Abstract
Eukaryotic cells employ different types of extracellular vesicles (EVs) to exchange proteins, mRNAs, non-coding regulatory RNAs, carbohydrates, and lipids. Cells of the immune system, in particular antigen (Ag)-presenting cells (APCs), acquire Major Histocompatibility Complex (MHC) class-I and class-II molecules loaded with antigenic peptides from leukocytes and tissue parenchymal and stromal cells, through a mechanism known as MHC cross-dressing. Increasing evidence indicates that cross-dressing of APCs with pre-formed Ag-peptide : MHC complexes (pMHCs) is mediated via passage of clusters of EVs with characteristics of exosomes. A percentage of the transferred EVs remain attached to the acceptor APCs, with the appropriate orientation, at sufficient concentration within localized areas of the plasma membrane, and for sufficient time, so the preformed pMHCs carried by the EVs are presented without further processing, to cognate T cells. Although its biological relevance is not fully understood, numerous studies have demonstrated that MHC cross-dressing of APCs represents a pathway of Ag-presentation of acquired pre-formed pMHCs to T cells -alternative to direct and cross-presentation-, participate in immune-homeostasis and T-cell tolerance, cross-regulate allo-reactive T cells with different MHC restricted specificities, and is a mechanism of Ag spreading for autologous, allogeneic, microbial, tumor, or vaccine-delivered Ags. Here, we compare MHC cross-dressing with other mechanisms and terminologies used for pMHC transfer, including trogocytosis. We discuss the experimental evidence, mostly from in vitro and ex vivo studies, of the role of MHC cross-dressing of APCs via EVs in positive or negative regulation of T-cell immunity in the steady-state, transplantation, microbial diseases, and cancer.
Keywords: Extracellular vesicles, exosomes, cross-dressing, MHC, antigen-presenting cells, dendritic cells
Introduction
Extracellular vesicles (EVs) constitute a mechanism of transfer of proteins, RNAs, lipids and carbohydrates between eukaryotic cells. The increasing family of EVs includes vesicles of different biogenesis, size, composition, and possibly distinct functions (1, 2). Exosomes are nanovesicles (50–120nm) originated as intraluminal vesicles within the endocytic compartment of the cell via inward invagination followed by cleavage of the limiting membrane of late endosomes, which are then termed as multi-vesicular bodies (MVBs) (2, 3). The resulting MVBs either merge with lysosomes following a degradative pathway, or fuse their limiting membrane with the plasma membrane of the parent cell and release its content of intraluminal vesicles (now termed as exosomes) to the extracellular space or bodily fluids (2, 3). Other types of EVs include microvesicles (also known as microparticles) of approximately 200–1000 nm in size that are shed directly from the plasma membrane, and apoptotic cell-derived EVs. Increasing evidence mostly from in vitro and ex vivo models has demonstrated that EVs regulate physiological functions, including innate and adaptive immunity (2, 3). One of the intriguing phenomena mediated by EV transfer between non-immune or immune cells and antigen (Ag)-presenting cells (APCs) is the passage of intact Major Histocompatibility Complex (MHC) molecules pre-loaded with Ag-derived peptides, a phenomenon known as MHC cross-dressing. The biological relevance of MHC cross-dressing is beginning to be elucidated (4).
Transfer of MHC molecules: different names for related phenomena?
Cells, in particular leukocytes, exchange cell surface MHC molecules and other glycoproteins, and intracellular components through mechanisms that have been termed trogocytosis, cell nibbling, or cross-dressing, depending on the cell types involved in the exchange and the experimental model (5–7).
Trogocytosis (from “trogo” meaning “gnaw” in ancient Greek) was originally described as the acquisition by lymphocytes of cognate Ags, which are the peptide : MHC complex (pMHC) for T cells and native Ag for B cells, from APCs or target cells. This phenomenon depends on cell-to-cell contact, is triggered by Ag receptor–signaling, requires immune synapse formation, takes place within minutes after cell conjugate formation, and is carried out by CD4 T, CD8 T, γδ T, NK and B lymphocytes (8–10). By this mechanism, CD4 and CD8 T cells acquire respectively MHC class -I (MHC-I) and class-II (MHC-II) molecules from APCs or target cells in an Ag-specific fashion (8, 9, 11, 12). Passage of pMHCs to T cells has been shown to be mediated via transfer of EVs with characteristics of exosomes (13–16). During Ag-presentation, engagement of pMHC-II on APCs by the T-cell receptor (TcR) on Ag-specific CD4 T cells promotes release of exosomes by the APCs (17). Only activated T cells capture efficiently exogenous exosomes released by dendritic cells (DCs) (16). This process is mediated via binding of the adhesion molecule CD54 on the EVs to high affinity state CD11a/CD18 on activated T cells (16). CD4 T cells also release TCR-enriched EVs from the plasma membrane to the center of the immunological synapse (18). Binding of these TCR-enriched EVs to pMHC-II on B cells increases cytosolic calcium concentration in B cells, which suggests that recognition followed by internalization of TCR-enriched EVs may be one of the mechanisms by which CD4 T cells provide cognate help to B cells (18).
Trogocytosis is not the passage of exclusively those molecules that trigger the phenomenon, but of other accompanying membrane and intracellular material contained in the package transferred form the APC to the T cell, including other MHC and T-cell costimulatory molecules (9, 19). Indeed, CD8 T cells that trigger trogocytosis through recognition of pMHC-I on APCs, also acquire non-specifically pMHC-II (20). Acquisition of pMHCs by T cells could limit clonal T-cell expansion by fratricide killing (i.e. killing by T cells specific for the transferred pMHCs) or by promoting anergy. Unlike human CD4 T cells, mouse CD4 T cells do not express MHC-II upon activation. Presentation by mouse activated CD4 T cells of acquired pMHC-II triggers apoptosis in other activated CD4 T cells in a cognate fashion, a phenomenon that could participate in the resolution of the T-cell response, and induction/maintenance of T-cell peripheral tolerance (11, 13, 21). Similarly, mouse CD8 T cells become targets of cytotoxic T cells after acquiring pMHC-I from APCs, and receive cognate help from CD4 T cells following acquisition of pMHC-II (8, 22). In other in vitro models, human CD4 T cells with acquired pMHC-II, CD80 and CD86 from allogeneic DCs have been shown to function as APCs, by maintaining proliferation of activated CD4 T cells and triggering cell division of resting CD4 T cells (19). Trogocytosis not only occurs during interaction of naïve T cells with APCs in secondary lymphoid organs (SLOs), but also during contact of effector T lymphocytes with target cells in peripheral tissues (23).
The term trogocytosis is currently applied more broadly, to intercellular transfer triggered also by non-Ag receptors (20, 24), and to acquisition of material from living cells by leukocytes other than lymphocytes (7). Although the biological relevance of trogocytosis is still matter of debate, its principles have been applied to the development of analytic tools. Analysis by flow cytometry of the ability of lymphocytes to capture by trogocytosis fragments of APCs surface labeled with lipophilic dyes or biotin and bearing cognate Ags has been used as an alternative to tetramer/multimer labeling to detect Ag-specific T lymphocytes (25). Transfer of membrane fragments between tumor cells pre-labeled with lipophilic dyes and tumor-specific CD8 T cells has been employed to characterize melanoma-specific CD8 T clones (26–29).
The term cell nibbling was introduced to define the uptake of plasma membrane and intracellular components by DCs from other types of living leukocytes through a mechanism(s) that is temperature -, cytoskeleton -, scavenger receptor-, and intercellular contact–dependent (5, 30). Tumor Ag transferred via cell nibbling is internalized, processed, and cross-presented by the acceptor DCs to CD8 T cells (5).
MHC cross-dressing, a term coined by Yewdell and Haeryfar, refers to the acquisition by leukocytes, in particular APCs, of foreign (i.e. not endogenously synthesized by the acceptor cell) intact MHC molecules loaded with peptides (31). The transferred pMHCs must remain on the surface of the acceptor APC with the right topology, at sufficient concentration within localized domains of the plasma membrane, and for long enough to be presented directly without further Ag processing to cognate T cells. DC cross-dressing with pMHCs is dependent on energy, temperature, and cell-to-cell contact (6, 32, 33). The transferred pMHCs can be provided by cells from the same individual or not (i.e. allo- or xenogeneic MHC from grafts).
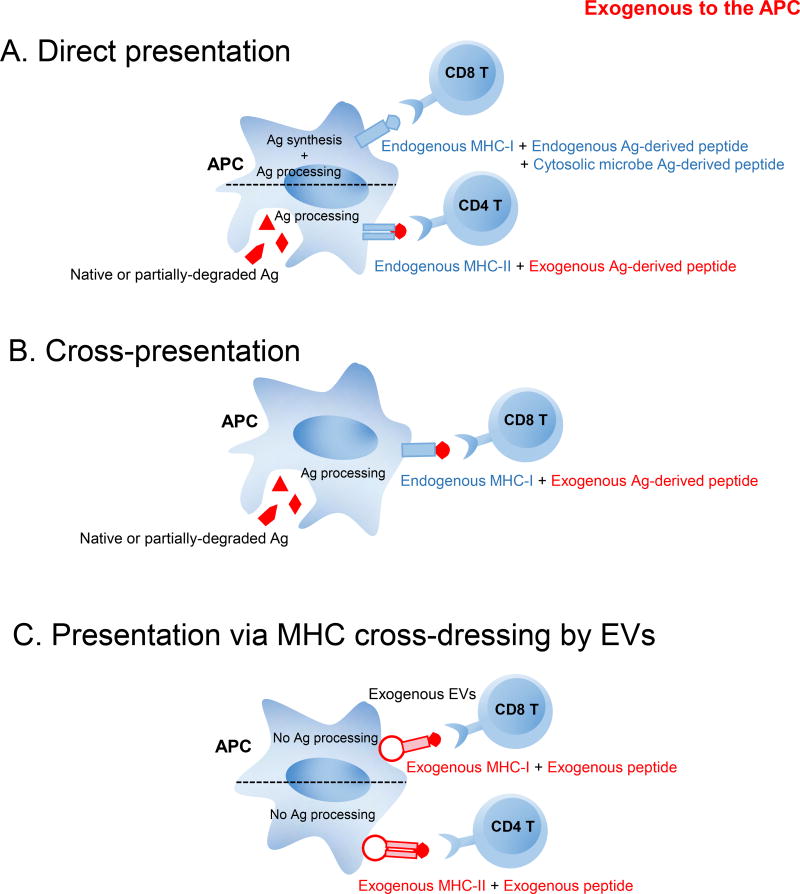
MHC cross-dressing is an alternative mode of Ag presentation that, unlike direct presentation and cross-presentation, eliminates the need for Ag processing by the acceptor APC, since the latter acquires preformed pMHCs. By contrast, through direct presentation CD8 T cells recognize on APCs endogenous MHC-I loaded with peptides derived from endogenous proteins synthesized by the APCs or from proteins of microbes that replicate in the cytosol or continuous nuclear compartment; and CD4 T cells recognize on the APCs endogenous MHC-II loaded with peptides generated by processing of internalized Ags (Figure 1). Alternatively, via cross-presentation, CD8 T cells recognize on the APCs endogenous MHC-I loaded with peptides resulting from processing of internalized Ags (Figure 1) (34). For CD8 T-cell activation, Ag presentation via cross-dressing is more efficient than cross-priming for low levels of soluble Ags (33). T-cell cognate activation via direct presentation, cross-presentation, and presentation by pMHC cross-dressed APCs may differ depending on the APC subset, the type of Ag and its concentration. It is likely that a combination of these complementary ways of Ag-presentation be required for optimal T-cell activation (33).
Fig. 1.
Mechanisms of presentation of pMHCs by APCs to cognate T cells include direct presentation, cross-presentation, and presentation via MHC cross-dressed APCs. A) Through direct presentation, APCs present endogenous MHC-II molecules loaded with peptides resulting from processing of internalized Ags to CD4 T cells; or endogenous MHC-I molecules loaded with peptides derived from endogenous proteins synthesized by the APCs or from proteins of microbes that replicate in the cytosol (i.e. from virally-infected cells, tumor cells) to CD8 T cells. B) APCs cross-present to CD8 T cells endogenous MHC-I molecules loaded with peptides resulting from processing of internalized Ags. C) APCs cross-dressed with exogenous EVs carrying pMHCs on the vesicle surface present the pre-formed pMHC-I and pMHC-II to CD8 T cells and CD4 T cells, respectively, without further Ag-processing of the acquired pMHCs by the APCs.
It still unknown whether transfer of pMHCs via trogocytosis, cell nibbling, and cross-dressing represent related phenomena. Different mechanisms have been proposed for the transfer of surface MHC molecules and other cell surface and intra-cellular components between cells. They include passage of membrane patches uprooted from the plasma membrane of the donor cell, membrane bridges or nanotubes connecting transiently donor and acceptor cells, and transfer of EVs from donor to acceptor cells (35–38).
Role of EVs in MHC cross-dressing of APCs
The seminal observation by Raposo et al that exosomes released by B-cell lines carry surface pMHC-II and stimulate Ag-specific CD4 T-cell clones and hybrids in vitro, led to the idea that free EVs could function as Ag-presenting vesicles (39). Subsequent studies confirmed that exosomes released by DCs and B cells express surface pMHC-I, pMHC-II, CD86, and adhesion molecules, and that APC-derived exosomes, at relatively high concentrations, activate T-cell clones, lines and hybrids, and already primed T cells in vitro (16, 17, 40–42). The amount of pMHCs, CD86 and CD54 is higher on exosomes released by activated DCs (i.e. mature exosomes) than that on exosomes secreted by immature DCs (i.e. immature exosomes) (40, 42, 43). However, the T-cell stimulatory ability of APC-derived exosomes is 10–20 lower than that of the parent APCs as compared at equivalent ratios, and the capacity of free exosomes to prime naïve T cells is rather weak, likely because naïve T cells require higher levels of TcR cross-linking and co-stimulation than that of T-cell clones or effector/memory T cells (17, 39, 44, 45). The poor stimulatory capacity of free exosomes for naïve T cells may be also caused by vesicle dispersion by Brownian motion. In fact, APC-derived free exosomes increase their T-cell stimulatory ability in vitro when the EVs are immobilized at high density on latex beads, or when the density of pMHCs per EV is augmented by direct peptide loading of the vesicles (43, 44, 46). In the absence of APCs, DC-derived exosomes are much less T-cell stimulatory than membrane vesicles prepared from DC sonicates (47).
Interestingly, the T-cell stimulatory ability of APC-derived exosomes, including for naïve T cells, increases substantially when the EVs are bound to DCs, a phenomenon that may explain the biological relevance of MHC cross-dressing of APCs (40, 42, 44, 45, 48–52). Ligands present on the exosome surface including milk fat globule E8 (i.e. lactadherin), tetraspanins, externalized phosphatidylserine, C-type lectins, and CD54 bind directly or indirectly, through bridging molecules, to receptors on target APCs (49, 53, 54). Once the exogenous exosomes are bound to DCs, a percentage of the EVs remains on the APC surface and the rest is internalized for Ag-processing (42, 53, 55, 56). Mature DCs retain most of the acquired exosomes on the cell surface, whereas immature DCs internalize the EVs more efficiently (42). The internalized exosomes can transfer Ag-peptides bound to their MHC-II to MHC-II molecules expressed by the acceptor DCs (42, 50). Alternatively, the pMHCs carried on the exogenous EVs that are retained on the surface of cross-dressed DCs can be presented directly, without further processing, to cognate T cells (42, 45, 54). In MHC cross-dressed DCs, the exogenous EVs provide the pMHCs whereas the acceptor APCs provide the required T-cell costimulatory molecules (45). Several variables control the efficiency of MHC cross-dressing of APCs through EVs, including the stage of maturation of both the APCs that release the exosomes (i.e. immature vs. mature exosomes) and the acceptor APCs, with maximal T-cell stimulatory capacity when mature exosomes are transferred to mature DCs (42, 45). Thus, the acceptor APCs function as platforms that amplify the ability of the acquired exosomes to present their pMHCs directly to T cells.
MHC cross-dressing of APCs in immune homeostasis
T-cell central tolerance is mediated in the thymus via clonal deletion of auto-reactive thymocytes, and production of FoxP3+ CD4+ natural regulatory T cells. Medullary thymic epithelial cells (TECs) and thymic DCs are the main APCs in charge of central tolerance. Medullary TECs, unlike thymic DCs, express ectopically many peripheral tissue-restricted Ags (PTAs) driven by the transcription factor autoimmune regulator known as AIRE. Interestingly, only 1–3 % of medullary TECs express a determined PTA, and medullary TECs are not as efficient as thymic DCs at deleting auto-reactive thymocytes (57, 58). This leads to the question of how such low percentages of medullary TECs bearing a given PTA are sufficient to trigger deletion of auto-reactive T cells and promote differentiation of self-reactive natural CD4 Treg so efficiently. A possibility is that self-Ags, pre-formed self-pMHCs, or both, are spread from medullary TECs to neighboring TECs or thymic DCs, a phenomenon that would increase the pool of APCs able to present self pMHCs to the developing thymocytes. Indeed, studies in bone marrow (BM) chimeric mice demonstrated that TECs transfer pMHCs to other TECs and thymic DCs (59). In mice, thymic CD8α+ and SIRPα+ DCs, but not plasmacytoid DCs, acquire avidly pMHC-I and pMHC-II from TECs (60). Transfer of pMHCs requires cell-to-cell contact and correlates with expression by the acceptor thymic DCs of EpCAM, an adhesion molecule that mediates homophilic interactions between TECs and thymic DCs (60, 61). To our knowledge, whether thymic DCs become cross-dressed with pMHCs through acquisition of EVs has not been formally demonstrated.
Thus, the role of medullary TECs in T-cell central tolerance is to some extent mediated indirectly through thymic DCs by means of two mechanisms: (i) transfer to thymic DCs of pre-processed self pMHCs; and (ii) internalization by thymic DCs of native or partially degraded self-Ags released by TECs, for their processing and presentation in MHC-II or cross-presentation in MHC-I (57, 59, 61, 62). In addition, self-Ags from periphery reach the thymus through capture by thymic DCs of soluble Ags transported via blood, or migration to the thymus of peripheral SIRPα+ conventional DCs (cDCs) and plasmacytoid DCs (63–67).
Auto-reactive T cells that evade thymic selection must be maintained in check through peripheral tolerance. Under steady-state, cDCs transfer pMHC-II to lymph node stromal cells (LNSCs) - which include lymphatic and blood endothelial cells and fibroblastic reticular cells-, by a cell contact-dependent mechanism in which passage of EVs is likely involved (68). LNSCs cross-dressed with pMHC-II impair proliferation of Ag-specific CD4 T cells (68). Since LNSCs are strategically located to interact with T cells, lack co-stimulatory molecules, and express co-inhibitory molecules, presentation of acquired pMHC-II by cross-dressed LNSCs to CD4 T cells may be a mechanism to maintain peripheral tolerance in SLOs (68).
Donor MHC cross-dressing of recipient APCs in transplantation
(a) The 3-cell model
In the absence of immunosuppression, allografts are rapidly recognized as non-self by B and T lymphocytes of the recipient. Alloreactive B cells recognize polymorphic domains on the surface of donor (non-self) intact MHC molecules. Alloreactive T cells detect donor Ag through two distinct mechanisms: the direct and indirect pathways of allorecognition. Through the direct pathway, host T cells recognize donor intact MHC molecules on the surface of donor cells (Figure 2). Via the indirect pathway, recipient T cells recognize donor-derived peptides originated in polymorphic regions of allogeneic MHC molecules or non-MHC proteins (i.e. minor histocompatibility Ags), presented within recipient MHC molecules by recipient APCs (Figure 2).
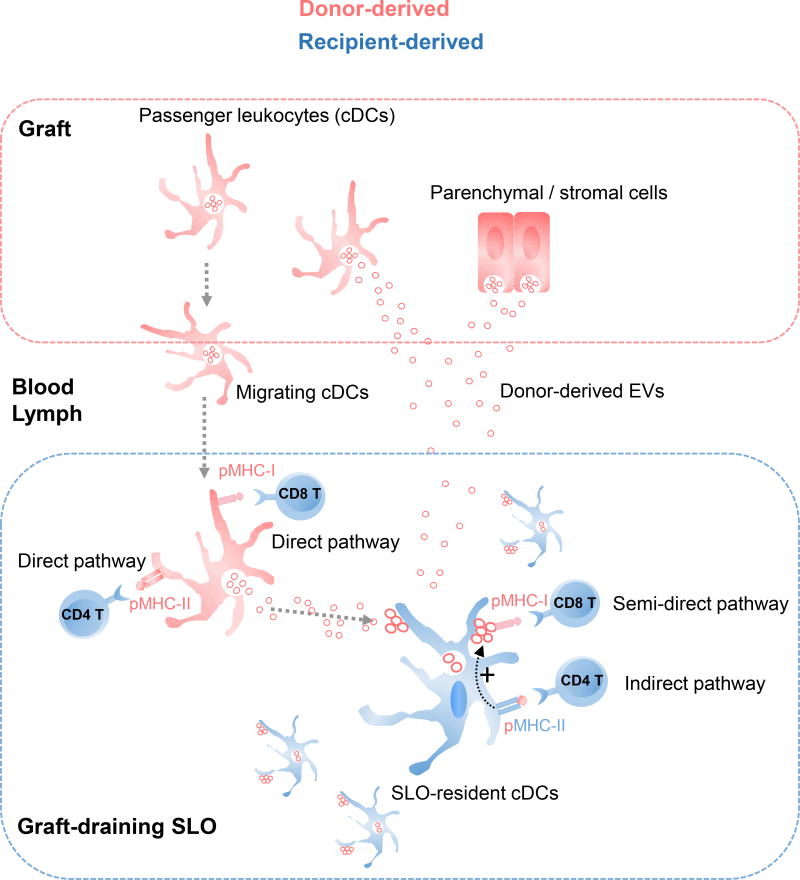
Fig. 2.
Donor MHC cross-dressing of recipient APCs in transplantation. After transplantation of skin or heart allografts in mice, recipient APCs (mainly cDCs) resident in graft-draining SLOs become cross-dressed with donor intact MHC molecules, which are acquired via capture of clusters of donor-derived EVs with characteristic of exosomes that carry donor intact MHC molecules on the vesicle surface. The donor-derived EVs are released directly by cells from the grafts or by the relatively few donor migrating DCs that reach the graft-draining SLOs. The 3-cell model predicts that individual recipient cDCs cross-dressed with donor-derived EVs present: (i) host MHC-II molecules loaded with donor allo-peptides to CD4 T cells, through the indirect pathway (i.e. canonical pathway of Ag-presentation); and (ii) donor intact MHC molecules on the surface of the attached EVs to directly alloreactive CD8 T cells, via the semi-direct pathway. The indirect pathway CD4 T cells promotes maturation of the MHC cross-dressed recipient cDCs, which then stimulate the direct pathway CD8 T cells.
The direct pathway is characterized by the high frequency of T cells recognizing donor intact MHC molecules (1 to 10% of the T cell population), since directly alloreactive T cells are not selected in the recipient thymus against or in favor of a given allogeneic intact MHC molecule. The indirect pathway is basically the canonical mechanism by which antigenic peptides are presented to T cells via self MHC molecules by self APCs, since allogeneic or semi-allogeneic cells are only present following allograft transplantation or during pregnancy. Thus, the frequency of indirectly alloreactive T cells against a given allopeptide is extremely low and similar to that against any nominal peptide (< 1/100,000). Depending on the experimental model, either directly or indirectly alloreactive T cells can trigger by themselves acute allograft rejection. The indirect CD4 T cells also provide the T-B cell-help required for allo-antibody production and participate in chronic rejection.
Accumulated evidence in transplantation models has shown that indirect pathway CD4 T cells regulate positively or negatively the function of directly alloreactive T cells (69, 70). In 2004, Lechler’s group proposed the 3-cell model to explain how indirect pathway CD4 T cells cross-regulate the function of directly alloreactive CD8 T cells on the same recipient APC that acts as a bridge between indirect pathway T cells and directly alloreactive T cells (6). In this model, indirect pathway CD4 T cells interact and regulate the APC-function of recipient APCs that present, simultaneously or sequentially, donor intact MHC molecules –acquired from donor cells- to direct pathway T cells (Figure 2). The recognition by directly alloreactive T cells of donor intact MHC molecules on the surface of recipient APCs cross-dressed with donor intact MHC molecules has been termed as the semi-direct pathway of allorecognition (Figure 2) (6).
In recent years, the 3-cell model and the existence of the semi-direct pathway in vivo have been confirmed in experimental models. Following transplantation of heart, kidney, pancreatic islet, or skin allografts in mice, donor intact MHC molecules have been detected on recipient DCs, and to a lesser extent on macrophages and B cells, within graft-draining SLOs (38, 71, 72). The recipient APCs cross-dressed with donor MHC molecules also co-expressed self MHC-II molecules loaded with donor allopeptides, which can be presented to indirect pathway CD4 T cells (73). Elegant experiments by Pettigrew’s group have demonstrated that DCs isolated from spleens of mice grafted with heart allografts carry donor MHC-I and when transferred into naïve hosts of the original recipient strain, prime directly alloreactive CD8 T cells (74). Importantly, the phenomenon was dependent on licensing of the recipient APCs by indirect pathway CD4 T cells, since direct CD8 T cell priming did not occur when the transferred recipient DCs cross-dressed with donor MHC-I were deficient in endogenous MHC-II (74). Interestingly, differentiation in graft-draining SLOs of direct pathway CD8 T cells through the semi-direct pathway also occurred after transplantation of heart allografts devoid of passenger leukocytes, which suggests that is dependent on shedding of cell-free donor MHC molecules from the parenchymal cells of the grafts to the draining SLOs (75). Once there, the recipient APCs processed the internalized donor Ag for presentation as donor-derived allopeptides to indirect CD4 T cells, and become cross-dressed with donor pMHC-I for its presentation to directly alloreactive CD8 T cells via the semidirect pathway (75). Although still unknown, it is likely that different types of parenchymal and stromal cells (migratory or not) from the allografts are the source of the cell-free donor pMHCs transferred to the recipient APCs. In co-cultures of mouse cells, DCs, endothelial and epithelial cells are capable of transferring allogeneic MHC molecules to DCs (6, 76). Acquisition by DCs of pMHC-I in sufficient quantities to prime CD8 T cells even occurs under conditions in which the number of pMHCs is limited on the donor cells (76). Acceptor DCs cross-dressed with pMHC-I are three orders of magnitude less efficient than the donor DCs at eliciting naïve or memory CD8 T-cell division (52). In mice, splenic CD8αpos and CD8αneg cDCs are both capable of acquiring pMHCs via cross-dressing, and CD8αneg cDCs are superior than CD8αpos cDCs at Ag-presentation to CD8 T cells through cross-dressing (52, 76).
One of the remaining questions in the 3-cell model is why recipient APCs cross-dressed with donor pMHC-I must be licensed by indirect pathway CD4 T cells, instead of directly allo-reactive CD4 T cells, to prime directly alloreactive CD8 T cells via the semi-direct pathway (Figure 2). This is particularly intriguing considering that recipient APCs also become cross-dressed with donor pMHC-II and that the frequency of directly alloreactive CD4 T cells is much higher than that of indirect pathway CD4 T cells. For reasons still not well understood, DCs cross-dressed with allogeneic pMHC-II do not seem to prime efficiently directly alloreactive CD4 T cells via the semi-direct pathway (77).
Passage of pMHCs between leukocytes also occurs in a reverse fashion, from recipient to donor cells. After BM transplantation, donor DCs become cross-dressed with recipient MHC-I and MHC-II captured from recipient hematopoietic cells (77, 78). Cross-dressing of donor DCs is transient after BM transplantation following myeloablative regimens, but is persistent after non-myeloablative conditioning, in which recipient hematopoietic cells remain in elevated numbers (77). Importantly, donor DCs cross-dressed in vivo with recipient pMHCs primed ex vivo CD8 T cells against the transferred host pMHCs (78). MHC cross-dressing from recipient cells to donor APCs may be relevant at later stages after BM or stem cell transplantation, when host APCs are replaced by donor APCs that via cross-dressing continue stimulating the donor-derived pathogenic CD8 T cells against the recipient pMHCs that cause graft-versus-host disease (78).
Although MHC cross-dressing of recipient APCs, the semi-direct pathway, and the 3-cell model provide satisfactory explanations for several unexplained phenomena in transplant immunology, until recently there was not clear understanding of how donor MHC molecules are transferred intact from donor to recipient cells in vivo. Recent evidence has demonstrated that recipient APC cross-dressing with donor MHC molecules after transplantation occurs through capture of donor-derived EVs.
(b) Donor EVs mediate donor MHC cross-dressing of recipient APCs after transplantation
The ultrastructural basis behind APC cross-dressing with donor MHC molecules after transplantation remained a mystery until recently. Analysis by immuno-electron microscopy demonstrated that after transplantation of cardiac allografts in mice, recipient cDCs acquire donor MHC molecules in graft-draining SLOs by capturing clusters of donor-derived EVs of the size of exosomes and carrying the exosome-associated marker CD63 (38). The donor EVs remain bound to the surface of recipient DCs or are internalized, but do not fuse with the plasma membrane of the acceptor DCs (38). The donor EVs can be released by donor passenger leukocytes mobilized to the graft-draining SLOs, by the graft itself, or both (Figure 2) (38, 42, 72). Passage of allogeneic MHC molecules by EV transfer between cDCs is detectable up to a ratio of 1 donor to 32 acceptor cDCs, and is reduced by inhibitors of exosome secretion, but not by blockers of other types of EVs (38). Different studies have shown that mature DCs transfer more pMHCs than immature DCs, and that passage of pMHCs is severely affected when donor and acceptor cells are separated by 0.4-µm pore transwells (6, 38, 52, 74). The latter agrees with the finding that the pMHCs are transferred via clusters of EVs that due to their hydrodynamic size (i.e. Stokes radius) and Brownian motion do not cross efficiently the filter pores. It may also indicate that contact between donor and acceptor cells is necessary for release or intercellular transfer of EVs. Indeed, clusters of donor-derived EVs are released into the gap between donor and acceptor DCs that are in close apposition (38). Analysis by confocal microscopy has shown that donor-derived red fluorescent protein (RFP)-tagged EVs transferred to recipient cDCs in SLOs, remain on the surface of the recipient APCs for up to at least 4 hours, which is a requirement for recognition of the transferred pMHCs by directly alloreactive T cells (38). Acquisition of exosomes released by allogeneic mature DCs (i.e. mature exosomes), unlike other types of EVs, promotes maturation and T-cell allostimulatory ability of recipient cDCs (38). Accordingly, flow cytometry-sorted cDCs cross-dressed with donor-derived EVs prime directly alloreactive naïve CD8 T cells and indirect pathway naïve CD4 T cells, as predicted by the 3-cell model (38). In agreement with the hypothesis that recipient APCs cross-dressed with donor pMHCs are the main initiators of direct T-cell allorecognition in the graft-draining SLOs, depletion of recipient cDCs in vivo reduces drastically priming of directly alloreactive T cells and delays acute rejection of cardiac allografts in mice (38, 75). Based on in vivo models using exosomes loaded with intact ovalbumin (OVA), the donor intact MHC molecules carried on the surface of donor-derived EVs should be also accessible for recognition by anti-donor B cells, which subsequently provide help for indirect pathway CD4 T cells (43, 79).
Cross-dressing of recipient APCs with donor MHC not only elicits allorecognition in vivo, but also in the mixed leukocyte reaction (MLR), a classic assay considered the in vitro allograft reaction. In the MLR a substantial component of the response is generated via presentation of pMHCs acquired from stimulatory APCs by the APCs of responder origin that are present in low percentages along with the responding T cells (80, 81).
(c) Donor-derived EVs spread donor Ag after transplantation
Increasing evidence indicates that release of donor-derived EVs that traffic passively to the graft-draining SLOs, instead of active migration of donor passenger leukocytes, triggers early activation of T cells by recipient APCs cross-dressed with donor-derived EVs (Figure 2) (72). It has been classically assumed that passenger leukocytes transplanted with the grafts migrate to the draining SLOs early after surgery, where they present by themselves donor Ag to directly alloreactive T cells (82). However, following transplantation of heart or pancreatic islets allografts in untreated mice, relatively few donor passenger leukocytes reach the graft-draining SLOs during the first week after transplantation, a critical period when T-cell allosensitization occurs (38, 72, 83). In the heart transplant model, a relative small percentage of the passenger leukocytes reach the spleen through peripheral blood (38, 72). By contrast, donor passenger leukocytes are undetectable by flow cytometric analysis, ImageStream technology, and RT-PCR in graft-draining SLOs up to 15 days after transplantation of fully-mismatch skin allografts in mice (38, 72). T-cell allo-sensitization against skin (non-vascularized) allografts occurs through the dermal lymphatic vessels, which are sectioned during surgery and do not fully reconnect until 5 to 7 days after transplantation (84). Thus, although donor passenger leukocytes are unable to migrate from the skin graft to the draining lymph nodes during that period, T-cell allo-sensitization against skin allografts is maximal between 4–6 days after surgery (85). A possible solution to that problem is that donor-derived EVs, instead of leukocytes, released by the allografts into the gap between the transplanted skin and the recipient dermis, enter into the open endings of the severed lymphatic vessels of the recipient dermis and traffic passively via lymph to the draining lymph nodes (72). Unlike blood vessels, lymphatics ends remain open for several days after being sectioned (86). The donor-derived EVs can be released by graft parenchymal cells, endothelium, or donor passenger leukocytes trapped on their way out of the graft. The exosome surface bears glycoproteins decorated with linked sialic acid that bind to sialoadehsin (i.e. CD169) expressed on specialized macrophages in the subcapsular sinus of lymph nodes and in the marginal zone of the spleen (87). Once inside the graft-draining SLOs, the donor EVs can be internalized and processed for presentation as allo-peptides via the indirect pathway, or cross-dress the surface of recipient APCs for presentation of donor intact MHC molecules through the semi-direct pathway. Dissemination of donor pMHCs within graft-draining SLOs through donor EVs seems to be more efficient than via donor passenger leukocytes (Figure 2). Indeed, after transplantation of heart allografts in mice, the quantity of recipient APCs cross-dressed with donor EVs was 100-fold higher than the number of passenger leukocytes detected at the same time in the graft-draining SLOs (38).
In mice, cross-dressing of recipients cDCs in graft-draining SLOs with donor MHC-I released by graft cells occurs throughout the life span of skin transplants, a phenomenon that is enhanced by graft inflammation (88). Since donor passenger DCs are rapidly eliminated after transplantation, this latter mechanism could maintain for long-term the response of directly allo-reactive CD8 T cells against allografts.
Donor MHC cross-dressing of recipient APCs also takes place within grafts. Following cardiac transplantation in mice, the donor passenger DCs transplanted with the grafts are rapidly replaced by recipient DCs stemmed from non-classical monocytes from the blood (89). The graft-infiltrating DCs cross-dressed with donor pMHC-I trigger proliferation of directly allo-reactive CD8 T cells ex vivo (89). Thus, graft-infiltrating DCs cross-dressed with donor pMHC-I amplify the response of directly allo-reactive CD8 T cells inside the allografts. Donor MHC cross-dressing of graft-infiltrating DCs has also been described during allograft tolerance, a phenomenon that occurs spontaneously after transplantation of liver allografts in mice. Ono et al have shown that recipient DCs that infiltrate hepatic allografts and become cross-dressed with donor MHC molecules express higher levels of program death-ligand 1 (PDL-1) and interleukin (IL)-10 than non-cross-dressed recipient DCs (90). The cross-dressed DCs exhibited impaired T-cell allostimulatory capacity compared to the non-cross-dressed DCs, and suppressed host T-cell proliferation against donor allo-Ags (90).
Dissemination of allo-Ags via MHC cross-dressing through EVs also occurs during maternal micro-chimerism in which non-(genetically) inherited maternal Ags (NIMAs) are transferred from maternal chimeric cells to the offspring DCs (91). Burlingham’s group has demonstrated in mice that offspring DCs cross-dressed with EVs carrying maternal MHC molecules and CD86 activate directly allo-reactive CD4 T cells against NIMAs (91). As a consequence of the maternal EV passage, the acceptor DCs upregulated expression of PD-L1 uniformly on the cell membrane and induced PD-L1-dependent abortive proliferation of indirect pathway CD4 T cells against NIMA-derived allo-peptides presented by host (offspring) MHC molecules (91). These findings explain the association between maternal microchimerism and split tolerance, a phenomenon in which directly alloreactive CD4 T cells against NIMAs can be stimulated, while CD4 T cells responding to NIMA-derived allopeptides in a self MHC-restricted fashion are silenced.
MHC cross-dressing of APCs in infectious diseases
Intercellular passage of preformed pMHCs through EVs, directly from non-migratory cells of peripheral tissues or from few migrating DCs, to a relatively high number of APCs resident in draining SLOs could also amplify T-cell activation following infections, allergies, or vaccinations. CD8 T-cell priming against microbial Ags occurs when the T cells recognize pathogen pMHCs on the surface of infected APCs. However, for those pathogens that do not infect or replicate poorly in APCs, CD8 T cells can still be primed by non-infected APCs that have acquired exogenous Ags from infected cells, via internalization cell fragments derived from live or dying infected cells. The endocytosed pathogen Ags are then processed by the acceptor APCs for cross-presentation within endogenous MHC-I to CD8 T cells (Figure 1).
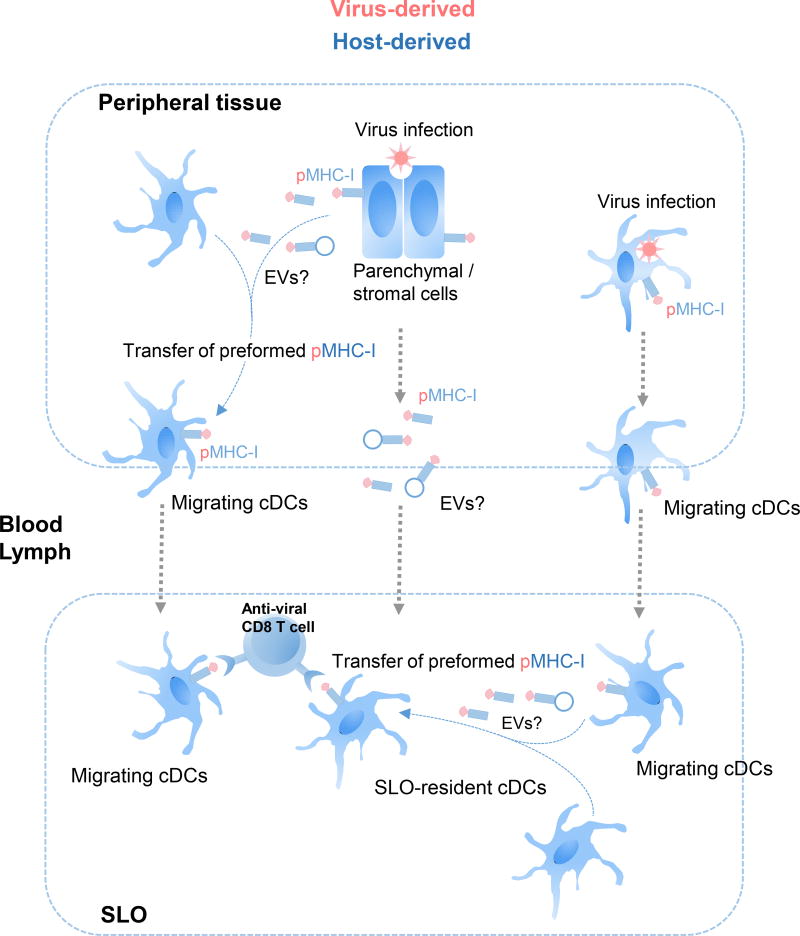
Besides direct presentation and cross-presentation, CD8 T cells can also recognize pathogen pMHC-I transferred via cross-dressing to APCs. In this scenario, peripheral tissue-resident DCs acquire pre-formed pathogen pMHC-I from infected parenchymal cells, before trafficking to SLOs (Figure 3). Consistent with this idea, DCs acquire pMHC-I from epithelial cells in vitro (76). Alternatively, migrating DCs mobilized from infected tissues can transfer pre-formed pathogen pMHC-I to APCs resident in SLOs (Figure 3) (92). This phenomenon could be relevant for those APCs poorly equipped for cross-priming, such as mouse CD8αneg cDCs in SLOs, which have been shown to prime CD8 T cells effectively following acquisition of pMHC-I (76, 93). Thus, passage of pathogen pMHC-I to APCs might overcome poor direct presentation and cross-presentation to CD8 T cells during microbial infections, an idea that is supported by increasing experimental evidence (92, 93). In this regard, mouse splenic cDCs that acquired pre-formed pMHC-I from adoptively transferred DCs previously infected with recombinant adenovirus, elicit CD8 T-cell immunity against virus-encoded Ags (93). Human monocyte-derived DCs cross-dressed with influenza pMHC-I stimulate Ag-specific CD8 T cells to release IFN-γ in vitro (92). In vivo in mice, cross-dressed cDCs drive activation of memory CD8 T cells against the model Ag OVA encoded in recombinant vesicular stomatitis virus, without increasing the numbers of naïve CD8 T cells against OVA (52). In that model, the distinct responses of naïve versus memory CD8 T cells may be explained by their different requirements in epitope density on the cross-dressed APCs, which likely bear the transferred pMHCs at a density lower than the required to trigger activation of naïve CD8 T cells. Indeed, it has been shown in other mouse models that cDCs cross-dressed with pMHC-I stimulate both naïve and memory CD8 T cells (94).
Fig. 3.
Pathways of MHC-I cross-dressing of APCs during viral infections. For viruses that do not infect or infect poorly APCs, virus-derived pMHC-I can be transferred from peripheral tissue parenchymal or stromal cells to tissue-resident cDCs before cDC migration to SLOs, where the pMHC-I cross-dressed migrating cDCs prime anti-viral cytotoxic CD8 T cells. Alternatively, tissue-resident cDCs that become infected with virus in periphery mobilize to SLOs and transfer virus-derived pMHC-I to SLO-resident cDCs, which initiate the anti-viral CD8 T cell response. Alternatively, virus-derived pMHC-I could be transferred via a cell-free mechanism, likely mediated through EVs, to SLO-resident APCs.
The way the EVs bearing pMHCs are acquired by the acceptor APCs may be relevant for the efficiency of MHC cross-dressing. In vitro, EVs added exogenously to the culture medium bind as individual vesicles throughout the surface of the APCs whereas in vivo, EVs are transferred in clusters to localized spots on the acceptor APC within the area of contact between donor and acceptor cells (38, 95). In support of this idea, mouse DCs supplemented in vitro with free exosomes carrying lymphocyte choriomeningitis virus (LCMV) pMHC-I were unable to stimulate, via cross-priming or MHC-I cross-dressing, naïve or memory CD8 T cells against LCMV (96).
Unlike what has been classically assumed, DCs mobilized from skin infected with herpes simplex virus 1 (HSV) do not stimulate by themselves HSV-specific cytotoxic T cells in lymph nodes (97, 98). Rather, priming of HSV-specific cytotoxic T cells requires of lymph node-resident CD8α+ DCs (97, 98). In this model, passage between the skin migrating DCs and lymph node CD8α+ DCs, of EVs carrying native HSV Ag (to be processed for cross-priming) or preformed HSV pMHC-I (to be used for cross-dressing) have been suggested as potential mechanisms (54, 98).
Group 2 innate lymphoid cells (ILC2s) support type-2 biased responses and release IL-13 that promote protective immunity against helminth infections, or detrimental effects in asthma and allergies. ILC2s bear endogenous MHC-II, but also acquire exogenous MHC-II via cross-dressing (99). ILC2s also express co-stimulatory molecules, which allows ILC2s to present pMHC-II to naïve CD4 T cells (99). As a result of the ILC2s : CD4 T cell crosstalk, the ILC2s stimulate proliferation and Th2-bias of the CD4 T cells through T-cell derived IL-2, a cytokine that induces proliferation and IL-13 secretion by ILC2s (99).
MHC cross-dressing of APCs in cancer
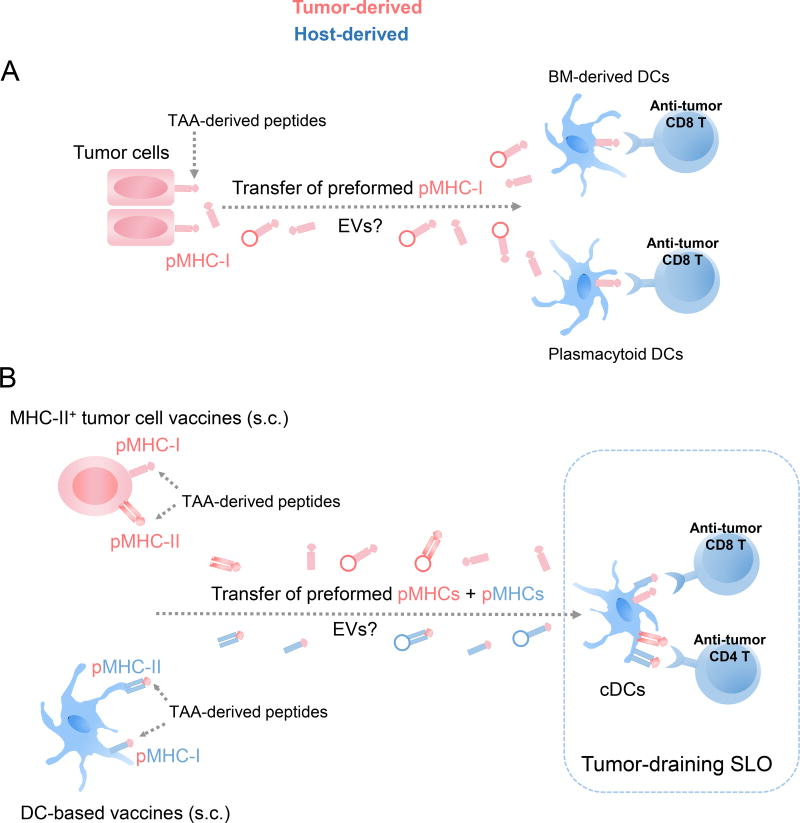
EVs, in particular exosomes, have been used as carriers of native or partially degraded tumor Ag, or tumor-derived peptides for delivery to APCs for cross-priming of anti-tumor CD8 T cells (48, 100–103). There is increasing evidence that tumor cells may also employ EVs to transfer MHC molecules loaded with tumor-derived peptides to APCs (Figure 4). In mice, BM-derived DCs cross-dressed with pMHC-I acquired from cancer cells, primed Ag-specific cytotoxic T cells in vivo (7). Compared to DCs of myeloid lineage, plasmacytoid DCs are much less proficient at engulfing soluble Ags and at processing them for cross-presentation to CD8 T cells. However, plasmacytoid DCs efficiently acquire pMHC-I from tumor cells through a cell-to-cell dependent mechanism that requires CD54 – CD11a/CD18 interaction (104). The transferred pMHC-I remain on the surface of plasmacytod DCs up to 48 hours, and pDCs cross-dressed with tumor pMHC-I stimulate CD8 T-cell clones in vitro (104). Interestingly, tumor associated Ags have been detected on the surface of plasmacytoid DCs isolated from human colorectal carcinomas (104). Thus, cross-dressing of plasmacytoid DCs with tumor pMHC-I may allow plasmacytoid DCs to present tumor Ags to CD8 T cells despite their limited endocytic and cross-presentation capabilities.
Fig. 4.
Acquisition by APCs of MHC molecules loaded with tumor-associated Ag (TAA)-derived peptides released from tumor cells or cell-based anti-tumor vaccines. A) Tumor cells transfer MHC-I molecules loaded with TAA-derived peptides, likely via EVs, to DCs of myeloid lineage and to plasmacytoid DCs, a phenomenon that overcomes the limited capability of plasmacytoid DCs for cross-presentation to CD8 T cells. B) MHC-II+ tumor cell- and DC-based vaccines injected s.c. prime anti-tumor T cells through SLO-resident cDCs cross-dressed with MHC molecules loaded with TAA-derived peptides released, possibly through EVs, by the injected cells.
Passage of preformed pMHCs from tumor cells to APCs also occurs after vaccination with tumor cells (Figure 4). Following s.c. inoculation of mice with tumor cell-based vaccines genetically engineered to express host MHC-II, most of the tumor-specific CD4 T cell response was elicited by host cDCs cross-dressed with pMHC-II acquired from the injected cells (33). In this setting, the tumor pMHC-II that are presented are generated by the vaccine cells. Unlike the cells of MHC-II+ tumor vaccines, the host cDCs express invariant chain (Ii) that skews the selection of peptides presented to those derived mainly from internalized Ags, and HLA-DM molecules that edits the repertoire of peptides as they bind to MHC-II. Thus, host DCs cross-dressed with tumor pMHC-II acquired from MHC-II+ tumor cell vaccines may present to CD4 T cells novel tumor Ag epitopes that are not endogenously generated by the acceptor DCs (33).
In vitro generated DCs loaded with tumor Ag or tumor-derived peptides are used as cellular vaccines against cancer. Surprisingly, following s.c. administration, only a small percentage of the injected DCs (< 1%) home in draining SLOs (105, 106). In mice, passage of pre-formed pMHC complexes from s.c. injected DCs to lymph node-resident DCs has been proposed as a mechanism of amplification of the CD4 T cell response (Figure 4) (107, 108). This phenomenon is of importance regarding the applicability of DC-based vaccines in cancer patients, where the immunostimulatory function of endogenous DCs can be affected by the tumor microenvironment or chemotherapy. Studies in mice have shown that cross-dressing of CD8α+ CD103+ DCs with pMHC-I plays a key role in CD8 T-cell priming following DNA and cellular vaccination (94).
Concluding remarks
During the past 3 decades, advances in the fields of EVs, mechanisms of Ag–presentation, mouse models, and biological imagining allowed scientists to partially unveil the basis of MHC cross-dressing via EVs, and its importance in immune-homeostasis, and the immune response against tumors, pathogens, or allografts. From being considered originally a laboratory curiosity, MHC cross-dressing of APCs via EVs has been shown to be a mechanism of Ag-spreading, alternative mechanism of presentation of pre-formed pMHCs to T cells, and cross-regulation of T cells with different MHC restricted specificities, among other functions. Poor consistency between laboratories in the methods of purification and identification of different types of EVs, difficulties in detection at subcellular level the endogenous EVs by intra-vital microscopy or ultra-structural analysis, and lack of appropriate gain-of-function or loss-of-function mouse models, are some of the main problems that need to be overcome to fully address the relevance of EV-mediated MHC cross-dressing of APCs in the steady-state and disease, and its potential therapeutic applications.
Acknowledgments
Supported by the National Institutes of Health grant R01 HL130191 (to A.E.M.).
Footnotes
Conflict of interest statement: The authors have declared no conflict of interest exists.
References
- 1.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 2.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 3.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakayama M. Antigen Presentation by MHC-Dressed Cells. Front Immunol. 2014;5:672. doi: 10.3389/fimmu.2014.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harshyne LA, Watkins SC, Gambotto A, Barratt-Boyes SM. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J Immunol. 2001;166:3717–23. doi: 10.4049/jimmunol.166.6.3717. [DOI] [PubMed] [Google Scholar]
- 6.Herrera OB, Golshayan D, Tibbott R, Salcido Ochoa F, James MJ, Marelli-Berg FM, Lechler RI. A novel pathway of alloantigen presentation by dendritic cells. J Immunol. 2004;173:4828–37. doi: 10.4049/jimmunol.173.8.4828. [DOI] [PubMed] [Google Scholar]
- 7.Zhang QJ, Li XL, Wang D, Huang XC, Mathis JM, Duan WM, Knight D, Shi R, Glass J, Zhang DQ, Eisenbach L, Jefferies WA. Trogocytosis of MHC-I/peptide complexes derived from tumors and infected cells enhances dendritic cell cross-priming and promotes adaptive T cell responses. PLoS One. 2008;3:e3097. doi: 10.1371/journal.pone.0003097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang JF, Yang Y, Sepulveda H, Shi W, Hwang I, Peterson PA, Jackson MR, Sprent J, Cai Z. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science. 1999;286:952–4. doi: 10.1126/science.286.5441.952. [DOI] [PubMed] [Google Scholar]
- 9.Hudrisier D, Riond J, Mazarguil H, Gairin JE, Joly E. Cutting edge: CTLs rapidly capture membrane fragments from target cells in a TCR signaling-dependent manner. J Immunol. 2001;166:3645–9. doi: 10.4049/jimmunol.166.6.3645. [DOI] [PubMed] [Google Scholar]
- 10.Batista FD, Iber D, Neuberger MS. B cells acquire antigen from target cells after synapse formation. Nature. 2001;411:489–94. doi: 10.1038/35078099. [DOI] [PubMed] [Google Scholar]
- 11.Patel DM, Arnold PY, White GA, Nardella JP, Mannie MD. Class II MHC/peptide complexes are released from APC and are acquired by T cell responders during specific antigen recognition. J Immunol. 1999;163:5201–10. [PubMed] [Google Scholar]
- 12.Wetzel SA, McKeithan TW, Parker DC. Peptide-specific intercellular transfer of MHC class II to CD4+ T cells directly from the immunological synapse upon cellular dissociation. J Immunol. 2005;174:80–9. doi: 10.4049/jimmunol.174.1.80. [DOI] [PubMed] [Google Scholar]
- 13.Arnold PY, Mannie MD. Vesicles bearing MHC class II molecules mediate transfer of antigen from antigen-presenting cells to CD4+ T cells. Eur J Immunol. 1999;29:1363–73. doi: 10.1002/(SICI)1521-4141(199904)29:04<1363::AID-IMMU1363>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Patel DM, Mannie MD. Intercellular exchange of class II major histocompatibility complex/peptide complexes is a conserved process that requires activation of T cells but is constitutive in other types of antigen presenting cell. Cell Immunol. 2001;214:165–72. doi: 10.1006/cimm.2001.1897. [DOI] [PubMed] [Google Scholar]
- 15.Hwang I, Shen X, Sprent J. Direct stimulation of naive T cells by membrane vesicles from antigen-presenting cells: distinct roles for CD54 and B7 molecules. Proc Natl Acad Sci U S A. 2003;100:6670–5. doi: 10.1073/pnas.1131852100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nolte-'t Hoen EN, Buschow SI, Anderton SM, Stoorvogel W, Wauben MH. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009;113:1977–81. doi: 10.1182/blood-2008-08-174094. [DOI] [PubMed] [Google Scholar]
- 17.Muntasell A, Berger AC, Roche PA. T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. EMBO J. 2007;26:4263–72. doi: 10.1038/sj.emboj.7601842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choudhuri K, Llodra J, Roth EW, Tsai J, Gordo S, Wucherpfennig KW, Kam LC, Stokes DL, Dustin ML. Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature. 2014;507:118–23. doi: 10.1038/nature12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Game DS, Rogers NJ, Lechler RI. Acquisition of HLA-DR and costimulatory molecules by T cells from allogeneic antigen presenting cells. Am J Transplant. 2005;5:1614–25. doi: 10.1111/j.1600-6143.2005.00916.x. [DOI] [PubMed] [Google Scholar]
- 20.Hwang I, Huang JF, Kishimoto H, Brunmark A, Peterson PA, Jackson MR, Surh CD, Cai Z, Sprent J. T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J Exp Med. 2000;191:1137–48. doi: 10.1084/jem.191.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsang JY, Chai JG, Lechler R. Antigen presentation by mouse CD4+ T cells involving acquired MHC class II. peptide complexes: another mechanism to limit clonal expansion? Blood. 2003;101:2704–10. doi: 10.1182/blood-2002-04-1230. [DOI] [PubMed] [Google Scholar]
- 22.Romagnoli PA, Premenko-Lanier MF, Loria GD, Altman JD. CD8 T cell memory recall is enhanced by novel direct interactions with CD4 T cells enabled by MHC class II transferred from APCs. PLoS One. 2013;8:e56999. doi: 10.1371/journal.pone.0056999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riond J, Elhmouzi J, Hudrisier D, Gairin JE. Capture of membrane components via trogocytosis occurs in vivo during both dendritic cells and target cells encounter by CD8(+) T cells. Scand J Immunol. 2007;66:441–50. doi: 10.1111/j.1365-3083.2007.01996.x. [DOI] [PubMed] [Google Scholar]
- 24.Hudrisier D, Aucher A, Puaux AL, Bordier C, Joly E. Capture of target cell membrane components via trogocytosis is triggered by a selected set of surface molecules on T or B cells. J Immunol. 2007;178:3637–47. doi: 10.4049/jimmunol.178.6.3637. [DOI] [PubMed] [Google Scholar]
- 25.Daubeuf S, Puaux AL, Joly E, Hudrisier D. A simple trogocytosis-based method to detect, quantify, characterize and purify antigen-specific live lymphocytes by flow cytometry, via their capture of membrane fragments from antigen-presenting cells. Nat Protoc. 2006;1:2536–42. doi: 10.1038/nprot.2006.400. [DOI] [PubMed] [Google Scholar]
- 26.Machlenkin A, Uzana R, Frankenburg S, Eisenberg G, Eisenbach L, Pitcovski J, Gorodetsky R, Nissan A, Peretz T, Lotem M. Capture of tumor cell membranes by trogocytosis facilitates detection and isolation of tumor-specific functional CTLs. Cancer Res. 2008;68:2006–13. doi: 10.1158/0008-5472.CAN-07-3119. [DOI] [PubMed] [Google Scholar]
- 27.Uzana R, Eisenberg G, Sagi Y, Frankenburg S, Merims S, Amariglio N, Yefenof E, Peretz T, Machlenkin A, Lotem M. Trogocytosis is a gateway to characterize functional diversity in melanoma-specific CD8+ T cell clones. J Immunol. 2012;188:632–40. doi: 10.4049/jimmunol.1101429. [DOI] [PubMed] [Google Scholar]
- 28.Eisenberg G, Uzana R, Pato A, Frankenburg S, Merims S, Yefenof E, Ferrone S, Peretz T, Machlenkin A, Lotem M. Imprinting of lymphocytes with melanoma antigens acquired by trogocytosis facilitates identification of tumor-reactive T cells. J Immunol. 2013;190:5856–65. doi: 10.4049/jimmunol.1202879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uzana R, Eisenberg G, Merims S, Frankenburg S, Pato A, Yefenof E, Engelstein R, Peretz T, Machlenkin A, Lotem M. Human T cell crosstalk is induced by tumor membrane transfer. PLoS One. 2015;10:e0118244. doi: 10.1371/journal.pone.0118244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harshyne LA, Zimmer MI, Watkins SC, Barratt-Boyes SM. A role for class A scavenger receptor in dendritic cell nibbling from live cells. J Immunol. 2003;170:2302–9. doi: 10.4049/jimmunol.170.5.2302. [DOI] [PubMed] [Google Scholar]
- 31.Yewdell JW, Haeryfar SM. Understanding presentation of viral antigens to CD8+ T cells in vivo: the key to rational vaccine design. Annu Rev Immunol. 2005;23:651–82. doi: 10.1146/annurev.immunol.23.021704.115702. [DOI] [PubMed] [Google Scholar]
- 32.Russo V, Zhou D, Sartirana C, Rovere P, Villa A, Rossini S, Traversari C, Bordignon C. Acquisition of intact allogeneic human leukocyte antigen molecules by human dendritic cells. Blood. 2000;95:3473–7. [PubMed] [Google Scholar]
- 33.Dolan BP, Gibbs KD, Jr, Ostrand-Rosenberg S. Dendritic cells cross-dressed with peptide MHC class I complexes prime CD8+ T cells. J Immunol. 2006;177:6018–24. doi: 10.4049/jimmunol.177.9.6018. [DOI] [PubMed] [Google Scholar]
- 34.Thacker RI, Janssen EM. Cross-presentation of cell-associated antigens by mouse splenic dendritic cell populations. Front Immunol. 2012;3:41. doi: 10.3389/fimmu.2012.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–61. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 36.Onfelt B, Nedvetzki S, Yanagi K, Davis DM. Cutting edge: Membrane nanotubes connect immune cells. J Immunol. 2004;173:1511–3. doi: 10.4049/jimmunol.173.3.1511. [DOI] [PubMed] [Google Scholar]
- 37.Davis DM. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nat Rev Immunol. 2007;7:238–43. doi: 10.1038/nri2020. [DOI] [PubMed] [Google Scholar]
- 38.Liu Q, Rojas-Canales DM, Divito SJ, Shufesky WJ, Stolz DB, Erdos G, Sullivan ML, Gibson GA, Watkins SC, Larregina AT, Morelli AE. Donor dendritic cell-derived exosomes promote allograft-targeting immune response. J Clin Invest. 2016;126:2805–20. doi: 10.1172/JCI84577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–72. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Segura E, Nicco C, Lombard B, Veron P, Raposo G, Batteux F, Amigorena S, Thery C. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106:216–23. doi: 10.1182/blood-2005-01-0220. [DOI] [PubMed] [Google Scholar]
- 41.Admyre C, Bohle B, Johansson SM, Focke-Tejkl M, Valenta R, Scheynius A, Gabrielsson S. B cell-derived exosomes can present allergen peptides and activate allergen-specific T cells to proliferate and produce TH2-like cytokines. J Allergy Clin Immunol. 2007;120:1418–24. doi: 10.1016/j.jaci.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 42.Montecalvo A, Shufesky WJ, Stolz DB, Sullivan MG, Wang Z, Divito SJ, Papworth GD, Watkins SC, Robbins PD, Larregina AT, Morelli AE. Exosomes as a short-range mechanism to spread alloantigen between dendritic cells during T cell allorecognition. J Immunol. 2008;180:3081–90. doi: 10.4049/jimmunol.180.5.3081. [DOI] [PubMed] [Google Scholar]
- 43.Qazi KR, Gehrmann U, Domange Jordo E, Karlsson MC, Gabrielsson S. Antigen-loaded exosomes alone induce Th1-type memory through a B-cell-dependent mechanism. Blood. 2009;113:2673–83. doi: 10.1182/blood-2008-04-153536. [DOI] [PubMed] [Google Scholar]
- 44.Vincent-Schneider H, Stumptner-Cuvelette P, Lankar D, Pain S, Raposo G, Benaroch P, Bonnerot C. Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int Immunol. 2002;14:713–22. doi: 10.1093/intimm/dxf048. [DOI] [PubMed] [Google Scholar]
- 45.Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–62. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 46.Hsu DH, Paz P, Villaflor G, Rivas A, Mehta-Damani A, Angevin E, Zitvogel L, Le Pecq JB. Exosomes as a tumor vaccine: enhancing potency through direct loading of antigenic peptides. J Immunother. 2003;26:440–50. doi: 10.1097/00002371-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Kovar M, Boyman O, Shen X, Hwang I, Kohler R, Sprent J. Direct stimulation of T cells by membrane vesicles from antigen-presenting cells. Proc Natl Acad Sci U S A. 2006;103:11671–6. doi: 10.1073/pnas.0603466103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andre F, Chaput N, Schartz NE, Flament C, Aubert N, Bernard J, Lemonnier F, Raposo G, Escudier B, Hsu DH, Tursz T, Amigorena S, Angevin E, Zitvogel L. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172:2126–36. doi: 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- 49.Hao S, Bai O, Li F, Yuan J, Laferte S, Xiang J. Mature dendritic cells pulsed with exosomes stimulate efficient cytotoxic T-lymphocyte responses and antitumour immunity. Immunology. 2007;120:90–102. doi: 10.1111/j.1365-2567.2006.02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mallegol J, Van Niel G, Lebreton C, Lepelletier Y, Candalh C, Dugave C, Heath JK, Raposo G, Cerf-Bensussan N, Heyman M. T84-intestinal epithelial exosomes bear MHC class II/peptide complexes potentiating antigen presentation by dendritic cells. Gastroenterology. 2007;132:1866–76. doi: 10.1053/j.gastro.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 51.Giri PK, Schorey JS. Exosomes derived from M. Bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T cells in vitro and in vivo. PLoS One. 2008;3:e2461. doi: 10.1371/journal.pone.0002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wakim LM, Bevan MJ. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature. 2011;471:629–32. doi: 10.1038/nature09863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC, Falo LD, Jr, Thomson AW. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–66. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 54.Segura E, Guerin C, Hogg N, Amigorena S, Thery C. CD8+ dendritic cells use LFA-1 to capture MHC-peptide complexes from exosomes in vivo. J Immunol. 2007;179:1489–96. doi: 10.4049/jimmunol.179.3.1489. [DOI] [PubMed] [Google Scholar]
- 55.Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, Zhou Q, Sui SF. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11:675–87. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- 56.Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M, Regen T, Hanisch UK, Simons M. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124:447–58. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- 57.Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med. 2004;200:1039–49. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Derbinski J, Pinto S, Rosch S, Hexel K, Kyewski B. Promiscuous gene expression patterns in single medullary thymic epithelial cells argue for a stochastic mechanism. Proc Natl Acad Sci U S A. 2008;105:657–62. doi: 10.1073/pnas.0707486105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Millet V, Naquet P, Guinamard RR. Intercellular MHC transfer between thymic epithelial and dendritic cells. Eur J Immunol. 2008;38:1257–63. doi: 10.1002/eji.200737982. [DOI] [PubMed] [Google Scholar]
- 60.Kroger CJ, Spidale NA, Wang B, Tisch R. Thymic Dendritic Cell Subsets Display Distinct Efficiencies and Mechanisms of Intercellular MHC Transfer. J Immunol. 2017;198:249–56. doi: 10.4049/jimmunol.1601516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koble C, Kyewski B. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. J Exp Med. 2009;206:1505–13. doi: 10.1084/jem.20082449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hubert FX, Kinkel SA, Davey GM, Phipson B, Mueller SN, Liston A, Proietto AI, Cannon PZ, Forehan S, Smyth GK, Wu L, Goodnow CC, Carbone FR, Scott HS, Heath WR. Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood. 2011;118:2462–72. doi: 10.1182/blood-2010-06-286393. [DOI] [PubMed] [Google Scholar]
- 63.Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol. 2006;7:1092–100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 64.Atibalentja DF, Byersdorfer CA, Unanue ER. Thymus-blood protein interactions are highly effective in negative selection and regulatory T cell induction. J Immunol. 2009;183:7909–18. doi: 10.4049/jimmunol.0902632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li J, Park J, Foss D, Goldschneider I. Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. J Exp Med. 2009;206:607–22. doi: 10.1084/jem.20082232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Atibalentja DF, Murphy KM, Unanue ER. Functional redundancy between thymic CD8alpha+ and Sirpalpha+ conventional dendritic cells in presentation of blood-derived lysozyme by MHC class II proteins. J Immunol. 2011;186:1421–31. doi: 10.4049/jimmunol.1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hadeiba H, Lahl K, Edalati A, Oderup C, Habtezion A, Pachynski R, Nguyen L, Ghodsi A, Adler S, Butcher EC. Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity. 2012;36:438–50. doi: 10.1016/j.immuni.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dubrot J, Duraes FV, Potin L, Capotosti F, Brighouse D, Suter T, LeibundGut-Landmann S, Garbi N, Reith W, Swartz MA, Hugues S. Lymph node stromal cells acquire peptide-MHCII complexes from dendritic cells and induce antigen-specific CD4(+) T cell tolerance. J Exp Med. 2014;211:1153–66. doi: 10.1084/jem.20132000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee RS, Grusby MJ, Glimcher LH, Winn HJ, Auchincloss H., Jr Indirect recognition by helper cells can induce donor-specific cytotoxic T lymphocytes in vivo. J Exp Med. 1994;179:865–72. doi: 10.1084/jem.179.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wise MP, Bemelman F, Cobbold SP, Waldmann H. Linked suppression of skin graft rejection can operate through indirect recognition. J Immunol. 1998;161:5813–6. [PubMed] [Google Scholar]
- 71.Brown K, Sacks SH, Wong W. Extensive and bidirectional transfer of major histocompatibility complex class II molecules between donor and recipient cells in vivo following solid organ transplantation. FASEB J. 2008;22:3776–84. doi: 10.1096/fj.08-107441. [DOI] [PubMed] [Google Scholar]
- 72.Marino JMB-MMH, Crosby-Bertorini P, Paster JT, LeGuern C, Germana S, Abdi R, Uehara M, Kin JI, Markmann JF, Tocco G, Benichou G. Donor exosomes rather than passenger leukocytes initiate alloreactive T cell responses after transplantation. Science Immunology. 2016;1 doi: 10.1126/sciimmunol.aaf8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brown K, Sacks SH, Wong W. Coexpression of donor peptide/recipient MHC complex and intact donor MHC: evidence for a link between the direct and indirect pathways. Am J Transplant. 2011;11:826–31. doi: 10.1111/j.1600-6143.2011.03437.x. [DOI] [PubMed] [Google Scholar]
- 74.Sivaganesh S, Harper SJ, Conlon TM, Callaghan CJ, Saeb-Parsy K, Negus MC, Motallebzadeh R, Bolton EM, Bradley JA, Pettigrew GJ. Copresentation of intact and processed MHC alloantigen by recipient dendritic cells enables delivery of linked help to alloreactive CD8 T cells by indirect-pathway CD4 T cells. J Immunol. 2013;190:5829–38. doi: 10.4049/jimmunol.1300458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harper SJ, Ali JM, Wlodek E, Negus MC, Harper IG, Chhabra M, Qureshi MS, Mallik M, Bolton E, Bradley JA, Pettigrew GJ. CD8 T-cell recognition of acquired alloantigen promotes acute allograft rejection. Proc Natl Acad Sci U S A. 2015;112:12788–93. doi: 10.1073/pnas.1513533112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smyth LA, Harker N, Turnbull W, El-Doueik H, Klavinskis L, Kioussis D, Lombardi G, Lechler R. The relative efficiency of acquisition of MHC:peptide complexes and cross-presentation depends on dendritic cell type. J Immunol. 2008;181:3212–20. doi: 10.4049/jimmunol.181.5.3212. [DOI] [PubMed] [Google Scholar]
- 77.Markey KA, Koyama M, Gartlan KH, Leveque L, Kuns RD, Lineburg KE, Teal BE, MacDonald KP, Hill GR. Cross-dressing by donor dendritic cells after allogeneic bone marrow transplantation contributes to formation of the immunological synapse and maximizes responses to indirectly presented antigen. J Immunol. 2014;192:5426–33. doi: 10.4049/jimmunol.1302490. [DOI] [PubMed] [Google Scholar]
- 78.Wang X, Li H, Matte-Martone C, Cui W, Li N, Tan HS, Roopenian D, Shlomchik WD. Mechanisms of antigen presentation to T cells in murine graft-versus-host disease: cross-presentation and the appearance of cross-presentation. Blood. 2011;118:6426–37. doi: 10.1182/blood-2011-06-358747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Naslund TI, Gehrmann U, Qazi KR, Karlsson MC, Gabrielsson S. Dendritic cell-derived exosomes need to activate both T and B cells to induce antitumor immunity. J Immunol. 2013;190:2712–9. doi: 10.4049/jimmunol.1203082. [DOI] [PubMed] [Google Scholar]
- 80.Bedford P, Garner K, Knight SC. MHC class II molecules transferred between allogeneic dendritic cells stimulate primary mixed leukocyte reactions. Int Immunol. 1999;11:1739–44. doi: 10.1093/intimm/11.11.1739. [DOI] [PubMed] [Google Scholar]
- 81.Bedford PA, Burke F, Stagg AJ, Knight SC. Dendritic cells derived from bone marrow cells fail to acquire and present major histocompatibility complex antigens from other dendritic cells. Immunology. 2008;124:542–52. doi: 10.1111/j.1365-2567.2008.02808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Snell GD. The homograft reaction. Annu Rev Microbiol. 1957;11:439–58. doi: 10.1146/annurev.mi.11.100157.002255. [DOI] [PubMed] [Google Scholar]
- 83.Celli S, Albert ML, Bousso P. Visualizing the innate and adaptive immune responses underlying allograft rejection by two-photon microscopy. Nat Med. 2011;17:744–9. doi: 10.1038/nm.2376. [DOI] [PubMed] [Google Scholar]
- 84.Barker CF, Billingham RE. The role of afferent lymphatics in the rejection of skin homografts. J Exp Med. 1968;128:197–221. doi: 10.1084/jem.128.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Steinmuller D. Passenger leukocytes and the immunogenicity of skin allografts. J Invest Dermatol. 1980;75:107–15. doi: 10.1111/1523-1747.ep12521331. [DOI] [PubMed] [Google Scholar]
- 86.McMaster PD, Hudack SS. The Participation of Skin Lymphatics in Repair of the Lesions Due to Incisions and Burns. J Exp Med. 1934;60:479–501. doi: 10.1084/jem.60.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saunderson SC, Dunn AC, Crocker PR, McLellan AD. CD169 mediates the capture of exosomes in spleen and lymph node. Blood. 2014;123:208–16. doi: 10.1182/blood-2013-03-489732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smyth LA, Lechler RI, Lombardi G. Continuous Acquisition of MHC:Peptide Complexes by Recipient Cells Contributes to the Generation of Anti-Graft CD8(+) T Cell Immunity. Am J Transplant. 2017;17:60–8. doi: 10.1111/ajt.13996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhuang Q, Liu Q, Divito SJ, Zeng Q, Yatim KM, Hughes AD, Rojas-Canales DM, Nakao A, Shufesky WJ, Williams AL, Humar R, Hoffman RA, Shlomchik WD, Oberbarnscheidt MH, Lakkis FG, Morelli AE. Graft-infiltrating host dendritic cells play a key role in organ transplant rejection. Nat Commun. 2016;7:12623. doi: 10.1038/ncomms12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ono Y, Perez-Gutierrez A, Nakao T, Dai H, Camirand G, Yoshida O, Yokota S, Stolz DB, Ross MA, Morelli AE, Geller DA, Thomson AW. Graft-infiltrating PD-L1(hi) cross-dressed dendritic cells regulate anti-donor T cell responses in mouse liver transplant tolerance. Hepatology. 2017 doi: 10.1002/hep.29529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bracamonte-Baran W, Florentin J, Zhou Y, Jankowska-Gan E, Haynes WJ, Zhong W, Brennan TV, Dutta P, Claas FH, van Rood JJ, Burlingham WJ. Modification of host dendritic cells by microchimerism-derived extracellular vesicles generates split tolerance. Proc Natl Acad Sci U S A. 2017;114:1099–104. doi: 10.1073/pnas.1618364114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Qu C, Nguyen VA, Merad M, Randolph GJ. MHC class I/peptide transfer between dendritic cells overcomes poor cross-presentation by monocyte-derived APCs that engulf dying cells. J Immunol. 2009;182:3650–9. doi: 10.4049/jimmunol.0801532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smyth LA, Hervouet C, Hayday T, Becker PD, Ellis R, Lechler RI, Lombardi G, Klavinskis LS. Acquisition of MHC:peptide complexes by dendritic cells contributes to the generation of antiviral CD8+ T cell immunity in vivo. J Immunol. 2012;189:2274–82. doi: 10.4049/jimmunol.1200664. [DOI] [PubMed] [Google Scholar]
- 94.Li L, Kim S, Herndon JM, Goedegebuure P, Belt BA, Satpathy AT, Fleming TP, Hansen TH, Murphy KM, Gillanders WE. Cross-dressed CD8alpha+/CD103+ dendritic cells prime CD8+ T cells following vaccination. Proc Natl Acad Sci U S A. 2012;109:12716–21. doi: 10.1073/pnas.1203468109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tian T, Zhu YL, Hu FH, Wang YY, Huang NP, Xiao ZD. Dynamics of exosome internalization and trafficking. J Cell Physiol. 2013;228:1487–95. doi: 10.1002/jcp.24304. [DOI] [PubMed] [Google Scholar]
- 96.Coppieters K, Barral AM, Juedes A, Wolfe T, Rodrigo E, Thery C, Amigorena S, von Herrath MG. No significant CTL cross-priming by dendritic cell-derived exosomes during murine lymphocytic choriomeningitis virus infection. J Immunol. 2009;182:2213–20. doi: 10.4049/jimmunol.0802578. [DOI] [PubMed] [Google Scholar]
- 97.Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–8. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 98.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–62. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 99.Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM, Englezakis A, Barlow JL, Hams E, Scanlon ST, Ogg GS, Fallon PG, McKenzie AN. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–95. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 101.Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, Tursz T, Amigorena S, Raposo G, Angevin E, Zitvogel L. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360:295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 102.Chaput N, Schartz NE, Andre F, Taieb J, Novault S, Bonnaventure P, Aubert N, Bernard J, Lemonnier F, Merad M, Adema G, Adams M, Ferrantini M, Carpentier AF, Escudier B, Tursz T, Angevin E, Zitvogel L. Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection. J Immunol. 2004;172:2137–46. doi: 10.4049/jimmunol.172.4.2137. [DOI] [PubMed] [Google Scholar]
- 103.Hao S, Bai O, Yuan J, Qureshi M, Xiang J. Dendritic cell-derived exosomes stimulate stronger CD8+ CTL responses and antitumor immunity than tumor cell-derived exosomes. Cell Mol Immunol. 2006;3:205–11. [PubMed] [Google Scholar]
- 104.Bonaccorsi I, Morandi B, Antsiferova O, Costa G, Oliveri D, Conte R, Pezzino G, Vermiglio G, Anastasi GP, Navarra G, Munz C, Di Carlo E, Mingari MC, Ferlazzo G. Membrane transfer from tumor cells overcomes deficient phagocytic ability of plasmacytoid dendritic cells for the acquisition and presentation of tumor antigens. J Immunol. 2014;192:824–32. doi: 10.4049/jimmunol.1301039. [DOI] [PubMed] [Google Scholar]
- 105.Josien R, Li HL, Ingulli E, Sarma S, Wong BR, Vologodskaia M, Steinman RM, Choi Y. TRANCE, a tumor necrosis factor family member, enhances the longevity and adjuvant properties of dendritic cells in vivo. J Exp Med. 2000;191:495–502. doi: 10.1084/jem.191.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barratt-Boyes SM, Zimmer MI, Harshyne LA, Meyer EM, Watkins SC, Capuano S, 3rd, Murphey-Corb M, Falo LD, Jr, Donnenberg AD. Maturation and trafficking of monocyte-derived dendritic cells in monkeys: implications for dendritic cell-based vaccines. J Immunol. 2000;164:2487–95. doi: 10.4049/jimmunol.164.5.2487. [DOI] [PubMed] [Google Scholar]
- 107.Smith AL, Fazekas de St Groth B. Antigen-pulsed CD8alpha+ dendritic cells generate an immune response after subcutaneous injection without homing to the draining lymph node. J Exp Med. 1999;189:593–8. doi: 10.1084/jem.189.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kleindienst P, Brocker T. Endogenous dendritic cells are required for amplification of T cell responses induced by dendritic cell vaccines in vivo. J Immunol. 2003;170:2817–23. doi: 10.4049/jimmunol.170.6.2817. [DOI] [PubMed] [Google Scholar]