Abstract
Quercetin and rutin, two flavonoids were examined for antimycobacterial activities against M. tuberculosis H37Rv (ATCC 27294). The quercetin exhibited (99.30 ± 0.268%) in (LRP) assay at 200 µg/ml and 56.21 ± 0.97% inhibition in (BMD) at 50 µg/ml, whereas rutin exhibited (90.40 ± 0.68%) in LRP assay at 200 µg/ml and 56.10 ± 0.67% inhibition in BMD at 50 µg/ml. The minimum inhibitory concentration (MIC) was found to be 6.25 µg ml−1 and 25 µg ml−1 respectively. The current investigation suggests that quercetin has better inhibitory activity than rutin.
Keywords: Antimicrobials, Mycobacteria, Biopharmaceutical, Rapid methods, Cytotoxicity
Introduction
Medicinal plants, animals, microbes and marine organisms have historically proven their value as a source of bioactive molecules with therapeutic potentials and natural products as secondary metabolites have played a key role in drug discovery and constituted a prolific source of novel lead compounds or pharmacophores for medicinal chemistry to treat human diseases (Cragg and Newman 2013). Approximately 40% of natural products and their derivatives were approved by FDA as developed therapeutic agents during a period from 1981 to 2010 (Newman and Cragg 2012). Tuberculosis (TB), an old, highly infectious disease which was declared a global health emergency by the World Health Organization (WHO) in 1993, is still the second leading killer in the world (WHO 2014). TB drugs are more toxic and less efficient, with cure rates in the range of 60–75%. Unfortunately, there is lack of adherence to prescribed TB treatment procedures and inefficient healthcare structures have contributed to the development of multi-drug-resistant TB (MDR-TB, defined as resistance to at least isoniazid and rifampin, the two most potent TB drugs), Extensively drug-resistant TB (XDR-TB) is a rare type of multi-drug-resistant tuberculosis (MDR-TB) that is resistant to isoniazid, rifampin, all fluoroquinolones and at least one of three injectable second-line drugs (i.e., amikacin, kanamycin, or capreomycin) (Lienhardt et al. 2012). The increasing frequency of MDR-TB, XDR-TB and currently, totally drug-resistant tuberculosis (TDR-TB), and limited therapeutic options emphasize the urgent need for novel drugs from natural products with fewer side effects, so as to overcome the problem of drug resistance and to finally eradicate TB (Nguta et al. 2015).
In Ayurvedic system of medicine, TB is known as ‘Kshya Kasha’ or ‘Raja Yaksha’. Sixty Indian medicinal plant species have been reported to be used in different Ayurvedic formulations for TB (Anon 2003) and also several plant species used for treatment of TB and related diseases has been described in various Indian traditional systems of medicine (Kirtikar and Basu 1935). Approximately 60% of world’s population still rely on plant based drugs for the treatment of various diseases and disorders. Phenolic compounds from few plant extracts possess a high amount of antimycobacterial flavonoids and most of them belong to classes of flavones and flavonones (Lin et al. 2002). Flavonoids are secondary poly phenolic plant metabolites with several health promoting effects including anticancer, antiinflammatory, analgesic, antimicrobial, antioxidant and many others. Quercetin (PubChem CID: 5280343) and rutin (PubChem CID: 5280805) are two simple flavonoids commonly found in vegetables, fruits, herbs, leaves, seeds, red wine, tea, coffee, beer, and several medicinal plants (Dai et al. 2010). The average human intake of the flavonoids has been estimated to be ~ 25 mg/day (Yang et al. 2000).
The growth inhibitory property of the flavonoids (quercetin and rutin) against slow-growing mycobacteria (M. tuberculosis H37Rv) was determined using the microplate alamar blue assay (MABA), luciferase reporter phage assay (LRPA) and broth-microdilution method (BMDM) respectively. There are no much in vitro studies on antimycobacterial activity of rutin and this is first report of quercetin in luciferase reporter phage assay.
Materials and methods
In vitro antituberculosis bioassays
A well characterized slow growing, virulent strain, M. tuberculosis H37Rv (ATCC 27294) was used for the drug susceptibility studies. In the current study, quercetin and rutin were used as test compounds and pyrazinamide (PZA), ciprofloxacin (CIP) and streptomycin (STM) were used as standard drug.
Microplate alamar blue assay (MABA)
Minimum inhibitory concentrations (MIC) of rutin and quercetin against M. tuberculosis H37Rv were determined in Middlebrook 7H9 broth by the standard micro dilution method, with some modifications (Sharma et al. 2014). Briefly, 50 µl of test compound, standard drugs (positive control) like pyrazinamide, ciprofloxacin and streptomycin in DMSO were serially diluted to yield a final concentrations ranging from 100 to 0.2 µg/ml were added to a 96-well microtitre plate. Control wells included medium and culture controls. Forty micro-litre of culture (at 3 × 105 CFU/ml) was added to all the wells except the medium control wells. The plates were sealed with parafilm and incubated at 37 °C for 6 days. 25 µl of a freshly prepared 1:1 mixture of Alamar Blue reagent and 10% Tween 80 were added to all the wells and re-incubated for 24 h at 37 °C and the colour conversion of all wells was recorded. A blue colour in the well was interpreted as no growth, and a pink colour was scored as growth. The MIC was defined as the lowest concentration of compound which yielded 100% inhibition of growth of organisms, where no colour change from blue to pink was observed.
Luciferase reporter phage assay (LRPA)
Antimycobacterial activity of the rutin and quercetin were evaluated by luciferase reporter phage (LRP) assay. LRP assay for standard strain H37Rv, a clinical sensitive strain was grown in Middlebrook 7H9 complete medium with and without test compounds for 3 days at 37 °C. LRP assay was done using different concentration of test compounds. DMSO was used as the solvent control. LRP phage AETRC21 was added and the samples were incubated for 4 h. Equal volume of the cell phage mixture was mixed with 0.3 Mm d-Luciferin in 0.05 M sodium citrate buffer of pH 4.5 and light output was immediately measured as RLU (Relative light units) in the luminometer at 10 s integration. Cultures showing more than 50% reduction in RLU in ‘test’ compared to ‘control’ were classified as sensitive and less than 50% reduction were considered as resistant. These LRP assays offer an elegant means of detecting viable mycobacteria and provide a rapid tool for drug susceptibility screening (Dusthackeer et al. 2008):
Broth-micro-dilution method (BMDM)
Anti-TB potentials of rutin and quercetin against M. tuberculosis H37Rv were determined by the standard broth-micro-dilution method (Tekwu et al. 2012). Bioactive compound rutin and quercetin were dissolved in 1 ml DMSO and further dilution will be made to obtain the concentration 50 µg/ml using Middlebrook 7H9 with 0.2% glycerol, 0.5% BSA, 0.2% dextrose, 0.085% NaCl and 0.05% Tween 80. Bacterial suspensions were prepared and adjusted to 0.01 OD at 580 nm (0.5 × 106 cfu/ml) in 7H9 medium and subjected to the action of the bioactive compound separately for 4 days. After the exposure, 50 µl of each of the drug exposed and the unexposed culture suspension were diluted tenfold times with 7H9 medium and 3 µl from each of the dilution was spotted on Middlebrook 7H11 plates containing 0.5% glycerol and 10% OADC (oleic acid/albumin/dextrose/catalase supplement) (Difco). The plates were placed in polythene bags and incubated at 37 °C. The plates were read after 4 week incubation and countable number of colonies was recorded and the colony forming units per millilitre (cfu/ml) were calculated for each of the dilutions. The results were represented, as the mean of the quadruplicates of the cultures for every drug concentration and for the control cultures it was mean of duplicates:
Statistical analysis
All experiments were carried out in triplicates and expressed as Mean ± SD. The statistical analysis for each experiment was done using one-way ANOVA at significance level < 0.05.
Results and discussion
Antimycobacterial potentials of quercetin and rutin
The minimum inhibitory concentration (MIC) of quercetin and rutin were determined using MABA on a drug sensitive strain of M. tuberculosis H37Rv and was found to be 6.25 µg/ml and 25 µg/ml respectively. The standard drugs PZA, CIP and STM were used as positive control and the results are shown in Table 1. Quercetin was found very potent and showed significant inhibition compared to rutin (quercetin-3-O-rutinoside). M. tuberculosis is a slow-growing intracellular pathogen which has a complex cell envelope containing mycolic acids and a diversity of other lipids, many of which are unique for mycobacteria (Hoffmann et al. 2008). The evaluation of anti-TB potential of flavonoids using 96-well microplate alamar blue assay offers the advantages of less sample requirements and low cost (Balouiri et al. 2016). Alamar blue (an oxidation–reduction indicator dye) reagent has been used to study both metabolism and viability in microorganisms and their detection is more sensitive and rapid. Alamar blue is blue in colour in the oxidized state, but it turns pink when reduced due to bacterial metabolism. The colours can easily be differentiated with the naked eye (Rampersad 2012). A sample with an MIC value < 250 µg ml−1 was defined as active against M. tuberculosis (Araujo et al. 2014). In MABA assay, 10% Tween 80 was used in the preparation of resazurin indicator which reduces the ability of the mycobacterial cells to adhere to one another and hence increases the available surface area of mycobacterial cells (Schafer et al. 1999) which allows for greater contact, absorption and metabolism of the resazurin solution, thereby facilitating its reduction to resorufin. The resazurin indicator solution was prepared with concentration range of 0.02% to but not more than 25% Tween 80. Above this concentration of Tween 80 in resazurin indicator were neglected to use in MABA protocols (O’Neill et al. 2014).
Table 1.
Antimycobacterial activity of flavonoids (quercetin and rutin)
| Strain | Minimum inhibition concentration µg/mL (µM) | ||||
|---|---|---|---|---|---|
| Quercetin | Rutin | Pyrazinamide | Ciprofloxacin | Streptomycin | |
| M. tuberculosis H37RV | 6.25 (20.67) | 25 (40.94) | 3.125 (2.53) | 3.125 (0.94) | 6.25 (10.74) |
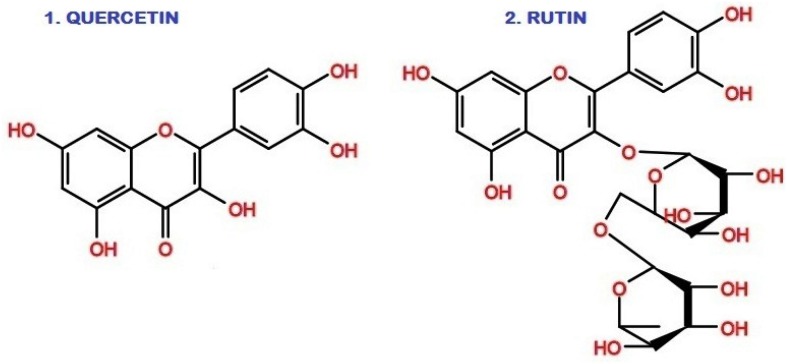
Antimycobacterial activity of quercetin using radiometric assay of BACTEC 460 against M. tuberculosis showed variations in MIC values found at 100 µg ml−1 (Villaume et al. 2017) and also 50 µg ml−1. The MIC of other flavonoids, viz., luteolin (25 µg ml−1), myricetin (50 µg ml−1) and hispidulin (100 µg ml−1) were found against M. tuberculosis H37Rv strain radiometrically by BACTEC 460 assay. A possible structure–activity relationship concluded that the presence of hydroxyl the 3, 4 positions (luteolin, quercetin, and myricetin) were the prerequisites for the inhibition of growth of M. tuberculosis (Yadav et al. 2013). Quercetin with glycoside is called rutin (quercetin-3-O-rutinoside) and quercetin (aglycone) has similar physical and chemical properties but quercetin exhibit significant inhibition against M. tuberculosis than rutin (Fig. 1). This may be due to the structure rigidity and binding specificity of the compounds towards mycobacterium.
Fig. 1.
Flavonoids with good inhibitory potentials on the Mtb H37Rv and each at 50 µg/ml in LRP assay showed quercetin 91%, rutin 78% inhibition and both showed 56% similar inhibition effect on BMM. The common structural features are quercetin—hydroxyl group at the C rings in C-3 position; rutin—at C-3 hydroxylation with disaccharide rutinose (α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranose)
The results of luciferase phage assay are shown in Table 2. Both quercetin and rutin possessed 50% above inhibition of mycobacterium at all concentrations (50, 100, 200 µg/ml) tested. The maximum inhibition of quercetin was found to be 99.30 ± 0.268%, whereas rutin showed 90.40 ± 0.685% at the concentration of 200 µg/ml. LRP assay is also used for rapid determination of bacterial viability by measuring the expression of an introduced fluorescent or luminescent protein. These fluorescent proteins do not require an exogenous substrate, thus simplifying quantitation and enabling easy determination of growth and/or inhibition kinetics (Nguta et al. 2015). Since reporter gene assays use for commercial applications is often limited by patent restrictions and very few laboratories reported to be using this technology for primary screens of natural products (Sanchez and Kouznetsov 2010). Compounds showing relative light unit (RLU) reduction by 50% or more when compared to control will be considered as having antimycobacterial potentials (Dusthackeer et al. 2008).
Table 2.
Susceptibilities of M. tuberculosis H37Rv against flavonoids (quercetin and rutin) by luciferase phage assay (LRP) and broth-micro-dilution method (BMD)
| Flavonoids | % Inhibition of M. tuberculosis H37Rv | |||||
|---|---|---|---|---|---|---|
| Rutin | Quercetin | |||||
| Dose (µg/mL) | 50 | 100 | 200 | 50 | 100 | 200 |
| LRP assay | 77.70 ± 0.78 | 84.12 ± 0.93 | 90.40 ± 0.68 | 91.87 ± 0.21 | 98.78 ± 0.90 | 99.30 ± 0.26 |
| BMD method | 56.10 ± 0.67 | N/D | N/D | 56.21 ± 0.97 | N/D | N/D |
N/D not determined
The broth dilution was performed for quercetin and rutin at 50 µg/mL of each and aliquots of 100 µL from the MIC wells were spread onto agar plates to detect minimum bactericidal concentration (MBC) values for the active substances. The MBC was defined as the concentration resulting in a sharp reduction (> 99%) in the growth of mycobacteria after incubation. In this study, both the compounds showed similar percentage of inhibition of mycobacterium around 56.21 ± 0.97% for quercetin, whereas 56.10 ± 0.67% for rutin. The bactericidal concentrations of the quercetin and rutin at 50 µg/mL against M. tuberculosis showed that the both had similar inhibition compared to control. Quercetin and rutin were endowed with lower MIC by microplate resazurin assay method than by CFU method (broth-micro-dilution). This has been reported based on the determination techniques of MIC values exhibited by various compounds. In liquid medium MICs obtained are lower than from a solid medium (Nair et al. 2015) and also reported that variations in MICs values of aporphine alkaloids were found in solid and liquid medium growth inhibition assay for M. bovis BCG (Guzman et al. 2010). Difference in MIC were observed with variations in inoculum size for M. tuberculosis H37Rv on agar or in broth, in protein concentration and labware plastics on agar, and with variations in pH and Tween 80 concentrations in broth. Bedaquiline showed MIC in 7H9 liquid medium was more susceptible to changes than the MIC determined on 7H11 agar medium. This may be due to the partial inhibition of the condition by the agar and/or to the more rapid growth of M. tuberculosis in 7H9 liquid medium (Lounis et al. 2016).
Quercetin and rutin (derivative of rutin) are dietary flavonoid, which widely exists in nature and both exhibits an excellent other pharmacological potentials like antibacterial, antifungal, antimalarial, antioxidant, antiinflammatory, anticancer, anticardiovascular and many others (Ganeshpurkar and Saluja 2017; Wang et al. 2016; Dubey et al. 2013). In a previous study with flavonoids, chalcones showed high antituberculosis activity and flavones, flavonones and flavonols moderate activity against M. tuberculosis H37Rv and M. bovis BCG (Lin et al. 2002). Biological assay studies and quantitative structure–activity relationship (QSAR) studies of chalcones, flavones, flavanones and their derivatives show very good inhibition potentials against M. tuberculosis H37Rv strain (Sivakumar et al. 2007). The free hydroxyl group and overall lipophylicity of the compounds were the two most important structural characteristics responsible for in vitro anti-TB activity (Rajab et al. 1998). In reported articles, rutin was displayed antimycobacterial activity of crude extracts including rutin using single assay and there is not much activity performed (Jesus et al. 2018). The secondary metabolites, 5-hydroxy-3,7,4′-trimethoxyflavone, 5,7-dihydroxy-3,4-dimethoxyflavone, and 5,4-dihydroxy-3,7- dimethoxyflavone, from Haplopappus sonorensis exhibits significant antimycobacterial potentials (Murillo et al. 2003). Several proposed mechanism of action for quercetin against Mycobacterium spp were involved in inhibition of the mycobacterial cell wall biosynthesis via enzyme inhibition. In M. smegmatis and M. tuberculosis, quercetin inhibits the subunit B of DNA gyrase (Suriyanarayanan et al. 2013). Through docking studies, quercetin was shown to inhibit beta-ketoacyl ACP synthase III, which is involved in the synthesis of mycolic acid (Jacob et al. 2014) and also docking analysis suggested that quercetin-3-O-β-d-glucoside inhibits the M. tuberculosis glutamine synthetase (MtGS) enzyme (MtGS enzyme play an essential role in its pathogenesis and was identified as a potential drug target) and related to its role in the production of the poly-l-glutamate–glutamine: a major component of the cell wall in pathogenic mycobacteria (Safwat et al. 2018). Quercetin also inhibits isocitratelyase, a key enzyme of the glyoxylate shunt, crucial for the intracellular survival of bacteria in macrophages (Shukla et al. 2015). Quercetin exhibited good inhibition and affinities for flavoenzyme of uridine 5′-diphosphategalactopyranosemutase (UGM). This enzyme is an essential biocatalyst involved in the cell wall biosynthesis of M. tuberculosis (Villaume et al. 2017). Furthermore, it also regulates the ammonia levels within infected host cells and so helps the pathogen in the inhibition of the phagosome acidification and phagosome–lysosome fusion (Gising et al. 2012). It also has been reported that quercetin inhibits 74.40% of mycobacteria proteasomes with IC50 71.29 µM, whereas rutin did not inhibit (Zheng et al. 2014). The quercetin suppressed the hyaluronan-dependent growth of M. tuberculosis complex in the lungs by inhibiting the hyaluronidase enzyme which utilizes hyaluronan as a carbon source for multiplication (Hirayama et al. 2009). Furthermore, natural products have specific selectivity for cellular targets like selective ligand for disease-related targets (Gu et al. 2013). Sulfotranferase plays key role in sulfur metabolic pathways which are essential for survival and the expression of virulence in pathogenic bacteria, M tuberculosis. Reduced sulfur-containing metabolite, coenzyme A (CoA), is heavily utilized for lipid metabolism and biosynthesis of mycolic acid, which is an important constituent in mycobacterial cell wall. Quercetin inhibits the sulfotransferase enzyme which leads to the blocking of sulfur metabolism (Bhave et al. 2007).
Flavonoids can inhibit enzymes involved in the biosynthesis of fatty acid and mycolic acid which is essential for mycobacterium survival. Interestingly, the common structural feature of the potent fatty acid synthase (FAS) II inhibitors, i.e., flavonoids became understood that the ketone group borne of the flavonoids imitate the carbonyl group of a substrate fatty acid, they all possess a 2, 3 double bond compatible with a product mimic and FAS-II do not bear the oxygen containing cycle possessed flavonoids like fisetin and quercetin (Brown et al. 2007). The anti-TB potentials of flavonoids against Mycobacterium clearly represent an important potential target for future drug development studies.
Tuberculosis is a very serious neglected disease in the world and one-third of the world’s population, especially from low- and middle income developing countries, is infected with M. tuberculosis. It is clearly evident from the above data that the flavonoids from medicinal plants have great potential to be used as anti-TB agents. These flavonoids have displayed very high activity, not only by inhibiting but also by killing all of the Mycobacteria strains. This indicates that the phenolics and flavonoids might represent promising sources of anti-TB drugs and might be “a model” for the drug design. Identification of the antimycobacterial mechanism of action might be the key to the further development of these compounds.
Acknowledgements
We are grateful to Dr. Uma Devi, HOD, Department of Bacteriology, National Institute for Research in Tuberculosis (ICMR), Chennai, India for performing antituberculosis assays. Authors are also thankful to the management of VIT University for their support.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest in the present study.
References
- Anon (2003) Ayurvedic Formulary of India. Part I. Department of ISM & H, Ministry of Health and Family Welfare, Govt. of India
- Araujo RC, Neves FA, Formagio AS, Kassuya CA, Stefanello ME, Souza VV, Pavan FR, Croda J. Evaluation of the anti-mycobacterium tuberculosis activity and in vivo acute toxicity of Annona sylvatic. BMC Complement Altern Med. 2014;14:209. doi: 10.1186/1472-6882-14-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave DP, III WBM, Carroll KS. Drug targets in mycobacterial sulfur metabolism. Infect Disord Drug Targets. 2007;7:140–158. doi: 10.2174/187152607781001772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AK, Papaemmanouil A, Bhowruth V, Bhatt A, Dover LG, Besra GS. Flavonoid inhibitors as novel antimycobacterial agents targeting Rv0636, a putative dehydratase enzyme involved in Mycobacterium tuberculosis fatty acid synthase II. Microbiology. 2007;153:3314–3322. doi: 10.1099/mic.0.2007/009936-0. [DOI] [PubMed] [Google Scholar]
- Cragg GM, Newman DJ. Natural products: a continuing source of novel drug leads. Biochem Biophys Acta. 2013;1830:3670–95. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey S, Ganeshpurkar A, Bansal D, Dubey N. Experimental studies on bioactive potential of rutin. Chron Young Sci. 2013;4(2):153–157. doi: 10.4103/2229-5186.115556. [DOI] [Google Scholar]
- Dusthackeer A, Kumar V, Subbianb S, Sivaramakrishnan G, Zhu G, Subramanyam B, Hassan S, Nagamaiah S, Chan J, Rama NP. Construction and evaluation of luciferase reporter phages for the detection of active and non-replicating tuberculi bacilli. J Microbiol Methods. 2008;73:18–25. doi: 10.1016/j.mimet.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeshpurkar A, Saluja AK. The pharmacological potential of rutin. Saudi Pharm J. 2017;25(2):149–164. doi: 10.1016/j.jsps.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gising J, Nilsson MT, OdellL R, Yahiaoui S, Lindh M, Iyer H, Sinha AM, Srinivasa BR, Larhed M, Mowbray SL, Karlen A. Trisubstitutedimidazoles as Mycobacterium tuberculosis glutamine synthetase inhibitors. J Med Chem. 2012;22:2894–2898. doi: 10.1021/jm201212h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Gui Y, Chen L, Yuan G, Lu HZ, Xu X. Use of natural products as chemical library for drug discovery and network pharmacology. PLoS One. 2013;8:e62839. doi: 10.1371/journal.pone.0062839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman JD, Gupta A, Evangelopoulos D, Basavannacharya C, Pabon LC, Plazas EA, Munoz DR, Delgado WA, Cuca LE, Ribon W, Gibbons S, Bhakta S. Anti-tubercular screening of natural products from Colombian plants: 3-methoxynordomesticine, an inhibitor of MurE ligase of Mycobacterium tuberculosis. J Antimicrob Chemother. 2010;65:2101–2107. doi: 10.1093/jac/dkq313. [DOI] [PubMed] [Google Scholar]
- Hirayama Y, Yoshimura M, Ozeki Y, Sugawara I, Udagawa T, Mizuno S, Itano N, Kimata K, Tamaru A, Ogura H, Kobayashi K, Matsumoto S. Mycobacteria exploit host hyaluronan for efficient extracellular replication. PLoS Pathog. 2009;5(10):e1000643. doi: 10.1371/journal.ppat.1000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C, Leis A, Niederweis M, Plitzko JM, Engelhardt H. Disclosure of the mycobacterial outer membrane: cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc Natl Acad Sci USA. 2008;105:3963–3967. doi: 10.1073/pnas.0709530105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob SJP, Jothivanan E, Vamsi BYV. Molecular docking analysis of bioflavonoids against Mycobacterium tuberculosis drug target beta-ketoacyl-acp synthase III. Intern J Pharm Res Dev. 2014;6:48–52. [Google Scholar]
- Jesus RS, Piana M, Freitas RB, Brum TF, Alves CFS, Belka BV, Mossmann NJ, Cruz CC, Santos RCV, Dalmolin TV, Bianchini BV, Campos MA, Bauermann LF. Invitro antimicrobial and antimycobacterial activity and HPLC–DAD screening of phenolics from Chenopodium ambrosioides L. Brazilian J Microbiol. 2018;49(2):296–302. doi: 10.1016/j.bjm.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtikar KR, Basu BD. Indian Medicinal Plants, vols 1–4. Allahabad: Lalit Mohan Basu; 1935. [Google Scholar]
- Lienhardt C, Raviglione M, Spigelman M, Hafner R, Jaramillo E, Hoelscher M, Zumla A, Gheuens J. New drugs for the treatment of tuberculosis: needs, challenges, promise and prospects for the future. J Infect Dis. 2012;205:S241–S249. doi: 10.1093/infdis/jis034. [DOI] [PubMed] [Google Scholar]
- Lin YM, Zhou Y, Flavin MT, Zhou LM, Nie W, Chen FC. Chalcones and flavonoids as anti-tuberculosis agents. Bioorz Med Chem. 2002;10:2795–2802. doi: 10.1016/S0968-0896(02)00094-9. [DOI] [PubMed] [Google Scholar]
- Lounis N, Vranckx L, Gevers T, Kaniga K, Andries K. In vitro culture conditions affecting minimal inhibitory concentration ofbedaquiline against M. tuberculosis. Médecineet Maladies Infectieuses. 2016;46:220–225. doi: 10.1016/j.medmal.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Murillo JI, Dimayuga RE, Malmstrom J, Christophersen C, Franzblau SG. Antimycobacterial flavones from Haplopappus sonorensis. Fitoterapia. 2003;74:226–230. doi: 10.1016/S0367-326X(03)00033-9. [DOI] [PubMed] [Google Scholar]
- Nair SS, Pharande RR, Bannalikar AS, Mukne AP. In vitro anti-mycobacterial activity of acetone extract of GLYCYRRHIZA GLABRA. J Pharm Pharmacogn Res. 2015;3:80–86. [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguta JM, Appiah-Opong R, Nyarko AK, Yeboah-Manu D, Addo PG. Current perspectives in drug discovery against tuberculosis from natural products. Int J Mycobacteriol. 2015;4:165–183. doi: 10.1016/j.ijmyco.2015.05.004. [DOI] [PubMed] [Google Scholar]
- O’Neill TE, Li H, Colquhoun CD, Johnson JA, Webster D, Gray CA. Optimization of the microplate resazurin assay for screening and bioassay-guided fractionation of phytochemical extracts against Mycobacterium tuberculosis. Phytochem Anal. 2014;25(5):461–467. doi: 10.1002/pca.2516. [DOI] [PubMed] [Google Scholar]
- Rajab MS, Cantrell CL, Franzblau SG, Fischer NH. Antimycobacterial activity of (E)-phytol and derivatives: a preliminary structure-activity study. Planta Med. 1998;64:2–4. doi: 10.1055/s-2006-957354. [DOI] [PubMed] [Google Scholar]
- Rampersad SN. Multiple applications of alamar blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors. 2012;12:12347–12360. doi: 10.3390/s120912347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safwat NA, Kashef MT, Aziz RK, Amer KF, Ramadan MA. Quercetin 3-O-glucoside recovered from the wild Egyptian Sahara plant, Euphorbia paralias L., inhibits glutamine synthetase and has antimycobacterial activity. Tuberculosis. 2018;108:106–113. doi: 10.1016/j.tube.2017.11.005. [DOI] [PubMed] [Google Scholar]
- Sanchez JGB, Kouznetsov VV. Antimycobacterial susceptibility testing methods for natural products research. Braz J Microbiol. 2010;41:270–277. doi: 10.1590/S1517-83822010000200001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MP, Fernback JE, Ernst MK. Detection and characterization of airborne Mycobacterium tuberculosis H37Ra particles, a surrogate for airborne pathogenic, M. tuberculosis. Aerosol Sci Technol. 1999;30(2):161–173. doi: 10.1080/027868299304750. [DOI] [Google Scholar]
- Sharma S, Gelman E, Narayan C, Bhattacharjee D, Achar V, Humnabakar V, Balasubramanian V, Ramachandran V, Dhar N, Dinesh N. Simple and rapid method to determine antimycobacterial potency of compounds by using auto-luminescent Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2014;58:5801–5808. doi: 10.1128/AAC.03205-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla H, Kumar V, Singh AK, Rastogi S, Khan SR, Siddiqi MI, et al. Isocitratelyase of Mycobacterium tuberculosis is inhibited by quercetin through binding at N-terminus. Intern J Biol Macromol. 2015;78::137–41. doi: 10.1016/j.ijbiomac.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Sivakumar PM, Babu SKG, Mukesh D. QSAR studies on chalcones and flavonoids as anti-tuberculosis agentsusing genetic function approximation (GFA) method. Chem Pharm Bull. 2007;55(1):44–49. doi: 10.1248/cpb.55.44. [DOI] [PubMed] [Google Scholar]
- Suriyanarayanan B, Shanmugam K, Santhosh RS. Synthetic quercetin inhibits mycobacterial growth possibly by interacting with DNA gyrase. Rom Biotechnol Lett. 2013;18:1587–1593. [Google Scholar]
- Tekwu EM, Askun T, Kuete V, Nkengfack AE, Nyasse B, Etoa FX, Beng VP. Antibacterial activity of selected Cameroonian dietary spices ethno-medically used against strains of Mycobacterium tuberculosis. J Ethnopharmacol. 2012;142:374–382. doi: 10.1016/j.jep.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Villaume SA, Fu J, Go IN, Liang H, Lou H, Kremer L, Pan W, Vincent SP. Natural and synthetic flavonoids as potent Mycobacterium tuberculosis UGM inhibitors. Chem Eur J. 2017;23:10423–10429. doi: 10.1002/chem.201701812. [DOI] [PubMed] [Google Scholar]
- Wang W, Sun C, Mao L, Ma P, Liu F, Yang J, Gao Y. The biological activities, chemical stability, metabolism and delivery system of quercetin: a review. Trends Food Sci Technol. 2016;56:21–38. doi: 10.1016/j.tifs.2016.07.004. [DOI] [Google Scholar]
- World Health Organization, WHO Global Tuberculosis Report (2014) http://www.who.int/tdr/news/2014/global-TB-report/en/
- Yadav AK, Thakur J, Prakash O, Khan F, Saikia D, Gupta MM. Screening of flavonoids for antitubercular activity and their structure–activity relationships. Med Chem Res. 2013;22:2706–2716. doi: 10.1007/s00044-012-0268-7. [DOI] [Google Scholar]
- Yang K, Lamprechet SA, Liu Y, Shinozaki H, Fan K, Leung D, Newmark H, Steele VE, Kelloff GJ, Liipkin M. Chemoprevention studies of the flavonoids quercetin and rutin in normal and azoxymethane-treated mouse colon. Carcinogenesis. 2000;21:1655–1660. doi: 10.1093/carcin/21.9.1655. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Jiang X, Gao F, Song J, Sun J, Wang L, Sun X, Lu Z, Zhang H. Identification of plant-derived natural products as potential inhibitors of the Mycobacterium tuberculosis proteasome. BMC Complement Altern Med. 2014;14:400. doi: 10.1186/1472-6882-14-400. [DOI] [PMC free article] [PubMed] [Google Scholar]