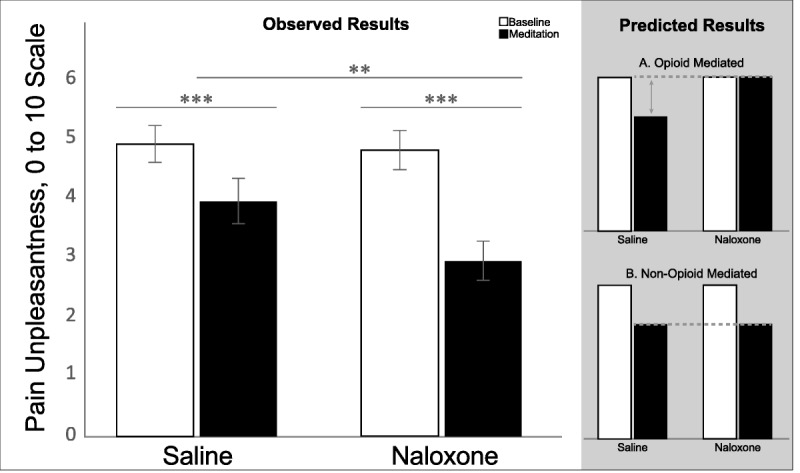
Observed results of mean pain unpleasantness for baseline and meditation conditions in the saline and naloxone sessions do not align with either predicted pattern of results. N = 32. Error bars represent standard error. All statistical comparisons are within-subject. *p < .05, **p < .01, ***p < .001. The results for pain intensity follow the same pattern as pain unpleasantness.
METHODS
Overview
This study tested opioidergic involvement in meditation analgesia by using electrical pain induction and randomized, double-blind, cross-over administration of naloxone with healthy, experienced meditation practitioners across three sessions. Meditation analgesia was operationalized in each session by comparing participants' pain at a baseline measurement to their pain during a standardized open monitoring meditation.
The first session (screening session) identified participants who exhibited meditation analgesia. Because the goal of the project was to determine opioidergic involvement in meditation analgesia, it was necessary to first verify the existence of meditation analgesia in each participant. This was especially important because no empirical work has documented the prevalence of meditation analgesia among experienced meditators. Participants who demonstrated 15% or more meditation analgesia in the screening session were invited to participate in sessions 2 and 3, in which naloxone and saline were administered in a randomized, double-blind, cross-over study design.
Participants
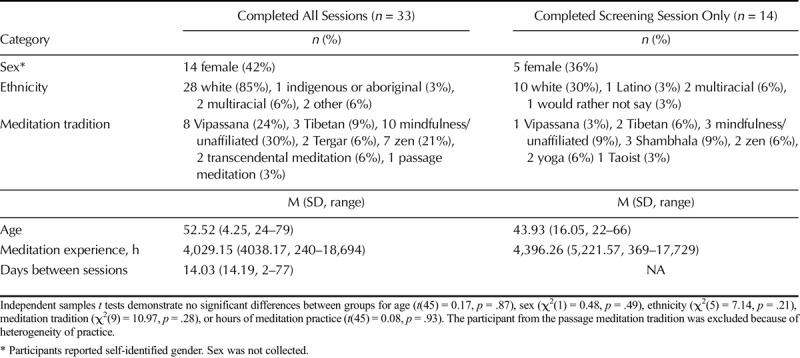
From an initial group of 49 healthy, pain-free adults with established meditation practices from the Eugene, Oregon, community who provided consent between March and November 2015 we obtained a final sample of 32 (Figure 1). The University of Oregon's institutional review board approved all study procedures. Participants were not pregnant or breastfeeding, not smoking more than five cigarettes per day or taking medications that affected pain perception or cognitive function, and had no history of significant neurological disorder, cardiac disorder, chronic pain, or alcohol or drug abuse. Participants were screened via urine test for use of opiate medications. Two individuals were excluded during session 1, one because of a urine test positive for opiates and another because of a pain tolerance too high to safely measure. Of the 47 participants, 40 (85%) who completed the screening session were eligible for sessions 2 and 3, and 35 of those 40 chose to participate. One participant withdrew from session 2 because of lightheadedness at intravenous (IV) needle insertion, and another because of nausea from naloxone. A total of 33 participants (Table 1, demographics) completed all study procedures. These 33 participants came from a wide variety of meditation traditions; the participant who practiced passage meditation was excluded (leaving 32 participants) because of heterogeneity of practice relative to other participants, all of whom reported proficiency with open monitoring meditation. With 32 participants, the results reported are unchanged when this participant is included.
FIGURE 1.
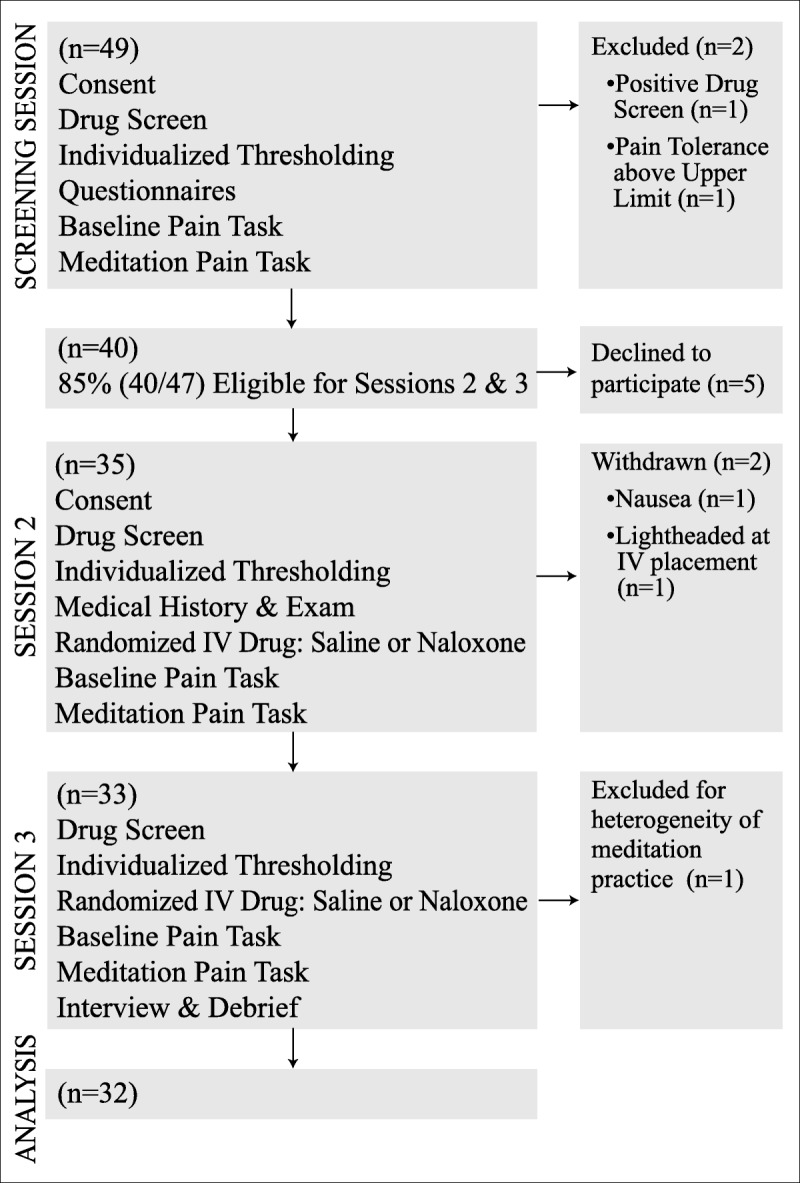
Flow chart describing study recruitment and participation.
TABLE 1.
Demographics
Sample Size Determination
An a priori power analysis (G*Power 3.1) indicated that a sample size of 21 would be sufficient to detect a medium (d = 0.3) drug by condition (within by within) interaction, assuming a moderate repeated-measures correlation of 0.8. We overrecruited to ensure adequate power even with possible data loss.
Pain Induction
Pain was evaluated using noninvasive, atraumatic application of constant AC current electrical stimulation (5 Hz; neurometer CPT diagnostic stimulator; Neurotron Inc, Baltimore, MD). Electrical stimulation has been used effectively in studies of meditation analgesia (2,30) and in studies of opioid-mediated analgesia with naloxone (31–34). Electrical current was delivered to the skin via a pair of 1-cm diameter gold electrodes on the medial and lateral surfaces of the distal phalange of the middle or ring finger of the nondominant hand.
Individualized Thresholding Procedure
To equate sensory experience across participants, the ascending method of limits (2,3,30) was used to determine the stimulus intensities associated with nonpainful sensation (0 on a 10-point visual scale), low pain (3 on a 10-point scale), and high pain (7 on a 10-point scale) for each participant. Stimulus intensities were delivered for 3-second intervals, beginning below the sensory threshold and progressively increasing. Subsequent stimulus intensities were delivered in randomized order. At the end of each 3-second stimulus, participants verbally reported perceived intensity on a scale of 0 to 10, with 0 indicating nonpainful sensation and 10 indicating maximum tolerable pain. Individual thresholds were set when a given stimulus was rated as the same perceived intensity across four deliveries. All measurements were collected with the participant blinded to current magnitude.
Pain Task
Four repetitions of each level (nonpainful sensation, low pain, high pain) were randomly delivered and participant blind, for a total of twelve 3-second stimuli in each pain task. Participants verbally reported pain intensity (described as the degree to which the pain was strong or intense) and pain unpleasantness (the degree to which the pain was aversive or unpleasant) for each 3-second stimulus, based on 11-point numerical rating scales in view (0 = not intense/not unpleasant; 3 = mild intensity/unpleasantness; 10 = maximum intensity/unpleasantness).
Baseline Pain Task
Participants were instructed to respond to pain in a way similar to how they would deal with an unexpected minor injury in everyday life (e.g., stubbed toe, paper cut) and not to meditate.
Meditation Pain Task
Standard meditation instructions were used for all participants to equate meditation practice across participants. Participants were instructed to listen to an open monitoring prompt (Cultivation of Open Presence, as in (5); see Supplemental Digital Content 1 for full text, http://links.lww.com/PSYMED/A462) and then to meditate for 10 minutes, at which time they would be notified that the pain task would begin. They were instructed to continue to meditate during the pain task, maintaining their mind in a state of total openness without engaging with, or rejecting, the stimulation.
Drug Administration
A registered nurse administered either naloxone hydrochloride (half-life: 64 ± 12 min; 0.15-mg/kg bolus, then 0.2-mg/kg per hour infusion) or normal saline (0.9% sodium chloride, 0.1 mL/kg) under the supervision of a board-certified anesthesiologist. Positron emission tomography data indicate that this dose of naloxone is sufficient to completely block central opioid receptors (35). Equal or slightly smaller doses of naloxone have been repeatedly shown to block endogenous opioid-mediated processes (27,36–39).
Procedure
Potential participants were telephone screened for eligibility; meditation experience hours were assessed. Those eligible were scheduled for the screening session, which involved pain testing at baseline and during meditation without drug administration. Participants who demonstrated 15% or more reduction in pain from the baseline measurement to the meditation measurement in low pain or high pain, as seen in pain intensity or pain unpleasantness, were invited to participate in sessions 2 and 3. Sessions 2 and 3 involved the same pain testing, along with saline and naloxone administration, one per day, in a randomized and double-blind manner. Participants were informed that they would receive both naloxone and saline in a double-blind, randomized manner. They were advised that naloxone is a medication that might or might not affect their pain perception, and normal saline would not affect their pain perception.
All sessions included gratitude and distraction conditions that always took place after the baseline and meditation conditions; these conditions are not discussed further here.
Session 1
Screening for Meditation Analgesia
In session 1, the researcher described the protocol and participants gave informed consent for session 1. They then underwent a urine screen (CLIA waived IDTC 12 panel) to confirm absence of opioidergic substances, and the individualized thresholding procedure was performed. Participants completed computerized questionnaires and then the baseline pain task. Participants engaged in meditation for 10 minutes, followed by the meditation pain task. Data collection software displayed eligibility for sessions 2 and 3 after the final pain measurement. Those eligible were invited to participate. Those who were ineligible or declined to participate in sessions 2 and 3 were debriefed. All participants were paid US $40 for session 1.
Sessions 2 and 3
Randomized, Double-Blind Drug Administration
In session 2, the researcher described the new aspects of the procedure to the participant and obtained informed consent. Because eligibility for these sessions was unknown before the end of session 1, we implemented this additional consent in session 2 to ensure that eligible and willing participants were adequately educated on the invasive nature of these procedures before consent. A urine screen was again completed, and the individualized thresholding procedure was conducted. After a brief medical history and physician's examination, an IV was placed and either naloxone or saline was randomly administered in a double-blind setting. Pain testing was initiated at a minimum of 5 minutes after drug administration, well after usual naloxone onset of action (40). Next, participants completed the baseline pain task. Participants then engaged in meditation for 10 minutes, followed by the meditation pain task. After the final pain measurement, the IV was discontinued and the RN conducted a short interview/examination to ensure that the participant was experiencing no ill effects. The participant received the session payment of US $60. The next appointment time was then confirmed (session 2), or the participant was interviewed and debriefed (session 3).
Postsession Interview
In a short interview at the end of session 3, participants were asked in which session they believed they received naloxone. This was the only question asked of the first five participants, after which a more in-depth interview procedure was implemented. The subsequent 27 participants were asked to report what subjective experience, if any, led them to their conclusion. They were also asked, “In your understanding, what does naloxone do?”
Analysis
Data were analyzed with SPSS 22 (IBM, Armonk, New York, NY). Mean scores were computed for each level of pain intensity/unpleasantness, yielding mean scores at baseline and during meditation for the saline and naloxone sessions. Repeated-measures analyses of variance were calculated for pain intensity/unpleasantness, followed by a priori two-tailed paired sample t tests. One-way analyses of variance were calculated to examine the impact of belief about naloxone on naloxone's effect. There were no extreme outliers of greater than ± 3 SD from the mean in dependent variables, and winsorizing outliers 1.5 to 3 SD did not change results. One participant reported meditation experience of great than 3 SD and was winsorized to 1.5 SD above the mean. The preregistered analysis plan and data are available at https://osf.io/92rth/.
RESULTS
First, we want to highlight that 85% participants (40/47) demonstrated meditation analgesia of 15% or more in session 1. To our knowledge, this is the first estimate of the prevalence of meditation analgesia in a sample of experienced meditators.
We have limited our results reported here to the high pain level for simplicity; the pattern of results is similar for low pain and is reported in Supplemental Digital Content 1, http://links.lww.com/PSYMED/A462. Mean pain intensity and pain unpleasantness scores were subjected to separate repeated-measures analyses of variance having two levels of condition (baseline, meditation) and two levels of drug (saline, naloxone). There were significant main effects of condition and drug, and both were qualified by an interaction. There was a main effect of condition for pain intensity, F(1,31) = 34.40, p < .001, 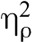 = 0.53, and for pain unpleasantness, F(1,31) = 44.19, p < .001,
= 0.53, and for pain unpleasantness, F(1,31) = 44.19, p < .001, 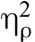 = 0.59, indicating that pain intensity and pain unpleasantness during meditation were significantly different than at baseline. Paired sample t tests confirmed the presence of meditation analgesia under saline and naloxone for pain intensity and pain unpleasantness (Table 2, Figure 2). There was a main effect of drug for pain intensity, F(1,31) = 4.17, p = .050,
= 0.59, indicating that pain intensity and pain unpleasantness during meditation were significantly different than at baseline. Paired sample t tests confirmed the presence of meditation analgesia under saline and naloxone for pain intensity and pain unpleasantness (Table 2, Figure 2). There was a main effect of drug for pain intensity, F(1,31) = 4.17, p = .050, 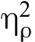 = 0.12, but not for pain unpleasantness, F(1,31) = 3.11, p = .088,
= 0.12, but not for pain unpleasantness, F(1,31) = 3.11, p = .088, 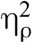 = 0.09, indicating that naloxone had a significant effect across conditions for pain intensity but not for pain unpleasantness.
= 0.09, indicating that naloxone had a significant effect across conditions for pain intensity but not for pain unpleasantness.
TABLE 2.
The Effect of Naloxone on Meditation Analgesia, High Pain Level
The interaction between drug and condition was significant for both pain intensity, F(1,31) = 8.42, p = .007, 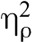 = 0.21, and pain unpleasantness, F(1,31) = 12.329, p = .001,
= 0.21, and pain unpleasantness, F(1,31) = 12.329, p = .001, 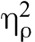 = 0.29 indicating that the effect of naloxone on pain was different during meditation than at baseline for both pain intensity and pain unpleasantness. Paired-samples t tests (Table 2, Figure 2) comparing saline and naloxone revealed no significant differences at baseline for pain intensity, t(31) = 0.454, p = .65, d = 0.08, or for pain unpleasantness, t(31) = 0.25, p = .80, d = 0.04, but significantly lower pain intensity, t(31) = 3.12, p = .004, d = 0.56, and pain unpleasantness, t(31) = 3.47, p = .002, d = 0.62, during meditation under naloxone than under saline. These effects persisted when controlling for session order, age, and gender.1 Naloxone not only failed to eliminate meditation analgesia but also made meditation analgesia stronger.
= 0.29 indicating that the effect of naloxone on pain was different during meditation than at baseline for both pain intensity and pain unpleasantness. Paired-samples t tests (Table 2, Figure 2) comparing saline and naloxone revealed no significant differences at baseline for pain intensity, t(31) = 0.454, p = .65, d = 0.08, or for pain unpleasantness, t(31) = 0.25, p = .80, d = 0.04, but significantly lower pain intensity, t(31) = 3.12, p = .004, d = 0.56, and pain unpleasantness, t(31) = 3.47, p = .002, d = 0.62, during meditation under naloxone than under saline. These effects persisted when controlling for session order, age, and gender.1 Naloxone not only failed to eliminate meditation analgesia but also made meditation analgesia stronger.
A potential confound is whether participants were aware of which drug they received in which session and their expectation of the drug's effect. Six participants stated that they did not have a belief about in which session they had received naloxone, and 21 of 32 participants (66%) correctly guessed in which session they had received naloxone. This rate is not different from chance, χ2(1) = 3.13, p = .077. When asked about the perceptions upon which participants based their guesses, the most common responses were related to cognitive functioning (44%, e.g., feeling more “spacey” during meditation) and awareness of physical sensations (19%, e.g., more aware of body sensations during meditation). Critically, the effect of naloxone on meditation analgesia did not differ significantly between participants with an accurate belief [Δ pain intensity, M (SD) = 0.83 (1.29); Δ pain unpleasantness, M (SD) = 0.82 (1.30)] and those with an inaccurate belief or no belief about which session naloxone had been administered (Δ pain intensity, M (SD) = 0.98 (2.14), t[14] = 0.20, p = .84; Δ pain unpleasantness, M (SD) = 1.41 (2.24), t[14] = 0.80, p = .44).
Of the 26 participants who were asked about their belief of the effect of naloxone (naloxone belief), 10 had no belief, nine thought that naloxone would reduce their pain, and seven thought that naloxone would increase their pain. The effect of naloxone did not differ significantly by naloxone belief: Δ pain intensity, F(2, 23) = 0.49, p = .62, η2 = 0.04; Δ pain unpleasantness, F(2, 23) = 0.56, p = .58, η2= 0.05.
DISCUSSION
This study tested endogenous opioid involvement in meditation analgesia in a double-blind, placebo-controlled, cross-over design. Experienced meditation practitioners underwent electrical pain induction during meditation while being administered the opioid antagonist naloxone or a placebo. Naloxone failed to antagonize meditation analgesia; instead, naloxone enhanced meditation analgesia, which is an unprecedented result.
We had hypothesized that experienced practitioners, in addition to the nonopioid pathway seen in novices (25), might have an opioid component to their analgesia resulting from contextual factors, such as expectation, conditioning, or social learning. However, contrary to our hypothesis, pain was not higher during meditation under naloxone versus saline. This is a clear demonstration that meditation analgesia in experienced practitioners also does not rely on endogenous opioids.
These results have implications for health contexts outside of pain management. If meditation reduces pain via a nonopioid pathway, its effects on other health outcomes are likely nonopioid as well. This may be particularly useful in understanding meditation's impact on health issues when the opioid/nonopioid distinction is highly relevant, including addiction and posttraumatic stress disorder (41,42). For example, therapies that rely on the opioid system may not be as effective for posttraumatic stress disorder when disordered opioid function has been implicated; our results suggest that meditation is a potential candidate treatment because it does not rely on opioids.
Our alternative hypothesis, which meditation analgesia is not mediated through endogenous opioids, suggested that naloxone would have a null effect on pain ratings. This hypothesis yielded to an unexpected third possibility. Our data show that meditation analgesia is enhanced by naloxone: We observed significantly greater meditation analgesia in the naloxone session than in the saline session. To our knowledge, this is the first report of high-dose naloxone-enhancing psychological analgesia, despite naloxone's use in pain research for more than 4 decades.
We entertained several explanations for why opioid blockade might enhance analgesia. First, we considered whether opioid activity in any receptor could explain enhancement. Naloxone has the highest binding affinity for mu opioid receptors but also fair affinity for δ and κ opioid receptors (43). The dose administered here was double the dose shown sufficient for complete inhibition of mu opioid receptor binding (35) and therefore likely blocked all subtypes of opioid receptors. Inhibition of glial cell activity (44) is also not likely. Analgesia resulting from antagonism of glial receptors should be independent of psychological manipulation, but we observed naloxone-related analgesia only during meditation; there was no effect of naloxone at baseline. We also considered altered opioid receptor function akin to the nociceptive sensitization caused by changes in G-protein coupling (45,46) with opioid medication and early life stress (47), but this explanation seems unlikely because (1) changes in G-protein coupling should also be independent of psychological manipulation instead of the meditation-specific effect we observed and (2) this type of nociceptive sensitization is associated with negative health outcomes such as those seen with opioid-induced hyperalgesia (48) instead of the positive health outcomes associated with meditation.
Second, we considered whether naloxone's meditation analgesia enhancement could result from behavioral upregulation during meditation, when participants change their meditation behavior as a reaction to naloxone-induced difference in meditation experience. For example, meditation might have felt less potent under naloxone, cueing participants to increase effort toward their meditation practice in our study. Opioid blockade has been shown to induce increases in opiate-mediated behaviors in primates (49) but is not perfectly analogous to what we are reporting here. In addition, for this explanation to be viable, participants would have needed to know when they received naloxone and to expect it to exacerbate pain. Our participants were not better than chance in guessing their drug condition and had a variety of expectations of the drug effect. Even if naloxone-induced meditation analgesia enhancement is the result of differences in effort, the resulting reductions in pain could not have been mediated by endogenous opioids, which also points to a nonopioid mechanism for meditation analgesia.
Third, we considered whether meditation analgesia enhancement could be associated with an interaction between opioid and nonopioid pathways. The endogenous opioid system interacts with other neurochemical pathways, so it is possible that an opioid blockade causes changes in nonopioid pathways that in turn cause the enhancement of analgesia during meditation. A cholecystokinin antagonist can interact with the opioid pathway to enhance analgesia (50–52). An opioid antagonist could interact with another pain modulatory pathway to enhance analgesia. Reciprocal alteration of receptor density and function has been shown between opioid and cannabinoid systems (53,54). Bidirectional interactions with opioids are known for cannabinoid, TRPV1 (24,55), norepinephrine, and dopamine pathways (55). To our knowledge, there is no evidence that opioid antagonism causes upregulation of analgesia via these systems, but it remains a biologically feasible explanation. Now that opioid noninvolvement in meditation analgesia has been demonstrated in novices and experienced practitioners, investigation of this nonopioid pathway's identity is a logical next step. The cannabinoid pathway is a promising candidate, given its known involvement in psychological analgesia (56) and possible interaction with opioids (57).
A recent study investigated opioid-mediated meditation analgesia in experienced practitioners, and its authors concluded that naloxone reduced meditation analgesia (58), an effect that is opposite of what we report here. However, this study's small sample size (n = 14), as well as the report's omission of effect sizes and the critical direct comparisons between naloxone and saline at baseline and after meditation, makes it difficult to evaluate the authors' conclusion. Despite requests, a debate that recently appeared in press ended without further evidence or analyses being presented by the study's authors (59,60).
Our study provides the first estimate of the prevalence of meditation analgesia in a sample of experienced meditators. We observed meditation analgesia, operationalized as a reduction of 15% or more in pain intensity or unpleasantness, in 85% of our sample; most participants received analgesia from meditation practice. Similar estimates are needed for clinical populations, but this is an important first step in understanding for whom meditation is an effective pain management strategy. A measure of prevalence paves the way for studies to examine biological or psychological markers of effectiveness.
This study is the first to demonstrate that meditation analgesia is not caused by opioids in experienced meditation practitioners and presents a naloxone-induced enhancement effect that may be explained by the impact of opioid receptor blockade on nonopioid systems. These results highlight the unique nature of meditation neurochemically and psychologically and suggest a nonopioid pathway for meditation's impact on other health outcomes.
Supplementary Material
Acknowledgments
The authors thank Terry Mitchell, RN, Sierra Hovdey, RN, and the staff of Pain Consultants of Oregon for their assistance in data collection.
Source of Funding and Conflicts of Interest: This work was supported by a grant awarded to Lisa May from the John Templeton Foundation, which funded the Expanding the Science and Practice of Gratitude project run by UC Berkeley's Greater Good Science Center, and by a Varela award awarded to Lisa May from the Mind and Life Institute. For the remaining authors none are declared. Any views, findings, conclusions, or recommendations expressed in this article do not necessarily reflect those of the John Templeton Foundation or the Mind & Life Institute. The authors declare no conflict of interest.
Footnotes
1Including hours of meditation experience as a covariate reduced the magnitude of the drug by condition interaction for both pain intensity, F(1,30) = 1.15, p = .29, 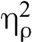 = 0.04, and pain unpleasantness, F(1, 30) = 0.13, p = .72,
= 0.04, and pain unpleasantness, F(1, 30) = 0.13, p = .72, 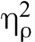 = 0.004, but did not change the direction of the interaction. This reduction in the magnitude of the effect was driven by significant negative correlations between hours of meditation experience and saline meditation analgesia in Δ pain intensity (r = −0.42, p = .02) and Δ pain unpleasantness (r = −0.41, p = .02); in other words, more meditation experience was related to smaller drops in pain after meditating in the saline condition. The relationship between hours of meditation experience and meditation analgesia was not replicated in the naloxone session (Δ pain intensity, r = −0.11, p = .54; Δ pain unpleasantness, r = 0.07, p = .72) or in the screening session (Δ pain intensity, r = −0.14, p = .46; Δ pain unpleasantness, r = −18, p = .33), suggesting the correlation to be unstable.
= 0.004, but did not change the direction of the interaction. This reduction in the magnitude of the effect was driven by significant negative correlations between hours of meditation experience and saline meditation analgesia in Δ pain intensity (r = −0.42, p = .02) and Δ pain unpleasantness (r = −0.41, p = .02); in other words, more meditation experience was related to smaller drops in pain after meditating in the saline condition. The relationship between hours of meditation experience and meditation analgesia was not replicated in the naloxone session (Δ pain intensity, r = −0.11, p = .54; Δ pain unpleasantness, r = 0.07, p = .72) or in the screening session (Δ pain intensity, r = −0.14, p = .46; Δ pain unpleasantness, r = −18, p = .33), suggesting the correlation to be unstable.
Supplemental Content
REFERENCES
- 1.Brown C, Jones AK. Meditation experience predicts less negative appraisal of pain: electrophysiological evidence for the involvement of anticipatory neural responses. Pain 2010;150:428–38. [DOI] [PubMed] [Google Scholar]
- 2.Gard T, Hölzel BK, Sack AT, Hempel H, Lazar SW, Vaitl D, Ott U. Pain attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cereb Cortex 2012;22:2692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant J, Rainville P. Pain sensitivity and analgesic effects of mindful states in Zen meditators: a cross-sectional study. Psychosom Med 2009;71:106–14. [DOI] [PubMed] [Google Scholar]
- 4.Lutz A, McFarlin DR, Perlman DM, Salomons TV, Davidson RJ. Altered anterior insula activation during anticipation and experience of painful stimuli in expert meditators. Neuroimage 2013;64C:538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perlman DM, Salomons TV, Davidson RJ, Lutz A. Differential effects on pain intensity and unpleasantness of two meditation practices. Emotion 2010;10:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaasbøll J, Lydersen S, Indredavik M. Psychological symptoms in children of parents with chronic pain—the HUNT study. Pain 2012;153:1054–62. [DOI] [PubMed] [Google Scholar]
- 7.Loeser JD. Relieving pain in America. Clin J Pain 2012;28:185–6. [DOI] [PubMed] [Google Scholar]
- 8.Poole H, White S, Blake C, Murphy P, Bramwell R. Depression in chronic pain patients: prevalence and measurement. Pain Pract 2009;9:173–80. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR Morb Mortal Wkly Rep 2011;60:1487–92. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Vital signs: prescription painkiller overdoses, a growing epidemic, especially among women. 2013:4–7. Available at: https://www.cdc.gov/vitalsigns/prescriptionpainkilleroverdoses/index.html. Accessed May 11, 2018. [Google Scholar]
- 11.Højsted J, Sjøgren P. Addiction to opioids in chronic pain patients: a literature review. Eur J Pain 2007;11:490–518. [DOI] [PubMed] [Google Scholar]
- 12.Manchikanti L, Helm S, Fellows B, Janata JW, Pampati V, Grider JS, Boswell MV. Opioid epidemic in the United States. Pain Physician 2012;15:ES9–38. [PubMed] [Google Scholar]
- 13.Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet 1978;2:654–57. [DOI] [PubMed] [Google Scholar]
- 14.Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci 2005;25:7754–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta J-K. Neurobiological mechanisms of the placebo effect. J Neurosci 2005;25:10390–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King CD, Goodin B, Kindler LL, Caudle RM, Edwards RR, Gravenstein N, Riley JL, Fillingim RB. Reduction of conditioned pain modulation in humans by naltrexone: an exploratory study of the effects of pain catastrophizing. J Behav Med 2013;36:315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sprenger C, Eippert F, Finsterbusch J, Bingel U, Rose M, Büchel C. Attention modulates spinal cord responses to pain. Curr Biol 2012;22:1019–22. [DOI] [PubMed] [Google Scholar]
- 18.Benedetti F, Carlino E, Pollo A. How placebos change the patient's brain. Neuropsychopharmacology 2011;36:339–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albrecht DS, MacKie PJ, Kareken DA, Hutchins GD, Chumin EJ, Christian BT, Yoder KK. Differential dopamine function in fibromyalgia. Brain Imaging Behav 2016;10:829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karjalainen T, Karlsson HK, Lahnakoski JM, Glerean E, Nuutila P, Jääskeläinen IP, Hari R, Sams M, Nummenmaa L. Dissociable roles of cerebral μ-opioid and type 2 dopamine receptors in vicarious pain: a combined PET–fMRI study. Cereb Cortex 2017;27:4257–66. [DOI] [PubMed] [Google Scholar]
- 21.Konno S, Sekiguchi M. Association between brain and low back pain. J Orthop Sci 2018;23:3–7. [DOI] [PubMed] [Google Scholar]
- 22.Gamaleddin IH. Role of the endogenous cannabinoid system in nicotine addiction: novel insights. Front Psych 2015;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirilly E, Gonda X, Bagdy G. CB1 receptor antagonists: new discoveries leading to new perspectives. Acta Physiol (Oxf) 2012;205:41–60. [DOI] [PubMed] [Google Scholar]
- 24.Lutz A, Slagter H, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends Cogn Sci 2008;12:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeidan F, Adler-Neal AL, Wells RE, Stagnaro E, May LM, Eisenach JC, McHaffie JG, Coghill RC. Mindfulness-meditation-based pain relief is not mediated by endogenous opioids. J Neurosci 2016;36:3391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grant J, Courtemanche J, Rainville P. A non-elaborative mental stance and decoupling of executive and pain-related cortices predicts low pain sensitivity in Zen meditators. Pain 2011;152:150–6. [DOI] [PubMed] [Google Scholar]
- 27.Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Büchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 2009;63:533–43. [DOI] [PubMed] [Google Scholar]
- 28.Grevert P, Albert LH, Goldstein A. Partial antagonism of placebo analgesia by naloxone. Pain 1983;16:129–43. [DOI] [PubMed] [Google Scholar]
- 29.Grevert P, Goldstein A. Endorphins: naloxone fails to alter experimental pain or mood in humans. Science 1978;199:1093–5. [DOI] [PubMed] [Google Scholar]
- 30.Zeidan F, Gordon N, Merchant J, Goolkasian P. The effects of brief mindfulness meditation training on experimentally induced pain. J Pain 2010;11:199–209. [DOI] [PubMed] [Google Scholar]
- 31.Cruz S, Villarreal J, Volkow N. Further evidence that naloxone acts as an inverse opiate agonist: implications for drug dependence and withdrawal. Life Sci 1996;56:381–9. [DOI] [PubMed] [Google Scholar]
- 32.Koppert W, Angst MS, Alsheimer M, Sittl R, Albrecht S, Schüttler J, Schmelz M. Naloxone provokes similar pain facilitation as observed after short-term infusion of remifentanil in humans. Pain 2003;106:91–9. [DOI] [PubMed] [Google Scholar]
- 33.Liu SS, Gerancher JC, Bainton BG, Kopacz DJ, Carpenter RL. The effects of electrical stimulation at different frequencies on perception and pain in human volunteers: epidural versus intravenous administration of fentanyl. Anesth Analg 1996;82:98–102. [DOI] [PubMed] [Google Scholar]
- 34.Nabeshima T, Matsuno K, Kamei H, Noda Y, Kameyama T. Electric footshock-induced changes in behavior and opioid receptor function. Pharmacol Biochem Behav 1985;23:769–75. [DOI] [PubMed] [Google Scholar]
- 35.Mayberg HS, Frost JJ. Opiate receptors. In: Frost JJ, Wagner HN, Jr, eds. Quantitative Imaging: Neuroreceptors, Neurotransmitters, and Enzymes, New York: Raven Press; 1990;90–3.30. [Google Scholar]
- 36.Amanzio M, Pollo A, Maggi G, Benedetti F. Response variability to analgesics: a role for non-specific activation of endogenous opioids. Pain 2001;90:205–15. [DOI] [PubMed] [Google Scholar]
- 37.Benedetti F, Arduino C, Amanzio M. Somatotopic activation of opioid systems by target-directed expectations of analgesia. J Neurosci 1999;19:3639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrovic P, Pleger B, Seymour B, Kloppel S, De Martino B, Critchley H, Dolan RJ. Blocking central opiate function modulates hedonic impact and anterior cingulate response to rewards and losses. J Neurosci 2008;28:10509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stern J, Candia V, Porchet RI, Krummenacher P, Folkers G, Schedlowski M, Ettlin DA, Schönbächler G. Placebo-mediated, Naloxone-sensitive suggestibility of short-term memory performance. Neurobiol Learn Mem 2011;95:326–34. [DOI] [PubMed] [Google Scholar]
- 40.Handal K, Schauben J, Salamone F. Naloxone. Ann Emerg Med 1983;12:438–45. [DOI] [PubMed] [Google Scholar]
- 41.Bodnar R. Endogenous opiates and behavior: 2009. Peptides 2010;31:2325–59. [DOI] [PubMed] [Google Scholar]
- 42.Carr DJ. The role of endogenous opioids and their receptors in the immune system. Proc Soc Exp Biol Med 1991;198:710–20. [DOI] [PubMed] [Google Scholar]
- 43.Magnan J, Paterson SJ, Tavani A, Kosterlitz HW. The binding spectrum of narcotic analgesic drugs with different agonist and antagonist properties. Naunyn Schmiedebergs Arch Pharmacol 1982;319:197–205. [DOI] [PubMed] [Google Scholar]
- 44.Watkins LR, Hutchinson MR, Rice KC, Maier SF. The "toll" of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci 2010;30:581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crain SM, Shen KF. Antagonists of excitatory opioid receptor functions enhance morphine's analgesic potency and attenuate opioid tolerance/dependence liability. Pain 2000;84(2–3):121–31. [DOI] [PubMed] [Google Scholar]
- 46.Wang H, Friedman E, Olmstead MC, Burns LH. Ultra-low-dose naloxone suppresses opioid tolerance, dependence and associated changes in mu opioid receptor-G protein coupling and Gbetagamma signaling. Neuroscience 2005;135(1):247–61. [DOI] [PubMed] [Google Scholar]
- 47.Le Roy C, Laboureyras E, Gavello-Baudy S, Chateauraynaud J, Laulin JP, Simonnet G. Endogenous opioids released during non-nociceptive environmental stress induce latent pain sensitization Via a NMDA-dependent process. J Pain 2011;12(10):1069–79. [DOI] [PubMed] [Google Scholar]
- 48.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 2006;104(3):570–87. [DOI] [PubMed] [Google Scholar]
- 49.Keverne EB, Martensz ND, Tuite B. Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology 1989;14:155–161. [DOI] [PubMed] [Google Scholar]
- 50.Benedetti F. The opposite effects of the opiate antagonist naloxone and the cholecystokinin antagonist proglumide on placebo analgesia. Pain 1996;64:535–43. [DOI] [PubMed] [Google Scholar]
- 51.Benedetti F, Amanzio M, Maggi G. Potentiation of placebo analgesia by proglumide. Lancet 1995;346:1231. [DOI] [PubMed] [Google Scholar]
- 52.Benedetti F, Amanzio M, Thoen W. Disruption of opioid-induced placebo responses by activation of cholecystokinin type-2 receptors. Psychopharmacology (Berl) 2011;213:791–7. [DOI] [PubMed] [Google Scholar]
- 53.Viganò D, Rubino T, Parolaro D. Molecular and cellular basis of cannabinoid and opioid interactions. Pharmacol Biochem Behav 2005;81:360–8. [DOI] [PubMed] [Google Scholar]
- 54.Zádor F, Wollemann M. Receptome: interactions between three pain-related receptors or the “Triumvirate” of cannabinoid, opioid and TRPV1 receptors. Pharmacol Res 2015;102:254–63. [DOI] [PubMed] [Google Scholar]
- 55.Wiskerke J, Schetters D, van Es IE, van Mourik Y, den Hollander BR, Schoffelmeer AN, Pattij T. μ-Opioid receptors in the nucleus accumbens shell region mediate the effects of amphetamine on inhibitory control but not impulsive choice. J Neurosci 2011;31:262–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carlino E, Benedetti F. Different contexts, different pains, different experiences. J Neurosci 2016;338:19–26. [DOI] [PubMed] [Google Scholar]
- 57.Benedetti F, Thoen W, Blanchard C, Vighetti S, Arduino C. Pain as a reward: changing the meaning of pain from negative to positive co-activates opioid and cannabinoid systems. Pain 2013;154:361–7. [DOI] [PubMed] [Google Scholar]
- 58.Sharon H, Maron-Katz A, Ben Simon E, Flusser Y, Hendler T, Tarrasch R, Brill S. Mindfulness meditation modulates pain through endogenous opioids. Am J Med 2016;129:755–8. [DOI] [PubMed] [Google Scholar]
- 59.Sharon H, Ben Simon E, Tarrasch R. The reply. Am J Med 2016;129:e299. [DOI] [PubMed] [Google Scholar]
- 60.Zeidan F. No, mindfulness meditation-based analgesia is not mediated by endogenous opioids. Am J Med 2016;129:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


