Abstract
This study was conducted to elucidate the inherent potential of Bacillus sp. MR-1/2, which was isolated from root zone of maize crop grown on a textile wastewater-irrigated soil. The isolated strain was identified through its ribosomal RNA sequence. Under in vitro conditions, the strain demonstrated its tolerance for high concentrations of various heavy metal ions as determined by minimum inhibitory concentration. Moreover, the strain MR-1/2 exhibited many important phytobeneficial traits such as inorganic P solubilization and 1-aminocyclopropane-1-carboxylate (ACC) deaminase ability even under high metal and salt stress. Results showed that the strain proficiently decolorizes various azo dye compounds, e.g., reactive black-5, reactive red-120, and direct blue-1 and congo red, in broth culture. The bioremediation potential of the strain MR-1/2 was further confirmed by analyzing the retrieved azoreductase gene sequence through bioinformatics tools, whereby a subsequent prediction revealed that the azoreductase enzyme activity was involved in decolorization process. When mung bean seeds were grown in pots under various concentrations of decolorized and non-decolorized azo dye, the Bacillus sp. MR-1/2 not only alleviated the azo dye toxicity, but also increased the plant growth parameters. In conclusion, the strain MR-1/2 efficiently decolorized the azo dyes and helped in mung bean plant growth by alleviating azo dye toxicity.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1442-5) contains supplementary material, which is available to authorized users.
Keywords: Azo dyes, Bacillus, Decolorization, Metals, Plant growth, Wastewater
Introduction
Water is the most important substance on earth and is an integral part of all living organisms. However, water resources face a serious threat due to pollution originating from various sources. Freshwater in urban areas is becoming polluted due to different effluents produced by industries, sewage, and runoff from farmland and cities (Stavenhagen et al. 2018). Being one of the largest industries of Pakistan, the textile industry is responsible for the release of enormous quantities of effluents loaded with salts, metal ions, and synthetic dyes (Maqbool et al. 2016). Among the commercial synthetic dyes, azo dyes are an important group of available chemicals having aromatic compounds (Cui et al. 2011). About 70% of the total dyes used in various industries consist of azo dyes thanks to their low prices, high stability, and a large range of colors (Saratale et al. 2013). The wastewater produced from textile industries contain a high concentration of azo dyes due to inefficient dyeing process (Chacko and Subramaniam 2011). The discharge of azo dye-loaded effluents increases biological oxygen demand (BOD), chemical oxygen demand (COD), and salinity in addition to causing limited penetration of light in water systems due to colors (Saratale et al. 2013). In developing countries including Pakistan, it is a common practice to use textile wastewater for irrigating arable lands near the drains due to the unsuitability of ground water. This practice poses negative effects on soil health, nutrient cycling, microbial communities, and crop yield (Hussain et al. 2013). Therefore, it is important to devise cost-effective and environmental friendly strategies to remove these dyes from the environment.
Several physiochemical methods including specific coagulation, filtration, chemical flocculation, use of active carbon, etc. have been reported for the removal of dyes (Chacko and Subramaniam 2011). However, most of them are highly expensive and carry alternative risks. During the last decade, use of biological approaches for removal of dyes has attracted global attention because of their cost competitiveness and environmentally friendly nature. Microorganisms have been reported for their potential of degradation and removal of various textile dyes (Abbas et al. 2016; Maqbool et al. 2016; Hussain et al. 2017). The use of microbes is preferred because of the several advantages, e.g., their low cost, effectiveness against recalcitrant compounds, reduced sludge production under given ecological conditions and easy handling. However, the efficiency of microbial degradation of dyes is based on many factors such as their survival, movement, adaptability, and the chemical composition (Ahmed et al. 2016). Hence, novel microbial strains with better adaptability, survival, and activity are needed for degradation of azo dyes.
Plant growth-promoting rhizobacteria (PGPR) are considered as important agents for enhancing crop productivity (Hilda and Fraga 1999; Arif et al. 2017). Directly, they can increase nutrient solubility and induce phytohormones’ production and higher nitrogen fixation (Bhattacharyya and Jha 2012). Indirectly, they are able to control phytopathogens by producing antibiotics, different enzymes, etc. (Rashid et al. 2016). In addition to their plant growth-promoting (PGP) traits, some PGPR harbor environmentally beneficial attributes. For example, Azospirillum species can enhance the bioremediation of textile effluents by promoting algal proliferation and metabolism (Bashan et al. 2008). Some PGPR may also sequester heavy metals ions and pesticides (Tak et al. 2013). Isolation of such multifunctional bacteria expands the range of potential bioresources which might be exploited for concurrent agricultural production and remediation of pollutants in the soils under stress due to industrial effluents. Therefore, this study was conducted to physiologically and genetically characterize a PGPR strain Bacillus sp. MR-1/2 harboring dye decolorization potential. The strain was evaluated for combined bioremediation and PGPR potential under varying levels of different abiotic stresses. Moreover, bioinformatics analysis predicted that an NAD(P)H-dependent azoreductase enzyme was involved in azo dye decolorizing process.
Materials and methods
Soil sampling and bacterial isolation
Soil samples from rhizosphere were collected from maize plants growing on a soil contaminated with a high concentration of textile dyes because of being irrigated with Paharang Drain effluents from Faisalabad, Pakistan, over several years (31.467°North and 73.051°East). Soil samples were brought to the laboratory in pre-sterilized bags. Bacterial isolation was carried out by serial dilution technique (Somasegaran and Hoben 1994) in mineral salt medium having yeast extract as carbon source and 25 mg L−1 each of reactive red-120 (RR120), blue direct-1 (BD1), congo red (CR), and reactive black-5 (RB5). One gram soil was added to 9 mL saline solution and serially diluted. Then, 100 µL aliquot from each of two dilutions (10−4 and 10−6) was spread on mineral salt agar plates and incubated at temperature 28 ± 2 °C for 48 h for the colony growth. In total, 17 isolates were purified by repeated-streaking and subjected to screening based on relative decolorization of the selected dyes, viz., RR120, BD1, CR, and RB5, in mineral salt medium. Owing to the maximum potential for the decolorization of selected dyes, the isolate (designated as MR-1/2) was selected and stored at − 80 °C in 20% (v/v) glycerol solution. All morphological parameters of growth, colony formation, and cell morphology were observed through light microscope. Physico-chemical characteristics of the soil were determined at Ayub Agricultural Research Institute (AARI), Faisalabad, Pakistan (Table S1).
Characterization of bacterial isolate for plant growth-promoting characteristics
Phosphate solubilization
Phosphate solubilization index for the isolate MR-1/2 was determined using the halo-zone method on Pikovskaya’s agar plates (Pradhan and Sukla 2005). Quantification of P solubilization by the isolate MR-1/2 was carried out colorimetrically by growing pure culture of the isolate (MR-1/2) in Pikovskaya’s broth media (Pradhan and Sukla 2005). It was tested with different metal ions (Ni2+, Cd2+, Pb2+, Cu2+, Zn2+, and Cr6+) at varying concentrations, viz., 100, 500, 1000, 2000, and 3000 mg L−1. Phosphate solubilization was also quantified in Pikovskaya’s broth media amended with different concentrations of NaCl, viz., 0%, 2%, 4%, 6%, 8%, and 10%. The cultures in Pikovskaya’s media were incubated with different amendments in shaking conditions (150 rpm) at 28 ± 2 °C for 240 h. From each flask, a 20 mL sample was collected, centrifuged at 13,000g for 10 min and the supernatant was separated. Soluble P in the culture supernatant was measured by phosphomolybdate method (Murphy and Riley 1962). Double beam scanning spectrophotometer at a wavelength of 882 nm was used for this purpose.
Synthesis of indole-3-acetic acid (IAA)
Synthesis of the IAA by the isolate MR-1/2 was measured by Gordon and Weber (1951) method. A 100 mL nutrient broth medium was amended with 100 mg L−1 of tryptophan as a precursor of IAA and inoculated with the isolate MR-1/2. Different concentrations of metal ions and NaCl were also used in media as experimental variants as previously described. Thereafter, the cultures were allowed to grow on an orbital shaker (150 rpm) at 28 ± 2 °C for a 48 h. Aliquots from all cultures were obtained and subjected to centrifuge at 13,000g. Quantification of IAA was carried out spectrophotometrically (Camspec M350, UK) using Salkowski reagent, in which 1 mL of supernatant was mixed with 2 mL of Salkowski reagent. For quantitative measurements, different concentrations (0, 5, 10, 50, 100, 200, or 500 µg mL−1) of IAA solutions were used for drawing the standard curve.
ACC deaminase activity
The isolate MR-1/2 was subjected to ACC deaminase estimation by adding ACC as a sole nitrogen source in the culture medium having 3 µL of 0.5 M ACC (Penrose and Glick 2003). Several levels of heavy metal ions and NaCl concentrations were used as experimental variants as previously described. The cultures were grown in DF salt minimal medium under shaking (150 rpm) at 28 ± 2 °C for 24 h. The comparison between the turbidity of the inoculated (both with and without ACC) and non-inoculated cultures was marked as ACC deaminase activity.
Characterization of bioremediation potential
Azo dye decolonization
Potential of the bacterial isolate MR-1/2 to decolorize RR120, BD1, RB5, and CR was estimated in different experimental conditions. Briefly, MR-1/2 was cultured in MS media, containing yeast extract as carbon source, with varying pH values (5, 6, 7, 8, and 9), temperatures (20 °C, 25 °C, 30 °C, 35 °C, and 45 °C) and NaCl concentrations (0%, 2.5%, 5%, and 7.5%). Each medium was amended with individual selected azo dye (150 mg L−1) and inoculation was done with pre-grown MR-1/2 culture (OD600 = 0.05) along with an un-inoculated control. After the completion of incubation at 28 ± 2 °C under static conditions for 72 h, the aliquots were harvested at 24, 48, and 72 h, and centrifuged at 6000g for 10 min. Quantitative decolonization was measured using spectrophotometer (Camspec M350, UK) at highest absorbance wavelength (λmax) of respective dyes. Estimation of dye decolorization (%) was carried out using the following formula:
Here, A and B indicate the absorbance of the negative control and inoculation of samples with isolate MR-1/2, respectively.
Heavy metal resistance
The ability of isolate MR-1/2 to resist various metal ions in culture media was estimated by calculating the minimum inhibitory concentration (MIC) of different metal ions, viz., Ni2+, Cd2+, Pb2+, Cu2+, Zn2+, and Cr6+, for MR-1/2. For this purpose, different concentrations, viz., 50 mg L−1, 100 mg L−1, 500 mg L−1, 1000 mg L−1, and 2000 mg L−1, of each individual metal ion were added in nutrient agar media plates using their source salts (e.g., NiCl2·6H2O, CdCl2, Pb(NO3)2, CuSO4, ZnSO4, and K2Cr2O7, respectively). The strain MR-1/2 was grown on these plates, and for MIC estimation, the growth of isolates was observed after 7 days of incubation at 28 ± 2 °C.
Molecular identification and characterization of bacterial isolates
Sequencing of 16S rRNA gene
Genomic DNA of bacterial strain MR-1/2 was isolated using the method of Maniatis et al. (1982). The DNA was quantified by NanoDrop™ 2000/2000c (Thermo Fisher Scientific, Waltham, MA, USA). Universal pair of primers using primers, forward fD1 (5′ AGAGTTTGATCCTGGCTCAG 3′) and reverse rD1 (5′ AAGGAGGTGATCCAGCC 3′) (Weisburg et al. 1991), was used for the amplification of 16S rRNA gene of isolate MR-1/2. Similar PCR given conditions were used as reported by Shahid et al. (2015). The amplicon was purified through Qiaquick PCR purification kit (Qiagen, Germany) and cloned in pTZ57R/T vector (Thermo Fisher Scientific, USA). The E. coli strain DH5α was transformed with a recombinant vector which was subsequently sent for sequencing from Macrogen, South Korea. The 16S rRNA gene was sequenced on both sides by the same primers used for amplification. The raw sequences were trimmed for the presence of bad sequences at the 3′ and the 5′ end, and the forward and reverse sequences were joined using the CAP3 assembly online tool. The BLASTn and phylogenetic analysis was carried out as previously reported by Shahid et al. (2017) with additional sequence similarity search using Eztaxon Cloud online server (https://www.ezbiocloud.net/). The sequence was deposited in the database and GenBank accession number, i.e., MG548383 was obtained. To further confirm the taxonomy of MR-1/2, a phylogenetic tree was constructed with type strains belonging to genus Bacillus using MEGA (ver. 7) software package in which multiple sequence alignment was constructed through CLUSTALW software.
Azoreductase gene amplification and sequencing
Azoreductase gene was amplified using AZR1F (5′-ATGAAACTAGTCGTTATTAAC-3′) and AZR1R (5′-TCACTCCACTCCTAGTTGTTTTTT-3′) primers. For this purpose, the reaction mixture was prepared by mixing primers and 40 ng of template DNA in green PCR mix (Thermo Fisher Scientific, USA) according to the manufacturer’s protocol. The PCR reaction was carried out in a thermocycler (advanced Primus 96; PeQLab Biotechnologie, Germany) using the conditions reported by Mahmood et al. (2017). The amplified PCR product was ligated in pTZ57R/T vector, transformed in E. coli strain DH5α as described in the previous section, and sequenced from Macrogen, Korea.
Bioinformatics prediction of MR-1/2 azoreductase gene
The ExPASy translate tool, with the standard genetic code, was used to translate azoreductase gene of strain MR-1/2. The functional identification of the deduced protein sequence was obtained using NCBI conserved domain search tool and compared (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) with the database (CDD v3.16-50369 PSSMs) and e value (0.010000). To perform a comparative study, the translated protein sequence was searched in protein data bank (PDB) with BLASTp tool. For identification of conserved and functional motifs, MEME motif discovery server version 4.12.0 was used with default parameters. The multiple sequence alignment of PDB protein sequences with deduced sequence of MR-1/2 was accomplished by the CLC biosoftware. Gene Ontology annotation of MR-1/2 was performed using blast2GO software with default parameters (Götz et al. 2011). For sequence homology searching NCBI non-redundant (nr) protein, NCBI nucleotide sequence, Swiss-Prot protein, Gene Ontology (GO), and Kyoto Encyclopedia of Genes and Genomes were used. To predict 3D structure of translated protein, several bioinformatics tools were employed like RaptorX, Phyr2 with pre-defined parameters. For protein structure prediction, modular command software was also used using the known protein structure (PDB ID: 3GFR_A, 2GSW_A, 3GFS_A, 1NNI_1, and 3GFQA).
Phytotoxicity evaluation of azo dyes
Different concentrations (0, 100, and 500 mg L−1) of RR120 were mixed in MS media inoculated with the strain MR-1/2 and incubated at 30 °C for 72 h for decolorization of the dye. After incubation, decolorization (%) was estimated by the method described in “Azo dye decolonization”, followed by the estimation of total organic carbon (TOC) using Nelson and Sommers (1982) method. Surface sterilized mung bean seeds of NM-2006 cultivar were sown in Petri plates (10 seeds per plate) filled with sterilized sand (30 g per plate). The Petri plates were grown in a plant growth chamber at a temperature of 25 °C, 16/8 h light/dark periods, and moistened (3 mL per day) with the above-mentioned three concentrations of decolorized RR120 solutions along with their respective un-inoculated non-decolorized dye solutions. The growth and germination data were collected after 7 days of growth.
Statistical analysis
Data for azo dye decolorization and phytotoxicity were evaluated statistically by the one-way analysis of variance (Steel et al. 1997) using the Statistix (ver. 8.1) software. All the treatments were compared with 95% confidence level by Fisher’s least significant difference test. The phylogenetic tree was constructed with MEGA (ver. 7) software (Tamura et al. 2013). Each experimental treatment was repeated three times to calculate the statistical errors in all the experiments related to plant beneficial traits, bioremediation potential, and phytotoxicity evaluation of strain MR-1/2.
Results
Plant growth-promoting traits
Phosphate solubilization and IAA production
The isolate MR-1/2 demonstrated excellent phosphate solubilization potential that varied according to type and concentrations of heavy metal ions as well as salinity levels (Table 1). No inorganic P solubilization was found when Co2+ and Cr6+ were added in the culture medium. For all the other metals tested in the study, increasing the metal concentration decreased the P solubilization. The highest P solubilization (25.57 µg mL−1 and 21.66 µg mL−1) was observed with Cd2+ at 100 mg L−1 and 200 mg L−1 concentrations, respectively, followed by the Pb2+ (21.35 µg L−1) at 100 mg L−1 concentration (Table 1). At a maximum concentration (i.e., 1000 mg L−1) of all metal concentrations, the isolate showed a negative response in terms of P solubilization. The isolate MR-1/2 also demonstrated P solubilization in the presence of NaCl medium (up to 6%) (Table 3). The strain MR-1/2 was unable to synthesize IAA in the presence or absence of tryptophan.
Table 1.
P solubilization potential of Bacillus sp. MR-1/2 in the presence of various concentrations of metals
| Metal | P solubilization (µg mL−1) | ||
|---|---|---|---|
| 100 mg L−1 | 200 mg L−1 | 500 mg L−1 | |
| Ni2+ | 22.1 (3.16)a | 20.89 (2.32) | −ve |
| Cd2+ | 25.57 (3.20)a | 21.66 (2.05) | 12.14 (1.35) |
| Pb2+ | 21.35 (2.37)a | 17.46 (2.49) | −ve |
| Co2+ | −ve | −ve | −ve |
| Zn2+ | 11.66 (1.46)a | 11.4 (1.08) | 9.46 (1.35) |
| Cr6+ | −ve | −ve | −ve |
aEach metal concentration is repeated three times and standard errors are presented in parentheses
Table 3.
P solubilization and ACC deaminase activity of Bacillus sp. MR-1/2 in the presence of various salt concentrations
| PGP-trait | NaCl concentrations (%) | |||||
|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | |
| Phosphate solubilization | 57.09 (5.39)a | 40.99 (6.25) | 38.76 (3.24) | 22.61 (4.32) | −ve | −ve |
| ACC deaminase activity | + | + | + | −ve | −ve | −ve |
aEach NaCl concentration is repeated three times and the standard errors are presented in parentheses
ACC deaminase activity
ACC deaminase activity of the isolate MR-1/2 at different metal concentrations is presented in Table 2. This strain exhibited ACC deaminase activity in the presence of up to 500 mg L−1 of each of Cd2+, Co2+, and Cr6+ in the medium. However, in the presence of Ni2+ and Pb2+ in the medium, this enzyme activity was found positive only up to 200 mg L−1 concentrations. Interestingly, no ACC deaminase activity was observed in the presence of Zn2+ ions even at the lowest provided concentrations. ACC deaminase activity of the isolate MR-1/2 was positive in the presence of NaCl up to 4% in the medium (Table 3).
Table 2.
ACC deaminase activity of Bacillus sp. MR-1/2 in the presence of various metal concentrations
| Metal | Concentration (mg L−1) | |||
|---|---|---|---|---|
| 100 | 200 | 500 | 1000 | |
| Ni2+ | +ve | +ve | −ve | −ve |
| Cd2+ | +ve | +ve | +ve | −ve |
| Pb2+ | +ve | +ve | −ve | −ve |
| Co2+ | +ve | +ve | +ve | −ve |
| Zn2+ | −ve | −ve | −ve | −ve |
| Cr6+ | +ve | +ve | +ve | −ve |
Bioremediation potential
Metal tolerance
Bacterial isolate MR-1/2 was tested for its growth at varying concentrations of different metal ions. The MIC of studied metal ions for the isolate MR-1/2 is presented in Table S2. The isolate MR-1/2 showed a varying extent of tolerance for the selected metals. The growth of the isolate MR-1/2 was inhibited at a concentration of 2000 mg L−1 of each metal. However, it tolerated 1000 mg L−1 concentration of Ni2+, Cd2+, and Cr6+. It is noteworthy that, for some metals like Pb2+, Co2+, and Zn2+, the isolate could resist only up to 100 mg L−1 concentration.
Azo dye decolorization
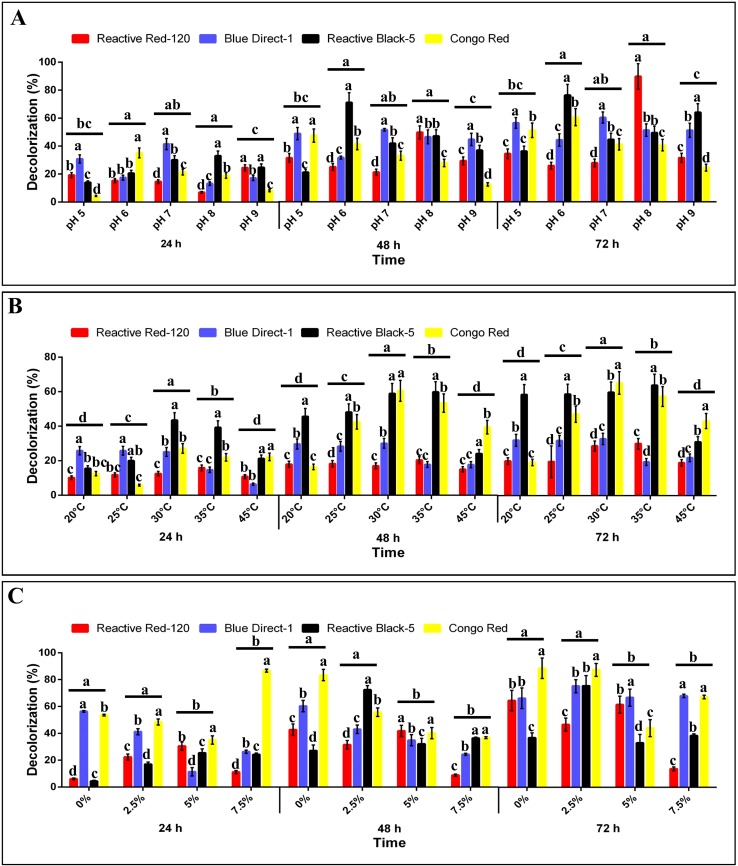
Decolorization of all selected dyes under all conditions was observed after 72 h of incubation, and hence, azo dye decolorization followed an increasing trend with time. Decolorization of dyes by isolate MR-1/2 was determined at varying pH values of 5–9. The pH substantially affected the decolorization potential of the strain MR-1/2 (Fig. 1a). Over 72 h incubation, the maximum decolorization of RR120, BD1, RB5, and CR was observed at pH values of 8, 7, 6, and 6, respectively.
Fig. 1.
Decolorization of dyes (reactive red-120, blue direct-1, reactive black-5, and congo red) by Bacillus sp. MR-1/2 after 24, 48 and 72 h. a At varying pH values, b at different temperatures, c: in the presence of varying levels of NaCl salt. Different lower case letters on the bars and lines represent significance between the dyes, pH levels, temperatures, and salt concentrations, respectively (Fisher’s LSD; P ≤ 0.05). Error bars represent the standard errors (n = 3)
Variation in temperature was also found to affect the magnitude of decolorization (Fig. 1b). In general, maximum decolorization of dyes was obtained at 30 °C. However, over 72 h incubation, the maximum decolorization of RR120, RB5, and CR was almost similar at 30 °C and 35 °C.
Similarly, the presence of NaCl, at varying concentrations in the medium also affected the decolorization (Fig. 1c). In general, the decolorization (%) of RR120, DB1, and CR was maximum in the absence of salt (0% NaCl). However, the decolorization of various dyes was changed differently under varying concentrations of NaCl. The maximum decolorization of RB5 under saline conditions was found in the treatment containing 2.5% of NaCl. In general, a salt concentration up to 2.5% did not significantly reduced decolorization percentage of tested dyes. The NaCl concentrations above and below this level decreased the decolorization (%).
Molecular identification
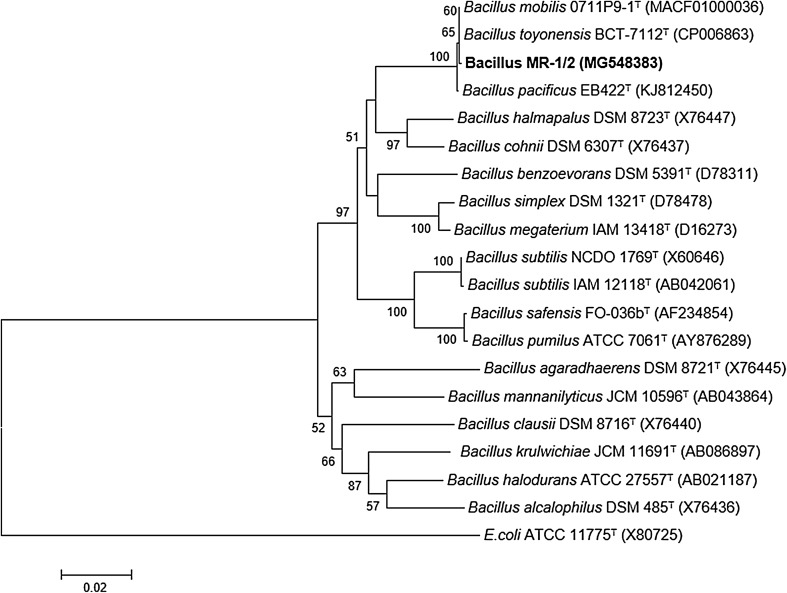
The BLASTn analysis of ribosomal RNA gene sequence of the strain MR-1/2 depicted that it had the highest identity (99%) with B. thuringiensis strain M4 (MG758026). The same level of identity was also observed with B. thuringiensis strain VCRC B-17 (MG745385) and B. subtilis strain CHAPGPBS-076B (KY495216). On the other hand, the 16S rRNA gene sequence was found 99.4% similar with the type strains B. mobilis 0711P9-1 (MACF01000036) and B. toyonensis BCT-7112 (CP006863) when searched using Eztaxon Cloud server. In phylogenetic tree, it formed a cluster with B. toyonensis BCT-7112T (CP006863) and B. mobilis 0711P9-1T (MACF01000036) (Fig. 2). Based on the BLASTn, Eztaxon Cloud, and phylogenetic analyses, the molecular identity of the strain was confirmed as genus Bacillus and hence given name as Bacillus sp. MR-1/2.
Fig. 2.
Phylogenetic relationships of Bacillus sp. MR-1/2 established by constructing a phylogenetic tree with other type strains of genus Bacillus using neighbor-joining method. The percentages (≥ 50%) of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown
Bioinformatics analysis of azoreductase gene
The MR-1/2 azo gene sequence was translated into a protein-containing 100 amino acids. By the comparative bioinformatics analysis, it was assumed that the deduced protein decolorized tested azo dyes through NAD(P)H-dependent oxidoreductase activity. The deduced protein showed closest homology with FMN-dependent NADH-azoreductase proteins (3GFR, 2GSW, 3GFS, 1NNI, and 3GFQ) of PDB (Table 4). The conserved domain (CD) analysis of deduced protein sequence depicted that it had NAD(P)H-dependent azoreductase activity. The Blast2GO-mediated analysis indicated that the deduced protein of MR-1/2 had distinctive attributes in contrast to other PDB proteins. The gene ontology (GO) analysis predicted that MR-1/2 protein has roles in azobenzene reductase activity, oxidoreductase activity, and oxidation reduction reactions, whereas other closely related proteins of PDB also had similar functions. Moreover, a distinct feature of MR-1/2 deduced azoreductase protein was to encode enzyme EC: 1.7.1.6 which was found absent in other closely related proteins of PDB (Table 4). Similarly, multiple sequence alignment also revealed difference in deduced amino acid sequence of MR-1/2 with other PDB proteins (Figure S2). Moreover, the 3D structure of predicted protein of MR-1/2 azo gene was also found different from the PDB proteins (Figure S3). The Blast2GO-mediated species distribution analysis indicated that Bacillus sp. MR-1/2 was closely related to Bacillus cereus and other Bacillus species based on deduced protein (Figure S4). The motif analysis generated through MEME web server demonstrated that the motifs were located at different positions in MR-1/2 azoreductase protein and other PDB proteins (Figure S5). MR-1/2 azoreductase protein was found to contain seven motifs (Figure S6), whereas other known proteins (except 2GSW which have 6 motifs) have 5 motifs in their protein sequence. Moreover, MR-1/2 azoreductase protein has 4 unique motifs (7, 8, 9, and 10) which were not found in other closely related proteins of PDB.
Table 4.
Comparative bioinformatics analysis of Bacillus sp. MR-1/2 azoreductase protein with closely related PDB proteins
| Sequence name | Description | Activities | No. of amino acids | No. of hits | e value | Similarity mean | Encoded enzyme |
|---|---|---|---|---|---|---|---|
| MR-1/2 | NAD(P)H-dependent oxidoreductase | P: Oxidation–reduction process, F: azobenzene reductase activity | 100 | 20 | 6.83876E−53 | 98.95 | EC:1.7.1.6 |
| 3GFR | FMN-dependent NADH-azoreductase | F: Identical protein binding; F: oxidoreductase activity; P: oxidation–reduction process | 174 | 20 | 1.15477E−106 | 98.6 | – |
| 2GSW | FMN-dependent NADH-azoreductase | F: Identical protein binding, F: oxidoreductase activity, P: oxidation–reduction process | 182 | 20 | 5.12367E−101 | 96.8 | – |
| 3GFS | FMN-dependent NADH-azoreductase | F: Identical protein binding, F: oxidoreductase activity, P: oxidation–reduction process | 174 | 20 | 8.40121E−107 | 98.1 | – |
| 1NNI | FMN-dependent NADH-azoreductase | F: Identical protein binding, F: oxidoreductase activity, P: oxidation–reduction process | 174 | 20 | 5.26538E−101 | 96.8 | – |
| 3GFQ | FMN-dependent NADH-azoreductase | F: Identical protein binding, F: oxidoreductase activity, P: oxidation–reduction process | 174 | 20 | 2.51611E−106 | 98.55 | – |
Phytotoxicity evaluation
Inoculation of the media-containing RR120 at varying concentrations resulted into a significant decrease in color as well as TOC (Table 5). The phytotoxicity evaluation experiment depicted that germination (%), root length, and shoot length of mungbean have been significantly decreased when seeds were irrigated with the solutions containing the dyes at both levels, viz., 100 mg L−1 and 500 mg L−1 (Table 5). However, it is noteworthy that irrigation of the seeds with the RR120 solutions decolorized by the strain MR-1/2 resulted in increase in growth characteristics as compared to their respective counterparts irrigated with un-inoculated solutions.
Table 5.
Phytotoxicity evaluation of decolorized reactive red-120 with mungbean seeds
| Treatments | Decolorization (%) | TOC reduction (%) | Germination (%) | Root length (cm) | Shoot length (cm) |
|---|---|---|---|---|---|
| NRR0 | – | – | 90 (10)a** | 2.09b (0.31)b | 6.16 (0.74)ab |
| NRR100 | – | – | 40.98 (8.4)b | 1.33 (0.25)c | 3.63 (0.61)c |
| NRR500 | – | – | 36.09 (4.17)b | 1.33 (0.26)c | 2.73 (0.36)c |
| WRR0 | – | – | 86.56 (9.5)a | 2.77 (0.19)a | 6.83 (0.28)a |
| WRR100 | 77.67 (7.57)a | 26.36 (2.23)a | 80.06 (3.99)ab | 2.43 (0.22)ab | 6.30 (0.83)a |
| WRR500 | 50.66 (6.42)b | 18.72 (6.06)b | 83.78 (5.57)a | 2.06 (0.33)b | 5.33 (0.57)b |
NRR0 non-inoculated with no reactive red-120, NRR100 non-inoculated with 100 mg L−1 reactive red-120, NRR500 non-inoculated with 500 mg L−1 reactive red-120, WRR0 inoculated with MR-1/2 and no reactive red-120, WRR100 inoculated with MR-1/2 and 100 mg L−1 reactive red-120, WRR500 inoculated with MR-1/2 and 500 mg L−1 reactive red-120
**Different lower case letters represent significance between the treatments means (Fisher’s LSD; P ≤ 0.05). Error bars represent the standard errors (n = 3)
Discussion
This study highlights the potential of a multifarious bacterial strain MR-1/2, isolated from rhizosphere of maize plant, to neutralize deleterious effects of textile wastewater and heavy metal pollution in agricultural. The strain demonstrated various PGP as well as bioremediation attributes. The 16S rRNA sequence, as well as the phylogenetic analysis, revealed that this strain belongs to genus Bacillus. As the 16S rRNA gene sequence showed the same level of identity (99%) with B. thuringiensis, B. cereus, and B. proteolyticus in BLASTn analysis, and hence, it was named as Bacillus sp. MR-1/2. The Bacillus species have been documented to harbor plant growth-promoting (PGP) characteristics (Shahid et al. 2015) and to affect plant growth and physiology under heavy metal and salt concentrations (Akram et al. 2016). A few Bacillus strains play their role for plant growth promotion and bioremediation of environmental pollutants simultaneously (Shinwari et al. 2015; Mahmood et al. 2017). Recently, an IAA-producing endophyte Bacillus altitudinis WR10 alleviated the iron stress and promoted wheat growth by upregulating the stress-responsive genetic mechanisms (Sun et al. 2017). Hence, the bacterial strain MR-1/2 can also serve as a potential bioresource for plant growth promotion and remediation of dyes in the soils under wastewater irrigation.
The PGP potential of bacterial strains is mainly attributed to their inorganic P solubilization ability, which facilitates acquisition of P from soil due to soil acidosis caused mainly by the microbial synthesis of organic acids (Trivedi and Sa 2008). Thus, organic acids produced in the rhizosphere environment lower the pH of the micro-environment facilitating the cations (Al2+, Fe2+, and Ca2+) detachment from their phosphate molecules. Hence, cation-free phosphates become available to plants in the form of primary (HPO4) and secondary (H2PO4) ortho-phosphate. The strain MR-1/2 was observed to solubilize the inorganic phosphate in the presence of up to 6% NaCl. Moreover, the strain was also found to exhibit inorganic P solubilization potential when tested with varying concentrations of different metal ions, viz., Ni2+, Cd2+, Pb2+, and Zn2+. However, no P solubilization was found with Co2+ and Cr6+ even at their lowest concentrations. The absence of P solubilization with Co2+ and Cr6+ may be due to the toxic nature of these heavy metals. Despite several in vitro studies deciphering the capability of PGP bacteria for P solubilization (Shahid et al. 2015; Akram et al. 2016), strain MR-1/2 is unique in the sense that it demonstrated this potential in simultaneous presence of heavy metal and salt stress. Previously, Akram et al. (2016) reported a Staphylococcus sciuri strain SAT-17 which had the potential for P solubilization in the presence of 2 M NaCl salt. Another PGP characteristic which enables bacterial strains to be used as bioinoculants is ACC deaminase activity. The deamination ethylene precursor ACC in plants is caused by bacterially synthesized enzyme ACC deaminase. Thus, association of bacteria producing ACC deaminase with plant roots becomes significantly vital, especially under stress conditions. The bacterial isolate MR-1/2 was also tested for ACC deaminase activity under different concentrations of selected heavy metals and salt. Results showed that the isolate exhibits the ACC deaminase activity at various metals and salts stress levels, except the Zn2+ where no enzyme activity was found. The ability of the isolate to retain its ACC deaminase activity under metals and salt stress made the strain comparatively potent (Akram et al. 2016). It has also been reported that PGPR-bearing ACC deaminase activity is able to trigger abiotic stress tolerance mechanisms in plants for stress alleviation (Heydarian et al. 2018).
The bioremediation potential of the strain MR-1/2 was attributed to its metal tolerance and ability to decolorize azo dyes under varying conditions including varying temperatures, pH values, and NaCl concentrations. Temperature affects the metabolic activity of microorganisms including azo dyes decolorization. Maximum decolorization of selected dyes by the strain MR-1/2 was observed at 30 and 35 °C. This temperature range has been shown as optimum for azo dye decolorization by different bacterial strains (Wang et al. 2009; Hussain et al. 2013). Varying ranges of decolorization with changes in temperature can be explained by changes in different enzyme production systems or cell viability and activity which are controlled by the change in temperature values (Hussain et al. 2013). Like temperature, different pH values also have a significant effect on decolorization of azo dyes, because highly acidic and alkaline conditions may hinder microbial metabolic activity (Maqbool et al. 2016). The isolate MR-1/2 showed the maximum decolorization at a pH range of 6–8. It is common that the azo dye decolorization activity of bacterial strains is maximum near neutral to alkaline pH (Hussain et al. 2013; Najme et al. 2015; Abbas et al. 2016). The percent deviation in decolorization with changes in pH values might be due to modification in the azoreductase enzyme (Imran et al. 2016a, b).
Textile wastewaters mainly contain the azo dyes; however, a large amount of salt and metal ions can also be found. The presence of salts and metal ions hinders the microbial enzyme activity (Mahmood et al. 2013). In the current study, the isolate MR-1/2 efficiently decolorized the selected dyes with high concentration of 7.5% of NaCl in the medium. This indicates that this strain has the potential of dye decolorization while tolerating the salt. Moreover, the strain MR-1/2 was also found to tolerate the considerable concentrations of different metal ions, including Ni2+, Cd2+, and Cr6+ in the medium. A promising feature of this strain to perform its activity in the wastewaters is the onset of metabolic activity under abiotic stress conditions. Such metal tolerance in dye decolorizing bacterial strains was also reported in several previous studies (Hussain et al. 2013; Najme et al. 2015; Abbas et al. 2016). Recently, it was found that thermophilic microflora were able to decolorize direct black G dye up to 97% in 8 h by simultaneously employing laccase, manganese peroxidase, lignin peroxidase, and azoreductase enzymes (Chen et al. 2018). In another study, a yeast strain Pichia occidentalis G1 efficiently decolorized acid red B dye by the enzymatic degradation, deamination, and desulfonation processes (Song et al. 2017).
The known genetic mechanism for azo dye degradation is the catalysis of azo dyes into aromatic amines by the bacterial enzyme azoreductase (Imran et al. 2016b). This protein is very important and carries out the reduction cleavage of azo bond (–N=N–). Hence, the azoreductase-encoding gene was sequenced and translated to the corresponding protein to predict its function. The bioinformatics analysis revealed that deduced protein of MR-1/2 azoreductase gene was supposed to decolorize azo dyes through NAD(P)H-dependent oxidoreductase activity. This activity has also been recently predicted in another Bacillus strain in terms of azo dye decolorization (Mahmood et al. 2017). However, the deduced protein showed some distinct features which were not present in its closely related proteins in the PDB as depicted through multiple sequence alignment, motif analysis, species distribution, etc. (Figures S2–S6). For instance, the activity of the closely related proteins of PDB was FMN-dependent, while the activity of MR-1/2 azoreductase protein was predicted to be NAD(P)H-dependent. Moreover, motif analysis revealed some distinct motifs in the predicted protein of MR-1/2 strain, which were found absent in closely related proteins of PDB. These comparative structural differences in predicted azoreductase protein may contribute to the greater physiological potential of Bacillus sp. MR-1/2 in terms of azo dye decolorization. Several reports have been published, indicating that both NADH-dependent oxidoreductase and FMN-dependent NADH-azoreductase activities are linked with azo dye decolorization process. Furthermore, the activity of azoreductase enzyme in azo dye decolorization has been reported earlier (Imran et al. 2016a, b). The azoreductase enzyme-encoding genes can be obtained from bacterial strains and expressed in host systems to over-express the protein. For instance, recently, twofold increase in azo dye decolorization has been achieved after the isolation of azoA gene from Enterococcus sp. L2 and over-expression in E. coli DH5α (Rathod et al. 2017).
Degradation products of azo dyes should be safe for plant growth to harness the actual potential of PGP strains. For phytotoxicity evaluation, the decolorized dye solutions were directly applied to mungbean plants. The plants treated with decolorized RR120 showed a better growth as compared to those treated with intact dye solutions. This depicted that the decolorized dye has no phytotoxic effects. Similar results have been reported by Ebency et al. (2013), who showed non-toxic effects of decolorized blue indigo dye on kidney beans. Moreover, a significant decrease in the TOC values was measured in toxicity evaluation experiment. Lade et al. (2015) described that TOC value reduction is one of the important quality variables for wastewater detoxification.
Conclusion
The Bacillus sp. MR-1/2 successfully degraded the harmful azo dyes under in vitro conditions in stress free as well as under heavy metal and salt stress. In addition, this strain demonstrated substantial potential for in vitro phosphate solubilization and ACC deaminase activity when exposed to increasing concentrations of salt and heavy metals. The strain MR-1/2 decolorized azo dyes such as RR120, BD1, CR, and RB5 in the culture medium and tolerated high concentrations of heavy metal ions. Bioinformatics analysis of azoreductase-encoding gene sequence predicted that the dye decolorization ability was due to NAD(P)H-dependent oxidoreductase activity. The predicted azoreductase protein carried unique motifs as compared to most closely related database proteins. Decolorized azo dyes had no toxic effects on inoculated mung bean plants.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are indebted to the financial contribution of Department of Bioinformatics and Biotechnology and Department of Environmental Sciences and Engineering, Government College University, Faisalabad, Pakistan, from the departmental annual grant.
Author contributions
MS: experiment designing, conducting the experiments, and manuscript preparation; FM: experiment designing, conducting the experiments, and manuscript preparation; SH: manuscript preparation and Data analysis, TS: manuscript preparation and data analysis, MZH: manuscript preparation and data analysis, MN: conduction of experiments and manuscript preparation, AM: conduction of experiments and manuscript preparation, QF: conduction of experiments and manuscript preparation, TA: conduction of experiments and manuscript preparation, and GM: conduction of experiments and manuscript preparation
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest related to this study.
References
- Abbas N, Hussain S, Azeem F, Shahzad T, Bhatti SH, Imran M, Ahmad Z, Maqbool Z, Abid M. Characterization of a slat resistant bacterial strain Proteus sp. NA6 capable of decolorizing reactive dyes in presence of multi-metal stress. World J Microbiol Biotechnol. 2016;32:181. doi: 10.1007/s11274-016-2141-1. [DOI] [PubMed] [Google Scholar]
- Ahmed F, Arshad M, Ditta A, Hussain A, Naveed M, Hasnain M, Nazir Q. Combining textile effluent wastewater with organic fertilizer for improved growth and productivity of wheat and soil health. J Environ Agric Sci. 2016;8:14–20. [Google Scholar]
- Akram MS, Shahid M, Tariq M, Azeem M, Javed MT, Saleem S, Riaz S. Deciphering Staphylococcus sciuri SAT-17 mediated anti-oxidative defense mechanisms and growth modulations in salt stressed maize (Zea mays L.) Front Microbiol. 2016;7:867. doi: 10.3389/fmicb.2016.00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif MS, Shahzad SM, Riaz M, Yasmeen T, Shahzad T, Akhtar MJ, Bragazza L, Buttler A. Nitrogen-enriched compost application combined with plant growth-promoting rhizobacteria (PGPR) improves seed quality and nutrient use efficiency of sunflower. J Plant Nutr Soil Sci. 2017;180:464–473. doi: 10.1002/jpln.201600615. [DOI] [Google Scholar]
- Bashan Y, Puente ME, de Bashan LE, Hernandez JP. Environmental uses of plant growth-promoting bacteria. Plant Microb Interact. 2008;661:69–93. [Google Scholar]
- Bhattacharyya PN, Jha DK. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol. 2012;28:1327–1350. doi: 10.1007/s11274-011-0979-9. [DOI] [PubMed] [Google Scholar]
- Chacko JT, Subramaniam K. Enzymatic degradation of azo dyes—a review. Int J Environ Sci. 2011;1:1250–1260. [Google Scholar]
- Chen Y, Feng L, Li H, Wang Y, Chen G, Zhang Q. Biodegradation and detoxification of Direct Black G textile dye by a newly isolated thermophilic microflora. Bioresour Technol. 2018;250:650–657. doi: 10.1016/j.biortech.2017.11.092. [DOI] [PubMed] [Google Scholar]
- Cui D, Kong FY, Liang B, Cheng HY, Liu D, Sun Q, Wang AJ. Decolorization of azo dyes in dual-chamber biocatalyzed electrolysis systems seeding with enriched inoculum. J Environ Anal Toxicol. 2011;3:001. [Google Scholar]
- Ebency CL, Rajan S, Murugesan AG, Rajesh A, Elayarajah B. Biodegradation of textile azo dyes and its bioremediation potential using seed germination efficiency. Int J Curr Microbiol Appl Sci. 2013;2:496–505. [Google Scholar]
- Götz S, Arnold Arnold, Sebastián-León P, Martín-Rodríguez S, Tischler P, Jehl MA, Dopazo J, Rattei T, Conesa A. B2G-FAR, a species-centered GO annotation repository. Bioinformatics. 2011;27(7):919–924. doi: 10.1093/bioinformatics/btr059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SA, Weber RP. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951;26:192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydarian Z, Gruber M, Glick BR, Hegedus DD. Gene expression patterns in roots of Camelina sativa with enhanced salinity tolerance arising from inoculation of soil with plant growth promoting bacteria producing 1-aminocyclopropane-1-carboxylate deaminase or expression the corresponding acds gene. Front Microbiol. 2018;9:1297. doi: 10.3389/fmicb.2018.01297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilda R, Fraga R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv. 1999;17:319–339. doi: 10.1016/S0734-9750(99)00014-2. [DOI] [PubMed] [Google Scholar]
- Hussain S, Maqbool Z, Ali S, Yasmeen T, Imran M, Mahmood F, Abbas F. Biodecolorization of reactive black-5 by a metal and salt tolerant bacterial strain Pseudomonas sp. RA20 isolated from Paharang drain effluents in Pakistan. Ecotoxicol Environ Saf. 2013;98:331–338. doi: 10.1016/j.ecoenv.2013.09.018. [DOI] [PubMed] [Google Scholar]
- Hussain S, Quinn L, Li J, Casey E, Murphy CD. Simultaneous removal of malachite green and hexavalent chromium by Cunninghamella elegans biofilm in a semi-continuous system. Int Biodeterior Biodegrad. 2017;125:142–149. doi: 10.1016/j.ibiod.2017.09.003. [DOI] [Google Scholar]
- Imran M, Arshad M, Negm F, Khalid A, Shaharoona B, Hussain S, Nadeem SM, Crowley DE. Yeast extract promotes decolorization of azo dyes by stimulating azoreductase activity in Shewanella sp. strain IFN4. Ecotoxicol Environ Saf. 2016;124:42–49. doi: 10.1016/j.ecoenv.2015.09.041. [DOI] [PubMed] [Google Scholar]
- Imran M, Negm F, Hussain S, Ashraf M, Ahmad Z, Arshad M, Crowley DE. Characterization and purification of membrane-bound azoreductase from azo dye degrading Shewanella sp. Strain IFN4. Clean Soil Air Water. 2016;44:1523–1530. doi: 10.1002/clen.201501007. [DOI] [Google Scholar]
- Lade H, Kadam A, Paul D, Govindwar S. Biodegradation and detoxification of textile azo dyes by bacterial consortium under sequential microaerophilic/aerobic processes. EXCLI J. 2015;14:158–174. doi: 10.17179/excli2014-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood S, Khalid A, Mahmood T, Arshad M, Ahmad R. Potential of newly isolated bacterial strains for simultaneous removal of hexavalent chromium and reactive black-5 azo dye from tannery effluent. J Chem Technol Biotechnol. 2013;88:1506–1513. doi: 10.1002/jctb.3994. [DOI] [Google Scholar]
- Mahmood F, Shahid M, Hussain S, Shahzad T, Tahir M, Ijaz M, Hussain A, Mahmood K, Imran M, Babar SAK. Potential plant growth-promoting strain Bacillus sp. SR-2-1/1 decolorized azo dyes through NADH-ubiquinone: oxidoreductase activity. Bioresour Technol. 2017;235:176–184. doi: 10.1016/j.biortech.2017.03.098. [DOI] [PubMed] [Google Scholar]
- Maniatis T, Fritsch EF, Sambrook J. Molecular cloning: a laboratory manual. New York: Cold Spring Harbor Laboratory; 1982. p. 545. [Google Scholar]
- Maqbool Z, Hussain S, Ahmad T, Nadeem H, Imran M, Khalid A, Abid M, Martin-Laurent F. Use of RSM modeling for optimizing decolorization of simulated textile wastewater by Pseudomonas aeruginosa strain ZM130 capable of simultaneous removal of reactive dyes and hexavalent chromium. Environ Sci Pollut Res. 2016;23:11224–11239. doi: 10.1007/s11356-016-6275-3. [DOI] [PubMed] [Google Scholar]
- Murphy J, Riley JP. Modified solution method for determination of phosphate in natural water. Anal Chim Acta. 1962;27:31–36. doi: 10.1016/S0003-2670(00)88444-5. [DOI] [Google Scholar]
- Najme R, Hussain S, Maqbool Z, Imran M, Mahmood F, Manzoor H, Yasmeen T, Shahzad T. Biodecolorization of reactive yellow-2 by Serratia sp. RN34 isolated from textile wastewater. Water Environ Res. 2015;87:2065–2075. doi: 10.2175/106143015X14362865226031. [DOI] [PubMed] [Google Scholar]
- Nelson DW, Sommers L. Total carbon, organic carbon, and organic matter 1. Methods of soil analysis. Part 2. Chemical and microbiological properties. Madison: American Society of Agronomy; 1982. pp. 539–579. [Google Scholar]
- Penrose DM, Glick BR. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant. 2003;118:10–15. doi: 10.1034/j.1399-3054.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- Pradhan N, Sukla LB. Solubilization of inorganic phosphates by fungi isolated from agriculture soil. Afr J Biotechnol. 2005;5:850–854. [Google Scholar]
- Rashid MI, Mujawar LH, Shahzad T, Almeelbi T, Ismail IMI, Oves M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol Res. 2016;183:26–24. doi: 10.1016/j.micres.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Rathod J, Dhebar S, Archana G. Efficient approach to enhance whole cell azo dye decolorization by heterologous overexpression of Enterococcus sp. L2 azoreductase (azoA) and Mycobacterium vaccae formate dehydrogenase (fdh) in different bacterial systems. Int Biodeterior Biodegrad. 2017;124:91–100. doi: 10.1016/j.ibiod.2017.04.023. [DOI] [Google Scholar]
- Saratale RG, Gandhi SS, Purankar MV, Kurade MB, Govindwar SP, Oh SE, Saratale GD. Decolorization and detoxification of sulfonated azo dye CI Remazol Red and textile effluent by isolated Lysinibacillus sp. RGS J Biosci Bioeng. 2013;115:658–667. doi: 10.1016/j.jbiosc.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Shahid M, Hameed S, Tariq M, Zafar M, Ali A, Ahmad N. Characterization of mineral phosphate-solubilizing bacteria for enhanced sunflower growth and yield-attributing traits. Ann Microbiol. 2015;65:1525–1536. doi: 10.1007/s13213-014-0991-z. [DOI] [Google Scholar]
- Shahid M, Hussain B, Riaz D, Khurshid M, Ismail M, Tariq M. Identification and partial characterization of potential probiotic lactic acid bacteria in freshwater Labeo rohita and Cirrhinus mrigala. Aquac Res. 2017;48:1688–1698. doi: 10.1111/are.13006. [DOI] [Google Scholar]
- Shinwari KI, Shah AU, Afridi MI, Zeeshan M, Hussain H, Hussain J, Ahmad O. Application of plant growth promoting rhizobacteria in bioremediation of heavy metal polluted soil. Asian J Multidiscip Stud. 2015;3:179–185. [Google Scholar]
- Somasegaran P, Hoben HJ. Handbook for rhizobia: methods in legume rhizobium technology. Berlin: Springer; 1994. [Google Scholar]
- Song L, Shao Y, Ning S, Tan L. Performance of a newly isolated salt-tolerant yeast strain Pichia occidentalis G1 for degrading and detoxifying azo dyes. Bioresour Technol. 2017;233:21–29. doi: 10.1016/j.biortech.2017.02.065. [DOI] [PubMed] [Google Scholar]
- Stavenhagen M, Buurman J, Tortajada C. Saving water in cities: assessing policies for residential water demand management in four cities in Europe. Cities. 2018;79:187–195. doi: 10.1016/j.cities.2018.03.008. [DOI] [Google Scholar]
- Steel RG, Torrie JH, Dickey D. Principles and procedures of statistics, a biometrical approach. New York: The McGraw-Hill Co. Inc.; 1997. [Google Scholar]
- Sun Z, Liu K, Zhang J, Zhang Y, Xu K, Yu D, Wang J, Hu L, Chen L, Li C. IAA producing Bacillus altitudinis alleviates iron stress in Triticum aestivum L. seedling by both bioleaching of iron and up-regulation of genes encoding ferritins. Plant Soil. 2017;419:1–11. doi: 10.1007/s11104-017-3218-9. [DOI] [Google Scholar]
- Tak HI, Ahmad F, Babalola OO. Advances in the application of plant growth-promoting rhizobacteria in phytoremediation of heavy metals. Rev Environ Contam Toxicol. 2013;223:33–52. doi: 10.1007/978-1-4614-5577-6_2. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi P, Sa T. Pseudomonas corrugata (NRRL B-30409) mutants increased phosphate solubilization, organic acid production, and plant growth at lower temperatures. Curr Microbiol. 2008;56:140–144. doi: 10.1007/s00284-007-9058-8. [DOI] [PubMed] [Google Scholar]
- Wang H, Su JQ, Zheng XW, Tian Y, Xiong XJ, Zheng TL. Bacterial decolorization and degradation of the reactive dye Reactive Red 180 by Citrobacter sp. CK3. Int Biodeterior Biodegrad. 2009;63:395–399. doi: 10.1016/j.ibiod.2008.11.006. [DOI] [Google Scholar]
- Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.