Abstract
BACKGROUND
Cannabis is the most widely used illicit drug, but knowledge of the neurological consequences of cannabis use is deficient. Two primary components of cannabis are Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), and we established a THC+CBD model of self-administration and reinstated drug seeking to determine if like other addictive drugs, cannabis produces enduring synaptic changes in nucleus accumbens core (NAcore) thought to contribute vulnerability to drug reinstatement.
METHODS
Sprague-Dawley rats were trained to self-administer THC+CBD (n=165) or used as vehicle self-administering controls (n=24). Reinstatement was initiated by context, cues, drug priming and stress (yohimbine injection). Enduring neuroadaptations produced by THC+CBD self-administration were assayed using four measures: dendritic spine morphology, long-term depression (LTD), AMPA/NMDA ratios and behavioral pharmacology.
RESULTS
We describe a novel rodent model of cannabis relapse involving intravenous THC+CBD self-administration and drug seeking induced by conditioned context, cues and stress. Cued reinstatement of THC+CBD seeking depended on a sequence of events implicated in relapse to other addictive drugs since reinstatement was prevented by daily treatment with N-acetylcysteine, or acute intra-NAcore pretreatment with an nNOS or MMP-9 inhibitor, all of which normalize impaired glutamate homeostasis. The capacity to induce NMDA-LTD in NAcore medium spiny neurons was abolished and dendritic spine density was reduced but AMPA/NMDA ratio was unaltered in THC+CBD-trained animals akin to opioids, but not psychostimulants.
CONCLUSIONS
We report enduring consequences of THC+CBD use on critical relapse circuitry and synaptic physiology in NAcore following rat self-administration and provide the first report of cue- and stress-induced reinstatement with this model.
Keywords: Δ9-Tetrahyrdocannabinol, cannabidiol, drug abuse, synaptic plasticity, nucleus accumbens, reinstatement
Introduction
Cannabis is the most widely used illicit drug in the United States with 19.8 million current users, and is decriminalized or legalized in 26 states plus the District of Columbia. Despite high recreational usage, understanding of the neurobiological consequences of long-term cannabis use is lacking. The major psychoactive component of cannabis is Δ9-tetrahydrocannabinol (THC), producing its primary effects as a partial agonist at cannabinoid receptors, CB1 and CB2 (2). Activation of presynaptic CB1 receptors, the main neuronal subtype, inhibits glutamate and GABA release in nucleus accumbens, a key brain structure that mediates drug relapse (3, 4). Moreover, CB1 receptors participate in several forms of short- and long-term synaptic plasticity (5, 6).
We established a reliable and scalable model of rat THC self-administration, extinction and reinstatement by modifying specific parameters of standard operant self-administration. Intravenous THC provides a pharmacokinetic time course mirroring inhaled cannabis and limits between-subject variation in bioavailability (7, 8). In an effort to overcome the low reinforcing and anxiogenic qualities of THC, we implemented passive THC pre-exposure before operant self-administration, utilized lower unit doses of THC per infusion than previously reported (9–11), and combined THC with another cannabis constituent, cannabidiol (CBD), in a 10:1 ratio reported to alleviate the aversive properties of THC (12–15). The enduring propensity to relapse is a cardinal feature of drug addiction, and clinical studies of cannabis use disorder report high rates of relapse akin to other abused drugs (16, 17); although, much lower rates have been reported in some community samples (18). Here we established a relapse model of reinstated drug seeking following THC+CBD self-administration via exposure to conditioned context, cues or yohimbine injection (stress-induced reinstatement). We also examined enduring THC+CBD- dependent adaptations in nucleus accumbens core (NAcore). Long-lasting synaptic adaptations in NAcore are thought to contribute vulnerability to relapse for all addictive drugs. Psychostimulants like cocaine and nicotine potentiate glutamatergic synapses, while opioids like heroin or morphine induce synaptic depression (19–22). Rats extinguished from THC+CBD self-administration showed loss of LTD akin to both psychostimulant and opioid drugs, and a heroin-like reduction in dendritic spine density. Finally, to assess the predictive validity of our model we examined three pharmacological treatments known to reduce cued reinstatement across all classes of addictive drug tested. Inhibition of neuronal nitric oxide synthase (nNOS), matrix metalloprotease-9 (MMP-9) activity or treatment with the antioxidant N-acetylcysteine (NAC) prevented cue-induced THC+CBD seeking.
Methods and materials
Animals
Male Sprague–Dawley rats (250–300 g, Charles River Laboratories) were maintained on a 12–12 hr reverse light-dark cycle, and experiments were performed during the dark cycle. After 1 week of vivarium acclimation, rats were implanted with indwelling jugular catheters (Supplemental Procedures). Food was restricted to 25 g standard chow before food training and throughout self-administration. Procedures were approved by the Animal Care and Use Committee of Medical University of South Carolina and performed in accordance with National Institutes of Health guidelines.
THC vapor, food training and self-administration
Following surgery, 5 days of recovery occurred before involuntary vapor exposure to THC+CBD (5 mg THC with 0.5 mg CBD per liquid pad) for 10 minutes per day × 5 days. THC+CBD was delivered to rats in plastic boxes (42×30×15 cm3) using a Volcano vaporizer (Storz and Bickel). A 1-hour food training session occurred prior to self-administration. No cues are delivered during food training to avoid confounds during later cue reinstatement testing. Rats were trained on a fixed-ratio 1 schedule to lever press for intravenous infusion of THC+CBD or vehicle paired with cue light and tone (78 dB, 4.5 kHz). During extinction, active lever presses no longer delivered infusions or cues. A dose-response curve was generated by averaging three consecutive days of self-administration per dose (1.27, 2, 4, and 12.64 μg/kg) using a random crossover design after self-administration stabilized on 4+0.4 μg/kg THC+CBD. In another study, rimonabant (1,3 or 10 mg/kg, ip) or vehicle was delivered 30 min prior to THC+CBD self-administration for two consecutive days. Rimonabant doses were compared in a between-subjects design with a randomized within-subject crossover with saline for those animals receiving the 10 mg/kg dose.
Discrimination index
We calculated lever discrimination index = (active lever presses − inactive lever presses)/(active lever presses + inactive lever presses) where 0 equals no discrimination and 1 equals complete discrimination (23). Using this formula, a 2:1 ratio of active to inactive lever pressing is signified by a value of 0.33. This level is indicated by a dotted line on all graphs displaying this measure for illustrative clarity. The formula was chosen over a simple ratio of active to inactive lever to avoid the mathematically untenable situation when no inactive lever presses creates a denominator equal to zero. Discrimination index was assessed for drug seeking during the maintenance phase of self-administration (average of days 6–10).
Reinstatement
Cue-induced reinstatement entailed drug-free reintroduction of drug-paired cues contingent upon lever pressing. THC-primed reinstatement consisted of monitoring lever presses under extinction conditions 10 min after noncontingent THC injection (1 mg/kg, ip) or THC+CBD (1 mg+ 0.1 mg/kg, ip). To control for the effect of ip injection we performed a fully randomized within subject cross over with Vehicle and THC+CBD injection for this priming experiment. Saline habituation injections were given for two days prior to reinstatement testing and on intervening extinction sessions. Stress-induced reinstatement was elicited with yohimbine (2.5 mg/kg, ip) 30 min prior to testing under cue-induced reinstatement conditions.
Antagonism of cue-induced reinstatement
Rats were pretreated with N-acetylcysteine (60 mg/kg, ip X 5 days) 2 hrs before extinction or reinstatement. Alternatively, rats were pretreated 10 min prior to cued reinstatement with NPLA (0.1 nmol/side; nNOS Ki = 57 nM. 3158-fold selectivity over iNOS, 149-fold specificity over eNOS), or 15 min prior to reinstatement with MMP-9 inhibitor (0.1 nmol/side) or saline plus 1% DMSO intra-NAcore. These doses of NAC, NPLA and MMP-9-I were previously shown to reduce cue-induced reinstatement of cocaine seeking without altering cued sucrose seeking or locomotion (24–26).
Electrophysiology
General methods for patch clamp and extracellular recording are provided in supplemental materials.
Slice preparation
Rats were anesthetized with ketamine (100 mg/kg), decapitated, and coronal brain slices (250 μm) made using a vibratome (VT1200S, Leica). Cutting was performed in ice cold ACSF at 4° C (in mM: 126 NaCl, 1.4 NaH2PO4, 25 NaHCO3, 11 glucose, 1.2 MgCl2, 2.4 CaCl2, 2.5 KCl, 2.0 sodium pyruvate, 0.4 ascorbic acid, 5 kynurenic acid, 0.05 D-(−)-2-amino-5-phosphonopentanoic acid (D-AP5); bubbled with 95% O2 and 5% CO2). After cutting, slices were stored for ≥45 min at 25°C.
LTD
NMDAR-dependent LTD in MSNs was induced after ≥10 minutes of stable baseline recording using a pairing protocol consisting of three trains of stimulus at 5 Hz (train duration: 3 min, 5 minutes apart) while holding the cells at −50mV. The magnitude of NMDAR-LTD was estimated from EPSC recorded after 20–30 minutes LTD induction as percentage of baseline EPSC amplitudes. The glutamatergic nature of the fEPSP was confirmed by blocking with AMPA receptor antagonist DNQX (20 μM).
Dendritic spine quantification
Dendritic spine labeling procedures were based on Seabold et al. (27)(see Supplemental Procedures). DiI-labeled neurons and dendrites were imaged via optical sectioning by a 63× oil-immersion objective (numerical aperture=1.4) on a confocal microscope (Leica) using the Helium/Neon 543-nm laser line. Spines on dendrites beginning at >75 μm and ending at ≤200 μm distal to the soma and after the first branch point were quantified from NAcore MSNs using Imaris (Bitplane, Concord, MA). Seven to nine segments (45–55 μm each) were analyzed per animal. Minimum spine head diameter was set at ≥0.143 μm to reflect the microscope’s Nyquist frequency resolution limits.
Statistics
Statistics were performed using Prism (GraphPad Software, La Jolla, CA) or SPSS (IBM, Armonk, NY). Self-administration data were analyzed by one- or two-way ANOVA followed by Sidak’s multiple comparisons. Reinstatement was analyzed by two- or three-way repeated measures ANOVA. When only two groups were compared, statistical significance (p < 0.05) was determined by Student’s t test. Electrophysiological data were analyzed by cell using a one-way ANOVA with Dunnett’s post-hoc tests. Significance was set at p ≤ 0.05, and all data are presented as mean ± SEM.
Results
Self-administration and reinstatement of THC+CBD in rats
Historically, THC failed to sustain intravenous self-administration in rodents (10, 11), although there are a few reports of intracranial and intravenous self-administration in rats (28–31). To facilitate acquisition of THC use, we incorporated three protocol modifications (Fig. 1A). 1) Rats were passively pre-exposed to THC+CBD vapor (5+0.5 mg/day × 5 days over 10 minutes) because THC pre-exposure facilitates conditioned preference (CPP) in mice (32), and neutralizes aversion in rats (33). 2) Cannabidiol was combined with THC (1:10) because this combination neutralizes THC place aversion without altering reward (15). 3) Low unit doses were delivered compared to early investigations (10, 11). Vapor and intravenous THC+CBD produced hypothermia, indicative of a pharmacological effect (Fig S1A). Also, it is noteworthy that the number of infusions of THC+CBD during self-administration correlated with the plasma levels of THC and metabolites (Fig S1B), and that these levels (Fig S1C) are comparable to levels reported in human users (2, 8).
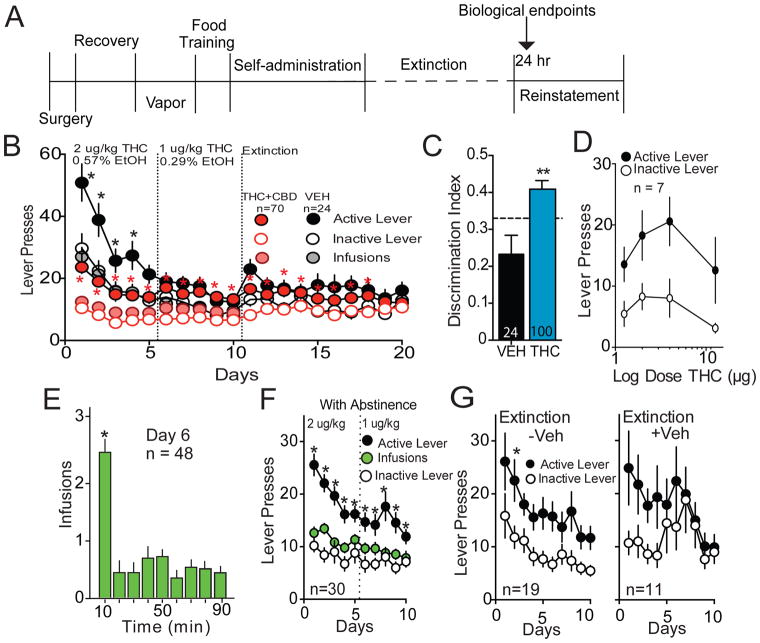
Figure 1. THC+CBD self-administration rats.
A) Time line of experimental protocol. Arrow indicates when tissue was harvested for in vitro measurements (24 hrs after the final extinction session). Note that in some experiments 7–10 days of withdrawal without extinction training was inserted between the last day of self-administration and the first day of extinction. B) Active and inactive lever pressing, during self-administration and extinction for THC+CBD and vehicle rats that proceeded directly to extinction following self-administration. Doses shown as THC μg/infusion, which was paired with CBD in a 10:1 ratio or % ethanol for vehicle self-administration. C) Lever discrimination ratios of the vehicle versus THC+CBD self-administering animals during the self-administration maintenance phase (mean+/− SEM of days 6–10). D) Dose-response function of lever pressing for various doses of THC+CBD (μg=THC/infusion). E) Infusions over 10 min bins from day 6 of self-administration in panel B showing most infusions occur during first 10 min of session. F) Active and inactive lever pressing, and infusions during THC+CBD self-administration for rats that proceeded into 7–10 days of homecage abstinence. G) Active and inactive lever responding for rats in panel F that were placed in 7–10 days of abstinence prior to extinction without (−Veh) or with (+Veh) availability of vehicle infusions upon active lever pressing.
*p< 0.05, comparing active and inactive lever pressing using a Sidak’s multiple comparisons analysis (panels B and G), or p<0.05, comparing 10 min with all other times using a Dunnett’s test (panel F).
Lever press and infusion data during self-administration and extinction for rats receiving THC +CBD or vehicle infusions is shown in Fig 1B. The vehicle self-administration group was run to control for ethanol diluent in THC solution (0.25–0.5%). Discrimination between active and inactive levers occurred throughout THC+CBD self-administration, and was gradually lost during extinction (Fig. 1B: two-way ANOVA revealed main effects of lever F(1,69)=132.3, p<0.001, day F(19,1311)=3.00, p<0.001, and interaction F(19,1311)=6.69 p<0.001). Noticeably, overall lever pressing was higher in vehicle self-administering rats, but in contrast with THC+CBD, lever discrimination was lost by day 5 of self-administration consistent with lack of vehicle-maintained reinforcement. Of note, substantial individual differences were present in the levels of THC+CBD self-administration (see Fig S2 heat map of pooled THC+CBD data from Figs 1B,F). The discrimination index over the last 5 days of self-administration was higher in THC+CBD compared to vehicle controls (t(123)=3.24, p=0.002) (Fig 1C). Food responding before self-administration did not differ between groups (vehicle: active/inactive 61.3±11.6/27.9±3.8; THC+CBD: active/inactive 66.9±7.1/34.1±7.2). We also examined the contribution of CBD, vapor pre-exposure and food training on the self-administration discrimination index. Eliminating any single component from the protocol produced a discrimination index intermediate between vehicle and THC+CBD (Fig. S3). Dose-response analysis of self-administered THC+CBD revealed a shallow biphasic response with maximum active lever pressing at 2 and 4 μg/kg and lesser responding at 1.3 and 12.6 μg/kg (main effect of lever F(1,6)=30.34, p=0.002) (Fig. 1D). Akin to other addictive drugs, maximum THC+CBD use occurred in a loading period during the first 10 min of self-administration (F(8,414)=19.61, p<0.001) (Fig. 1E).
We noted that Day 1 extinction lever pressing measured 24 hr after the last self-administration was relatively low (Fig 1B). This may have resulted from residual circulating THC or active metabolites measured in plasma 24 hr after the last self-administration session diminishing the motivation to press for THC+CBD (8, 34). In support of this possibility, we found measureable plasma levels of THC and its metabolites up to 5 days after discontinuing self-administration (Fig S1C). Accordingly, we established a separate group of rats trained to self-administer THC+CBD that underwent day 1 extinction after 7–10 days of homecage abstinence (Fig. 1F: two-way ANOVA revealed main effects of lever F(1,29)=113.6, p<0.001, day F(9,261)=5.54, p<0.001, and interaction F(9,261)=4,23, p<0.001). During extinction pressing the active lever either delivered vehicle (+Veh) or was without consequence (−Veh). Active lever pressing during extinction training with or without vehicle delivery was statistically equivalent (Fig 1G: F(1,28)=0.072, p=0.791), indicating that rats receiving vehicle detect the changing reinforcement conditions and that vehicle did not maintain responding. Introducing an abstinence period of 7–10 days potentiated day 1 extinction active lever pressing compared to rats entering extinction training immediately (e.g. compare day 1 THC+CBD extinction between Figs 1B and 1G; Table S1; two-way ANOVA for day 1 extinction: interaction F(2,196)=0.38, p=0.68; lever F(1,196)=16.32, p<0.001; abstinence vs. no abstinence F(2,196)=5.96, p=0.003). In all groups, main effect of lever was lost by day 10 extinction (interaction F(2,196)=2.85, p=0.06; lever F(1,196)=0.602, p=0.44; condition F(2,196)=1.57, p=0.21). Of note, an alternative interpretation to the increased active lever pressing on day 1 extinction after abstinence is that, akin to cocaine, responding may ‘incubate’ and increase with abstinence duration (35).
Conditioned cues and stress reinstated lever pressing following THC+CBD self-administration
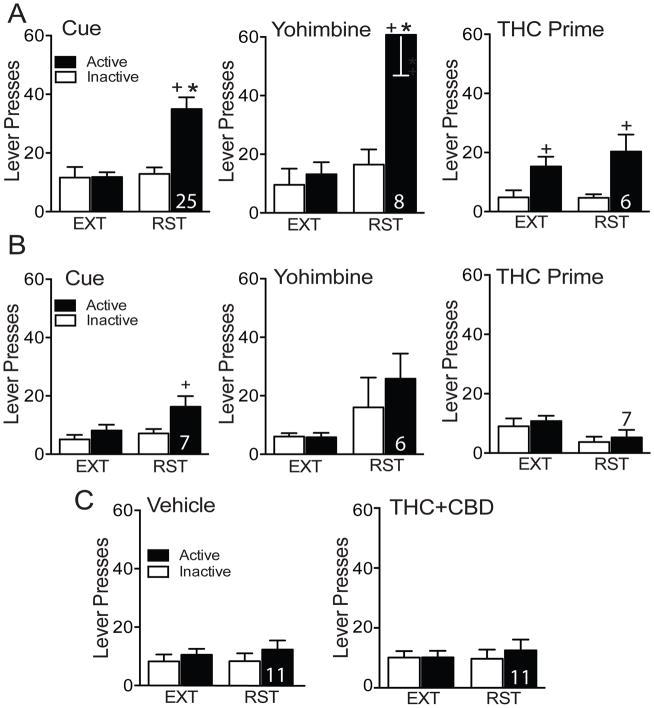
After extinction, lever pressing was reinstated by conditioned cues (restoring contingent light and tone cues), a noncontingent injection of THC (1 mg/kg, ip) or THC+CBD (1 mg + 0.1 mg/kg, ip), or a noncontingent injection of yohimbine (2.5 mg/kg, ip) to model stress-induced reinstatement. Each rat underwent 2–3 reinstatement modalities separated by a minimum 2 days of extinction. Robust reinstatement was elicited by conditioned cues (Fig 2A, left; two-way repeated measures ANOVA: interaction F(1,24)=28.02, p<0.001; ext vs. cue F(1,24)=18.94, p<0.002; lever F(1,24)=19.95, p<0.002) and yohimbine (Fig 2A, middle; two-way repeated measures ANOVA: interaction F(1,7)=11.32, p=0.010; ext vs. cue F(1,7)=14.66, p=0.007; lever F(1,7)=17.54, p=0.004) in THC+CBD-trained rats. However, THC priming did not reinstate lever pressing (Fig 2A, right). We hypothesized that this might be due to differences in the interoceptive experience between THC alone in the prime compared to THC+CBD experienced throughout self-administration. However, when THC+CBD was crossed over with vehicle priming injections neither THC+CBD injection nor vehicle elicited reinstatement (Fig 2C). Moreover, rats trained on vehicle self-administration did not reinstate to cues, yohimbine, or THC prime (Fig 2B). However, active lever pressing was significantly higher than inactive pressing for vehicle animals during cue reinstatement suggesting, as has been shown previously, that cues alone possess some rewarding value (36). For this reason, we compared cue reinstatement between vehicle and THC+CBD self-administering animals and report that cued reinstatement was greater in THC+CBD- than vehicle-trained rats (main effects of lever F(1,60)=11.98, p=0.001 and treatment F(1,60)=7.33, p=0.009, but no interaction F(1,60)=2.06, p=0.157). Full statistics for figure 2 are provided in Table S2. Additional self-administration, extinction and reinstatement experiments were conducted using other doses of THC+CBD self-administration (summarized in Supplemental Fig. S4 and Table S3).
Figure 2. Reinstatement following THC+CBD or Vehicle self-administration.
A) Cue- (left) and yohimbine-induced (2.5 mg/kg, ip) reinstatement in rats trained to self-administer THC+CBD +p<0.05, comparing active and inactive lever pressing. *p<0.05, comparing active lever pressing between reinstatement and extinction. THC-prime (right, 1 mg/kg, ip) did not reinstate lever pressing. B) Lack of cue-, yohimbine, and THC-primed reinstatement compared to extinction lever pressing in vehicle self-administering rats. +p<0.05, comparing active and inactive lever pressing. C) THC+CBD prime (1 + 0.1 mg/kg, ip) and vehicle did not reinstate lever pressing in THC+CBD extinguished rats here performed using within subject crossover of vehicle prime. N is shown over or within the bars. (See Table S2 for complete statistics).
Pharmacological assessment of THC self-administration and reinstatement
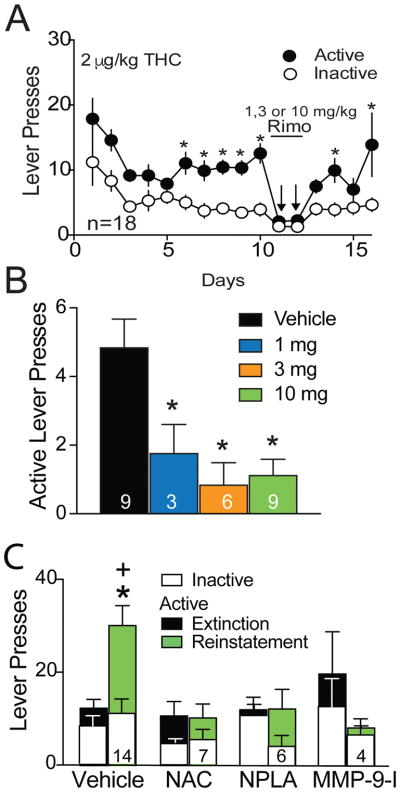
To examine the role of CB1 receptors in sustaining THC+CBD self-administration, rats were treated with CB1 receptor antagonist rimonabant (1, 3, or 10 mg/kg, ip) 30 min prior to self-administration. Rimonabant suppressed THC+CBD self-administration compared to vehicle pre-treatment (Fig. 3A,B; one-way ANOVA F(3,24)=7.60, p=0.001). Supporting a specific effect on self-administration, and confirming previous reports for doses ranging from 0.3–10 mg/kg, neither 3 nor 10 mg/kg rimonabant suppressed locomotion (Fig. S5)(37, 38). It should be noted that we did not test effects of rimonabant on locomotor activity or other behaviors in the presence of THC, and rimonabant could impact factors such as arousal in the presence of THC+CBD. For example, CB1 antagonist pre-treatment blocks both the acute psychological (high feeling) and physiological (heart rate) effects of smoked cannabis in a community sample of cannabis users (39).
Figure 3. Pharmacological assessment of THC self-administration and cue-induced reinstatement.
A) Pretreatment with the CB1 receptor antagonist rimonabant (Rim; 10, 3 or 1 mg/kg, ip) suppressed THC+CBD self-administration. Two-way ANOVA reveals main effect of day (F(1,478)=101.6, p<0.001) and lever (F(1,478)=7.308, p<0.001) but no interaction. *p< 0.05, comparing active and inactive lever pressing using a Sidak’s multiple comparisons analysis B) The rimonabant treatment separated by dose reveals significant suppression of active lever pressing during THC+CBD self-administration over a dose range of 1 to 10 mg/kg, ip. Data are shown as average of the first 30 min of self-administration. C) NAC, NPLA and MMP-9 inhibition (MMP-9-I) inhibit cue-induced reinstatement of THC+CBD seeking in independent experiments. Five daily injections of NAC were administered prior to the cued reinstatement session. NPLA and MMP-9-I were administered into the NAcore 10 or 15 min, respectively, prior to beginning the 90-minute reinstatement session. Vehicle data contains 7 rats that were controls for systemic NAC and received saline, ip, daily for 5 days prior to reinstatement, plus 7 rats microinjected with intracranial NAcore vehicle, saline plus 1% DMSO 15 min prior to reinstatement. The data were not significantly different in these two vehicle control groups and were pooled for presentation and statistical analysis. Data are shown as mean±sem and N is shown in bars. Each animal was reinstated once. *p< 0.05 comparing vehicle active lever pressing to the other treatments using a Sidak’s multiple comparison, +p< 0.05 comparing extinction active lever pressing to reinstated active lever pressing within each treatment group.
Reinstatement in rodents trained to self-administer different classes of addictive drug share a common mechanism in NAcore relative to animals trained to self-administer sucrose (40). For example, restoration of glutamate transport with N-acetylcysteine (NAC) reduces reinstated nicotine, heroin and cocaine seeking (41–43), and inhibiting nNOS or MMP-9 prevents cocaine and heroin reinstatement (25, 26). Likewise, cue-induced reinstatement of THC+CBD was inhibited by pretreatment with NAC, the nNOS inhibitor NPLA and an MMP-9 inhibitor, implicating involvement of mechanisms similar to other addictive drugs (Fig. 3C; two-way ANOVA for active lever revealed main effects of treatment F(3,52)=3.94, p=0.013 and interaction F(3,52)=5.14, p=0.004; no significant effects for inactive lever pressing). Although we did not determine effects of NAC, NPLA or MMP-9i in vehicle trained rats, previous studies show that NPLA and MMP-9i are without effect in sucrose-trained animals (25, 44), minimizing the possibility that they are producing nonspecific effects on reinstated behavior. However one study found that NAC treatment reduces reinstated food seeking (42).
Extinction from THC+CBD self-administration abolished NMDA-dependent LTD without altering AMPA/NMDA ratio
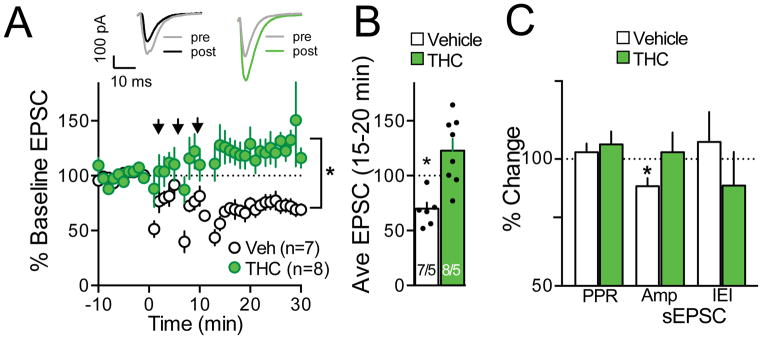
LTD is abolished in vitro in accumbens after withdrawal from cocaine self-administration (45, 46), after extinction from cocaine or heroin self-administration in vivo (20, 22), and in ethanol-dependent rats (47). We generated NMDA-dependent LTD using low frequency pairing in vehicle animals (1–5 Hz afferent stimulation in postsynaptic cells held at −50 mV) (46, 48). Extinction from THC+CBD self-administration abolished this form of LTD (Fig. 4A; treatment F(1,391)=161.10, p<0.001, time F(30,391)=1.84, p=0.005, interaction F(30,391)=3.14, p<0.001; Fig. 3B t13=4.17, p=0.001). Supporting a postsynaptic mechanism for the LTD loss, sEPSC amplitude was reduced after inducing LTD in vehicle rats, with no change measured in the THC+CBD group (Fig. 4C, t6=3.29, p=0.017). In contrast, two estimates of presynaptic changes, PPR and sEPSC inter-event interval (IEI) were unaltered by LTD induction in either group (Fig. 4C). Following extinction training we observed no difference in AMPA/NMDA between THC+CBD and vehicle groups (Fig S6A) (t11= 0.47, p= 0.650). A double exponential fit of decay revealed no effect of THC+CBD on fast, slow or dual decay times of isolated NMDA currents (Fig. S6B). Finally, neither amplitude nor frequency of spontaneous events differed between vehicle- and THC+CBD-extinguished animals (Figure S6C,D).
Figure 4. Extinction from THC+CBD self-administration abolishes NMDA-dependent LTD.
A) Sample pre and post current traces from the two treatment groups. Averaged data traces summarizing time course of synaptic response before, during, and after LTD induction protocol. *p<0.05, comparing vehicle (Veh) with THC+CBD trained. B) Boxplots show averaged responses, comparing baseline to the average of 21–30 min after LTD induction protocol. N is shown as cells recorded/animals. *p<0.05, comparing pre- to post-stimulation. C) Vehicle animals exhibit decreased sEPSC amplitude. *p<0.05, comparing pre- to post-stimulation.
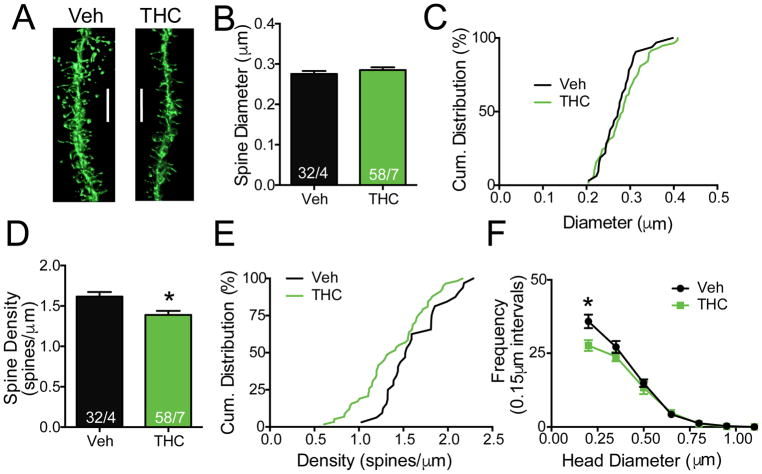
Extinction from THC+CBD self-administration decreased dendritic spine density
Dendritic spine density and spine head diameter in accumbens MSNs are synaptic adaptations that vary between addictive drugs, with potentiation produced following cocaine or nicotine self-administration, and heroin inducing morphological depotentiation (21, 49). Reconstructed confocal images of DiI labeled MSNs revealed no difference in spine diameter between THC+CBD extinguished rats and controls (Fig. 5A,B,C). However, dendritic spine density was decreased after extinction from THC+CBD self-administration. The reduction was apparent when data were analyzed as mean difference between neuron populations (Fig. 5A,D; t(88)=2.82, p=0.006), and by the left-shifted cumulative distribution of spine density (Fig. 5E) in the THC+CBD extinguished group (comparing elevations F(1,31)=20.38, p<0.001). The difference in spine number was attributed to a decrease in number of thin spines (< 0.25 μm)(Fig 5F; treatment F(1,63)=5.90, p=0.018; size bin F(6,63)=172.3, p<0.001; interaction F(6,63)=2.41, p=0.037).
Figure 5. Extinction from THC+CBD self-administration decreases dendritic spine density.
A) Representative diolistically-labeled segments from NAcore. Bar= 5 μm. B) There was no difference in dendritic spine head diameter between vehicle (Veh) and THC+CBD (THC) extinguished rats. Numbers of neurons quantified over number of animals shown in each bar. C) Cumulative distribution plot of data shown in panel B. D) Dendritic spine density was decreased in THC+CBD-extinguished compared to vehicle rats (p<0.05). E) Cumulative distribution plot of data shown in panel D reveals shift to the left in THC+CBD group. F) Frequency plot of dendritic spines binned according to spine head size. *p<0.05 comparing Veh to THC at diameter bin using a Sidak’s post hoc.
Discussion
We describe a novel rodent model of self-administration and relapse to the active ingredient of cannabis, THC. The self-administration behavior described here is reliable and reproducible across cohorts, but the relatively poor reinforcing properties of THC compared to other abused substances are apparent. A shallow biphasic dose-response curve indicates a limited reinforcing range with higher doses decreasing the number of infusions taken per session and a minimum reinforcing dose of THC+CBD below 0.5+0.05 μg/infusion (Fig. S4A). Literature-based strategies were implemented to maximize THC self-administration including passive vapor exposure before self-administration, combining THC with CBD in a 10:1 ratio, and food training prior to self-administration (15, 32, 50). The relative importance of each component was assessed, and if CBD, vapor treatment or food training was omitted the discrimination index was intermediate between vehicle and the complete THC+CBD protocol (Fig. S3). CBD inclusion was designed to counteract the aversive effects of THC. It was recently reported that CBD pre-treatment had no effect on THC self-administration in rats (30), but this experiment was performed in a small number of rats assessing only the effect of CBD on pre-established THC self-administration. Given the variability in THC and CBD content in different cannabis strains and the distinct effects of specific combinations in human populations (51), it is important to further examine additional doses and ratio combinations of THC+CBD. Although the ethanol vehicle did not sustain self-administration or result in cue or yohimbine-induced drug seeking, an additional caveat in the present study is that the co-delivery of THC/CBD plus ethanol may interact to produce effects distinct from either drug alone. While we did not set out to create a model of co-morbid cannabis and ethanol use, potential ethanol influences cannot be fully excluded. Given the high comorbidity between alcohol and cannabis use disorders it is important for future studies to systematically evaluate a co-administration model (52).
Relative Reinforcing Qualities of THC+CBD
Both VEH and THC+CBD animals were food trained at similar levels, but clear differences in maintenance responding emerged such that THC+CBD animals displayed greater lever discrimination ratios during self-administration maintenance, thereby mitigating lasting contributions of food training that were extinguished in VEH rats (Fig. 1F). Despite achieving discrimination ratios >2:1 THC+CBD trained rats displayed relatively high inactive lever pressing throughout self-administration compared to other abused drugs. These findings are in line with other reports showing similar deficits in response bias using a cannabinoid e-cigarette vapor inhalation model (53). Also, human studies show that THC impairs the ability to inhibit ongoing responses in the Stop task (54), as well as modulates brain activity in regions known to mediate response inhibition (e.g. inferior frontal gyrus, insula, and medial frontal cortex) (55).
With other addictive drugs, drug seeking can be initiated after a period of abstinence by returning animals to the drug-paired context or by presenting drug-conditioned discrete cues or stress exposure after extinguishing the drug-context association. THC+CBD use recapitulated vulnerability to relapse with these modalities. However, a noncontingent injection of THC or THC+CBD failed to reinstate THC+CBD seeking, consistent with the poor rewarding qualities of noncontingent THC injection in the CPP paradigm (15, 56, 57). Of note, reinstatement induced by noncontingent drug administration occurs with some addictive drugs (e.g. heroin and cocaine), but is less robust with others such as nicotine and alcohol (20, 58).
Pharmacological Regulation of THC+CBD Seeking
We show similarities with other addictive drugs in self-administration and reinstatement, and also found that compounds known to inhibit reinstated behavior in rats trained to self-administer other addictive drugs were effective at inhibiting cued reinstatement to THC+CBD. A series of studies show that cued reinstatement to addictive drugs depends on spillover of synaptic glutamate in accumbens that stimulates nNOS production of nitric oxide and S-nitrosylates MMPs in the nucleus accumbens during transient synaptic potentiation underlying cued drug seeking (26, 40). Similarly, cue-induced reinstatement of THC+CBD was prevented by pretreatment with daily injections of NAC, previously shown to restore glutamate transport and limit synaptic glutamate spillover, as well as pharmacological inhibition of nNOS and MMP-9. NAC is well-established in the reinstatement model of relapse to inhibit cue-induced reinstatement to many addictive drugs, and a clinical trial found that NAC reduces adolescent cannabis use (59).
Neurobiological Adaptations Produced by THC+CBD Self-administration
We endeavored to link THC+CBD to other addictive drugs through shared, enduring changes in NAcore MSN morphology and synaptic physiology. Cocaine (41, 45, 46), heroin (60) and alcohol (47) produce long-lasting impairments in the capacity to induce LTD at excitatory synapses on accumbens MSNs. Loss of LTD in the cortico-accumbens circuit is associated with compulsive drug seeking in addiction-vulnerable subpopulations (45). Rats extinguished from THC+CBD self-administration showed complete loss of LTD. THC presynaptically reduces glutamate synaptic transmission, and can alter the expression and function of glutamate receptors and transporters (61). Given the well-established mediation of reinstated drug seeking by cortico-accumbens synapses and the strong association between activating this pathway and drug craving in human imaging studies (62, 63), it seems probable that the enduring changes in cortico-accumbens signaling produced by addictive drugs, now including THC+CBD, contribute to loss of LTD. It is known that noncontingent THC can produce morphological and physiological adaptations in the accumbens (64, 65), and it is important to note that our study was not designed to discern contingent from noncontingent THC+CBD administration. However, the present study sets the stage for future studies to understand how conditioned cues can induce neurobiological changes that mediate reinstated THC+CBD, akin to what has been found with other drugs of abuse (40, 66).
THC+CBD also reduced dendritic spine density. This morphological evidence of enduring synaptic depotentiation after THC+CBD poses occlusion as a mechanism for impairment of LTD. The dendritic spine loss more closely resembles enduring adaptations produced by opioids since psychostimulants like cocaine or nicotine increase accumbens MSN dendritic spine density and/or spine head diameter (49). Also, similar to THC+CBD, heroin produces no change or reduces AMPA/NMDA in NAcore (22), while cocaine and nicotine withdrawal is associated with elevated AMPA/NMDA (19, 20). The similarity between THC+CBD and heroin is consistent with substantial neuroanatomical overlap between brain structures expressing high levels of Gi/o-coupled cannabinoid and opiate receptors (67) and functional heteromerization between CB1 and mu or delta opioid receptors (68).
Conclusions
Establishing an efficient, scalable rat model of THC+CBD self-administration and relapse is an important first step in identifying the neurological consequences of chronic cannabis use. We clearly show that THC+CBD self-administration elicited long-term changes in the cortico-accumbens circuit resembling impairments produced by better-studied addictive drugs, and in particular, most closely resembling the profile of long-term adaptations produced by heroin. Reversing drug-induced neuroadaptations in cortico-accumbens synapses reduces cocaine and heroin seeking in animal models (69–71), and we have demonstrated that these compounds have utility in this model. Thus, biological rationales for pharmacotherapies being developed for treating addiction to other drugs may apply to THC+CBD, and thereby facilitate developing cannabis addiction treatment protocols.
Supplementary Material
Figure S1. Validation of delivery of physiologically relevant THC levels. A) Hypothermia produced by vapor and intravenous THC+CBD (2.0+0.2 μg/infusion). Hab= habituation (no drug), Vap= at the end of vapor exposure, IV= at the end of a self-administration session (2.0+0.2 μg/infusion). *p<0.05, comparing pre- to post-THC+CBD exposure using a paired Student’s t-test. B) Amount of THC and metabolites detected in serum correlates with number of infusions achieved during a THC+CBD self-administration session. C) Measurement of THC and metabolites in the serum of rats following THC+CBD vapor, intravenous THC+CBD self-administration, or after one (E-1) or five (E-5) days of extinction training.
Figure S2. Individual differences in drug intake. A heat map showing individual variability in THC+CBD infusions throughout the 10 days of self-administration. Higher numbers of infusions are shown in green (max=45) and lower numbers of infusions are red (min=1). Note that 3 potential subgroups emerged based on drug intake with high users generally consuming >10 infusions per day throughout training and low users consuming <10. A subset of rats displayed a highly variable infusion rates between days.
Figure S3. The treatment protocol in Figure 1A results in a higher discrimination index than when CBD, vapor pretreatment or food training was eliminated. A) Left panel illustrates a frequency plot of the discrimination index showing that THC+CBD is shifted to the right relative to all other treatments, indicating greater reinforcing value of this combination. Right panel compares the mean lever preference ratio, verifying the higher lever preference ratio for THC+CBD relative to Vehicle, with the other combinations having intermediate values. The dotted line indicates a 2:1 ratio of active to inactive lever pressing. N shown in the bar. *p< 0.05 comparing all treatments to vehicle. One-way ANOVA followed by a Dunnett’s multiple comparisons test. B) Comparison of total infusion number between different treatment groups. There was a trend for THC+CBD combination rats to show the highest drug intake (one way ANOVA F(3,61)=2.17, p=0.101).
Figure S4. Reinstatement in rats extinguished from varying doses of THC self-administration. A) Comparison of lever pressing across doses of THC+CBD during the last three days of self-administration. Doses shown are for THC (CBD was co-administered at 10% the dose of THC). +p<0.05, comparing active and inactive lever pressing, using a paired Student’s t-test. B) Comparison of day 1 extinction lever pressing between rats placed in 7–10 days of abstinence (Abs) versus rats placed into extinction training the day after discontinuing self-administration (No Abs) for the 4 μg/kg/infusion dose. +p<0.05, comparing active and inactive lever pressing. C) Comparison of lever pressing across doses after cue-induced, THC-primed (1 mg/kg, i.p.), or yohimbine-primed (2.5 mg/kg, i.p.) drug seeking. Lighter colored bars refer to the average of the last two extinction (Ext) sessions just prior to each reinstatement. *p<0.05, comparing active lever pressing between reinstatement and extinction within each dose and reinstatement modality using a 2-way ANOVA with repeated measures over lever and extinction vs. reinstatement, followed by a Sidak post hoc test. +p<0.05, comparing active and inactive lever pressing. In all panels N is shown over the bars. (See Table S3 for complete statistics).
Figure S5. Lack of effect by rimonabant on locomotor activity and histological verification of inhibitor microinjections. A) Lack of effect by 10 or 3 mg/kg, ip of rimonabant on locomotor activity in an adapted open field. Rats were pretreated with rimonabant or vehicle in a crossover design using a 3 day inter-trial interval. Separate two-way ANOVA with repeated measures over time and treatment reveal an effect of time (10 mg/kg- F(17,119)=18.87, p<0.001; 3 mg/kg- F(17,102)=19.95, p<0.001), but no effect of treatment or interaction. B) Histologically determined location of microinjections of NPLA localized to the core subcompartment of the nucleus accumbens. Rats microinjected with NPLA are indicated by closed circles and rats microinjected with vehicle are indicated by open triangles. C) Histologically determined location of microinjections of MMP-9-I localized to the core subcompartment of the nucleus accumbens. Rats microinjected with MMP-9-I are indicated by closed circles and rats microinjected with vehicle are indicated by open triangles.
Figure S6. Extinction from THC+CBD self-administration does not change AMPA receptor signaling. A) Sample AMPA and NMDA current traces and averages showing that THC+CBD self-administration did not change AMPA/NMDA ratio. Calibration bars represent 100 pA and 10 ms. B) Sample traces of pharmacologically isolated NMDA currents and averages showing THC+CBD self-administration did not change the decay times of NMDA currents. Calibration bars represent 100 pA and 10 ms. C) Cumulative probability and mean values of amplitude for sEPSCs recorded from both treatment groups. D) Cumulative probability and mean values of Inter-Event-Intervals (IEI) for sEPSCs recorded from both treatment groups.
Table S1. Statistics for Figure 1B and D.
Day one and day ten of extinction- Comparing abstinent with nonabstinent (i.e. extinction conducted 24 hr after the last self-administration) lever pressing using a 2-way ANOVA with repeated measures over lever.
Table S2. Statistics for Figure 2.
Table S3. Statistics for Figure S4.
Acknowledgments
We thank Carmela Reichel, Madhura Athreya, Ana Clara Bobadilla and Christian Lombino for advice and technical assistance. This research was supported by USPHS grants DA003906, DA012513, DA015369 (PWK), US Department of Defense grant W81XWH-13-2-0075 (PWK), DA037722, DA016511, Burroughs Wellcome Fund (SS), and GM072643 (VC).
Footnotes
Financial Disclosures. The authors report no biomedical financial disclosures or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.SAMHSA. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: HHS; 2014. NSDUH Series H-48. [Google Scholar]
- 2.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. British Journal of Pharmacology. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manzoni OJ, Bockaert J. Cannabinoids inhibit GABAergic synaptic transmission in mice nucleus accumbens. European Journal of Pharmacology. 2001;412:R3–R5. doi: 10.1016/s0014-2999(01)00723-3. [DOI] [PubMed] [Google Scholar]
- 4.Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ. Localization and Mechanisms of Action of Cannabinoid Receptors at the Glutamatergic Synapses of the Mouse Nucleus Accumbens. The Journal of Neuroscience. 2001;21:109–116. doi: 10.1523/JNEUROSCI.21-01-00109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castillo Pablo E, Younts Thomas J, Chavez Andres E, Hashimotodani Y. Endocannabinoid Signaling and Synaptic Function. Neuron. 2012;76:70–81. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-Mediated Control of Synaptic Transmission. 2009 doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- 7.Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharamcokinet. 2003;42:34. doi: 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- 8.Huestis MA. Human cannabinoid pharmacokinetics. Chemistry & biodiversity. 2007;4:1770–1804. doi: 10.1002/cbdv.200790152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefever TW, Marusich JA, Antonazzo KR, Wiley JL. Evaluation of WIN 55,212-2 self-administration in rats as a potential cannabinoid abuse liability model. Pharmacology Biochemistry and Behavior. 2014;118:30–35. doi: 10.1016/j.pbb.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi R, Singer G. Self-administration of delta-9-tetrahydrocannabinol by rats. Pharmacology Biochemistry and Behavior. 1979;11:4. doi: 10.1016/0091-3057(79)90274-0. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi R, Singer G. Effects of body weight levels on cannabis self-injection. Pharmacology Biochemistry and Behavior. 1980;13:5. doi: 10.1016/0091-3057(80)90222-1. [DOI] [PubMed] [Google Scholar]
- 12.Greydanus D, Hawyer E, Greydanus M, Merrick J. Marijuana: Current Concepts. Frontiers in Public Health. 2013 doi: 10.3389/fpubh.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein C, Karanges E, Spiro A, Wong A, Spencer J, Huynh T, et al. Cannabidiol potentiates Δ9-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology. 2011;218:443–457. doi: 10.1007/s00213-011-2342-0. [DOI] [PubMed] [Google Scholar]
- 14.Russo E, Guy GW. A tale of two cannabinoids: The therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Medical Hypotheses. 2006;66:234–246. doi: 10.1016/j.mehy.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Vann RE, Gamage TF, Warner JA, Marshall EM, Taylor NL, Martin BR, et al. Divergent effects of cannabidiol on the discriminative stimulus and place conditioning effects of Δ9-tetrahydrocannabinol. Drug and Alcohol Dependence. 2008;94:191–198. doi: 10.1016/j.drugalcdep.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore BA, Budney AJ. Relapse in outpatient treatment for marijuana dependence. Journal of Substance Abuse Treatment. 2003;25:85–89. doi: 10.1016/s0740-5472(03)00083-7. [DOI] [PubMed] [Google Scholar]
- 17.Copeland J, Swift W, Roffman R, Stephens R. A randomized controlled trial of brief cognitive–behavioral interventions for cannabis use disorder. Journal of Substance Abuse Treatment. 2001;21:55–64. doi: 10.1016/s0740-5472(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 18.Florez-Salamanca L, Secades-Villa R, Budney AJ, Garcia-Rodriguez O, Wang S, Blanco C. Probability and predictors of cannabis use disorders relapse: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Drug Alcohol Depend. 2013;132:127–133. doi: 10.1016/j.drugalcdep.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gipson Cassandra D, Kupchik Yonatan M, Shen H, Reissner Kathryn J, Thomas Charles A, Kalivas Peter W. Relapse Induced by Cues Predicting Cocaine Depends on Rapid, Transient Synaptic Potentiation. Neuron. 2013;77:867–872. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, et al. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9124–9129. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russo SJ, Dietz DM, Dumitriu D, Malenka RC, Nestler EJ. The Addicted Synapse: Mechanisms of Synaptic and Structural Plasticity in Nucleus Accumbens. Trends in neurosciences. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen H, Moussawi K, Zhou W, Toda S, Kalivas PW. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proceedings of the National Academy of Sciences. 2011;108:19407–19412. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin R, Cao J, Webb SM, Ikemoto S. Amphetamine administration into the ventral striatum facilitates behavioral interaction with unconditioned visual signals in rats. PloS one. 2010;5:e8741. doi: 10.1371/journal.pone.0008741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 25.Smith AC, Kupchik YM, Scofield MD, Gipson CD, Wiggins A, Thomas CA, et al. Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nat Neurosci. 2014;17:1655–1657. doi: 10.1038/nn.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith AC, Scofield MD, Heinsbroek JA, Gipson CD, Neuhofer D, Roberts-Wolfe DJ, et al. Accumbens nNOS Interneurons Regulate Cocaine Relapse. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2017;37:742–756. doi: 10.1523/JNEUROSCI.2673-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seabold GK, Daunais JB, Rau A, Grant KA, Alvarez VA. DiOLISTIC Labeling of Neurons from Rodent and Non-human Primate Brain Slices. 2010:e2081. doi: 10.3791/2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braida D, Iosue S, Pegorini S, Sala M. Δ9-Tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. European Journal of Pharmacology. 2004;506:63–69. doi: 10.1016/j.ejphar.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 29.Melis M, Frau R, Kalivas PW, Spencer S, Chioma V, Zamberletti E, et al. New vistas on cannabis use disorder. Neuropharmacology. doi: 10.1016/j.neuropharm.2017.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wakeford AGP, Wetzell BB, Pomfrey RL, Clasen MM, Taylor WW, Hempel BJ, et al. The Effects of Cannabidiol (CBD) on Delta9-Tetrahydrocannabinol (THC) Self-Administration in Male and Female Long-Evans Rats. Experimental and clinical psychopharmacology. 2017 doi: 10.1037/pha0000135. [DOI] [PubMed] [Google Scholar]
- 31.Zangen A, Solinas M, Ikemoto S, Goldberg SR, Wise RA. Two brain sites for cannabinoid reward. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:4901–4907. doi: 10.1523/JNEUROSCI.3554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tzschentke TM. REVIEW ON CPP: Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addiction Biology. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 33.Hempel BJ, Wakeford AG, Clasen MM, Friar MA, Riley AL. Delta-9-tetrahydrocannabinol (THC) history fails to affect THC’s ability to induce place preferences in rats. Pharmacol Biochem Behav. 2016;144:1–6. doi: 10.1016/j.pbb.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Toennes SW, Ramaekers JG, Theunissen EL, Moeller MR, Kauert GF. Comparison of cannabinoid pharmacokinetic properties in occasional and heavy users smoking a marijuana or placebo joint. J Anal Toxicol. 2008;32:470–477. doi: 10.1093/jat/32.7.470. [DOI] [PubMed] [Google Scholar]
- 35.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cain ME, Green TA, Bardo MT. Environmental enrichment decreases responding for visual novelty. Behavioural processes. 2006;73:360–366. doi: 10.1016/j.beproc.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adamczyk P, Golda A, McCreary AC, Filip M, Przegalinski E. Activation of endocannabinoid transmission induces antidepressant-like effects in rats. Journal of physiology and pharmacology: an official journal of the Polish Physiological Society. 2008;59:217–228. [PubMed] [Google Scholar]
- 38.Verty AN, Allen AM, Oldfield BJ. The effects of rimonabant on brown adipose tissue in rat: implications for energy expenditure. Obesity (Silver Spring, Md) 2009;17:254–261. doi: 10.1038/oby.2008.509. [DOI] [PubMed] [Google Scholar]
- 39.Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, et al. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Archives of general psychiatry. 2001;58:322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- 40.Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, et al. The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacol Rev. 2016;68:816–871. doi: 10.1124/pr.116.012484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moussawi K, Pacchioni AM, Moran M, Olive MF, Gass JT, Lavin A, et al. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nature Neuroscience. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramirez-Nino AM, D’Souza MS, Markou A. N-acetylcysteine decreased nicotine self-administration and cue-induced reinstatement of nicotine seeking in rats: comparison with the effects of N-acetylcysteine on food responding and food seeking. Psychopharmacology. 2013;225:473–482. doi: 10.1007/s00213-012-2837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou W, Kalivas PW. N-Acetylcysteine Reduces Extinction Responding and Induces Enduring Reductions in Cue- and Heroin-Induced Drug-Seeking. Biol Psychiatry. 2007;63:338–340. doi: 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bobadilla AC, Garcia-Keller C, Heinsbroek JA, Scofield MD, Chareunsouk V, Monforton C, et al. Accumbens Mechanisms for Cued Sucrose Seeking. Neuropsychopharmacology. 2017;42:2377–2386. doi: 10.1038/npp.2017.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O, et al. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science. 2010;328:1709–1712. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- 46.Martin M, Chen BT, Hopf FW, Bowers MS, Bonci A. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat Neurosci. 2006;9:868–869. doi: 10.1038/nn1713. [DOI] [PubMed] [Google Scholar]
- 47.Spiga S, Talani G, Mulas G, Licheri V, Fois GR, Muggironi G, et al. Hampered long-term depression and thin spine loss in the nucleus accumbens of ethanol-dependent rats. Proc Natl Acad Sci U S A. 2014;111:E3745–3754. doi: 10.1073/pnas.1406768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- 49.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 50.Garcia KLP, Le AD, Tyndale RF. EFFECT OF FOOD TRAINING AND TRAINING DOSE ON NICOTINE SELF-ADMINISTRATION IN RATS. Behavioural brain research. 2014;0:10–18. doi: 10.1016/j.bbr.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atakan Z. Cannabis, a complex plant: different compounds and different effects on individuals. Therapeutic Advances in Psychopharmacology. 2012;2:241–254. doi: 10.1177/2045125312457586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pava MJ, Woodward JJ. A review of the interactions between alcohol and the endocannabinoid system: implications for alcohol dependence and future directions for research. Alcohol (Fayetteville, NY) 2012;46:185–204. doi: 10.1016/j.alcohol.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Setlow B, McLaughlin R, Taffe M, Torregrossa M. Rodent models of cannabinoid administration: novel approaches and surprising findings. Winter Conference on Brain Research; Whistler, BC. 2018. [Google Scholar]
- 54.McDonald J, Schleifer Laura, Richards Jerry B, de Wit Harriet. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28:1356–1365. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- 55.Borgwardt SJ, Allen P, Bhattacharyya S, Fusar-Poli P, Crippa JA, Seal ML, et al. Neural basis of Delta-9-tetrahydrocannabinol and cannabidiol: effects during response inhibition. Biol Psychiatry. 2008;64:966–973. doi: 10.1016/j.biopsych.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 56.Cheer J, Kendall D, Marsden C. Cannabinoid receptors and reward in the rat: a conditioned place preference study. Psychopharmacology. 2000;151:25–30. doi: 10.1007/s002130000481. [DOI] [PubMed] [Google Scholar]
- 57.Lepore M, Vorel SR, Lowinson J, Gardner EL. Conditioned place preference induced by delta 9-tetrahydrocannabinol: comparison with cocaine, morphine, and food reward. Life sciences. 1995;56:2073–2080. doi: 10.1016/0024-3205(95)00191-8. [DOI] [PubMed] [Google Scholar]
- 58.Finn DA, Mark GP, Fretwell AM, Gililland-Kaufman KR, Strong MN, Ford MM. Reinstatement of ethanol and sucrose seeking by the neurosteroid allopregnanolone in C57BL/6 mice. Psychopharmacology (Berl) 2008;201:423–433. doi: 10.1007/s00213-008-1303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gray K, Carpenter M, Baker N, DeSantis S, Kryway E, Hartwell K, et al. A double-blind randomized controlled trial of n-acetylcysteine in cannabis-dependent adolescents. American Journal of Psychiatry. 2012;169:8. doi: 10.1176/appi.ajp.2012.12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shen H, Kalivas PW. Reduced LTP and LTD in prefrontal cortex synapses in the nucleus accumbens after heroin self-administration. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2013;16:1165–1167. doi: 10.1017/S1461145712001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Colizzi M, McGuire P, Pertwee RG, Bhattacharyya S. Effect of cannabis on glutamate signalling in the brain: A systematic review of human and animal evidence. Neuroscience and biobehavioral reviews. 2016;64:359–381. doi: 10.1016/j.neubiorev.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 62.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature reviews Neuroscience. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoffman AF, Lycas MD, Kaczmarzyk JR, Spivak CE, Baumann MH, Lupica CR. Disruption of hippocampal synaptic transmission and long-term potentiation by psychoactive synthetic cannabinoid ‘Spice’ compounds: comparison with Delta9 -tetrahydrocannabinol. Addict Biol. 2017;22:390–399. doi: 10.1111/adb.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kolb B, Gorny G, Limebeer CL, Parker LA. Chronic treatment with Δ-9-tetrahydrocannabinol alters the structure of neurons in the nucleus accumbens shell and medial prefrontal cortex of rats. Synapse. 2006;60:429–436. doi: 10.1002/syn.20313. [DOI] [PubMed] [Google Scholar]
- 66.Mulholland PJ, Chandler LJ, Kalivas PW. Signals from the Fourth Dimension Regulate Drug Relapse. Trends Neurosci. 2016;39:472–485. doi: 10.1016/j.tins.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robledo P, Berrendero F, Ozaita A, Maldonado R. Advances in the field of cannabinoid–opioid cross-talk. Addiction Biology. 2008;13:213–224. doi: 10.1111/j.1369-1600.2008.00107.x. [DOI] [PubMed] [Google Scholar]
- 68.Massotte D. In vivo opioid receptor heteromerization: where do we stand? British Journal of Pharmacology. 2015;172:420–434. doi: 10.1111/bph.12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- 70.Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16:974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pascoli V, Terrier J, Espallergues J, Valjent E, O’Connor EC, Luscher C. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature. 2014;509:459–464. doi: 10.1038/nature13257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Validation of delivery of physiologically relevant THC levels. A) Hypothermia produced by vapor and intravenous THC+CBD (2.0+0.2 μg/infusion). Hab= habituation (no drug), Vap= at the end of vapor exposure, IV= at the end of a self-administration session (2.0+0.2 μg/infusion). *p<0.05, comparing pre- to post-THC+CBD exposure using a paired Student’s t-test. B) Amount of THC and metabolites detected in serum correlates with number of infusions achieved during a THC+CBD self-administration session. C) Measurement of THC and metabolites in the serum of rats following THC+CBD vapor, intravenous THC+CBD self-administration, or after one (E-1) or five (E-5) days of extinction training.
Figure S2. Individual differences in drug intake. A heat map showing individual variability in THC+CBD infusions throughout the 10 days of self-administration. Higher numbers of infusions are shown in green (max=45) and lower numbers of infusions are red (min=1). Note that 3 potential subgroups emerged based on drug intake with high users generally consuming >10 infusions per day throughout training and low users consuming <10. A subset of rats displayed a highly variable infusion rates between days.
Figure S3. The treatment protocol in Figure 1A results in a higher discrimination index than when CBD, vapor pretreatment or food training was eliminated. A) Left panel illustrates a frequency plot of the discrimination index showing that THC+CBD is shifted to the right relative to all other treatments, indicating greater reinforcing value of this combination. Right panel compares the mean lever preference ratio, verifying the higher lever preference ratio for THC+CBD relative to Vehicle, with the other combinations having intermediate values. The dotted line indicates a 2:1 ratio of active to inactive lever pressing. N shown in the bar. *p< 0.05 comparing all treatments to vehicle. One-way ANOVA followed by a Dunnett’s multiple comparisons test. B) Comparison of total infusion number between different treatment groups. There was a trend for THC+CBD combination rats to show the highest drug intake (one way ANOVA F(3,61)=2.17, p=0.101).
Figure S4. Reinstatement in rats extinguished from varying doses of THC self-administration. A) Comparison of lever pressing across doses of THC+CBD during the last three days of self-administration. Doses shown are for THC (CBD was co-administered at 10% the dose of THC). +p<0.05, comparing active and inactive lever pressing, using a paired Student’s t-test. B) Comparison of day 1 extinction lever pressing between rats placed in 7–10 days of abstinence (Abs) versus rats placed into extinction training the day after discontinuing self-administration (No Abs) for the 4 μg/kg/infusion dose. +p<0.05, comparing active and inactive lever pressing. C) Comparison of lever pressing across doses after cue-induced, THC-primed (1 mg/kg, i.p.), or yohimbine-primed (2.5 mg/kg, i.p.) drug seeking. Lighter colored bars refer to the average of the last two extinction (Ext) sessions just prior to each reinstatement. *p<0.05, comparing active lever pressing between reinstatement and extinction within each dose and reinstatement modality using a 2-way ANOVA with repeated measures over lever and extinction vs. reinstatement, followed by a Sidak post hoc test. +p<0.05, comparing active and inactive lever pressing. In all panels N is shown over the bars. (See Table S3 for complete statistics).
Figure S5. Lack of effect by rimonabant on locomotor activity and histological verification of inhibitor microinjections. A) Lack of effect by 10 or 3 mg/kg, ip of rimonabant on locomotor activity in an adapted open field. Rats were pretreated with rimonabant or vehicle in a crossover design using a 3 day inter-trial interval. Separate two-way ANOVA with repeated measures over time and treatment reveal an effect of time (10 mg/kg- F(17,119)=18.87, p<0.001; 3 mg/kg- F(17,102)=19.95, p<0.001), but no effect of treatment or interaction. B) Histologically determined location of microinjections of NPLA localized to the core subcompartment of the nucleus accumbens. Rats microinjected with NPLA are indicated by closed circles and rats microinjected with vehicle are indicated by open triangles. C) Histologically determined location of microinjections of MMP-9-I localized to the core subcompartment of the nucleus accumbens. Rats microinjected with MMP-9-I are indicated by closed circles and rats microinjected with vehicle are indicated by open triangles.
Figure S6. Extinction from THC+CBD self-administration does not change AMPA receptor signaling. A) Sample AMPA and NMDA current traces and averages showing that THC+CBD self-administration did not change AMPA/NMDA ratio. Calibration bars represent 100 pA and 10 ms. B) Sample traces of pharmacologically isolated NMDA currents and averages showing THC+CBD self-administration did not change the decay times of NMDA currents. Calibration bars represent 100 pA and 10 ms. C) Cumulative probability and mean values of amplitude for sEPSCs recorded from both treatment groups. D) Cumulative probability and mean values of Inter-Event-Intervals (IEI) for sEPSCs recorded from both treatment groups.
Table S1. Statistics for Figure 1B and D.
Day one and day ten of extinction- Comparing abstinent with nonabstinent (i.e. extinction conducted 24 hr after the last self-administration) lever pressing using a 2-way ANOVA with repeated measures over lever.
Table S2. Statistics for Figure 2.
Table S3. Statistics for Figure S4.