Abstract
Membrane lipid composition and remodeling influence the function of ion channels. Polyunsaturated fatty acids (PUFAs) and their derivatives modulate ion channel function; whether this effect occurs directly by binding to the protein or indirectly through alteration of membranes' mechanical properties has been difficult to distinguish. There are a large number of studies addressing the effect of fatty acids; recent structural and functional analyses have identified binding sites and provided further evidence for the role of the plasma membrane in ion channel function. Here, we review cation channels that do not share a common topology or lipid-binding signature sequence, but for which there are recent compelling data that support both direct and indirect modulation by PUFAs or their derivatives.
Introduction
Biological membranes are very heterogeneous, not only in composition but also in spatial and temporal distribution [1]. They carry out multiple physiological roles, including acting as a physical barrier for ions and solutes, regulating membrane protein function, and mediating signal transmission. Singer and Nicolson proposed the fluid mosaic model 45 years ago to describe the structure of cell membranes [2]. Even though our view of biological membranes has not stood still since 1972, the model has aged considerably well. New fundamental concepts have been established to further understand the dynamic relationships between membranes and the proteins embedded in them [3]. For instance, the arrangement between membrane proteins and the lipid bilayer allows communication in two directions: one in which the lipid composition influences protein function and the other in which proteins perturb the surrounding bilayer while undergoing conformational changes [4]. Polyunsaturated fatty acids (PUFAs) are among the membrane lipid components that dynamically regulate membrane protein function. PUFAs are essential molecules that regulate gene expression, receptor signaling, and plasma membrane remodeling [5]. Notably, membranes containing different levels of PUFAs feature distinct elastic properties [6,7]. PUFAs occur esterified or as free fatty acids cleaved from the plasma membrane by phospholipases (PL: A1, A2, C, and D). Once released, PUFAs can be further metabolized [8], act as second messengers [9], interact with membrane proteins [10], and/or alter the mechanical properties of the bilayer [11-15]. Yet distinguishing between some of these modalities has been quite challenging.
The “force-from-lipids” principle [16] establishes that changes in bilayer force provide the energy that is needed to drive or facilitate conformational rearrangements underlying ion channel opening and closing (i.e., gating). This principle was first demonstrated 30 years ago when the purified bacterial mechanosensitive channel of large conductance (MscL) remained mechanosensitive even after reconstitution into pure bilayers [17]. However, the force-from-lipids effect also applies to other families of ion channels. Importantly, recent structure-function studies have demonstrated that lipids could also contribute to channel gating via direct lipid-protein interaction. As the direct effect of PUFAs on voltage-gated Na+, K+, and Ca2+ channels has been reviewed elsewhere [10], we will focus on other cation channels (Table 1) that do not necessarily share a common topology or PUFA-binding signature sequence, such as: glutamate receptors (N-methyl-D-aspartate receptor, NMDA), transient receptor potential (TRP) channels, mechanotransduction channel complexes, pentameric ligand-gated ion channels (pLGICs), and Ca2+-activated large-conductance K+ (BK) channels.
Table 1. Ion channels modulated directly and/or indirectly by lipids.
| Type of Interaction | Ion channel | Lipid | Effect on function | Reference |
|---|---|---|---|---|
| Indirect | NMDA | AA | Potentiation | [23] |
| TRP and TRPL | PIP2 depletion | Activation | [12,25] | |
| C. elegans mechanoreceptor complex | AA-containing phospholipids | Enhance activation | [14] | |
| TRPV4 | EEQ- containing phospholipids | Enhance activation | [15] | |
| Direct | pLGIC | DHA | Enhance desensitization | [34,35] |
| BK | DHA | Activation | [38-40] | |
| BK | LTB4 | Enhance activation | [41] | |
| TRPV1 | LPA | Activation | [48,49] | |
| TRPV1 | Anandamide | Activation | [50,52,54] |
Ion channels whose function are influenced by the mechanical properties of the membrane
NMDA receptors and membrane tension
NMDA receptors are glutamategated ion channels crucial for neuronal communication, synaptic plasticity, and cognitive functions [18]. Nearly 25 years ago, it was shown in cultured neurons that NMDA receptor currents are potentiated by arachidonic acid (AA) [19], docosahexaenoic acid (DHA) [20], and osmotic pressure [21], but inhibited by lysophospholipids (LPLs) [22]. Interestingly, mutagenesis studies ruled out the direct interaction between AA or LPL and the putative NMDA receptor fatty acid binding domain [22]. Still, after dismissing a direct interaction between fat molecules and the channel, it was unknown whether the receptors' gating properties responded to changes in membrane tension due to the incorporation of fatty acids and LPL. Following a reductionist approach of reconstituting purified channels into liposomes, Kloda and colleagues demonstrated that NMDA receptor function is enhanced by increasing the membrane lateral tension with negative pressure or AA incorporation [23]. Thanks to the ensemble of ex vivo and in vitro experiments, it is now recognized that AA modulates NMDA receptor gating by changes in bilayer mechanical properties rather than by specific protein-binding events.
Light-sensitive TRP and TRP-like channels and phospholipid hydrolysis
Phototransduction in Drosophila melanogaster is mediated by phospholipase C (PLC) and the subsequent activation of two distinct ion channels, TRP and TRP-like (L) [24]. For many years, the leading hypothesis was that PLC-mediated hydrolysis of PIP2 yielded the second messengers diacylglycerol and IP3, and a proton that gated these channels [16]. This natural assumption was challenged by the remarkable finding from Hardie and Franze [12,25] in which PIP2 depletion evokes changes in the mechanical properties of the membrane that in turn activates TRP and TRPL channels [24] (Figure 1a). Furthermore, increasing lipid crowding with cationic amphiphiles inhibited the photoreceptor light responses, suggesting the membrane as a key modulator of channel gating. These results were also supported by experiments in which manipulating the fly's diet (e.g., food without PUFAs) to increase plasma membrane stiffness slowed down light-induced responses [26]. The current model highlights the contribution of the membrane by supporting the idea that reduction in area, volume, and phospholipid crowding following PIP2 hydrolysis ultimately favors the protonation of previously buried sites in TRP and TRPL channels, which in turn promote channel gating [24].
Figure 1.
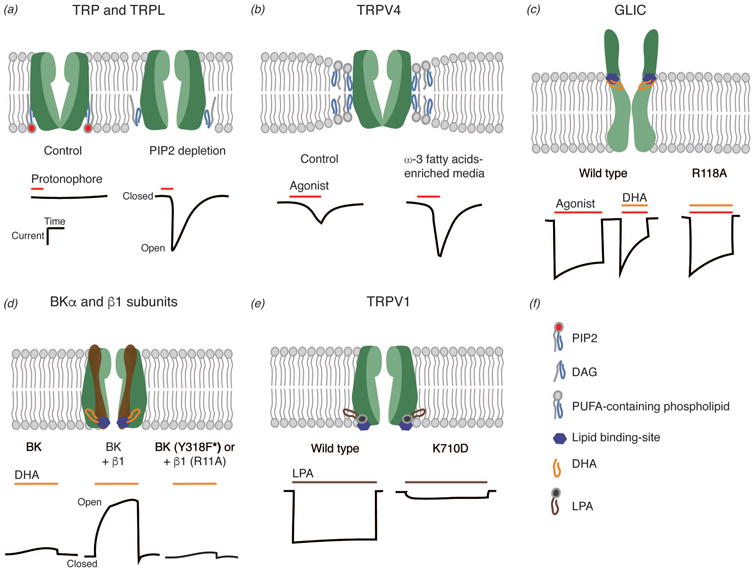
Mechanisms underlying ion channel regulation by lipids. (a) Light-sensitive TRP and TRP-like(L) channels. (b) Transient receptor potential cation channel subfamily V member 4 (TRPV4). (c) Prokaryotic pentameric ligand-gated ion channel from the cyanobacterium Gloeobacter violaceus (GLIC). (d) Ca2+-activated large-conductance K+ (BK) channel (α) and auxiliary subunit (β1). F*: phenylalanine-based unnatural amino acid residue. (e) Transient receptor potential cation channel subfamily V member 1 (TRPV1). (f) Schematic representation of phosphatidylinositol 4,5-bisphosphate (PIP2), diacylglycerol (DAG), polyunsaturated fatty acids (PUFAs)-containing phospholipids, docosahexaenoic acid (DHA), and lysophosphatidic acid (LPA).
C. elegans mechanoreceptor complex and AA-containing phospholipids
Mechanoelectrical transduction in C. elegans touch receptor neurons (TRNs) relies on at least 12 MEC proteins (MEC stands for proteins that when mutated confer mechanosensory abnormal phenotypes), including ion channels from the DEG/ENaC/ASIC family (MEC-4 and MEC-10) [27]. Recently, AA-containing phospholipids were shown to enhance the function of this multiprotein complex in the mechanical response of TRNs in vivo [14]. By examining the touch-elicited behavior of worms genetically unable to generate PUFAs, Vásquez et al. established that AA exerts its effect on mechanoelectrical transduction by influencing the viscoelastic properties of the plasma membrane rather than acting as a signaling molecule [14]. This was demonstrated by pulling membrane nanotubes with an atomic force microscopy (AFM) cantilever from the plasma membrane of native TRNs or TRNs genetically depleted of long PUFAs. In this context, one could imagine that the response of neurons to mechanical stimuli might be modulated by the presence of polyunsaturated bonds in the lipid acyl chains, creating a distinctive membrane environment that enhances the function of mechanoelectrical transduction channel complexes.
TRP vanilloid 4 and fatty acids
The TRP vanilloid 4 (TRPV4) channel contributes to intracellular Ca2+ homeostasis and is essential in mediating various physiological (e.g., vascular tone) and pathological conditions (e.g., neuromuscular diseases) [28]. TRPV4 is a polymodal ion channel activated by thermal, osmotic, and chemical stimuli [28]. Furthermore, ω-6 PUFAs, such as AA and its epoxyeicosatrienoic acid metabolites, have been shown to activate TRPV4 downstream of cell swelling [29,30]. Alternatively, it has been recently demonstrated that TRPV4 function greatly relies on the membrane environment. Plasma membranes enriched with ω-3 PUFA derivatives enhance TRPV4 activity when natively or transgenically expressed in human endothelial cells or C. elegans nociceptor neurons, respectively [15] (Figure 1b). Notably, among the fatty acids tested, the ω-3 eicosanoid derivative 17,18-EEQ (epoxyeicosatetraenoic acid) displayed the largest influence on TRPV4 activity, as well as in membrane fluidity, bending stiffness, and membrane structural disorder, as determined by differential scanning calorimetry and AFM [15]. The experimental results of Caires et al. support the notion that ω-3 PUFA derivatives enhance channel function mainly by altering membrane mechanics rather than through a direct activation mechanism.
Ion channels whose functions are modulated by direct lipid-protein interactions
pLGICs and DHA
The nicotinic acetylcholine receptor (nAChR) from Torpedo californica's electric organ is the archetypal ion channel of the pLGIC superfamily. Members of this family are responsible for fast intercellular communication at neuromuscular junctions in response to endogenous neurotransmitters [31]. Although the nAChR is a ligand-gated ion channel, its activation and desensitization rates are modulated by lipid composition [32]. Of particular importance is DHA, an ω-3 fatty acid, enriched in synaptic plasma membranes, that enhances neurotransmission [33]. Whether this fatty acid binds directly to pLGICs to alter their function was unknown until recently. Basak and colleagues determined the atomic resolution structure of a prokaryotic pLGIC (a proton-activated channel from the cyanobacterium Gloeobacter violaceus; GLIC) in the presence of DHA [34]. The crystal structure revealed a DHA molecule bound to the channel periphery (M4 transmembrane segment) interacting with Arg118 at the Cys-loop (in the extracellular domain; Figure 1c). Notably, DHA has the same effect enhancing GLIC desensitization rate as observed in the metazoan counterparts [35]. The interface between the transmembrane domain and the Cys-loop has been previously demonstrated to control desensitization in pLGICs [36]. Importantly, mutagenesis of GLIC R118A significantly reduced the effect of DHA on desensitization (Figure 1c, bottom). Although these results do not rule out that pLGIC gating may be also modified by the changes that free fatty acids exert on the bilayer elastic properties [11], they provide direct evidence that DHA binds to and alters the function of a pLGIC family member.
BK channels, DHA, and leukotrienes
BK channels are activated by transmembrane voltage and micromolar concentrations of Ca2+ [37]. These channels regulate excitability by translating local cytosolic Ca2+ increases into cell repolarization, a prominent feature for vascular tone and neurotransmission. Their function is significantly regulated by alternative splicing of a single gene (KCNMA1 in mammals) and other transcriptional and post-translational mechanisms, as well as association with auxiliary subunits (e.g., β1, β2, β3, and β4) [37]. A few years ago, Hoshi and colleagues determined that DHA also stimulates BK channels at nanomolar concentrations without requiring voltage activation or Ca2+ binding [38]. This effect on function was enhanced when BK channels were co-expressed with β1 or β4 subunits [39]. Notably, experimental evidence indicates that the effect of DHA on the BK-β1 channel complex is direct. For instance, mutagenesis of residues at the S6 helix of the BK channel (near the intracellular end of the conduction pathway, Tyr318 [40]) and the N-terminus of the β1 subunit (Arg11 and Cys18 [39]) abolished the effect of DHA on channel function (Figure 1d). Likewise, manipulation of PUFA derivatives and incorporation of unnatural amino acids led to the conclusion that the carboxylate group of DHA forms an ion-dipole bond with Tyr318 in BK [40]. These results support a model in which the effect of DHA on the BK-β1 channel complex function is mainly due to direct protein complex-fatty acid interactions.
Leukotrienes (LTs) are inflammatory eicosanoids derived from the oxidation of AA. It turns out that only LTB4, but no other LTs, enhances BK channel function at nanomolar concentrations when in complex with the β1 auxiliary subunit [41]. However, the mechanism seems to be different from the one depicted for DHA; LTB4 binds another region of the β1 subunit and requires an optimal Ca2+ concentration to exert its activatory effect. Combining computational docking experiments with site-directed mutagenesis, Bukiya and colleagues determined that LTB4 increases BK activity by binding to a cholane steroid-sensing site formed by residues Thr169, Ala176, and Lys179 in the second transmembrane segment of the β1 subunit [42]. Although DHA and LTB4 specifically interact with and enhance the function of the BK-β1 channel complex, it is important to note that membrane stretch also enhances the activation of BK channels expressed in vascular smooth muscle and HEK293 cells [43]. This underlines the supposition that changes in the mechanical properties of the membrane could also influence channel function, regardless of the direct interactions that the protein might have with specific fat molecules.
TRPV1, lysophosphatidic acid, and anandamide
The TRP vanilloid 1 (TRPV1) channel is an essential component of the cellular mechanism through which noxious stimuli evoke pain [44]. TRPV1 is a polymodal channel activated by heat, capsaicin, toxins, protons, and bioactive lipids [44]. Polymodal ion channels integrate many signals acting downstream of several enzymatic cascades. Hence, it is challenging to determine which stimuli act directly or indirectly on channel function. Here, we have chosen a couple of examples for which there is evidence pointing towards a direct interaction between TRPV1 and the bioactive lipids that regulate its function. For instance, lysophosphatidic acid (LPA) is a lipid metabolite – composed of a phosphate, a glycerol, and a fatty acid [45] – involved in the development of neuropathic pain [46]. Although LPA is best known for its role in activating G-protein-coupled receptors [47], Nieto-Posadas and colleagues showed that LPA activates TRPV1, as it elicits current magnitudes comparable to those obtained with the bona fide TRPV1 activator capsaicin [48,49]. Importantly, substitution of Lys710 at the C-terminal domain of the channel (Figure 1e) with neutral or negatively charged residues severely impairs the effect of LPA on binding as well as on channel function. These results provide strong evidence that TRPV1 is directly activated by LPA.
Anandamide, an endogenous metabolite derived from the non-oxidative metabolism of AA, activates TRPV1 on primary sensory neurons [50]. Even though anandamide modifies the membrane bilayer elastic properties [51], Jordt and Julius [52] determined that anandamide interacts with the TRPV1 vanilloid-binding pocket lined by residues Tyr511, Met547, and Thr550 [53]. Remarkably, mutant Y511A does not respond to anandamide or capsaicin, while keeping normal TRPV1 heat- and proton-evoked responses [52]. Later, using a reconstituted proteoliposome system, Cao and colleagues demonstrated that anandamide activation does not require other proteins or second messengers to evoke TRPV1 ionic currents [54]. We have previously reasoned here that direct binding does not rule out the influence that the membrane might have over channel function. However, there is compelling evidence to suggest that TRPV1 function is not modulated by membrane fluidity and/or curvature, since thermal activation does not change in cholesterol-containing membranes or when LPL is perfused to the membrane patch [54]. Moreover, capsaicin dose-response profiles of transgenic C. elegans expressing TRPV1 are indistinguishable between control worms and worms genetically depleted of long PUFAs [15,55]. Since it has been established that PUFA content alters the fluidity and bending stiffness of the bilayer [14], these results support the idea that TRPV1 function is not considerably modulated by the mechanical properties of the membrane.
Perspectives
The notion that ion channels only reside on a passive membrane is superseded by the fact that ion permeation relies on a dynamic cellular machine composed of both membrane proteins and lipids. However, identifying and determining the precise mechanism(s) by which lipids modulate (e.g., activate, enhance, desensitize, etc.) ion channel gating is a challenging task. For most of the ion channels reviewed here (Table 1), more than one experimental approach and laboratory were needed to establish direct and/or indirect protein-lipid interactions. Since these non-covalent interactions might not occur outside of the membrane environment and/or survive purification procedures, new approaches are required to determine their role in modulating ion channel gating. Of particular importance are nanodiscs, which are discoidal lipid bilayers stabilized by membrane scaffold proteins used to preserve the structure and function of recalcitrant membrane proteins [56,57]. New high-resolution structures of channels modulated by fatty acids in nanodiscs will provide definitive answers about this bipartite mechanism. Interestingly, new developments in mass spectrometry-based approaches have allowed quantification of lipid binding selectivity of some prokaryotic ion channels [58,59], which we hope will extend to mammalian receptors. Even if one could experimentally establish that a given ion channel is regulated by fatty acids and/or the membrane, it remains to be determined how impactful these are interactions in vivo.
Highlights.
- Fatty acids alter membrane mechanics and modify ion channel function.
- Fatty acids directly interact with ion channels and regulate their function.
- Structural studies reveal specific interactions between fatty acids and ion channels.
Acknowledgments
The authors thank Dr. Sudha Chakrapani, Dr. Alex M. Dopico, and Dr. Robert C. Foehring for critically reading the manuscript. Research in the authors' laboratories is supported by grants from the American Heart Association (15SDG25700146 to JFC-M and 16SDG26700010 to VV), National Institutes of Health (1-R01GM125629-01 to JFC-M), and United States-Israel Binational Science Foundation (2015221 to VV).
Footnotes
Conflict of interest statement: Nothing declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1••.Harayama T, Riezman H. Understanding the diversity of membrane lipid composition. Nat Rev Mol Cell Biol. 2018;1842:1–16. doi: 10.1038/nrm.2017.138. Reviews the current understanding of how membrane lipids diversity fulfills many biological functions. [DOI] [PubMed] [Google Scholar]
- 2.Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 3.Goñi FM. The basic structure and dynamics of cell membranes: An update of the Singer–Nicolson model. BBA - Biomembranes. 2014;1838:1467–1476. doi: 10.1016/j.bbamem.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen C, Goulian M, Andersen OS. Energetics of inclusion-induced bilayer deformations. Biophys J. 1998;74:1966–1983. doi: 10.1016/S0006-3495(98)77904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barden AE, Mas E, Mori TA. n-3 Fatty acid supplementation and proresolving mediators of inflammation. Curr Opin Lipidol. 2016;27:26–32. doi: 10.1097/MOL.0000000000000262. [DOI] [PubMed] [Google Scholar]
- 6.Rawicz W, Olbrich KC, McIntosh T, Needham D, Evans E. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys J. 2000;79:328–339. doi: 10.1016/S0006-3495(00)76295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajamoorthi K, Petrache HI, McIntosh TJ, Brown MF. Packing and viscoelasticity of polyunsaturated ω-3 and ω-6 lipid bilayers as seen by 2H NMR and X-ray diffraction. J Am Chem Soc. 2005;127:1576–1588. doi: 10.1021/ja046453b. [DOI] [PubMed] [Google Scholar]
- 8.Dennis EA, Rhee SG, Billah MM, Hannun YA. Role of phospholipase in generating lipid second messengers in signal transduction. The FASEB J. 1991;5:2068–2077. doi: 10.1096/fasebj.5.7.1901288. [DOI] [PubMed] [Google Scholar]
- 9.Khan WA, Blobe GC, Hannun YA. Arachidonic acid and free fatty acids as second messengers and the role of protein kinase C. Cell Signal. 1995;7:171–184. doi: 10.1016/0898-6568(94)00089-t. [DOI] [PubMed] [Google Scholar]
- 10•.Elinder F, Liin SI. Actions and mechanisms of polyunsaturated fatty acids on voltage-gated ion channels. Front Physiol. 2017;8:10–24. doi: 10.3389/fphys.2017.00043. Review of voltage-gated ion channels modulated by polyunsaturated fatty acids. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Bruno MJ, Koeppe RE, Andersen OS. Docosahexaenoic acid alters bilayer elastic properties. Proc Natl Acad Sci U S A. 2007;104:9638–9643. doi: 10.1073/pnas.0701015104. This study suggests that DHA may modulate membrane protein function by bilayer-mediated mechanisms that do not involve specific protein binding but rather changes in bilayer material properties. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12••.Hardie RC, Franze K. Photomechanical responses in Drosophila photoreceptors. Science. 2012;338:260–263. doi: 10.1126/science.1222376. First evidence that light-sensitive TRP and TRPL channels open in response to changes in membrane elastic properties. [DOI] [PubMed] [Google Scholar]
- 13.Charalambous K, Booth PJ, Woscholski R, Seddon JM, Templer RH, Law RV, Barter LMC, Ces O. Engineering de novo membrane-mediated protein-protein communication networks. J Am Chem Soc. 2012;134:5746–5749. doi: 10.1021/ja300523q. [DOI] [PubMed] [Google Scholar]
- 14.Vasquez V, Krieg M, Lockhead D, Goodman MB. Phospholipids that contain polyunsaturated fatty acids enhance neuronal cell mechanics and touch sensation. Cell Reports. 2014;6:70–80. doi: 10.1016/j.celrep.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15••.Caires R, Sierra-Valdez FJ, Millet JRM, Herwig JD, Roan E, Vasquez V, Cordero-Morales JF. Omega-3 fatty acids modulate TRPV4 function through plasma membrane remodeling. Cell Rep. 2017;21:246–258. doi: 10.1016/j.celrep.2017.09.029. First evidence that ω-3 fatty acids influence TRPV4 channel activity via membrane fluidity and bending stiffness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Teng J, Loukin S, Anishkin A, Kung C. The force-from-lipid (FFL) principle of mechanosensitivity, at large and in elements. Pflugers Arch. 2015;467:27–37. doi: 10.1007/s00424-014-1530-2. Review the mechanisms by which the functional response of membrane proteins is governed by bilayer force changes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinac B, Buechner M, Delcour AH, Adler J, Kung C. Pressure-sensitive ion channel in Escherichia coli. Proc Natl Acad Sci U S A. 1987;84:2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013 doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 19.Miller B, Sarantis M, Traynelis SF, Attwell D. Potentiation of NMDA receptor currents by arachidonic acid. Nature. 1992;355:722–725. doi: 10.1038/355722a0. [DOI] [PubMed] [Google Scholar]
- 20.Nishikawa M, Kimura S, Akaike N. Facilitatory effect of docosahexaenoic acid on N-methyl-D-aspartate response in pyramidal neurones of rat cerebral cortex. J Physiol (Lond) 1994;475:83–93. doi: 10.1113/jphysiol.1994.sp020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paoletti P, Ascher P. Mechanosensitivity of NMDA receptors in cultured mouse central neurons. Neuron. 1994;13:645–655. doi: 10.1016/0896-6273(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 22.Casado M, Ascher P. Opposite modulation of NMDA receptors by lysophospholipids and arachidonic acid: common features with mechanosensitivity. J Physiol (Lond) 1998;513(Pt 2):317–330. doi: 10.1111/j.1469-7793.1998.317bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Kloda A, Lua L, Hall R, Adams DJ, Martinac B. Liposome reconstitution and modulation of recombinant N-methyl-D-aspartate receptor channels by membrane stretch. Proc Natl Acad Sci U S A. 2007;104:1540–1545. doi: 10.1073/pnas.0609649104. This study reveals that NMDA receptors sense membrane stretch after purification and reconstitution in a liposome system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardie RC, Juusola M. Phototransduction in Drosophila. Curr Opin Neurobiol. 2015;34:37–45. doi: 10.1016/j.conb.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Huang J, Liu CH, Hughes SA, Postma M, Schwiening CJ, Hardie RC. Activation of TRP channels by protons and phosphoinositide depletion in Drosophila photoreceptors. Curr Biol. 2010;20:189–197. doi: 10.1016/j.cub.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 26.Randall AS, Liu CH, Chu B, Zhang Q, Dongre SA, Juusola M, Franze K, Wakelam MJO, Hardie RC. Speed and sensitivity of phototransduction in Drosophila depend on degree of saturation of membrane phospholipids. J Neurosci. 2015;35:2731–2746. doi: 10.1523/JNEUROSCI.1150-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodman MB. Mechanosensation. The C. elegans Research Community, editor. WormBook. 2006 doi: 10.1895/wormbook.1.62.1. http://www.wormbook.org. [DOI]
- 28.White JPM, Cibelli M, Urban L, Nilius B, McGeown JG, Nagy I. TRPV4: Molecular conductor of a diverse orchestra. Physiol Rev. 2016;96:911–973. doi: 10.1152/physrev.00016.2015. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 30.Berna-Erro A, Izquierdo-Serra M, Sepúlveda RV, Rubio-Moscardó F, Doñate-Macián P, Serra SA, Carrillo-Garcia J, Perálvarez-Marín A, González-Nilo F, Fernández-Fernández JM, et al. Structural determinants of 5″,6-″ epoxyeicosatrienoic acid binding to and activation of TRPV4 channel. Sci Rep. 2017;7:10522. doi: 10.1038/s41598-017-11274-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrantes FJ. Structural basis for lipid modulation of nicotinic acetylcholine receptor function. Brain Res Rev. 2004;47:71–95. doi: 10.1016/j.brainresrev.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Sunshine C, McNamee MG. Lipid modulation of nicotinic acetylcholine receptor function: the role of membrane lipid composition and fluidity. Biochim Biophys Acta. 1994;1191:59–64. doi: 10.1016/0005-2736(94)90233-x. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka K, Farooqui AA, Siddiqi NJ, Alhomida AS, Ong WY. Effects of docosahexaenoic acid on neurotransmission. Biomolecules and Therapeutics. 2012;20:152–157. doi: 10.4062/biomolther.2012.20.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34••.Basak S, Schmandt N, Gicheru Y, Chakrapani S. Crystal structure and dynamics of a lipid-induced potential desensitized-state of a pentameric ligand-gated channel. Elife. 2017;6:e23886. doi: 10.7554/eLife.23886. First crystal structure of a pLGIC revealing DHA binding site. Mutation on this site modifies desensitization; a long-established lipid-dependent feature of pLGIC channels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antollini SS, Barrantes FJ. Fatty acid regulation of voltage- and ligandgated ion channel function. Front Physiol. 2016;7:573. doi: 10.3389/fphys.2016.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouzat C, Bartos M, Corradi J, Sine SM. The interface between extracellular and transmembrane domains of homomeric Cys-loop receptors governs open-channel lifetime and rate of desensitization. J Neurosci. 2008;28:7808–7819. doi: 10.1523/JNEUROSCI.0448-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaczmarek LK, Aldrich RW, Chandy KG, Grissmer S, Wei AD, Wulff H. International Union of Basic and Clinical Pharmacology. C. Nomenclature and properties of calcium-activated and sodium-activated potassium channels. Pharmacol Rev. 2017;69:1–11. doi: 10.1124/pr.116.012864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoshi T, Wissuwa B, Tian Y, Tajima N, Xu R, Bauer M, Heinemann SH, Hou S. Omega-3 fatty acids lower blood pressure by directly activating large-conductance Ca2+-dependent K+ channels. Proc Natl Acad Sci U S A. 2013;110:4816–4821. doi: 10.1073/pnas.1221997110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.Hoshi T, Tian Y, Xu R, Heinemann SH, Hou S. Mechanism of the modulation of BK potassium channel complexes with different auxiliary subunit compositions by the omega-3 fatty acid DHA. Proc Natl Acad Sci USA. 2013;110:4822–4827. doi: 10.1073/pnas.1222003110. Together with Ref. 40 reveals that the BK-β1 complex interacts with DHA to robustly activate the channel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Tian Y, Aursnes M, Hansen TV, Tungen JE, Galpin JD, Leisle L, Ahern CA, Xu R, Heinemann SH, Hoshi T. Atomic determinants of BK channel activation by polyunsaturated fatty acids. Proc Natl Acad Sci U S A. 2016;113:13905–13910. doi: 10.1073/pnas.1615562113. Together with Ref. 39 reveals that the BK-β1 complex interacts with DHA to robustly activate the channel, using the para-group of a Tyr residue near the ion conduction pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•.Bukiya AN, McMillan J, Liu J, Shivakumar B, Parrill AL, Dopico AM. Activation of calcium- and voltage-gated potassium channels of large conductance by leukotriene B4. J Biol Chem. 2014;289:35314–35325. doi: 10.1074/jbc.M114.577825. First evidence that that the BK-β1 complex interacts with LTB4 to enhance channel activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bukiya AN, Singh AK, Parrill AL, Dopico AM. The steroid interaction site in transmembrane domain 2 of the large conductance, voltage- and calcium-gated potassium (BK) channel accessory β1 subunit. Proc Natl Acad Sci U S A. 2011;108:20207–20212. doi: 10.1073/pnas.1112901108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao HC, Agula H, Zhang W, Wang F, Sokabe M, Li LM. Membrane stretch and cytoplasmic Ca2+ independently modulate stretch-activated BK channel activity. J Biomech. 2010;43:3015–3019. doi: 10.1016/j.jbiomech.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 44.Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 45.Tigyi G, Parrill AL. Molecular mechanisms of lysophosphatidic acid action. Prog Lipid Res. 2003;42:498–526. doi: 10.1016/s0163-7827(03)00035-3. [DOI] [PubMed] [Google Scholar]
- 46.Inoue M, Rashid MH, Fujita R, Contos JJA, Chun J, Ueda H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med. 2004;10:712–718. doi: 10.1038/nm1060. [DOI] [PubMed] [Google Scholar]
- 47.Tigyi G. Lipids: LPA activates TRPV1—and it hurts. Nat Chem Biol. 2012;8:22–23. doi: 10.1038/nchembio.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48••.Nieto-Posadas A, Picazo-Juárez G, Llorente I, Jara-Oseguera A, Morales-Lázaro S, Escalante-Alcalde D, Islas LD, Rosenbaum T. Lysophosphatidic acid directly activates TRPV1 through a C-terminal binding site. Nat Chem Biol. 2011;8:78–85. doi: 10.1038/nchembio.712. First evidence that LPA directly activates TRPV1 by specifically binding to Lys710 at the C-terminal domain. [DOI] [PubMed] [Google Scholar]
- 49.Morales-Lazaro SL, Serrano-Flores B, Llorente I, Hernandez-Garcia E, Gonzalez-Ramirez R, Banerjee S, Miller D, Gududuru V, Fells J, Norman D, et al. Structural determinants of the transient receptor potential 1 (TRPV1) channel activation by phospholipid analogs. J Biol Chem. 2014;289:24079–24090. doi: 10.1074/jbc.M114.572503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, Julius D, Högestätt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 51.Medeiros D, da Costa Silva-Gonçalves L, da Silva AMB, Santos Cabrera dos MP, Arcisio-Miranda M. Membrane-mediated action of the endocannabinoid anandamide on membrane proteins: implications for understanding the receptor- independent mechanism. Sci Rep. 2017;7:1–9. doi: 10.1038/srep41362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52••.Jordt SE, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. First evidence that anandamide activates TRPV1 by binding to the vanilloid-binding site. [DOI] [PubMed] [Google Scholar]
- 53.Cao E, Liao M, Cheng Y, Julius D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature. 2013;504:113–118. doi: 10.1038/nature12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54•.Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D. TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron. 2013;77:667–679. doi: 10.1016/j.neuron.2012.12.016. This study reveals that heat and bioactive lipids like LPA and anandamide activate TRPV1 without the contribution of cellular factors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kahn-Kirby AH, Dantzker JLM, Apicella AJ, Schafer WR, Browse J, Bargmann CI, Watts JL. Specific polyunsaturated fatty acids drive TRPV-dependent sensory signaling in vivo. Cell. 2004;119:889–900. doi: 10.1016/j.cell.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Denisov IG, Sligar SG. Nanodiscs for structural and functional studies of membrane proteins. Nat Struct Mol Biol. 2016;23:481–486. doi: 10.1038/nsmb.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Denisov IG, Sligar SG. Nanodiscs in membrane biochemistry and biophysics. Chem Rev. 2017;117:4669–4713. doi: 10.1021/acs.chemrev.6b00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58•.Laganowsky A, Reading E, Allison TM, Ulmschneider MB, Degiacomi MT, Baldwin AJ, Robinson CV. Membrane proteins bind lipids selectively to modulate their structure and function. Nature. 2014;510:172–175. doi: 10.1038/nature13419. First report characterizing the selectivity of lipid binding to membrane protein complexes by mass spectrometry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y, Cong X, Liu W, Laganowsky A. Characterization of membrane protein-lipid interactions by mass spectrometry ion mobility mass spectrometry. J Am Soc Mass Spectrom. 2017;28:579–586. doi: 10.1007/s13361-016-1555-1. [DOI] [PubMed] [Google Scholar]


