Abstract
Rationale:
Drug addiction is a complex disease that is impacted by numerous factors. One such factor, time of day, influences drug intake, but there have been no investigations of how time of day affects the amount of drug taken and the development of addiction-like behavior. Previous data from our group show circadian disruption in rats given access to heroin during the light phase, which is important because circadian disruption, itself, can increase drug intake. Thus, the goal of this experiment was to determine how time of day of access affects heroin self-administration and the development of addiction-like behaviors including escalation of heroin intake, willingness to work for heroin on a progressive ratio schedule of reinforcement, seeking during extinction, incubation of seeking, and reinstatement of heroin seeking behavior.
Materials and Methods:
Male Sprague Dawley rats were given the opportunity to self-administer heroin for 6 hours per trial during the second half of either the light or dark phase for 18 trials, including one progressive ratio challenge. Rats then underwent 14 days of abstinence, with a five-hour extinction test occurring on both the first and the fourteenth days of abstinence. The second extinction test was followed by a heroin prime and 1 hour of reinstatement testing. On the following day, a subset of rats were tested in an additional extinction test where rats were tested either at the same time of day as their previous self-administration sessions or during the opposite light/dark phase.
Results:
Relative to Light Access rats, Dark Access rats took more heroin, exhibited more goal-directed behavior, exhibited more seeking during the dark phase, failed to extinguish seeking during the 5h extinction test in the dark phase, and exhibited greater incubation of heroin seeking following abstinence. However, Dark Access rats did not escalate drug taking over trials, work harder for drug, or seek more during drug-induced reinstatement than Light Access rats.
Conclusions:
These results show that time of access to heroin affects overall heroin intake and seeking in extinction, but does not affect other addiction-like behaviors in rats.
Introduction.
Addiction is a chronic disease characterized by uncontrolled drug use and multiple relapses (Edwards and Koob 2013; Leshner 1997; McLellan et al. 2000). Heroin use and overdose has risen to an epidemic level in the United States. In a study seeking to determine relative harmfulness of different drugs of abuse, heroin was determined to be the second most harmful drug to the user and to others (Nutt et al. 2010). In 2015, more than 300,000 people in the United States were using heroin and more than 800,000 people had used heroin within the last year (Center for Behavioral Health Statistics and Quality 2016). Most disturbingly, in 2015, more than 33,000 people died from opioid overdose (Rudd et al. 2016), and this number increased to over 42,000 in 2016 (Seth et al. 2018).
Previously, we have shown that self-administration of heroin during the light phase reverses sleep-wake patterns and disrupts the timing of REM sleep into the abstinence period (Coffey et al. 2016). A similar reversal of sleep-wake patterns was found in rats on the first day of abstinence from a one-day binge alcohol administration protocol (Sharma et al. 2014). Importantly, circadian disruption has been linked to increased intake of methamphetamine (Doyle et al. 2015), alcohol (Gauvin et al. 1997), and morphine (Garmabi et al. 2016). Repeated phase shifts, on the other hand, have been shown to decrease drinking in male, but slightly increase drinking in female, high-alcohol drinking rats (Clark et al. 2007). Importantly, there is evidence linking circadian shifts to decreased activation in the medial prefrontal cortex and nucleus accumbens in response to reward and reward anticipation (Hasler et al. 2012). Thus, previous data indicate that circadian disruption, induced in several different ways, can modulate intake of a variety of drugs.
Time of administration can affect the pharmacokinetics of a drug, which can influence the development of addiction (Allain et al. 2015). While time of day is not always reported in drug self-administration studies, there are several studies that examined how time of access affected drug intake. A previous study examining cocaine intake as a function of time of day reported that rats took more cocaine during the early active phase than the early inactive phase, but that intake was even higher halfway through the active phase and halfway through the inactive phase. Specifically, intake was highest mid-inactive phase, where rats took approximately 1.5 times more infusions compared to mid-active phase (Baird and Gauvin 2000). Similarly, rats given access to ethanol during the active phase initially drank more alcohol, but the rats with inactive-phase access ultimately increased their intake to a greater extent (Choi et al. 1990). When rats were given 23 h to self-administer heroin, their infusions initially were concentrated in the active phase but, over trials, became evenly spread across the entire day (Vendruscolo et al. 2011). Overall, previous data indicate that cocaine intake in rats is higher mid-inactive phase compared to the mid-active phase, and that increased intake in the inactive phase may be associated with the progression of addictive processes. To our knowledge, there have been no investigations of the effect of time of access on heroin self-administration.
Intake is important for the development of addiction, but animal models provide several other crucial indicators of addictive processes including escalation of intake, motivation to take drug, seeking once drug is no longer available, incubation, and relapse-like behavior. The goal of the current study was to investigate how time of access influences not only heroin self-administration, but also the development of addiction-like behaviors in rats. Our hypothesis was that the circadian disruption previously observed in Light Access rats (Coffey et al. 2016) would cause increased intake and addiction-like behaviors in rats given access to heroin during the light phase compared to those given access to heroin during the dark phase.
Methods.
Subjects.
The procedures described herein were approved by the Penn State College of Medicine Institutional Animal Care and Use Committee. The subjects were 45 adult male Sprague Dawley rats obtained from Charles River (7 rats were excluded from the study due to: injury, 2 rats; loss of catheter patency, 4 rats; or loss of intake data for three consecutive days, 1 rat). Rats weighed between 331 and 433 grams at the beginning of the experiment. They were housed individually in wire bottom cages in a temperature-controlled (21 °C) animal care facility with either a 12:12 hour light:dark cycle (lights on at 2:00 am; n=17) or a 12:12 hour reverse light:dark cycle (lights off at 2:30 am; n=21). Rats were given 2 weeks to adjust to the experimental light/dark cycle prior to the start of self-administration. This schedule allows ample time for re-entrainment (Marumoto et al. 1996; Takamure et al. 1991). Self-administration sessions began 6 h into the light or dark cycle. This start time was chosen to split the phase into two halves while still producing the circadian disruption seen previously when self-administration began 4 h into the light phase (Coffey et al. 2016). The rats were maintained with free access to dry Harlan Teklad rodent diet 6068 and water, except where noted otherwise. Rats were housed in the same room as the self-administration chambers in which they were tested. The windows on the doors to the room with the reverse light:dark cycle were covered in red film and black trash bags to prevent light contamination during the day.
Drugs.
Heroin was generously provided by the National Institute of Drug Abuse (Triangle Institute, Research Triangle Park, NC). Heroin was mixed with 0.9% sterile saline (0.0625 mg heroin/250 ml saline) weekly and covered with aluminum foil to protect the solution from light.
Self-administration chambers.
Each rat was trained in one of twenty-two identical operant conditioning chambers (MED Associates, St. Albans, VT) described previously (Grigson and Twining 2002; Twining et al. 2009). Each chamber measured 30.5 cm in length × 24.0 cm in width × 29.0 cm in height, and was individually housed in a light- and sound-attenuated cubicle. The chambers consisted of a clear Plexiglas top, front, and back wall. The side walls were made of aluminum. Grid floors consisted of nineteen 4.8-mm stainless steel rods, spaced 1.6 cm apart (center to center). Each chamber was equipped with two retractable empty sipper spouts that entered through 1.3-cm diameter holes, spaced 16.4 cm apart (center to center). A stimulus light was located 6.0 cm above each opening. Each chamber also was equipped with a house light (25 W), a tone generator (Sonalert Time Generator, 2900 Hz, Mallory, Indianapolis, IN), and a speaker for white noise (75 dB). Heroin reinforcement was controlled by a lickometer circuit that monitored empty spout contacts to operate a syringe pump (Model A, Razel Scientific Instruments, Stamford, CT). A coupling assembly attached the syringe pump to the catheter assembly on the back of each rat and entered through a 5.0-cm diameter hole in the top of the chamber. This assembly consisted of a metal spring attached to a metal spacer with Tygon tubing inserted down the center, protecting passage of the tubing from rat interference. The tubing was attached to a counterbalanced swivel assembly (Instech, Plymouth Meeting, PA) that, in turn, was attached to the syringe pump. Events in the chamber and collection of the data were controlled on-line with a Pentium computer that used programs written in the Medstate notation language (MED Associates).
Catheter Surgeries.
Intra-jugular catheters were custom-made in our laboratory and implanted as described previously (Twining et al. 2009). All animals received Carprofen (5 mg/kg) on the first postoperative day to ease pain. Any animals that exhibited signs of pain thereafter were given an additional dose of Carprofen (5mg/kg) daily, as needed. Following surgery, rats were allowed one week to recover. General maintenance of catheter patency involved daily examination and flushing of catheters with heparinized saline (0.2 ml of 30 IU/ml heparin). Catheter patency was verified, as needed, using 0.2 ml of propofol (Diprivan 1%) administered intravenously.
Habituation.
Water was removed from the rats 15 h prior to first habituation trial. Rats were habituated to the self-administration chambers for 2 days. During these 2 days the rats were given 1 h access to water in the self-administration chambers, first via the center spout (which would become the inactive spout) on Day 1, and then via the right hand spout (which would become the active spout) on Day 2. Immediately after removal from the chambers, the rats were given 25 ml_ of water overnight. Following completion of the second habituation trial, all rats were returned to water ad libitum.
Acquisition.
Beginning the next day, rats were given 6 h access to heroin for 16 non-consecutive days (5 days on/2 days off). The stimulus light was illuminated for the entire self-administration session, except during the 20s timeout following an infusion. This light was white for Light Access rats and red for Dark Access rats, thus maintaining room lighting for the groups during the self-administration sessions (McGuire et al. 1973). For “on” days, rats were placed in the self-administration chambers for 6 h, starting either 6 h into the light cycle (n=20) or 6 h into the dark cycle (n=24), and allowed to self-administer heroin (0.12 mg/kg/infusion) on a fixed ratio 10 (FR10) schedule of reinforcement. The FR10 schedule was chosen because the operant behavior (contacts on an empty spout) is a very natural behavior for rats. The FR10 schedule of reinforcement dissociates active from inactive responses, allowing for the observation of goal-directed behavior and assurance that the rats have acquired the operant response. Infusions were given over 4–7 seconds at an infusion rate of 2.154 ml/min. Once an infusion is earned, the spouts retract, the stimulus light above the right hand (active) spout turns off, the house light turns on, and a tone sounds for 20 seconds. After this 20 second timeout period, the house light and tone turn off, the stimulus light turns back on, and the spouts return to where they are accessible to the rat. Since the house light was an important cue, a white light, reflected toward the ceiling, was used for both groups. Light during the night is known to induce phase shifts (Honma et al. 1978); however, nonphotic cues suppress light-entraining mechanisms through inhibition of the suprachiasmatic nucleus (SCN), so heroin likely was preventing any effects of the house light on entrainment (Challet and Pévet 2003). Indeed, morphine injections during the subjective night have been shown to mitigate light-induced phase shifts (Mistlberger and Holmes 1999). In addition, previous data from our group indicate that the availability of heroin is a stronger timing signal than the light/dark cycle (Coffey et al. 2016) and that rats given access to heroin during the dark phase exhibit normal nocturnal sleep/wake patterns (Coffey et al., unpublished results). There were 18 self-administration trials. For Trials 1–16 and 18, the FR10 schedule of reinforcement was used. Trial 17 was the progressive ratio challenge (described below).
Progressive Ratio (PR) Challenge.
Twenty-four h after the sixteenth FR10 self-administration trial, rats were allowed to self-administer heroin on a PR schedule of reinforcement. Under this schedule, a rat is required to make an increasing number of operant responses on the empty spout to earn each subsequent infusion, beginning with the usual 10 lick requirement (Infusion requirements: 10, 12, 16, 22, 30, 40, 52, 66, 82, 100, 120, 142, 166, etc.) (Imperio and Grigson 2015; Imperio et al. 2016). Breakpoint is defined as the last ratio completed. The challenge ends when two h have passed since the rat’s last infusion or after 8 h, whichever comes first. Twenty-four h after the PR challenge, rats were given one additional maintenance trial (Trial 18) under the FR10 schedule of reinforcement.
Abstinence.
Immediately following the last day of acquisition, rats were maintained in the home cages for 14 days. During this time, the rats were not given access to heroin.
Extinction Test 1.
All rats were placed in the self-administration chambers for 5 h on the first day of abstinence for Extinction Test 1. Ten contacts with the empty “active” spout resulted in activation of all cues previously associated with heroin, but no infusions were received.
Extinction Test 2/Reinstatement.
All rats were placed back in the self-administration chambers on the fourteenth day of abstinence for a 5 h extinction test, during which time no drug was available. Again, 10 contacts on the active empty spout resulted in presentation of all of the cues previously associated with heroin, but no drug delivery. After 5 h, the rats received a single, computer-delivered, non-contingent infusion of heroin (0.12 mg/kg, i.v.) followed by another hour of extinction conditions to test for heroin-induced reinstatement of heroin-seeking behavior. All cues were presented, but no drug was available.
Extinction 3 and Light/Dark Access Switch.
On the day after the Extinction Test 2/Reinstatement test, all rats in the second replication underwent a third extinction test that was identical to the first. Time of test, however, was switched for half of the rats, creating four groups: Light Access rats with light phase extinction (Light-Light, n=4), Dark Access rats with dark phase extinction (Dark-Dark, n=5), Light Access rats with dark phase extinction (Light-Dark, n=5), and Dark Access rats with light phase extinction (Dark-Light, n=6). All 5 h extinction tests began 6 h into the light or dark phases.
Addiction-like behaviors.
For the purpose of this experiment, addiction-like behaviors were defined as: escalation of drug taking in the first hour of access (Ahmed et al. 2000; Edwards and Koob 2013), motivation to take heroin as measured by progressive ratio (Deroche-Gamonet et al. 2004; Richardson and Roberts 1996), incubation of seeking during extinction (Grimm et al. 2001; Kuntz et al. 2008), and seeking during reinstatement (de Wit and Stewart 1983; Deroche-Gamonet et al. 2004; Shaham et al. 2003). Of note, throughout this paper, “drug taking” refers specifically to heroin self-administration, either on FR10 or PR, and “drug seeking” refers to motivated attempts to take heroin during extinction and reinstatement, when heroin is no longer available.
Data Analysis.
The data were analyzed using IBM SPSS Statistics (Version 24, IBM) and Statistica (Version 7.1, StatSoft, Tulsa, OK), then graphed using Origin (Version 7, OriginLab Corporation, Northampton, MA). Any missing infusion data were filled in by taking the average of infusions taken the day before and the day after the missing value. Three rats had one missing value each and one rat had three missing values throughout acquisition. The data were then analyzed using 2 × 2 factorial analyses of variance (ANOVAs) varying drug taker (low vs. high) and Access (light vs. dark) or 2 × 2 × 16 mixed factorial ANOVAs varying drug taker (low vs. high drug taker), access (light vs. dark), and trial (1–16). Significant interactions were followed by post hoc assessment using Student’s Newman-Keuls tests with p < 0.05.
Results.
Low and high drug takers.
Rats were split into low and high drug takers based on the average number of infusions they self-administered on terminal FR10 trials 16 and 18. Rats that took an average of at least 24 infusions across Trials 16 and 18 were labeled “high drug takers” (n=12: n=9 Dark Access; n=3 Light Access), while rats that took fewer were “low drug takers” (n=26: n=12 Dark Access; n=14 Light Access). Using this cutoff, these groups were found to be significantly different in terminal heroin self-administration behavior, t(27)=2.45, p=0.02. Further, assessment of the behavior of these two populations of rats demonstrates that the self-administration procedure was sufficient to produce addiction-like behaviors in these rats. While there were more high drug takers in the Dark Access group (n=9) than in the Light Access group (n=3), a chi-square test confirmed that the proportion of low and high drug takers did not significantly differ as a function of access, X2=2.76, p=0.096.
Infusions.
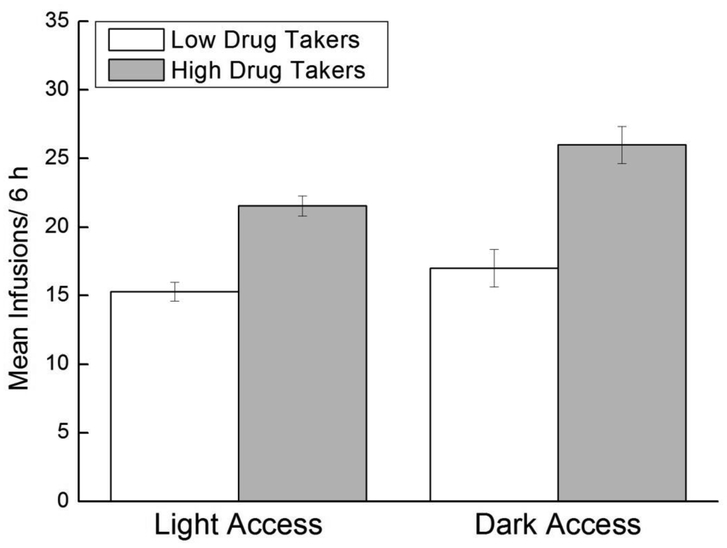
Across acquisition, Dark Access rats took more infusions than Light Access rats, overall, and high drug takers took more infusions than low drug takers, regardless of time of access (Figure 1). Support for these conclusions was provided by a 2 × 2 × 16 mixed factorial ANOVA varying Access (Light, Dark), Drug Taker (Low, High), and Trial (1–16). Results revealed significant main effects of Access, F(1,34)=4.98, p<0.02; Drug Taker, F(1,34)=25.87, p<0.0001; and Trial, F(15,510)=14.01, p<0.000001. There also was a significant Drug Taker x Trial interaction, F(15,510)=3.41, p<0.0001. Student Newman-Keuls (SNK) post hoc tests revealed that high drug takers took significantly more infusions on Trials 4–16 compared to Trial 1, overall, ps<0.05, indicating that high, but not low, drug takers increased their intake across trials regardless of time of access (data not shown). No other interaction effects were significant.
Figure 1. Drug-taking behavior in Light and Dark Access rats.
Mean (+/− SEM) number of heroin infusions across acquisition for Light Access low drug takers (n=14) and high drug takers (n=3) and Dark Access low drug takers (n=12) and high drug takers (n=9).
Escalation.
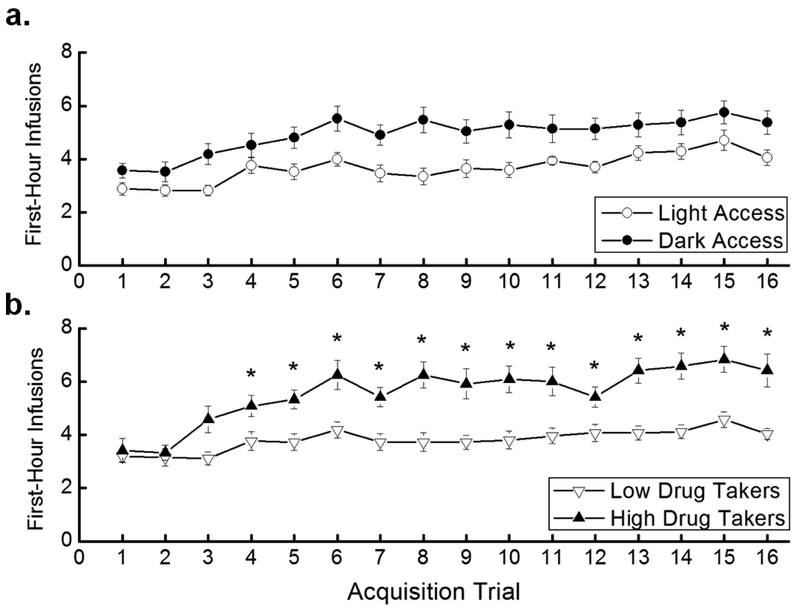
Ahmed and Koob argue that escalation of drug intake in rodents models the progression from controlled to uncontrolled drug use in humans (Ahmed et al. 2000). Here, we examined the number of infusions taken in the first hour of access across trials to assess escalation of intake. The results indicated that Dark Access rats took more first-hour infusions than did Light Access rats, overall, but that the groups did not differ in escalation (Figure 2a). In contrast, high drug takers significantly escalated their intake, whereas low drug takers did not (Figure 2b). In support of these conclusions, a 2 × 2 × 16 mixed factorial ANOVA varying Access (Light, Dark), Drug Taker (Low, High), and Trial (1–16) revealed a significant main effect of Access, F(1,34)=6.33, p<0.05, indicating that Dark Access rats took more first-hour infusions than Light Access rats overall. The main effect of Drug Taker, F(1,34)=14.52, p<0.001, also was significant, showing that high drug takers took more first-hour infusions of heroin than low drug takers, as was the main effect of Trial, F(15,510)=9.68, p<0.000001. The non-significant Access × Trial interaction demonstrated that there was no difference in escalation of heroin self-administration across trials between Dark and Light Access rats (Figure 2a). There was, however, a significant Drug Taker × Trial interaction, F(15,510)=2.62, p=0.0008. SNK post hoc tests of this interaction revealed that high drug takers took significantly more first-hour infusions on Trials 4–16 compared to Trial 1, p<0.05, confirming that high drug takers, but not low drug takers, escalated their heroin intake regardless of time of access (Figure 2b).
Figure 2. First-hour infusions.
a. Mean (+/− SEM) number of heroin infusions taken in the first hour of each trial for Light Access (n=17) and Dark Access (n=21) rats. b. Mean (+/− SEM) number of heroin infusions taken in the first hour of each trial for low drug takers (n=26) and high drug takers (n=12). *p<0.05 compared to Trial 1 for high drug takers.
Active vs. Inactive Contacts.
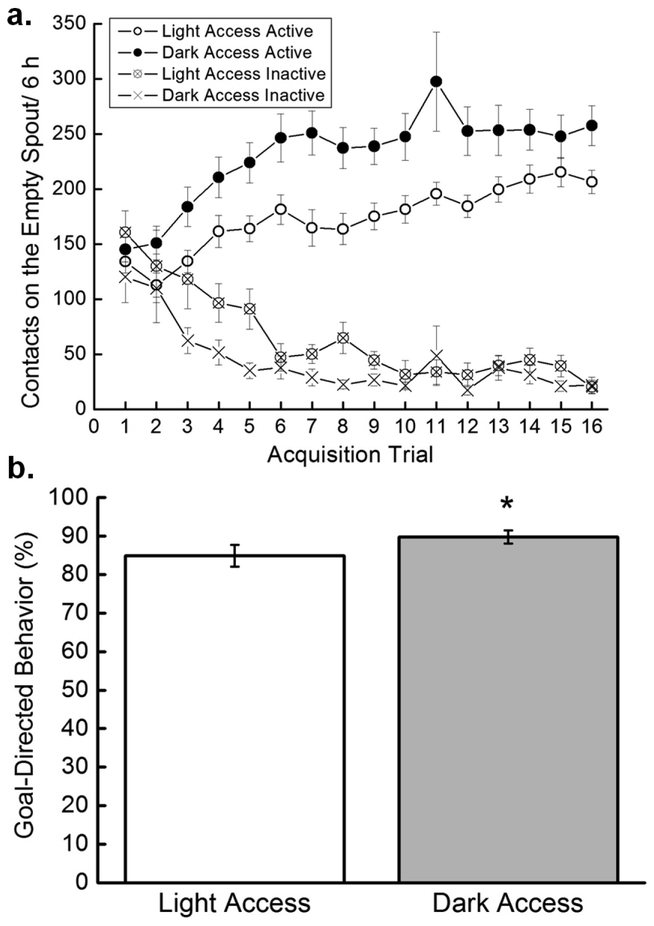
A 2 × 2 × 2 × 16 mixed factorial ANOVA varying Access (Light, Dark), Drug Taker (low, high), Spout (active, inactive), and Trial (1–16) was run to examine how rats differentiated between the active and inactive spouts. Results showed a significant main effect of Drug Taker, F(1,34)=17.78, p=0.0002, and a significant Drug Taker × Trial interaction, F(15,510)=3.03, p=0.0001. The main effect of Spout, F(1,34)=273.073, p<0.000001; the Drug Taker × Spout interaction, F(1,34)=19.65, p=0.00009; and the Access × Spout interaction, F(1,34)=10.15, p=0.003, were all significant. SNK post hoc tests on the Access × Spout interaction indicated that Light Access rats and Dark Access rats both made more active than inactive spout contacts, ps<0.05. Dark Access rats made more active spout contacts than did Light Access rats, p<0.05, but the two groups made the same number of inactive spout contacts, p>0.05. The Spout × Trial interaction also was significant, F(15,510)=21.02, p<0.000001, and SNK post hoc tests indicated that, overall, rats made more active than inactive spout contacts on Trials 3–16, ps<0.05. The Access × Contacts × Trial interaction was not significant (Figure 3a).
Figure 3. Goal-directed behavior.
a. Active and inactive licks. Mean (+/− SEM) contacts on the active and inactive spouts by acquisition trial for Light Access (n=17) and Dark Access (n=21) rats. b. Goal-directed behavior. Mean (+/− SEM) goal-directed behavior (active licks/active licks + inactive licks expressed as a percentage) averaged across the acquisition trials for Light Access (n=17) and Dark Access (n=21) rats.
Goal-Directed Behavior.
Goal-directed behavior was determined by dividing Active Licks/(Active + Inactive Licks) and multiplying by 100 to express the number as a percentage. Dark Access rats exhibited more goal-directed behavior than did Light Access rats (Figure 3b). In support, a 2 × 2 × 16 mixed factorial ANOVA varying Access (Light, Dark), Drug Taker (Low, High), and Trial (1–16) revealed a significant main effect of Access, F(1,34)=5.15, p<0.05. The main effect of Trial also was significant, F(15,510)=19.79, p<0.000001, while the main effect of Drug Taker was not.
Progressive Ratio.
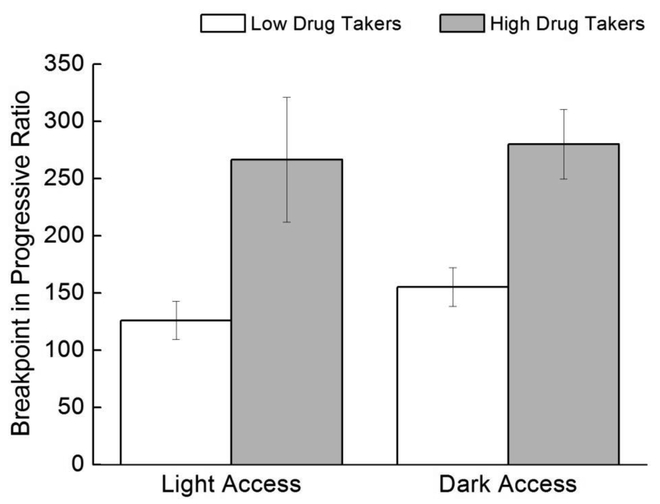
High drug takers worked harder for heroin on the progressive ratio challenge than did low drug takers, but time of access did not exert a significant impact (Figure 4). This conclusion was supported by a 2 × 2 ANOVA varying Access (Light, Dark) and Drug Taker (Low, High). Results revealed a nonsignificant main effect of Access, F<1.0, but a significant main effect of Drug Taker, F(1,34)=22.98, p=0.00003.
Figure 4. Progressive ratio.
Mean (+/− SEM) breakpoint for Light Access low drug takers (n=14), Light Access high drug takers (n=3), Dark Access low drug takers (n=12), and Dark Access high drug takers (n=9).
Extinction and Reinstatement Testing.
There were three extinction tests. Extinction Test 1 was a 5 h test held on the first day of abstinence, 18 h following the end of the last self-administration session. Extinction Test 2 was held on the 14th day of abstinence. Immediately following the fifth hour of Extinction 2 testing, all rats received a non-contingent infusion of heroin and responding was tested for an additional hour of reinstatement. A subset of rats (n=20) underwent Extinction Test 3, a 5 h test held on the 15th day of abstinence (18 h following the end of Extinction Test 2 and the drug-induced reinstatement test).
Extinction Test 1.
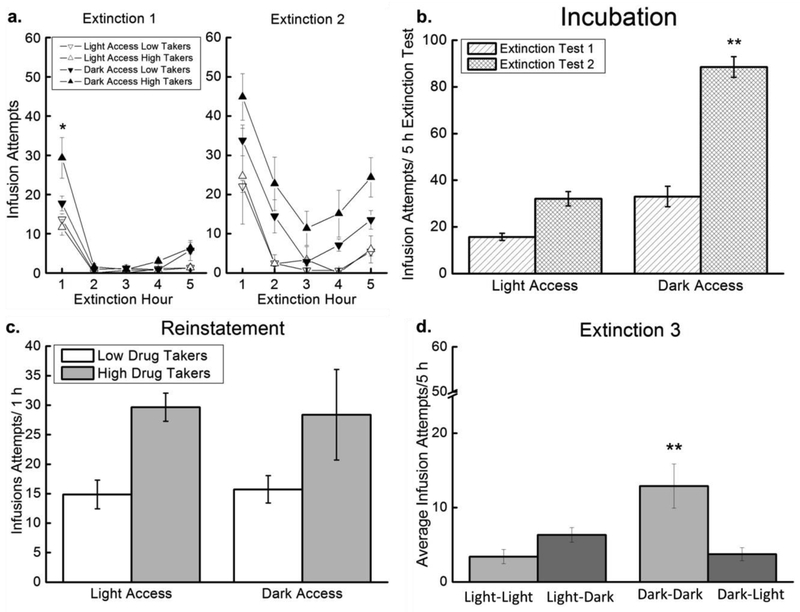
During Extinction Test 1, Dark Access rats made more infusion attempts than did Light Access rats, particularly in the first hour (Figure 5a, left panel). In support, a 2 × 2 × 5 mixed factorial ANOVA varying Access (Light, Dark), Drug Taker (Low, High), and Hour (1-5) revealed a significant main effect of Access, F(1,33)=10.90, p=0.002, indicating that Dark Access rats exhibited more seeking, overall, than did Light Access rats. The main effect of Hour also was significant, F(4,132)=74.53, p<0.000001, but the main effect of Drug Taker was not. There was a significant Access × Hour interaction, F(4,132)=6.21, p=0.0001, and a significant Access × Drug Taker × Hour interaction, F(4,132)=2.89, p=0.02. Post hoc tests on this 3-way interaction revealed that, in hour 1, Dark Access high drug takers exhibited more heroin seeking than rats in all other treatment conditions, p<0.05 (Figure 5a left panel).
Figure 5. Extinction/reinstatement.
There were three extinction tests: Extinction 1 (Abstinence Day 1), Extinction 2 (Abstinence Day 14; included reinstatement), and Extinction 3 (Abstinence Day 15). a. Extinction. Mean (+/− SEM) infusion attempts for Light Access low drug takers (n=14), Light Access high drug takers (n=3), Dark Access low drug takers (n=12), and Dark Access high drug takers (n=9) during the 5h Extinction Test 1 (left panel) and 5 h Extinction Test 2 (right panel). *p<0.05 comparing Dark Access high drug takers to all other groups. b. Incubation. Mean (+/− SEM) total infusion attempts during the 5h Extinction Test 1 and Extinction Test 2 for Light Access (n=17) and Dark Access (n=20) rats. **p<0.05 compared to all other data points. c. Reinstatement. Mean (+/− SEM) infusion attempts during the 1 h drug-induced reinstatement test for Light Access high drug takers (n=3), Light Access low drug takers (n=14), Dark Access high drug takers (n=9), and Dark Access low drug takers (n=12). d. Extinction 3. Mean (+/− SEM) infusion attempts across the 5 h Extinction Test 3 for Light Access rats tested in the light phase (Light-Light; n=4), Light Access rats tested in the dark phase (Light-Dark; n=5), Dark Access rats tested in the dark phase (Dark-Dark; n=5), and Dark Access rats tested in the light phase (Dark-Light; n=6). **p<0.05 compared to all other data points.
Extinction Test 2.
Dark Access rats also made more infusion attempts during the second extinction test than did Light Access rats (Figure 5a, right panel). In support, a 2 × 2 × 5 mixed factorial ANOVA varying Access (Light, Dark), Drug Taker (Low, High), and Hour (1–5) revealed a significant main effect of Access, F(1,32)=19.39, p=0.0001, showing that Dark Access rats made more infusion attempts than Light Access rats overall. The main effect of Hour also was significant, F(4,128)=42.22, p<0.000001, and SNK post hoc tests revealed that seeking during the first hour was greater than at all other time points, overall, p<0.05. Seeking during hours 2 and 5 also was greater than seeking during hours 3 and 4, p<0.05. Other main effects and interactions did not attain statistical significance, ps>0.05.
Incubation.
The two extinction tests, conducted on abstinence day 1 and abstinence day 14, allow for the assessment of incubation of heroin seeking. Heroin seeking increased across the 14 d period, and this effect was significant in Dark Access rats (Figure 5b). In support, a 2 × 2 × 2 mixed factorial ANOVA varying Access (Light, Dark), Drug Taker (Low, High), and Test (Extinction Test 1, Extinction Test 2) revealed significant main effects of Access, F(1,33)=15.76, p<0.001, and Test, F(1,33)=26.27, p<0.0001, on the total number of infusion attempts across each 5 h test. The Access × Test interaction also was significant, F(1,33)=6.4, p=0.016. Post hoc SNK tests revealed that there was a trend toward increased seeking during the second extinction test compared to the first in Light Access rats, p=0.06, but Dark Access rats exhibited increased seeking in the second extinction test compared to the first, p<0.05, and compared to the Light Access rats, ps<0.05 (Figure 5b). The main effect of Drug Taker was not significant, p>0.05.
Reinstatement.
High drug takers made more infusion attempts following the heroin prime than did low drug takers, regardless of time of access (Figure 5c). As such, a 2 × 2 ANOVA varying Access (Light, Dark) and Drug Taker (Low, High) revealed a nonsignificant main effect of Access, but a significant main effect of Drug Taker, F(1,32)=7.10, p<0.02. The Access × Drug Taker interaction was not significant (Figure 5c).
Extinction Test 3.
Extinction Test 3 was conducted either at the time of training (Light-Light and Dark-Dark) or at the opposite time of day (Light-Dark and Dark-Light) (Figure 5d). Testing Dark Access rats in the light phase (Dark-Light) caused these rats to exhibit less heroin seeking than Dark Access rats tested in the dark. Testing Light Access rats in the dark phase (Light-Dark) caused these rats to tend to exhibit more heroin seeking than Light Access rats tested in the light phase. In support, a 2 × 2 × 5 ANOVA varying Access (Light, Dark), Time of Testing (same, opposite), and Hour (1–5) revealed a significant Access × Time of Testing interaction, F(1,16)=12.39, p<0.01. SNK post hoc tests indicated that the Dark-Dark group exhibited more seeking, overall, than all other groups, p<0.05 (Figure 5d). There also was a significant main effect of Hour, F(4,64)=27.69, p<0.000001. The main effects of Access and Time of Testing were not significant. None of the other interactions were significant. There was only one high drug taker each in the Light-Light and Light-Dark groups for Extinction 3, so the variable “Drug Taker” could not be included in the analysis.
Discussion.
The goal of this study was to test whether time of daily access to heroin would impact heroin self-administration and addiction-like behaviors. The results showed that Dark Access to heroin increased intake, goal-directed behavior, incubation, and seeking during the dark phase, but did not affect other addiction-like behaviors for heroin (See Table 1). These findings do not support our hypothesis that the circadian disruption caused by Light Access to heroin increases addiction vulnerability. In contrast to our hypothesis, Dark Access rats took more heroin infusions than Light Access rats and exhibited more goal-directed behavior, although both groups dedicated more than 80% of their total spout contacts to the heroin-associated spout. Importantly, both Dark Access and Light Access groups reduced inactive licks across acquisition, indicating that the effects seen in Dark Access rats were not merely due to increased activity during the active phase. In Extinction Tests 1 and 2, Dark Access rats showed more drug-seeking behavior than Light Access rats and exhibited a surprising failure to extinguish over the second 5 h test. Further, while Light Access rats tended to exhibit incubation of heroin seeking across Extinction Test 1 and Extinction Test 2, this effect was very dramatic in Dark Access rats. Due to this continued seeking out to the fifth hour of extinction testing, the heroin prime did not elicit a clear increase in infusion attempts in Dark Access rats, although Light Access rats did reinstate seeking following the prime. There was no difference in escalation of first-hour intake across trials or in the motivation to take heroin on the progressive ratio challenge between the Dark and Light Access rats. Finally, a preliminary test switching the time of access for Extinction Test 3 tended to increase seeking in Light Access rats tested in the dark phase and significantly decreased seeking in Dark Access rats tested in the light phase. Thus, the effect in extinction appears to be due to the time of testing, rather than the time of training. Taken together, the above results show a dissociation of addiction-like behaviors that are usually seen together. Dark Access rats exhibit more heroin taking, heroin seeking, and incubation but similar escalation, motivation to take heroin, and drug-induced reinstatement of seeking compared to Light Access rats. This differs from high drug takers, which consistently exhibit more addiction-like behaviors than low drug takers. They exhibit increased heroin intake, first-hour intake escalation, high motivation to take heroin, and robust reinstatement of heroin seeking following the heroin prime compared to low drug takers (See Table 1). This pattern of data is largely in keeping with the individual differences reported previously for low and high heroin takers by Imperio and Grigson (2015). It should be noted that, while low drug takers were evenly split across Light and Dark Access groups (n=12 Dark Access low drug takers; n=14 Light Access low drug takers), there were 3 times more high drug takers in the Dark Access group (n=9) compared to the Light Access group (n=3). This may have been coincidental or it may have been a consequence of the fact that Dark Access rats take more heroin than Light Access rats. Since high drug takers are defined by higher intake of drug and generally show more addiction-like behaviors than low drug takers, it will be important going forward to investigate how Dark Access rats take more heroin without mirroring the full addiction-like behavior phenotype.
Table 1.
Summary of results.
| Light Access |
Dark Access |
Low Drug Takers |
High Drug Takers |
|
|---|---|---|---|---|
| Infusions | + | ++ | + | ++ |
| First Hour Infusions | + | + | + | ++ |
| Active Licks | + | ++ | + | ++ |
| Inactive Licks | - | - | - | - |
| Goal-Directed Behavior |
+ | ++ | + | + |
| Progressive Ratio | + | + | + | ++ |
| Extinction 1 and 2 | + | ++ | + | + |
| Incubation | + | ++ | + | + |
| Reinstatement | + | + | + | ++ |
| Extinction Switch | + | - | NA | NA |
+ reflects responding for drug.
++ reflects increased responding for drug significant at p<0.05 vs. +.
− reflects reduced responding for drug.
Since rats are nocturnal, it is possible that Light Access rats are simply sleeping instead of seeking or taking heroin. However, EEG data confirmed that rats with access to heroin during the light phase are consistently awake for the entire 6 hour self-administration session, regardless of whether they are high or low drug takers (Coffey et al. 2016). In contrast, we have previously seen that Light Access rats sleep for an average of ~2.9 h during 6 h extinction/reinstatement tests, when heroin is no longer available (unpublished observations from the data reported in Coffey et al. 2016). In this case, the rats slept for less than 30 minutes of the first 2 h of the test, when the majority of seeking occurs. Taken together, these data support sleep and sleepiness as a potential factor in reducing seeking in extinction, but not heroin intake during acquisition, when self-administration sessions are during the light phase.
Chronopharmacology refers to the study of how the time of day of drug administration affects a drug’s pharmacology (Labrecque and Belanger 1991; Ohdo 2003; Reinberg 1992). A recent study measured levels of morphine and its metabolite, morphine-3-glucoronide (M3G), in the plasma and brain as a function of time of administration, then used computer modeling to simulate the chronopharmacokinetics of morphine (Kervezee et al. 2017). Unfortunately, no such studies have been conducted for heroin, but these data are relevant to heroin since it is quickly metabolized into morphine and 6-acetylmorphine (Inturrisi et al. 1983). The models showed that brain morphine levels were higher at 4 h or 8 h into the light phase compared to 4 h or 8 h into the dark phase (Kervezee et al. 2017). These results would be consistent with the conclusion that Dark Access rats are self-administering more heroin, but reaching a similar high as the Light Access rats, thus explaining similarities in many addiction-like behaviors. Importantly, brain levels of morphine were highest at dark onset and higher at light onset than mid-light phase (Kervezee et al. 2017), indicating that follow-up experiments to investigate how heroin self-administration changes at these times of day are important.
In humans, there is a circadian rhythm in overdose likelihood (Gallerani et al. 2001; Manfredini et al. 1994). Gallerani et al. (2001) investigated in more detail how sensitivity to the effects of opioids may vary across the 24h period. While the peak in number of overdoses occurred in the late afternoon (15:00–21:00), the peak in lethal overdoses occurred in the early morning (3:00–8:59). Perhaps most telling, the necessary dose of naloxone for revival also peaked between 3:00–8:59, despite similar levels of serum morphine regardless of time of overdose in a subset of patients sampled. Thus, in the late inactive period, people are more likely to die from an overdose and require more naloxone for rescue despite similar levels of drug intake (Gallerani et al. 2001). These findings suggest that people are most sensitive to the effects of opioids during the late inactive to early active period, which would correspond to the late light phase to early dark phase in rodents. In accordance, cancer patients self-administer more doses of morphine between 10:00–22:00, suggesting that they need more of the drug during the active period to achieve the same analgesia as during the inactive period (Bruera et al. 1992). However, evidence from the analgesia literature should be considered carefully because they reflect a complicated interaction between morphine, endogenous opioids, and corticotropin-releasing hormone (Oliverio et al. 1982; Rasmussen and Farr 2003; Yoshida et al. 2005), rather than a difference in sensitivity to morphine’s effects. The above data indicate the possibility that Dark Access rats take more drug because they are less responsive to heroin’s effects at that time of day. Thus, Light Access and Dark Access rats may be reaching similar levels of intoxication, but Light Access rats take less drug than Dark Access rats due to circadian sensitivity. This interpretation is supported by the fact that increased intake in the Dark Access rats is not associated with a full array of addiction-like behaviors. Perhaps most telling is the similar levels of escalation between Light and Dark Access rats, which has been hailed as a cardinal sign of addiction (Edwards and Koob 2013). Importantly, there is a difference in addiction-like behaviors between high and low drug takers, revealing individual differences, and perhaps vulnerabilities, that transcend time of access.
Acknowledgements.
The authors thank Danielle Alexander, Joaquin Douton, Sarah Ballard, and Christopher Jenney for assistance with this study. This study was supported by funds from NIDA grant R37DA009815.
References
- Ahmed SH, Walker JR, Koob GF (2000) Persistent increase in the motivation to take heroin in rats with a history of drug escalation Neuropsychopharmacology 22:413–421 doi: 10.1016/S0893-133X(99)00133-5 [DOI] [PubMed] [Google Scholar]
- Allain F, Minogianis EA, Roberts DC, Samaha AN (2015) How fast and how often: The pharmacokinetics of drug use are decisive in addiction Neurosci Biobehav Rev 56:166–179 doi: 10.1016/j.neubiorev.2015.06.012 [DOI] [PubMed] [Google Scholar]
- Baird TJ, Gauvin D (2000) Characterization of cocaine self-administration and pharmacokinetics as a function of time of day in the rat Pharmacol Biochem Behav 65:289–299 [DOI] [PubMed] [Google Scholar]
- Bruera E, Macmillan K, Kuehn N, Miller MJ (1992) Circadian distribution of extra doses of narcotic analgesics in patients with cancer pain: a preliminary report Pain 49:311–314 [DOI] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality (2016) Key substance use and mental health indicators in the United States: Results from the 2015 National Survey on Drug Use and Health HHS Publication No SMA 16–4984, NSDUH Series H-51
- Challet E, Pévet P (2003) Interactions between photic and nonphotic stimuli to synchronize the master circadian clock in mammals Front Biosci 8:s246–s257 [DOI] [PubMed] [Google Scholar]
- Choi HW, Wild KD, Hubbell CL, Reid LD (1990) Chronically administered morphine, circadian cyclicity, and intake of an alcoholic beverage Alcohol 7:7–10 [DOI] [PubMed] [Google Scholar]
- Clark JW, Fixaris MC, Belanger GV, Rosenwasser AM (2007) Repeated light-dark phase shifts modulate voluntary ethanol intake in male and female high alcohol-drinking (HAD1) rats Alcohol Clin Exp Res 31:1699–1706 doi: 10.1111/j.1530-0277.2007.00476.x [DOI] [PubMed] [Google Scholar]
- Coffey AA, Guan Z, Grigson PS, Fang J (2016) Reversal of the sleep-wake cycle by heroin self-administration in rats Brain Res Bull 123:33–46 doi: 10.1016/j.brainresbull.2015.09.008 [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J (1983) Drug reinstatement of heroin-reinforced responding in the rat Psychopharmacology (Berl) 79:29–31 [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV (2004) Evidence for addiction-like behavior in the rat Science 305:1014–1017 doi: 10.1126/science.1099020 [DOI] [PubMed] [Google Scholar]
- Doyle SE, Feng H, Garber G, Menaker M, Lynch WJ (2015) Effects of circadian disruption on methamphetamine consumption in methamphetamine-exposed rats Psychopharmacology (Berl) 232:2169–2179 doi: 10.1007/s00213-014-3845-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Koob GF (2013) Escalation of drug self-administration as a hallmark of persistent addiction liability Behav Pharmacol 24:356–362 doi: 10.1097/FBP.0b013e3283644d15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallerani M, Manfredini R, Dal Monte D, Calo G, Brunaldi V, Simonato M (2001) Circadian differences in the individual sensitivity to opiate overdose Crit Care Med 29:96–101 [DOI] [PubMed] [Google Scholar]
- Garmabi B, Vousooghi N, Vosough M, Yoonessi A, Bakhtazad A, Zarrindast MR (2016) Effect of circadian rhythm disturbance on morphine preference and addiction in male rats: Involvement of period genes and dopamine D1 receptor Neuroscience 322:104–114 doi: 10.1016/j.neuroscience.2016.02.019 [DOI] [PubMed] [Google Scholar]
- Gauvin DV, Baird TJ, Vanecek SA, Briscoe RJ, Vallett M, Holloway FA (1997) Effects of time-of-day and photoperiod phase shifts on voluntary ethanol consumption in rats Alcohol Clin Exp Res 21:817–825 [PubMed] [Google Scholar]
- Grigson PS, Twining RC (2002) Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards Behav Neurosci 116:321–333 [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y (2001) Neuroadaptation. Incubation of cocaine craving after withdrawal Nature 412:141–142 doi: 10.1038/35084134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP et al. (2012) Weekend-weekday advances in sleep timing are associated with altered reward-related brain function in healthy adolescents Biol Psychol 91:334–341 doi: 10.1016/j.biopsycho.2012.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma K, Katabami F, Hiroshige T (1978) A phase response curve for the locomotor activity rhythm of the rat Experientia 34:1602–1603 [DOI] [PubMed] [Google Scholar]
- Imperio CG, Grigson PS (2015) Greater avoidance of a heroin-paired taste cue is associated with greater escalation of heroin self-administration in rats Behav Neurosci 129:380–388 doi: 10.1037/bne0000069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperio CG, McFalls AJ, Colechio EM, Masser DR, Vrana KE, Grigson PS, Freeman WM (2016) Assessment of individual differences in the rat nucleus accumbens transcriptome following taste-heroin extended access Brain Res Bull 123:71–80 doi: 10.1016/j.brainresbull.2015.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inturrisi CE, Schultz M, Shin S, Umans JG, Angel L, Simon EJ (1983) Evidence from opiate binding studies that heroin acts through its metabolites Life Sci 33 Suppl 1:773–776 [DOI] [PubMed] [Google Scholar]
- Kervezee L, Hartman R, van den Berg DJ, Meijer JH, de Lange ECM (2017) Diurnal variation in the pharmacokinetics and brain distribution of morphine and its major metabolite Eur J Pharm Sci 109S:S132–S139 doi: 10.1016/j.ejps.2017.05.048 [DOI] [PubMed] [Google Scholar]
- Kuntz KL, Twining RC, Baldwin AE, Vrana KE, Grigson PS (2008) Heroin self-administration: I. Incubation of goal-directed behavior in rats Pharmacol Biochem Behav 90:344–348 doi: 10.1016/j.pbb.2008.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrecque G, Belanger PM (1991) Biological rhythms in the absorption, distribution, metabolism and excretion of drugs Pharmacol Ther 52:95–107 [DOI] [PubMed] [Google Scholar]
- Leshner AI (1997) Addiction is a brain disease, and it matters Science 278:45–47 [DOI] [PubMed] [Google Scholar]
- Manfredini R, Gallerani M, Calo G, Pasin M, Govoni M, Fersini C (1994) Emergency admissions of opioid drug abusers for overdose: a chronobiological study of enhanced risk Ann Emerg Med 24:615–618 [DOI] [PubMed] [Google Scholar]
- Marumoto N, Murakami N, Kuroda H, Murakami T (1996) Melatonin accelerates reentrainment of circadian locomotor activity rhythms to new light-dark cycles in the rat The Japanese journal of physiology 46:347–351 [DOI] [PubMed] [Google Scholar]
- McGuire RA, Rand WM, Wurtman RJ (1973) Entrainment of the body temperature rhythm in rats: effect of color and intensity of environmental light Science 181:956–957 [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD (2000) Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation Jama 284:1689–1695 [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Holmes MM (1999) Morphine-induced activity attenuates phase shifts to light in C57BL/6 J mice Brain Res 829:113–119 [DOI] [PubMed] [Google Scholar]
- Nutt DJ, King LA, Phillips LD (2010) Drug harms in the UK: a multicriteria decision analysis Lancet 376:1558–1565 doi: 10.1016/S0140-6736(10)61462-6 [DOI] [PubMed] [Google Scholar]
- Ohdo S (2003) Changes in toxicity and effectiveness with timing of drug administration: implications for drug safety Drug Saf 26:999–1010 [DOI] [PubMed] [Google Scholar]
- Oliverio A, Castellano C, Puglisi-Allegra S (1982) Opiate analgesia: evidence for circadian rhythms in mice Brain Res 249:265–270 [DOI] [PubMed] [Google Scholar]
- Rasmussen NA, Farr LA (2003) Effects of morphine and time of day on pain and beta-endorphin Biol Res Nurs 5:105–116 doi: 10.1177/1099800403257166 [DOI] [PubMed] [Google Scholar]
- Reinberg AE (1992) Concepts in chronopharmacology Annu Rev Pharmacol Toxicol 32:51–66 doi: 10.1146/annurev.pa.32.040192.000411 [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy J Neurosci Methods 66:1–11 [DOI] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F, Scholl L (2016) Increases in Drug and Opioid-Involved Overdose Deaths - United States, 2010–2015 MMWR Morb Mortal Wkly Rep 65:1445–1452 doi: 10.15585/mmwr.mm655051e1 [DOI] [PubMed] [Google Scholar]
- Seth P, Rudd RA, Noonan RK, Haegerich TM (2018) Quantifying the Epidemic of Prescription Opioid Overdose Deaths Am J Public Health 108:500–502 doi: 10.2105/ajph.2017.304265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J (2003) The reinstatement model of drug relapse: history, methodology and major findings Psychopharmacology (Berl) 168:3–20 doi: 10.1007/s00213-002-1224-x [DOI] [PubMed] [Google Scholar]
- Sharma R, Bradshaw K, Sahota P, Thakkar MM (2014) Acute binge alcohol administration reverses sleep-wake cycle in Sprague Dawley rats Alcohol Clin Exp Res 38:1941–1946 doi: 10.1111/acer.12463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamure M, Murakami N, Takahashi K, Kuroda H, Etoh T (1991) Rapid reentrainment of the circadian clock itself, but not the measurable activity rhythms to a new light-dark cycle in the rat Physiol Behav 50:443–449 [DOI] [PubMed] [Google Scholar]
- Twining RC, Bolan M, Grigson PS (2009) Yoked delivery of cocaine is aversive and protects against the motivation for drug in rats Behav Neurosci 123:913–925 doi: 10.1037/a0016498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Schlosburg JE, Misra KK, Chen SA, Greenwell TN, Koob GF (2011) Escalation patterns of varying periods of heroin access Pharmacol Biochem Behav 98:570–574 doi: 10.1016/j.pbb.2011.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Koyanagi S, Matsuo A, Fujioka T, To H, Higuchi S, Ohdo S (2005) Glucocorticoid hormone regulates the circadian coordination of micro-opioid receptor expression in mouse brainstem J Pharmacol Exp Ther 315:1119–1124 doi: 10.1124/jpet.105.091488 [DOI] [PubMed] [Google Scholar]