Abstract
Background
Advances in congestive heart failure (CHF) management depend on biomarkers for monitoring disease progression and therapeutic response. During systole, intracellular Ca2+ is released from the sarcoplasmic reticulum (SR) into the cytoplasm through type 2 ryanodine receptor/Ca2+ release channels (RyR2). In CHF, chronically elevated circulating catecholamine levels cause pathologic remodeling of RyR2 resulting in diastolic SR Ca2+ leak, and decreased myocardial contractility. Similarly, skeletal muscle contraction requires SR Ca2+ release through type-1 ryanodine receptors (RyR1), and chronically elevated catecholamine levels in CHF cause RyR1 mediated SR Ca2+ leak, contributing to myopathy and weakness. Circulating B-lymphocytes express RyR1 and catecholamine responsive signaling cascades, making them a potential surrogate for defects in intracellular Ca2+ handling due to leaky RyR channels in CHF.
Methods
Whole blood was collected from patients with CHF, CHF status-post left-ventricular assist devices (LVAD), and controls. Blood was also collected from mice with ischemic CHF, ischemic CHF + S107 (a drug that specifically reduces RyR channel Ca2+ leak), and WT controls. Channel macromolecular complex was assessed by immunostaining RyR1 immunoprecipitated from lymphocyte enriched preparations. RyR1 Ca2+ leak was assessed using flow cytometry to measure Ca2+ fluorescence in B-lymphocytes, in the absence and presence of RyR1 agonists that empty RyR1 Ca2+ stores within the endoplasmic reticulum (ER).
Results
Circulating B-lymphocytes from humans and mice with CHF exhibited remodeled RyR1 and decreased ER Ca2+ stores, consistent with chronic intracellular Ca2+ leak. This Ca2+ leak correlated with circulating catecholamine levels. The intracellular Ca2+ leak was significantly reduced in mice treated with the Rycal S107. CHF patients treated with LVAD exhibited a heterogeneous response.
Conclusion
In CHF, B-lymphocytes exhibit remodeled leaky RyR1 channels and decreased ER Ca2+ stores consistent with chronic intracellular Ca2+ leak. RyR1 mediated Ca2+ leak in B-lymphocytes assessed using flow cytometry provides a surrogate measure of intracellular Ca2+ handling and systemic sympathetic burden, presenting a novel biomarker for monitoring response to pharmacologic and mechanical CHF therapy.
Keywords: Heart failure, biomarker, ion channels, calcium
Introduction
Congestive heart failure (CHF) is a leading cause of hospitalization and mortality in the United States 1. Left ventricular assist devices (LVAD) are indicated for patients with end-stage heart failure as either a bridge-to-transplant or destination therapy 2. However, with 1-year, post-implant survival reaching 80% there is a greater potential for ventricular reconditioning and pump explant 3. Biomarkers for pump recovery, and response to CHF therapies in general, are needed to help guide device and pharmacologic management.
Elevated circulating plasma norepinephrine (NE) levels are associated with CHF progression and mortality 4, 5 and reduced NE levels are observed in the setting of CHF therapy 6, 7. However, the complexities associated with handling and processing NE assays limits the clinical utility of direct serum measurements 8. Novel approaches to assaying the effect of sympathetic activity in patients with heart failure are needed.
NE binds to β-adrenergic receptors on myocytes in both the heart and skeletal muscle causing production of cyclic-AMP, which activates protein kinase A (PKA) 9 as well as other protein kinases that phosphorylate targets in the skeletal muscle 10 and heart, and regulate gene expression 11. The type 2 ryanodine receptor (RyR2) is an intracellular, sarcoplasmic reticulum (SR) based, Ca2+ release channel responsible for providing the Ca2+ necessary for myocardial contraction 12. In human myocardium PKA phosphorylates (RyR2) at Ser2809 (Ser2808 in murine RyR2) 13–15. In skeletal muscle PKA phosphorylates the type-1 ryanodine receptor (RyR1) at Ser2843 (Ser2844 in murine RyR1) 16, 17. Under physiologic conditions PKA phosphorylation of RyR2 in the heart and RyR1 in skeletal muscle, contributes to modulating SR Ca2+ release and muscle contractility 13, 16, 17. RyR channel phosphorylation by CaMKII also plays a role in rate-related increase in cardiac contractility 18, although non-phosphorylation mechanisms have also been described 19.
In CHF, persistently elevated circulating NE levels cause chronic pathologic phosphorylation of RyR2 14, 20 and RyR1 17, 21. This results in remodeling of the channel macromolecular complex, including, nitrosylation/oxidation, dissociation of the phosphodiesterase PDE4D3 (which exacerbates PKA phosphorylation), and decreased binding of the stabilizing subunit calstabin to the channel 10, 14, 17, 22–26. These events together cause localized “Ca2+ sparks” 27 and more generalized post-repolarization Ca2+ release events. 14, 15, 20, 25, 28, 29. In the heart, this results in diastolic SR Ca2+ leak, decreased SR Ca2+ load, and reduced contractility and cardiac output 20, 30. Moreover, chronic SR Ca2+ leak via RyR2 channels causes mitochondrial Ca2+ overload, perpetuating metabolic dysfunction in failing hearts 31. Additionally, the Ca2+ leak and resultant increase in cytosolic [Ca2+] generate a transient inward current through Na+/Ca2+ exchangers which partially depolarizes the membrane, increasing the propensity for ectopic activity and arrhythmias 32, 33.
In skeletal muscle, leaky RyR1 in CHF contributes to muscle weakness 21. None of the current treatments for CHF improve skeletal muscle function, including β-blockers. However, a novel experimental class of drugs called Rycals prevents stress-induced dissociation of the channel stabilizing subunit calstabin2 from RyR2 and calstabin1 from RyR1 thereby reducing RyR mediated SR Ca2+ leak 21, 28, 34–36 resulting in improved cardiac and skeletal muscle function 21. S107, the Rycal drug used in the present study is in the same chemical class as the drug currently in clinical testing and shares the same mechanism of action, fixing leaky RyR channels.
In addition to its pivotal role in muscle contraction, intracellular Ca2+ release and handling contributes to B-lymphocyte activation during an immune response 37. Store operated Ca2+ entry (SOCE) through the plasma membrane is a major source of intracellular Ca2+ during an immune response. This is triggered by emptying of the ER Ca2+ stores by activation of inositol 1,4,5-trisphosphate receptors (IP3R) which themselves are regulated by phosphorylation in lymphocytes 37–42. RyR1 expression has been observed in B-lymphocytes 43, 44. While RyR1 Ca2+ store contribution to immune function has not been elucidated, it can be assessed using flow cytometry 45. In addition to expressing RyR1, B-lymphocytes express the components of the β-adrenergic cascade necessary for activating PKA in response to circulating NE 46. In this study, we investigated whether the elevated NE levels in CHF cause pathologic RyR1 remodeling and Ca2+ leak in circulating B-lymphocytes in humans and mice and whether this could serve as a biomarker for S107 therapy and response to LVAD mechanical support.
Methods
All reagents and materials are available upon request.
Mouse CHF model and blood sample collection
All animal studies were performed according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Columbia University and according to NIH guidelines. A post-infarct mouse model of CHF was utilized. Studies were conducted over a 4-month period. Permanent occlusion of the mid left anterior descending (LAD) coronary artery was performed in 4-month-old male C57BL/6 mice, as described 21,25. Mid LAD, as opposed to proximal LAD, was chosen in order to induce mild-moderate myocardial dysfunction while limiting mortality. Male mice were utilized in order to limit any potential effect of hormonal cycles 47. Briefly, mice were anesthetized, intubated and mechanically ventilated under isoflurane anesthesia with buprenorphine subcutaneous 0.05 mg/Kg for analgesia. A left thoracotomy was performed via the third intercostal space, and muscles and pericardium were carefully dissected. The LAD coronary artery was identified using a dissecting microscope and ligated using an 8-0 non-absorbable suture. The intercostal space was closed using a 6-0 non-absorbable suture and the skin with a 5-0 absorbable suture. Mice received 100% oxygen until wakening after which the endotracheal tube was withdrawn. Animals were then given oxygen via nasal cone until full recovery of consciousness. Buprenorphine subcutaneous 0.05 mg/Kg was provided every 12h for 2–3 days for post-operative analgesia. Mice were randomly assigned to three groups (n=10 each): CHF+Vehicle, CHF+S107 (50 mg/kg/day in drinking water from time of surgery) and Sham (mice underwent the same surgical procedure except for LAD occlusion). Following randomization there were no readily detectable baseline differences between each group and baseline cardiac function as determined by echocardiography was similar. Starting eight weeks after surgery blood was collected from the retro-orbital plexus into 1.5ml tubes containing 7.5ul Heparin 1000u/ml for experiments. Red blood cells were eliminated using red blood cell lysis buffer (BioLegend, San Diego, CA) per manufacturer’s instructions. For biochemistry experiments the lymphocyte enriched pellets were frozen and stored at -80. Tubes were labeled using a number system and the experimenters were blinded to the contents. To ensure adequate cell counts lymphocyte enriched pellets from mice could be combined within their respective groups. Cardiac function was assessed by echocardiography, using a Visualsonic Vevo 770 ultrasound equipped with a 30-MHz transducer applied to the chest wall. Fractional shortening (FS) was assessed in the M-mode.
Patient enrollment and blood sample collection
All human studies were performed according to protocols approved by the Institutional Review Boards (IRB) at Albert Einstein College of Medicine and Columbia University - New York Presbyterian Hospital. CHF and control patients were recruited from the inpatient and outpatient services at Jacobi Medical Center, Bronx NY. CHF, control, and CHF+LVAD patients were recruited from the inpatient and outpatient services at Columbia University Medical Center-New York Presbyterian Hospital (CUMC). All subjects gave informed consent. When available, baseline characteristics including age, gender, ejection fraction, New York Heart Association (NYHA) CHF class, etiology of CHF (ischemic vs non-ischemic), hypertension, diabetes mellitus, hyperlipidemia, ACE inhibitor use, beta-blocker use, and blood pressure and heart rate were recorded. All LVAD patients had their devices implanted for at least 9 months and were hemodynamically stable. Samples were de-identified prior to testing. For samples acquired at Jacobi Hospital, lymphocyte enriched pellets were isolated from 7–8 ml of whole blood collected from patients using BD CPT vacutainers (containing premade Ficoll-Hypaque™ gradient) with Na-citrate as previously described 48. In brief, the tubes were centrifuged at 1700 G for 15 minutes, 1xHBSS was added to supernatant, final volume 15 ml. Samples were centrifuged at 300 G for 10 minutes. Pellets were washed twice with 10 ml HBSS and resuspended in HBSS. For samples acquired at CUMC, 2–4 ml of blood was collected using Li-heparin vacutainers and red blood cells were eliminated from a 400 μL aliquot using red blood cell lysis buffer (BioLegend, San Diego, CA) per manufacturer’s instructions.
Biochemistry
Immunoprecipitation of RyR from lymphocyte enriched cell lysates was performed using an RyR antibody (5029) 13, 49, 50 in 0.5 ml of a modified RIPA buffer (50 mM Tris-HCl pH 7.2, 0.9% NaCl, 5.0 mM NaF, 1.0 mM Na3VO4, 1% Triton- X100, and protease inhibitors) overnight at 4°C. RyR1 and RyR2 isoform specific rabbit polyclonal antibodies were used to immunoprecipitate (1:200) specific RyR isoforms. These antibodies were generated against either the peptide sequence KPEFNNHKDYAQEK, corresponding to amino acids 1367–1380 of mouse cardiac RyR2 (also 1367–1380 of human RyR2) or the peptide sequence AEPDTDYENLRRS, corresponding to amino acids 1327–1339 of the mouse skeletal muscle RyR1 (also 1325–1337 of human RyR1) 18, 25, 51. Rabbit cardiac and skeletal muscle SR lysates were used as controls. The immune complexes were incubated with protein A Sepharose beads (Sigma, St. Louis, MS) at 4°C for 1 hr and the beads were washed three times with RIPA buffer. The immunoprecipitates were size-fractionated on SDS-PAGE gels (6% for RyR, 15% for calstabin) and transferred onto nitrocellulose membranes for 2 hours at 200 mA. Immunoblots were developed using the following primary antibodies: anti-RyR 5029 (1:5000)13, anti-phospho-RyR1-pSer2843 (1:5,000) 13, 17, anti-calstabin (FKBP12 C-19, 1:1000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA). To determine channel oxidation, the carbonyl groups on the protein side chains of immunoprecipitated RyR channels were derivatized to 2,4-dinitrophenylhydrazone (DNP) by reaction with 2,4-dinitrophenylhydrazine. The DNP signal associated with RyR was determined using a specific anti-DNP antibody, according to the manufacturer’s instructions (Millipore, Billerica, MA) 52. All immunoblots were developed with the Odyssey system (LI-COR Biosciences, Lincoln, NE), using IR-labeled anti-mouse and anti-rabbit IgG (1:10000 dilution) secondary antibodies. For immunoblot analyses of SERCA2, phospholamban (PLB), and PLB phosphorylation at Serine16 (PLB-S16), lymphocyte pellets were resuspended in lysis buffer (50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 20 mM NaF, and protease inhibitors) and then size-fractionated on 15% SDS-PAGE gels, transferred onto nitrocellulose membranes, and stained with the following primary antibodies: SERCA2 1:1000 (Santa Cruz Biotech), total PLB1:1,000 (MA3-922; Thermo Scientific), phospho-specific PLB-Ser16 1:1,000 (20R-P121A; Fitzgerald), GAPDH 1:1000 (Abcam). Experiments were performed by blinded individuals.
Norepinephrine measurements
Blood samples were drawn into EDTA coated BD-vacutainers and plasma was isolated by centrifugation for 15 minutes at 2,000 × g. Plasma was aliquoted and frozen at −80°C within 1 hour of draw. NE was measured using the Epinephrine/Norepinephrine ELISA Kit (Abnova, Taiwan) following the manufacturer’s instructions.
Flow cytometry
In both the human and murine experiments, the washed lymphocyte enriched pellets were suspended in 100 microliters HBSS containing a fluorescent-tagged anti-CD19 antibody. Either phycoerythrin (PE) (human experiments: Abcam ab1168 mouse anti-human; Mouse experiments: Abcam ab90753 rat anti-mouse) or APC-tagged anti-CD19 antibodies was used (Invitrogen, SJ25-C1), concentrations per manufacturers recommendations. Following a 10-minute incubation, 400 microliters of 1 micromolar Fluo-4 AM in 1xHBSS was added. The lymphocytes were allowed to load with Fluo-4 AM for 30 minutes and then the pellets were washed twice with 1x HBSS to remove excess Fluo-4 AM and fluorescent antibody. Baseline fluorescence was analyzed using a Becton Dickinson LSR II flow cytometer. Side scatter and forward scatter light were used for gating the lymphocyte population. Crossover of phycoerythrin and Fluo-4 fluorescence was compensated using single fluorophore controls. Fluorescent measurements were acquired in the absence and presence of RyR agonists, either caffeine (50mM) or 4-CmC (0.5mM).
Statistics
Data are expressed as mean +/− SEM. Two tailed students T-test or ANOVA with Tukey’s multiple comparison test, were used for calculating significance when applicable. Chi-square test was used for calculating significance between patient groups. Quantification of immunoblots is expressed in arbitrary units. Sample size in the mouse study was calculated by assuming an absolute difference of 15% between groups with a standard deviation of 10%, with beta error 20%, alpha error 5%, and accounting for possible deaths prior to experimentation. Mortality for mouse studies was plotted using Kaplan-Meyer survival curves and statistical significance was calculated using Mantel–Cox log-rank test.
Results
RyR1 in B-lymphocytes is remodeled in humans and mice with CHF
Patients from two medical centers with and without CHF were recruited for a one-time donation of whole blood. Patients, at least 9 months status-post LVAD implant, and hemodynamically stable were recruited from one medical center. There were significantly fewer women in the CHF and LVADs group compared to the normal group (Table 1). The EF in the LVAD group was significantly reduced compared to both CHF and normal patients. About half of CHF and LVAD patients had ischemic cardiomyopathy and all four NYHA CHF classes were represented. While beta-blocker and ACE inhibitor/angiotensin receptor blocker use was significantly higher in the CHF group compared to the normal group there were no significant differences in the heart rates or blood pressures between the two groups. However, SBP was significantly lower in the LVAD group compared to both the CHF and normal groups (Table 1).
Table 1.
Patient characteristics
| Normal (N=38) | HF (N=33) | LVAD (N=14) | P | |
|---|---|---|---|---|
| Age | 57±2 | 65±2 | 63±3 | <0.05 |
| % female | 53 | 30 | 7 | <0.05 |
| EF | 63±1 | 23±4 | 16±1 | <0.05 |
| NYHA 1 | N/A | 7 (21%) | N/A | |
| NYHA 2 | N/A | 8 (24%) | N/A | |
| NYHA 3 | N/A | 10 (30%) | N/A | |
| NYHA 4 | N/A | 8 (24%) | N/A | |
| SBP avg | 133±4 | 126±4 | 95±4 | <0.05 |
| DBP avg | 74±2 | 69±2 | 69±3 | N.S. |
| HR avg | 77±3 | 81±3 | 79±4 | N.S. |
| ACE/ARB | 7 (19%) | 26 (78%) | 5 (36%) | <0.05 |
| BB | 12 (33%) | 28 (85%) | 13 (93%) | <0.05 |
| HTN | 24 (67%) | 25 (75%) | 11 (79%) | N.S. |
| DM | 11 (31%) | 16 (48%) | 4 (29%) | <0.05 |
| HLD | 14 (42%) | 18 (54%) | 8 (57%) | N.S. |
| ICM | N/A | 15 (45%) | 8 (57%) | <0.05 |
Baseline characteristics of all patients included in this study when available, including: age, gender, ejection fraction (EF), NYHA CHF class (1–4), etiology of CHF (ischemic [ICM] or non-ischemic), hypertension (HTN), diabetes mellitus (DM), hyperlipidemia (HLD), ACE inhibitor/angiotensin receptor blocker use (ACE inhibitor/ARB), beta-blocker use (BB), systolic (SBP) and diastolic (DBP) blood pressures, and heart rates (HR). Samples from patients were used for either flow cytometry or biochemistry.
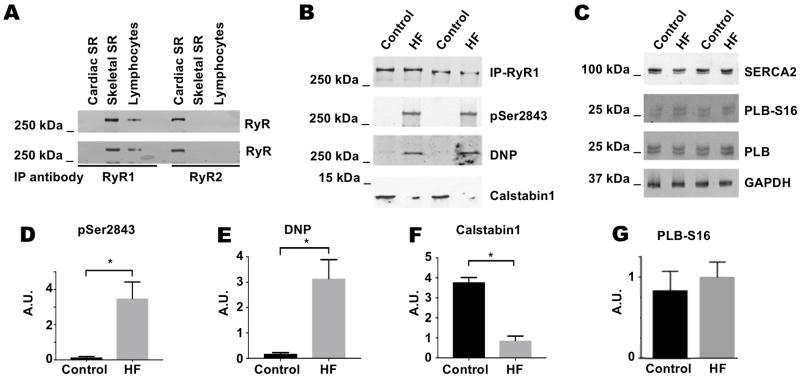
Immunoprecipitation using isoform specific anti-RyR1 and anti-RyR2 antibodies demonstrated that RyR1 but not RyR2 is expressed in circulating peripheral lymphocytes (Figure 1A), consistent with previous reports 43. RyR1 from CHF patients exhibited increased PKA phosphorylation at Ser2843, oxidation, and depletion of calstabin1 compared to control patients (Figure 1B, D-F. Also see supplemental Figure 1). These findings are consistent with previous reports that RyR2 from human myocardium 14, 34 and RyR1 from human 53 and canine 17 skeletal muscle are PKA phosphorylated and depleted of calstabin2 and calstabin1, respectively, in CHF. Levels of SERCA2, total PLB, and PKA phosphorylation of PLB at Ser16 were unchanged in the human lymphocyte CHF samples (Figure 1C, G). These data suggest that RyR1 and SERCA2 in B-lymphocytes are affected differently in CHF, and that RyR1 may exhibit increased opening and SR Ca2+ release without a corresponding increase in SR Ca2+ uptake via SERCA2.
Figure 1. RyR1 expressed in lymphocyte enriched cell population is pathologically remodeled in patients with CHF.
(A) RyR1 is immunoprecipitated from a lymphocyte enriched cell population isolated from normal patients using RyR1-specific antibodies but not with RyR2-specific antibodies. Experiment performed on lymphocytes isolated from two patients. (B) RyR1 immunoprecipitated from two CHF patients exhibits increased PKA phosphorylation at Ser2843 and oxidation (DNP), as well as depletion of calstabin1 compared to two control patients. (C) SERCA2 and PLB expression levels and PKA phosphorylation of PLB at Ser16 are unchanged in lymphocytes from two CHF patients and two control patients. (D–F) Quantification of data from 6 CHF and normal patients (includes data from Supplemental Figure 1). Results normalized to total RyR1 levels and expressed in arbitrary units. (G) Quantification of phosphorylation of PLB at Ser16 normalized to total PLB and GAPDH levels expressed in arbitrary units from western blot in (C). * represents P<0.05.
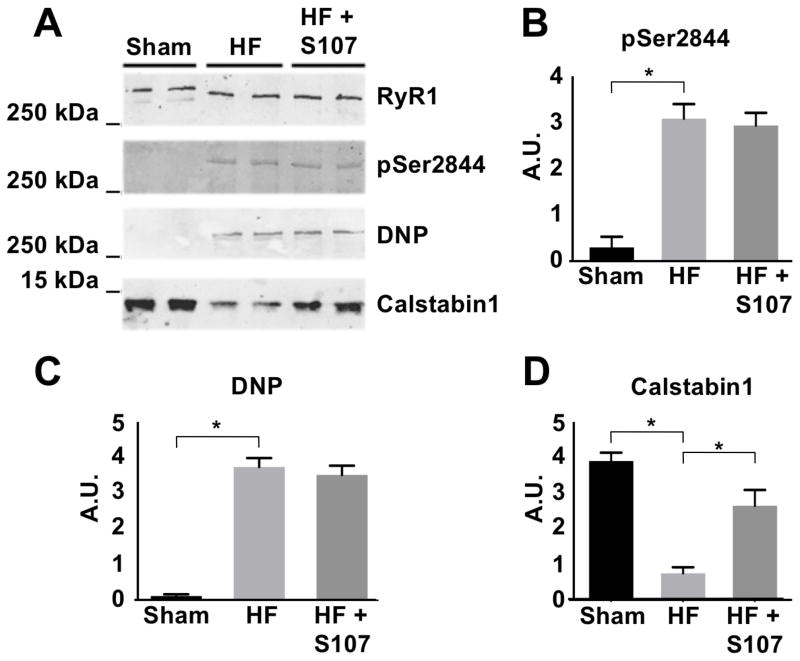
In order to validate that these results are specific to CHF, ischemic CHF was induced in mice by LAD occlusion as previously described 25. The CHF treatment group received S107, a drug that reduces Ca2+ leak through dysfunctional RyR1 51, via drinking water 35. RyR1 was immunoprecipitated from lymphocyte enriched pellets (Figure 2A). RyR1 from CHF mice exhibited increased PKA phosphorylation at Ser2844, oxidation, and depletion of calstabin1 compared to sham mice (Figure 2B–D. Also see supplemental Figure 2). Treatment with S107 did not affect PKA phosphorylation or oxidation of RyR1 but increased the level of calstabin1 that co-immunoprecipitates with the channel (i.e. is part of the channel macromolecular complex) as previously reported 22. These findings support our observations in RyR1 isolated from human lymphocytes, and are also consistent with previous reports that RyR2 and RyR1 21 are PKA phosphorylated, oxidized, and depleted of calstabin in mice with CHF 20, 26.
Figure 2. Pathologic remodeling of RyR1 in lymphocyte enriched cell population from mice with CHF, and reversal with S107.
(A) RyR1 immunoprecipitated from murine lymphocyte enriched cell population exhibits increased PKA phosphorylation at Ser2844, oxidation, and depletion of calstabin1 compared to WT mice. S107 inhibits the depletion of calstabin1 from the RyR1 complex without affecting PKA phosphorylation or oxidation levels. Experiments were performed from samples isolated from four mice in each group. (B–D) Quantification of western blot data (includes data from Supplemental Figure 2) expressed in arbitrary units. * P<0.05.
RyR1 in B-lymphocytes exhibits altered Ca2+ handling in humans and mice with CHF
In skeletal muscle, these biochemical changes in RyR1 (phosphorylation, depletion of clastabin1) are associated with channel dysfunction and Ca2+ leak 21. Flow cytometry has been previously used to assess RyR1 Ca2+ handling in B-lymphocytes from patients with malignant hyperthermia, a condition caused by dysfunctional RyR1 45. We used flow cytometry to assess ER Ca2+ load in B-lymphocytes as an indicator of chronic RyR1 Ca2+ leak resulting from exposure to elevated circulating catecholamine levels. B-lymphocytes were loaded with Fluo-4AM, a cytosolic-based fluorescent Ca2+ indicator. Baseline cytosolic Ca2+ fluorescence was measured. Afterwards, an RyR1 agonist that induces release of RyR1 mediated ER Ca2+ stores, either caffeine or 4-CmC 45, 54, was added to the cells and then Ca2+ fluorescence was re-measured. The % change of mean cytosolic Ca2+ fluorescence of CD-19+ cells following the addition of either RyR1 agonist, was calculated. Cells with reduced ER Ca2+ load secondary to chronic RyR1 Ca2+ leak would be expected to have a smaller % increase in cytosolic Ca2+ following the addition of either RyR1 agonist compared to cells without chronic RyR1 mediated ER Ca2+ leak.
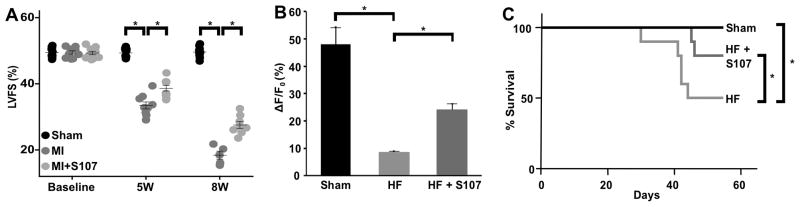
A mouse model of ischemic CHF was generated via mid LAD ligation. Mid LAD, as opposed to proximal LAD, was chosen in order to induce mild-moderate myocardial dysfunction while limiting mortality. FS, as assessed using echocardiography, revealed myocardial dysfunction in the post-MI mice at 5 and 8 weeks (Figure 3A). Mice treated with S107, a drug that improves cardiac and skeletal muscle function in CHF by reducing RyR mediated intracellular Ca2+ leak 20, 21, 35, 55, exhibited improved FS compared to untreated post-MI mice (Figure 3A). RyR1 Ca2+ leak in mouse peripheral, CD-19+, B-lymphocytes was assessed using flow cytometry. B-lymphocytes from CHF mice exhibited significantly reduced increase in cytosolic Ca2+ fluorescence compared to WT mice following the addition of the RyR1 agonist, caffeine (Figure 3B, WT, n=5, 48% ± 6% vs CHF, n=5, 9% ± 1%, P<0.01). These results are consistent with the presence of chronic RyR1 Ca2+ leak and reduced ER Ca2+ stores in the CHF lymphocytes. Mice with CHF treated with S107, exhibited significantly increased ER Ca2+ release following the addition of caffeine compared to untreated CHF mice (HF+S107, n=5, 24% ± 2% vs CHF, n=5, 9% ± 1%, P<0.01). The reduced RyR1 Ca2+ leak was associated with reduced 60-day mortality in the S107 treated CHF mice (Figure 3C). These findings support the validity of flow cytometry for specifically assessing RyR1 mediated intracellular Ca2+ leak in B-lymphocytes.
Figure 3. RyR1 Ca2+ leak in B-lymphocytes from mice with CHF, and improvement with S107 treatment.
(A) Left ventricular fractional shortening (LVFS) at baseline, 5 weeks, and 8 weeks in mice pre- and post-myocardial infarction, as measured by transthoracic echocardiography. N=10 mice in each group. (B) Quantification of the % change of mean Fluo-4 fluorescence of CD-19 positive cells in response to 50 mM caffeine. CHF mice exhibit a significantly reduced increase in cytosolic Ca2+ in response to caffeine, consistent with the presence of chronic RyR1 Ca2+ leak from intracellular ER Ca2+ stores. B-lymphocytes from mice treated with S107 exhibit increased cytosolic Ca2+ levels post caffeine consistent with reduced RyR1 Ca2+ leak and increased intracellular ER Ca2+ stores. Lymphocytes from 5 mice in each group were pooled within their respective groups. Graph represents mean data from 5 caffeine stimulations. (C) CHF mice treated with S107 in drinking water have decreased mortality compared to untreated mice: Sham (N=10), CHF (N=10), and CHF mice treated with S107 (N=10) were followed for 60 days post-MI. Untreated CHF mice exhibited significantly increased mortality at 60-days compared to the sham group. CHF mice treated with S107 have significantly reduced mortality compared to untreated CHF mice. In all studies significance (*) was defined as P<0.05
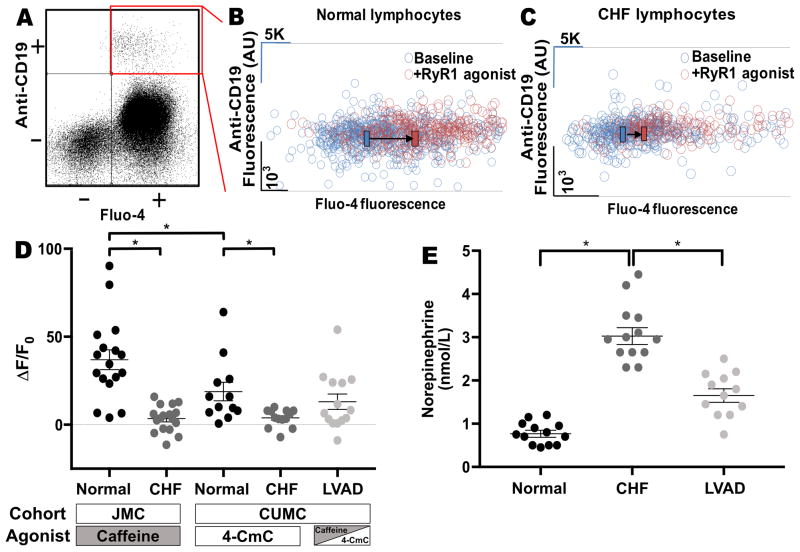
In order to assess whether intracellular Ca2+ handling in B-lymphocytes is dysfunctional in humans, patients from two independent centers (JMC and CUMC), with and without CHF, as well as CHF status-post LVAD implant, were recruited. Samples obtained from JMC were treated with the RyR agonist caffeine, while samples from normal and CHF patients recruited at CUMC were treated with the RyR agonist 4-CmC. LVAD samples were obtained from CUMC and treated with either caffeine or 4-CmC. B-lymphocytes from CHF patients at both centers exhibited a reduced increase in cytosolic Ca2+ levels following the administration of an RyR1 agonist compared to non-CHF patients (Figures 4A–D JMC normal, N=17, 37% ± 6% vs JMC CHF, N=14, 5% ± 2%, P<0.05; CUMC normal, N=13, 17% ± 5% vs CUMC CHF, N=13, 4% ± 1%, P<0.05). These results are consistent with the presence of chronic RyR1-mediated ER Ca2+ leak leading to reduced ER Ca2+ stores in the CHF cells. We observed that B-lymphocytes from control patients treated with the RyR agonist caffeine had a significantly increased response compared to samples treated with 4-CmC (JMC normal, N=17, 37% ± 6% vs CUMC normal, N=13, 17% ± 5%, P<0.05). This suggests that caffeine may have a greater effect on ryanodine releasable stores compared to 4-CmC. B-lymphocytes from the LVAD patients exhibited a significantly reduced response to caffeine compared to patients from the combined normal cohort, however there was no significant improvement between the LVAD group and combined CHF groups (Figure 4D, normal, N=30, 28% ± 4% vs CHF, N=27, 4% ± 1% vs LVAD, N=14, 13% ± 4%, P=N.S.). The LVAD group exhibited relatively larger heterogeneity compared to the CHF group, suggesting that some patients may have experienced improvement (Figure 4D, CHF STDEV=6%, LVAD STDEV=16%). Circulating NE levels were measured in normal, CHF, and LVAD patients. NE levels were significantly increased in CHF patients compared to normal and there was a significant reduction observed in the LVAD patients compared to CHF patients (Normal, N=13, 0.8 nmol/L ± 0.07 nmol/L vs CHF, N=13, 3 nmol/L ± 0.2 nmol/L vs LVAD, N=12, 1.7 nmol/L ± 0.15 nmol/L, P<0.05), (Figure 4E)
Figure 4. B-lymphocytes exhibit RyR1 Ca2+ leak in patients with CHF.
(A) Quadrants of flow cytometry assay. Scatter plot of Anti-CD19 fluorescence (B-cell specific antibody) on the Y-axis vs Fluo-4 fluorescence (fluorescent cytosolic Ca2+ marker) on the X-axis. Quadrant of interest (CD-19+ cells, loaded with Fluo4-AM) highlighted in red. (B–C) Representative graph of B-lymphocyte fluorescence pre- and post- addition of RyR1 agonist (caffeine or 4-CmC) as measured using flow-cytometry. Each circle represents a single cell. The cells displayed exhibit both Anti-CD19 and Fluo-4 fluorescence. Black bars and direction arrows represent the shift of the mean Fluo-4 fluorescence pre- and post- administration of RyR1 agonist. Post-RyR1 agonist data in both examples is equally offset in order to more clearly delineate the pre- and post- data. (D) Quantification of the % change of mean Fluo-4 fluorescence of CD-19 positive cells at baseline in response to 50 mM caffeine or 0.5 mM 4-CmC. Data segregated by cohort site (JMC and CUMC). JMC normal, N=17, 37% ± 6% vs JMC CHF, N=14, 5% ± 2%, P<0.05; CUMC normal, N=13, 17% ± 5% vs CUMC CHF, N=13, 4% ± 1, P<0.05; LVAD N=14, 13% ± 4%). Caffeine induces a greater response in the control population compared to 4-CmC. (E) Plasma norepinephrine levels measured in normal (N=13), CHF (N=13), and LVAD patients (N=12). Each sample tested in duplicate and average calculated. * represents P<0.05
Discussion
Circulating biomarkers that assess cardiac recovery in CHF are needed for monitoring response to pharmacologic and mechanical CHF therapy 56. Plasma catecholamine levels correlate with CHF severity and initiation of CHF therapy. However, they are difficult and complex to monitor 8. Persistently elevated circulating NE levels cause pathologic RyR2 Ca2+ leak in the heart and RyR1 Ca2+ leak in skeletal muscle, contributing to CHF progression and risk of sudden death 13, 14, 17, 21, 28, 29, 36. This study assessed whether elevated circulating NE levels in CHF are associated with pathological RyR1 remodeling and dysfunctional intracellular Ca2+ handling/metabolism in circulating B-lymphocytes and whether this improves with LVAD therapy.
Using a flow cytometry-based assay we observed that RyR1 mediated Ca2+ handling in circulating B-lymphocytes is dysfunctional in mice and humans with CHF. The specificity of this assay for measuring RyR1 mediated Ca2+ leak was demonstrated by testing two separate RyR1 agonists and by using CHF mice treated with S107, a drug that specifically targets RyR Ca2+ leak 51. The S107 treated CHF mice exhibited reduced intracellular Ca2+ leak and mortality compared to non-treated CHF controls. The association between decreased B-lymphocyte Ca2+ leak and mortality in the CHF mice suggests that this assay, once optimized, may have clinical utility for assessing the response to clinically approved CHF therapies and for prognosticating CHF patients.
We observed that CHF patients had significantly higher circulating NE levels compared to normal patients, and that these levels correlated with increased RyR1-mediated Ca2+ leak in B-lymphocytes. This association supports our hypothesis that chronically elevated circulating NE levels in CHF can cause RyR remodeling and dysfunction in any organ system where the channel is expressed. The specificity of this test for detecting cardiomyopathy, as opposed to co-morbid pathologies which are commonly present in patients with CHF is supported by the narrow range of responses to RyR1 agonists within the CHF population (−11% to 16%, STDEV 6), suggesting that unknown variables likely play a small role. However, larger studies are needed to adequately address this. While the LVAD patients exhibited decreased circulating NE levels due to improved cardiac output, the observed reduction in B-lymphocyte intracellular Ca2+ leak was not as robust. This finding is likely due to the heterogeneity of LVAD patients and raises the possibility that this assay may identify patients with dysfunctional intracellular Ca2+ handling despite improvement in circulating catecholamine levels. Future studies should focus on the prognosis of these different patient groups.
We have previously reported in a canine model of CHF that β-blocker therapy reverses PKA phosphorylation of RyR2 and depletion of calstabin2 28. However, in the present study despite the fact that that over 95% of CHF patients were prescribed β-blocker therapy (Table 1) the CHF samples tested exhibited PKA phosphorylation of RyR1 and depletion of calstabin1 compared to controls (Figure 1). Possible explanations for this discrepancy include poor compliance with medication and/or insufficient dosing or length of time on medication in the CHF patients. Indeed, the lack of differences in the heart rates (HR) or blood pressures (SBP, DBP) between the CHF and control groups (Table 1) support the hypothesis that these CHF patients were not physiologically β-blocked.
The contribution of RyR1 Ca2+ handling to B-lymphocyte function is poorly understood. Intracellular Ca2+ release in B-lymphocytes plays a key role in B-cell activation following antigen challenge 57. Following activation of the antigen receptor complex inositol trisphosphate (IP3) is produced and binds to IP3-receptors (IP3R) on the ER causing Ca2+ release from the ER 58. The ER Ca2+ sensors stromal interaction molecule (STIM)1 and STIM2 sense the drop in ER Ca2+ and activate plasma membrane Ca2+ channels Orai1-3 resulting in influx of extracellular Ca2+ into the cell 58. This process, known as store operated Ca2+ entry (SOCE), provides the necessary Ca2+ to drive gene transcription and the immune response 57. Ca2+ is then pumped back into the ER through SERCA2 and SERCA3.
RyR1 expression in B-lymphocytes has been previously described in studies employing a PCR-based restriction fragment length polymorphism method, [H3] ryanodine binding assay, and dose-dependent activation by the RyR specific activator 4-CmC 43. [H3]ryanodine binding studies have identified a single low affinity ryanodine binding site on B-lymphocyte RyR1, in contrast to the high and low affinity sites previously reported in skeletal muscle RyR1 59. It is intriguing that the low affinity ryanodine binding site on B-lymphocyte RyR1 behaves similar to the high affinity ryanodine binding site found in skeletal muscle RyR1, i.e. by increasing the Ca2+ release response to 4-CmC as opposed to inhibiting it 43. While the contribution of RyR1 to B-lymphocyte activation is not well understood, depletion of RyR1-gated stores significantly reduces the Ca2+ peak observed following antigen binding to B-cell receptor 43.
Studies have demonstrated that B-lymphocytes isolated from patients with malignant hyperthermia (MH), a disease characterized by leaky RyR1, exhibit increased sensitivity to caffeine induced ER Ca2+ release confirming the presence of functional RyR channels in B-lymphocytes 45. These studies, in addition to our animal data where mice with ischemic cardiomyopathy were treated with S107, a drug that specifically reduces RyR1 Ca2+ leak, support the specificity of our assay for assessing RyR1 Ca2+ leak in this cell population. The fact that we did not observe any significant differences with our assay between patients with ischemic or non-ischemic cardiomyopathy, suggests that the co-morbid conditions frequently associated with coronary artery disease were not responsible for our observations. The results of our study also raise the possibility that RyR1 dysfunction in B-lymphocytes may affect immune function in CHF.
The need for monitoring the effectiveness of CHF therapies highlights the importance of tests that can non-invasively assess intracellular Ca2+ handling, which is defective in cardiac and skeletal muscle, in CHF patients 21. In this study, we demonstrate the utility of a flow cytometry-based assay for measuring RyR1 intracellular Ca2+ leak in circulating B-lymphocytes as a surrogate for RyR2 function in cardiac and skeletal muscles. By testing the downstream molecular consequences of elevated NE levels this assay may have superior specificity for CHF and LVAD outcomes compared to available biomarkers such as brain naturetic peptide (BNP), whose levels correlate with existing ventricular dysfunction. In fact, we observed a correlation between decreased mortality and reduced B-lymphocyte RyR1 mediated intracellular Ca2+ leak in mice.
This B-lymphocyte Ca2+ leak assay is relatively simple and inexpensive to perform using standard reagents and equipment available in clinical laboratories. The assay may be useful for following patients serially over time or at a single time point. Larger studies are needed to assess the accuracy and reproducibility of these measurements and to eventually determine whether the degree of RyR1-mediated ER Ca2+ leak in B-lymphocytes correlates with CHF interventions and outcomes, and whether this test has prognostic significance in patients with LVAD.
Supplementary Material
Clinical Perspective.
1) What is new?
Systemic neurohormonal activation in heart failure causes dysfunctional intracellular calcium handling in cardiomyocytes, leading to disease progression.
It is not feasible to assess and monitor this in patients.
We demonstrate in humans and mice with heart failure that circulating B-lymphocytes exhibit dysfunctional intracellular calcium handling, in a manner similar to what is observed in cardiomyocytes.
A subgroup of heart failure patients status-post LVAD exhibited improved intracellular calcium handling.
This was assessed using flow cytometry, a readily available clinical laboratory technique.
2) What are the clinical implications?
Testing intracellular calcium handling in circulating B-lymphocytes is a potential novel biomarker for myocardial dysfunction and response to therapy in patients with heart failure and may help identify subgroups of LVAD patients with myocardial recovery.
Acknowledgments
Funding sources: This work was supported by a grant from the National Heart Lung and Blood Institute to ARM (R01HL061503), a grant from the NIH to RNK (5R01HL128071, 5R01HL130861, 1R01HL138475) and a grant from the American Medical Association Foundation to AK. GS is supported by a grant from the NIH (K99/R00DK107895). Research reported in this publication was performed in the CCTI Flow Cytometry Core, supported in part by the Office of the Director, National Institutes of Health under awards S10RR027050. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Research reported in this publication was performed at the Flow Cytometry Core Facility at Albert Einstein College of Medicine, supported in part by the Einstein National Cancer Institute’s cancer center support grant P30CA013330.
Footnotes
Disclosures: Andrew R. Marks is a consultant and member of the board for a start-up company, and has shares in ARMGO Pharma Inc. that is targeting RyR2 to prevent heart failure and sudden cardiac death.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gustafsson F, Rogers JG. Left ventricular assist device therapy in advanced heart failure: patient selection and outcomes. Eur J Heart Fail. 2017;19:595–602. doi: 10.1002/ejhf.779. [DOI] [PubMed] [Google Scholar]
- 3.Frazier OH, Baldwin AC, Demirozu ZT, Segura AM, Hernandez R, Taegtmeyer H, Mallidi H, Cohn WE. Ventricular reconditioning and pump explantation in patients supported by continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2015;34:766–772. doi: 10.1016/j.healun.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 5.Jackson G, Gibbs CR, Davies MK, Lip GY. ABC of heart failure. Pathophysiology. BMJ. 2000;320:167–170. doi: 10.1136/bmj.320.7228.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swedberg K, Eneroth P, Kjekshus J, Wilhelmsen L. Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. CONSENSUS Trial Study Group. Circulation. 1990;82:1730–1736. doi: 10.1161/01.cir.82.5.1730. [DOI] [PubMed] [Google Scholar]
- 7.Benedict CR, Francis GS, Shelton B, Johnstone DE, Kubo SH, Kirlin P, Nicklas J, Liang CS, Konstam MA, Greenberg B, Yusuf S. Effect of long-term enalapril therapy on neurohormones in patients with left ventricular dysfunction. SOLVD Investigators. Am J Cardiol. 1995;75:1151–1157. doi: 10.1016/s0002-9149(99)80748-6. [DOI] [PubMed] [Google Scholar]
- 8.Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, Fonarow GC, Greenberg B, Januzzi JL, Jr, Kiernan MS, Liu PP, Wang TJ, Yancy CW, Zile MR American Heart Association Clinical Pharmacology Committee of the Council on Clinical C, Council on Basic Cardiovascular S, Council on Cardiovascular Disease in the Y, Council on C, Stroke N, Council on Cardiopulmonary CCP, Resuscitation, Council on E, Prevention, Council on Functional G, Translational B, Council on Quality of C and Outcomes R. Role of Biomarkers for the Prevention, Assessment, and Management of Heart Failure: A Scientific Statement From the American Heart Association. Circulation. 2017;135:e1054–e1091. doi: 10.1161/CIR.0000000000000490. [DOI] [PubMed] [Google Scholar]
- 9.Wachter SB, Gilbert EM. Beta-adrenergic receptors, from their discovery and characterization through their manipulation to beneficial clinical application. Cardiology. 2012;122:104–112. doi: 10.1159/000339271. [DOI] [PubMed] [Google Scholar]
- 10.Bellinger AM, Mongillo M, Marks AR. Stressed out: the skeletal muscle ryanodine receptor as a target of stress. J Clin Invest. 2008;118:445–453. doi: 10.1172/JCI34006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maki T, Gruver EJ, Davidoff AJ, Izzo N, Toupin D, Colucci W, Marks AR, Marsh JD. Regulation of calcium channel expression in neonatal myocytes by catecholamines. J Clin Invest. 1996;97:656–663. doi: 10.1172/JCI118462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehnart SE, Wehrens XH, Kushnir A, Marks AR. Cardiac ryanodine receptor function and regulation in heart disease. Ann N Y Acad Sci. 2004;1015:144–159. doi: 10.1196/annals.1302.012. [DOI] [PubMed] [Google Scholar]
- 13.Shan J, Kushnir A, Betzenhauser MJ, Reiken S, Li J, Lehnart SE, Lindegger N, Mongillo M, Mohler PJ, Marks AR. Phosphorylation of the ryanodine receptor mediates the cardiac fight or flight response in mice. J Clin Invest. 2010;120:4388–4398. doi: 10.1172/JCI32726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 15.Reiken S, Gaburjakova M, Guatimosim S, Gomez AM, D’Armiento J, Burkhoff D, Wang J, Vassort G, Lederer WJ, Marks AR. Protein kinase A phosphorylation of the cardiac calcium release channel (ryanodine receptor) in normal and failing hearts. Role of phosphatases and response to isoproterenol. J Biol Chem. 2003;278:444–453. doi: 10.1074/jbc.M207028200. [DOI] [PubMed] [Google Scholar]
- 16.Andersson DC, Meli AC, Reiken S, Betzenhauser MJ, Umanskaya A, Shiomi T, D’Armiento J, Marks AR. Leaky ryanodine receptors in beta-sarcoglycan deficient mice: a potential common defect in muscular dystrophy. Skelet Muscle. 2012;2:9. doi: 10.1186/2044-5040-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiken S, Lacampagne A, Zhou H, Kherani A, Lehnart SE, Ward C, Huang F, Gaburjakova M, Gaburjakova J, Rosemblit N, Warren MS, He KL, Yi GH, Wang J, Burkhoff D, Vassort G, Marks AR. PKA phosphorylation activates the calcium release channel (ryanodine receptor) in skeletal muscle: defective regulation in heart failure. J Cell Biol. 2003;160:919–928. doi: 10.1083/jcb.200211012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kushnir A, Shan J, Betzenhauser MJ, Reiken S, Marks AR. Role of CaMKIIdelta phosphorylation of the cardiac ryanodine receptor in the force frequency relationship and heart failure. Proc Natl Acad Sci U S A. 2010;107:10274–10279. doi: 10.1073/pnas.1005843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HC, Clusin WT. Cytosolic calcium staircase in cultured myocardial cells. Circ Res. 1987;61:934–939. doi: 10.1161/01.res.61.6.934. [DOI] [PubMed] [Google Scholar]
- 20.Shan J, Betzenhauser MJ, Kushnir A, Reiken S, Meli AC, Wronska A, Dura M, Chen BX, Marks AR. Role of chronic ryanodine receptor phosphorylation in heart failure and beta-adrenergic receptor blockade in mice. J Clin Invest. 2010;120:4375–4387. doi: 10.1172/JCI37649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wehrens XH, Lehnart SE, Reiken S, van der Nagel R, Morales R, Sun J, Cheng Z, Deng SX, de Windt LJ, Landry DW, Marks AR. Enhancing calstabin binding to ryanodine receptors improves cardiac and skeletal muscle function in heart failure. Proc Natl Acad Sci U S A. 2005;102:9607–9612. doi: 10.1073/pnas.0500353102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersson DC, Betzenhauser MJ, Reiken S, Meli AC, Umanskaya A, Xie W, Shiomi T, Zalk R, Lacampagne A, Marks AR. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011;14:196–207. doi: 10.1016/j.cmet.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L, Matecki S, Lacampagne A, Marks AR. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med. 2009;15:325–330. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brillantes AB, Ondrias K, Scott A, Kobrinsky E, Ondriasova E, Moschella MC, Jayaraman T, Landers M, Ehrlich BE, Marks AR. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994;77:513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 25.Lehnart SE, Wehrens XH, Reiken S, Warrier S, Belevych AE, Harvey RD, Richter W, Jin SL, Conti M, Marks AR. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell. 2005;123:25–35. doi: 10.1016/j.cell.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci U S A. 2006;103:511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng H, Lederer WJ. Calcium sparks. Physiol Rev. 2008;88:1491–545. doi: 10.1152/physrev.00030.2007. [DOI] [PubMed] [Google Scholar]
- 28.Reiken S, Gaburjakova M, Gaburjakova J, He Kl KL, Prieto A, Becker E, Yi Gh GH, Wang J, Burkhoff D, Marks AR. beta-adrenergic receptor blockers restore cardiac calcium release channel (ryanodine receptor) structure and function in heart failure. Circulation. 2001;104:2843–2848. doi: 10.1161/hc4701.099578. [DOI] [PubMed] [Google Scholar]
- 29.Antos CL, Frey N, Marx SO, Reiken S, Gaburjakova M, Richardson JA, Marks AR, Olson EN. Dilated cardiomyopathy and sudden death resulting from constitutive activation of protein kinase a. Circ Res. 2001;89:997–1004. doi: 10.1161/hh2301.100003. [DOI] [PubMed] [Google Scholar]
- 30.Hobai IA, O’Rourke B. Decreased sarcoplasmic reticulum calcium content is responsible for defective excitation-contraction coupling in canine heart failure. Circulation. 2001;103:1577–1584. doi: 10.1161/01.cir.103.11.1577. [DOI] [PubMed] [Google Scholar]
- 31.Santulli G, Xie W, Reiken SR, Marks AR. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci U S A. 2015;112:11389–11394. doi: 10.1073/pnas.1513047112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulze D, Kofuji P, Hadley R, Kirby MS, Kieval RS, Doering A, Niggli E, Lederer WJ. Sodium/calcium exchanger in heart muscle: molecular biology, cellular function, and its special role in excitation-contraction coupling. Cardiovasc Res. 1993;27:1726–1734. doi: 10.1093/cvr/27.10.1726. [DOI] [PubMed] [Google Scholar]
- 33.Shan J, Xie W, Betzenhauser M, Reiken S, Chen BX, Wronska A, Marks AR. Calcium leak through ryanodine receptors leads to atrial fibrillation in 3 mouse models of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2012;111:708–717. doi: 10.1161/CIRCRESAHA.112.273342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klotz S, Barbone A, Reiken S, Holmes JW, Naka Y, Oz MC, Marks AR, Burkhoff D. Left ventricular assist device support normalizes left and right ventricular beta-adrenergic pathway properties. J Am Coll Cardiol. 2005;45:668–676. doi: 10.1016/j.jacc.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 35.Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, Coromilas J, Landry DW, Marks AR. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 36.Reiken S, Wehrens XH, Vest JA, Barbone A, Klotz S, Mancini D, Burkhoff D, Marks AR. Beta-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation. 2003;107:2459–2466. doi: 10.1161/01.CIR.0000068316.53218.49. [DOI] [PubMed] [Google Scholar]
- 37.Jayaraman T, Ondriasova E, Ondrias K, Harnick DJ, Marks AR. The inositol 1,4,5-trisphosphate receptor is essential for T-cell receptor signaling. Proc Natl Acad Sci U S A. 1995;92:6007–6011. doi: 10.1073/pnas.92.13.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harnick DJ, Jayaraman T, Ma Y, Mulieri P, Go LO, Marks AR. The human type 1 inositol 1,4,5-trisphosphate receptor from T lymphocytes. Structure, localization, and tyrosine phosphorylation. J Biol Chem. 1995;270:2833–2840. doi: 10.1074/jbc.270.6.2833. [DOI] [PubMed] [Google Scholar]
- 39.Jayaraman T, Ondrias K, Ondriasova E, Marks AR. Regulation of the inositol 1,4,5-trisphosphate receptor by tyrosine phosphorylation. Science. 1996;272:1492–1494. doi: 10.1126/science.272.5267.1492. [DOI] [PubMed] [Google Scholar]
- 40.DeSouza N, Reiken S, Ondrias K, Yang YM, Matkovich S, Marks AR. Protein kinase A and two phosphatases are components of the inositol 1,4,5-trisphosphate receptor macromolecular signaling complex. J Biol Chem. 2002;277:39397–39400. doi: 10.1074/jbc.M207059200. [DOI] [PubMed] [Google Scholar]
- 41.Cui J, Matkovich SJ, deSouza N, Li S, Rosemblit N, Marks AR. Regulation of the type 1 inositol 1,4,5-trisphosphate receptor by phosphorylation at tyrosine 353. J Biol Chem. 2004;279:16311–16316. doi: 10.1074/jbc.M400206200. [DOI] [PubMed] [Google Scholar]
- 42.deSouza N, Cui J, Dura M, McDonald TV, Marks AR. A function for tyrosine phosphorylation of type 1 inositol 1,4,5-trisphosphate receptor in lymphocyte activation. J Cell Biol. 2007;179:923–934. doi: 10.1083/jcb.200708200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sei Y, Gallagher KL, Basile AS. Skeletal muscle type ryanodine receptor is involved in calcium signaling in human B lymphocytes. J Biol Chem. 1999;274:5995–6002. doi: 10.1074/jbc.274.9.5995. [DOI] [PubMed] [Google Scholar]
- 44.Hosoi E, Nishizaki C, Gallagher KL, Wyre HW, Matsuo Y, Sei Y. Expression of the ryanodine receptor isoforms in immune cells. J Immunol. 2001;167:4887–4894. doi: 10.4049/jimmunol.167.9.4887. [DOI] [PubMed] [Google Scholar]
- 45.Sei Y, Brandom BW, Bina S, Hosoi E, Gallagher KL, Wyre HW, Pudimat PA, Holman SJ, Venzon DJ, Daly JW, Muldoon S. Patients with malignant hyperthermia demonstrate an altered calcium control mechanism in B lymphocytes. Anesthesiology. 2002;97:1052–1058. doi: 10.1097/00000542-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Thomas JA, Marks BH. Plasma norepinephrine in congestive heart failure. Am J Cardiol. 1978;41:233–243. doi: 10.1016/0002-9149(78)90162-5. [DOI] [PubMed] [Google Scholar]
- 47.Grimaldi CM, Cleary J, Dagtas AS, Moussai D, Diamond B. Estrogen alters thresholds for B cell apoptosis and activation. J Clin Invest. 2002;109:1625–1633. doi: 10.1172/JCI14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santulli G, Campanile A, Spinelli L, Assante di Panzillo E, Ciccarelli M, Trimarco B, Iaccarino G. G protein-coupled receptor kinase 2 in patients with acute myocardial infarction. Am J Cardiol. 2011;107:1125–1130. doi: 10.1016/j.amjcard.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 49.Callaway C, Seryshev A, Wang JP, Slavik KJ, Needleman DH, Cantu C, 3rd, Wu Y, Jayaraman T, Marks AR, Hamilton SL. Localization of the high and low affinity [3H]ryanodine binding sites on the skeletal muscle Ca2+ release channel. J Biol Chem. 1994;269:15876–15884. [PubMed] [Google Scholar]
- 50.Marks AR, Tempst P, Hwang KS, Taubman MB, Inui M, Chadwick C, Fleischer S, Nadal-Ginard B. Molecular cloning and characterization of the ryanodine receptor/junctional channel complex cDNA from skeletal muscle sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1989;86:8683–8687. doi: 10.1073/pnas.86.22.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bellinger AM, Reiken S, Dura M, Murphy PW, Deng SX, Landry DW, Nieman D, Lehnart SE, Samaru M, LaCampagne A, Marks AR. Remodeling of ryanodine receptor complex causes “leaky” channels: a molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci U S A. 2008;105:2198–2202. doi: 10.1073/pnas.0711074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin chim acta. 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 53.Rullman E, Andersson DC, Melin M, Reiken S, Mancini DM, Marks AR, Lund LH, Gustafsson T. Modifications of skeletal muscle ryanodine receptor type 1 and exercise intolerance in heart failure. J heart lung transplant. 2013;32:925–929. doi: 10.1016/j.healun.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sei Y, Gallagher KL, Daly JW. Multiple effects of caffeine on Ca2+ release and influx in human B lymphocytes. Cell Calcium. 2001;29:149–160. doi: 10.1054/ceca.2000.0175. [DOI] [PubMed] [Google Scholar]
- 55.Lehnart SE, Terrenoire C, Reiken S, Wehrens XH, Song LS, Tillman EJ, Mancarella S, Coromilas J, Lederer WJ, Kass RS, Marks AR. Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias. Proc Natl Acad Sci U S A. 2006;103:7906–7910. doi: 10.1073/pnas.0602133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bozkurt B, Mann DL. Use of biomarkers in the management of heart failure: are we there yet? Circulation. 2003;107:1231–1233. doi: 10.1161/01.cir.0000057608.97285.20. [DOI] [PubMed] [Google Scholar]
- 57.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 58.Baba Y, Kurosaki T. Impact of Ca2+ signaling on B cell function. Trends Immunol. 2011;32:589–594. doi: 10.1016/j.it.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 59.Zucchi R, Ronca-Testoni S. The sarcoplasmic reticulum Ca2+ channel/ryanodine receptor: modulation by endogenous effectors, drugs and disease states. Pharmacol Rev. 1997;49:1–51. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.