Abstract
Left ventricular myocardial fibrosis in patients with aortic stenosis (AS) confers worse prognosis. Plasma osteoprotegerin (OPG), a cytokine from the TNF receptor family, correlates with the degree of valve calcification in AS, reflecting the activity of the tissue RANKL/RANK/OPG (receptor activator of nuclear factor κΒ ligand/RANK/osteoprotegerin) axis, and is associated with poorer outcomes in AS. Its association with myocardial fibrosis is unknown. We hypothesised that OPG levels would reflect the extent of myocardial fibrosis in AS. We included 110 consecutive patients with AS who had undergone late-gadolinium contrast enhanced cardiovascular magnetic resonance (LGE-CMR). Patients were characterised according to pattern of fibrosis (no fibrosis, midwall fibrosis, or chronic myocardial infarction fibrosis). Serum OPG was measured with ELISA and compared between groups defined by valve stenosis severity. Some 36 patients had no fibrosis, 38 had midwall fibrosis, and 36 had chronic infarction. Patients with midwall fibrosis did not have higher levels of OPG compared to those without fibrosis (6.78 vs. 5.25 pmol/L, p = 0.12). There was no difference between those with midwall or chronic myocardial infarction fibrosis (6.78 vs. 6.97 pmol/L, p = 0.27). However, OPG levels in patients with chronic myocardial infarction fibrosis were significantly higher than those without fibrosis (p = 0.005).
Introduction
Calcification of the cardiovascular system occurs commonly with aging. Progressive aortic valve calcification occurs in some patients, with symptoms presenting once critical restriction of valve-opening and left ventricular pressure overload ensue. The mechanisms responsible for the pathogenesis of calcific aortic stenosis (AS) are unknown, and likely reflect the interplay of multiple cytokine pathways and genetic factors1,2. Determining the ideal timing for aortic valve replacement (AVR) in AS remains a challenge. Current guideline-based indications for intervention depend on the severity of valve narrowing, as usually assessed with echocardiography, the presence of symptoms relating to a narrowed valve, and impairment of systolic function, as assessed by left ventricular ejection fraction (LVEF)3,4. Unfortunately, stenosis severity on echocardiography does not correlate well with the risk of progression of LV hypertrophy to heart failure, which is primarily driven by the development of myocardial fibrosis5. Currently, cardiovascular magnetic resonance (CMR) remains the only non-invasive, radiation-free investigation enabling the visualisation of myocardial fibrosis in the late phase following gadolinium administration indicating myocardial scar6. However, the associated costs and limited access to CMR limit its routine use in all patients with AS. It is also recognised that patients with fibrosis have worse prognosis independently of LVEF7, and therefore formulating an easy, non-invasive, and cost-effective way of identifying patients with high prevalence of fibrosis would be clinically useful. Blood biomarkers have the potential to identify patients with fibrosis and lend themselves to serial monitoring of these patients. In this way, they may help to identify those patients in whom greatest benefit may be gained from early AVR. Recently, the RANKL/RANK/OPG (receptor activator of nuclear factor κΒ ligand/RANK/osteoprotegerin) system, known to play an important role in bone turnover and vascular calcification8, has gained much interest in AS9. Osteoprotegerin (OPG) is a member of the tumour necrosis factor (TNF) receptor family and inhibits the interaction between RANKL and RANK10,11, and therefore may represent one such biomarker.
Most studies of tissue levels of OPG within the diseased aortic valve report a reduction in tissue OPG compared to controls9,12,13, but increased mRNA levels13. In contrast, reported levels of serum OPG consistently show a rise in tandem with an increase in tissue RANKL/RANK activity (but not serum RANKL14), in patients with a range of cardiovascular conditions, including vascular calcification15–17, diabetes18, and heart failure19–21. The same has also been demonstrated in AS22–24. Recently, preoperative serum OPG was shown to correlate with a worse outcome after AVR in 124 patients with severe AS during a mean follow-up of 3.8 years23. Additionally in other studies22–24, OPG levels were associated with AS severity, pulmonary capillary wedge pressure, and NT-proBNP levels, indicating that the presence of HF may be an important determinant of serum OPG in AS. Interestingly, OPG levels also correlated with LV global longitudinal strain (GLS) and strain rate, but ejection fraction (EF) did not differ between OPG tertiles23. Subtle changes in LV longitudinal function often precede changes in EF25–27, and elevated OPG levels may therefore suggest underlying myocardial fibrosis, which drives the progression to HF5. Patients with severe AS and cardiovascular magnetic resonance (CMR)-derived measures of myocardial replacement fibrosis (either midwall or chronic myocardial infarction) have poorer outcomes28,29. This has also been confirmed in a large prospective observational cohort study of patients with less severe disease30. Inflammation is known to play a critical role in the development of LV myocardial fibrosis31, and can also activate the RANKL/RANK/OPG system32. In an isoproterenol-induced rat model of heart failure, RANKL/RANK were shown to be crucial mediators of interleukin-17 (IL-17) induced activation of matrix metalloproteinase-1 (MMP-1) in cardiac fibroblasts33. In this model, treatment with both OPG and inhibitors of IL-17 reduced myocardial fibrosis.
The association between OPG levels and myocardial replacement fibrosis in AS remains unknown. The main aim of our study was to determine whether serum OPG was associated with the presence of (1) myocardial midwall and (2) myocardial infarction fibrosis in patients with AS, and compare patients with mild/moderate and severe disease. Additionally, we investigated the prognostic role of OPG in patients with AS.
Methods
Consecutive patients with AS and late gadolinium contrast enhanced CMR (LGE-CMR) were included in this prospective substudy34 (ClinicalTrials.gov Identifier: NCT00930735). AS severity was assessed according to the most recent AHA/ACC clinical practice guidelines for management of patients with valvular heart disease3. Exclusion criteria included acute coronary syndrome, clinical suspicion or evidence of infection, disseminated malignancy, severe aortic regurgitation, more than moderate mitral regurgitation/stenosis, previous valve replacement, contraindication to CMR (including presence of non-conditional devices), and estimated glomerular filtration rate of <30 ml/min. This study was approved by the NHS England Research Ethics Committee, and was undertaken in line with the ethical standards of the Declaration of Helsinki. All patients provided written informed consent.
Cardiovascular Magnetic Resonance
CMR scans were performed on a 1.5 T scanner (Magnetom Sonata or Avanto, Siemens, Erlangen, Germany) using a standardised protocol as previously described34. In short, initial localiser images were used to guide acquisition of a vertical long axis (VLA) cine with balanced steady state free precession (SSFP) at end-expiration. This was then used to guide SSFP cines in the two-, three- and four-chamber views. Contiguous 10 mm short axis slices of the LV were then taken from base to apex. Retrospective ECG gating was preferred for the cine acquisition. In patients with arrhythmia, prospective triggering was utilised. Aortic valve planimetry and LV mass and volume were then calculated. After gadolinium contrast agent (Gadovist, Schering AG, Berlin, Germany) administration, inversion recovery-prepared spoiled gradient echo images were acquired in standard long- and short-axis views to detect areas of LGE28. Images were analysed offline on dedicated software for LV function, volumes, mass, and AS severity (CMR Tools, Cardiovascular Imaging Solutions., London, United Kingdom). LV myocardial fibrosis was quantified via separate dedicated software (CVI42, Circle Cardiovascular Imaging, Calgary, Canada). Two independent, blinded, expert observers analysed the images with a third blinded observer adjudicating in cases of disagreement.
Osteoprotegerin Levels
OPG measurement from stored plasma was undertaken using monoclonal anti-human OPG antibody. Detection was via biotin-labelled polyclonal anti-human OPG antibody and Streptavidin HRP conjugate (BioVendor, Research and Diagnostics Products). This produced a yellow colour whose absorbance was measured at 450 nm. A reference absorbance of 620 nm was also measured and subtracted from all samples before calculating concentrations. The absorbance was proportional to the concentration of OPG in the sample, allowing detection of OPG from 3–60 pmol/L.
Statistical analysis
Baseline data are presented as mean ± SD for continuous variables and number (proportion) for categorical variables. Mann-Whitney U tests were used to test for either an association between OPG levels and AS severity, or OPG levels and presence (or absence) of myocardial fibrosis. Univariable and multivariable linear regression analyses were performed to identify possible predictors of OPG levels. A p value of <0.05 was taken as significant. All analyses were undertaken using Stata version 14.0 (College Station, Texas, USA).
Results
Patient data are shown in Table 1. Mild/moderate AS was diagnosed in 35 (31.8%) patients, and severe AS in 75 (68.2%) patients. Compared to patients with mild/moderate disease, patients with severe disease were older (78 ± 9 years vs 71 ± 10, p < 0.001), but well-matched in terms of gender, ethnicity, body mass index (BMI), cardiovascular comorbidities and resting blood pressure. Patients with severe disease also had more severe symptoms by New York Heart Association (NYHA) symptom class (≥class II, 60 (81.1%) vs 19 (59.4%), p = 0.03). Drug therapy was very similar between groups, with the exception of calcium channel blocker therapy being more common in the patient group with mild/moderate disease (8/26.7%) compared to patients with severe disease (6/8.7%, p = 0.03). NT-proBNP levels were higher in patients with severe AS compared to those with mild/moderate disease (1232 pg/ml vs. 3813 pg/ml; p = 0.002); however serum CRP levels did not differ between the groups (12 mg/L vs. 15 mg/L; p = 0.52). Serum creatinine was also similar between mild/moderate and severe groups (93 μmol/L vs 102 μmol/L, p = 0.20). The correlations between demographic, biochemical, and CMR data with serum OPG are presented in Table 2. Age (p < 0.0001), weight (p < 0.0001), serum creatinine (p = 0.001), diuretic use (p = 0.009), serum NT-proBNP (p = 0.002), CRP (p = 0.038), valve area on CMR (p = 0.011), LV mass (p = 0.027), and LGE % (p = 0.01) correlated with serum OPG. Baseline patient data were also well-matched across fibrosis-pattern groups (Supplementary Data Table 1).
Table 1.
Baseline Patient Data.
| Demographics | Mild/Moderate (N = 35) | Severe (N = 75) | p Value |
|---|---|---|---|
| Age, years | 71 ± 10 | 78 ± 9 | <0.001* |
| Male, n (%) | 26 (74.3) | 51 (68.0) | 0.66 |
| Hypertension, n (%) | 13 (40.6) | 44 (58.7) | 0.096 |
| SBP, mmHg | 130 ± 19 | 130 ± 22 | 0.95 |
| DBP, mmHg | 71 ± 11 | 71 ± 13 | 0.46 |
| Diabetes mellitus, n (%) | 1 (3.8) | 4 (6.1) | 1.00 |
| Current smoker, n (%) | 2 (6.3) | 3 (4.1) | 0.64 |
| Any coronary artery disease, n (%) | 13 (37.1) | 27 (36.0) | 1.00 |
| Previous stroke, n (%) | 1 (2.9) | 2 (2.7) | 1.00 |
| Atrial Fibrillation, n (%) | 5 (14.3) | 6 (8.0) | 0.32 |
| Hypercholesterolaemia, n (%) | 18 (58.1) | 50 (67.6) | 0.38 |
| NYHA Class ≥ II | 19 (59.4) | 60 (81.1) | 0.03* |
| Caucasian | 33 (94.3) | 72 (96.0) | 0.65 |
| Height, cm | 172 ± 12 | 168 ± 10 | 0.03* |
| Weight, kg | 82 ± 18 | 75 ± 17 | 0.05 |
| BMI, kg/m2 | 28 ± 5 | 26 ± 5 | 0.17 |
| Pharmacotherapy | |||
| Aspirin, n (%) | 19 (61.3) | 44 (59.5) | 1.00 |
| Clopidogrel, n (%) | 4 (13.8) | 12 (16.7) | 1.00 |
| ACE-I/ARB, n (%) | 13 (43.3) | 38 (51.4) | 0.52 |
| Beta Blocker, n (%) | 14 (46.7) | 32 (44.4) | 1.00 |
| Calcium channel blocker, n (%) | 8 (26.7) | 6 (8.7) | 0.03* |
| Diuretic, n (%) | 14 (43.8) | 43 (58.1) | 0.21 |
| Warfarin, n (%) | 4 (14.3) | 6 (8.3) | 0.46 |
| Amiodarone, n (%) | 0 (0.0) | 4 (5.6) | 0.32 |
| Statin, n (%) | 20 (66.7) | 54 (72.0) | 0.64 |
| Biochemical data | |||
| Osteoprotegerin, pmol/L | 5.76 ± 1.96 | 7.91 ± 3.82 | 0.002* |
| Creatinine, μmol/L | 93 ± 30 | 102 ± 36 | 0.20 |
| NT-ProBNP (pg/mL) | 1232 ± 1698 | 3813 ± 5489 | 0.002* |
| CRP (mg/L) | 12 ± 22 | 15 ± 39 | 0.52 |
| CMR data | |||
| CMR aortic valve area, cm2 | 1.2 ± 0.3 | 0.7 ± 0.1 | <0.00001* |
| LVEF, % | 62 ± 14 | 57 ± 17 | 0.11 |
| LV Mass, g | 166 ± 47 | 168 ± 57 | 0.87 |
| No Myocardial Fibrosis, n (%) | 13 (37.1) | 23 (30.7) | 0.82 |
| Midwall Fibrosis, n (%) | 11 (31.4) | 27 (36.0) | 0.82 |
| Infarction Pattern Fibrosis, n (%) | 11 (31.4) | 25 (33.3) | 0.82 |
| LGE Mass | 5.7 (7.6) | 6.0 (7.9) | 0.72 |
| LGE Percent | 3.4 (4.8) | 3.8 (5.1) | 0.71 |
| Intervention | |||
| None | 21 (60.0) | 24 (32.0) | 0.01* |
| AVR | 9 (25.7) | 25 (33.3) | 0.01* |
| TAVR | 5 (14.3) | 26 (34.7) | 0.01* |
Values are mean ± SD unless otherwise stated. *p < 0.05. Baseline data for AS patients based on severity. ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; AVR, aortic valve replacement; BMI, body mass index; CMR, cardiovascular magnetic resonance; DBP, diastolic blood pressure; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SBP, systolic blood pressure; TAVR, transcatheter aortic valve replacement.
Table 2.
Correlations between Patient Data and Serum OPG levels.
| Variable | Spearman’s Correlation with OPG | P-Value |
|---|---|---|
| Patient Demographics | ||
| Age, years | 0.56 | <0.0001* |
| Male, n (%) | −0.025 | 0.80 |
| Hypertension, n (%) | −0.047 | 0.63 |
| SBP, mmHg | 0.015 | 0.88 |
| DBP, mmHg | −0.13 | 0.19 |
| Diabetes mellitus, n (%) | 0.024 | 0.82 |
| Current smoker, n (%) | 0.005 | 0.96 |
| Any coronary artery disease, n (%) | 0.10 | 0.29 |
| Previous stroke, n (%) | 0.0009 | 0.99 |
| Atrial Fibrillation, n (%) | −0.023 | 0.82 |
| Hypercholesterolaemia, n (%) | 0.028 | 0.78 |
| NYHA ≥ II | 0.033 | 0.73 |
| Height, cm | −0.17 | 0.077 |
| Weight, kg | −0.37 | <0.0001* |
| BMI, kg/m2 | −0.36 | <0.001* |
| Pharmacotherapy | ||
| Aspirin, n (%) | 0.006 | 0.95 |
| Clopidogrel, n (%) | 0.046 | 0.65 |
| ACE-I/ARB, n (%) | −0.11 | 0.26 |
| Beta Blocker, n (%) | −0.098 | 0.33 |
| Calcium channel blocker, n (%) | −0.25 | 0.014* |
| Diuretic, n (%) | 0.25 | 0.009* |
| Warfarin, n (%) | 0.061 | 0.55 |
| Amiodarone, n (%) | −0.071 | 0.48 |
| Statin, n (%) | −0.046 | 0.64 |
| Biochemical Data | ||
| Creatinine, μmol/L | 0.32 | 0.001* |
| NT-ProBNP (pg/mL) | 0.30 | 0.002* |
| CRP (mg/L) | 0.22 | 0.038* |
| CMR Data | ||
| CMR aortic valve area, cm2 | −0.24 | 0.011* |
| LVEF, % | −0.041 | 0.67 |
| LV Mass, g | −0.22 | 0.021* |
| LGE Mass | 0.22 | 0.027* |
| LGE Percent | 0.25 | 0.010* |
*p < 0.05. Correlation between Patient Data and Serum OPG Levels. ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CMR, cardiovascular magnetic resonance; DBP, diastolic blood pressure; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SBP, systolic blood pressure.
CMR Data
By definition, aortic valve orifice area on CMR was significantly reduced in the severe group compared to the mild/moderate group (0.7 ± 0.1 cm2 vs 1.2 ± 0.3 cm2, p < 0.00001). Ejection fraction and LV mass did not differ between these groups. Midwall fibrosis was detected in 11 (31.4%) patients with mild/moderate disease and 27 (36.0%) patients with severe disease (mild/moderate vs severe, p = 0.82). Chronic infarction pattern fibrosis was detected in 11 (31.4%) patients with mild/moderate disease and 25 (33.3%) patients with severe disease (mild/moderate vs severe, p = 0.82). Myocardial fibrosis was absent in 13 (37.1%) patients with mild/moderate disease and 23 (30.7%) patients with severe disease (mild/moderate vs severe, p = 0.82).
Surgical Intervention
Significantly fewer patients with mild/moderate disease underwent an intervention compared to patients with severe disease (14 patients vs 51 patients, 40% vs 68%, p = 0.01). Some 9/14 (64.3%) patients with moderate disease underwent aortic valve replacement surgery (AVR) concurrently with coronary artery bypass grafting (CABG) surgery for ischaemic heart disease, compared to 25/51 (49.0%) patients with severe disease (mild/moderate vs severe, p = 0.01).
Aortic Stenosis Severity and OPG Levels
Serum OPG levels in patients with mild/moderate disease were a median of 5.24 pmol/L (IQR 4.38–6.76 pmol/L). In patients with severe disease, OPG levels were a median of 7.43 pmol/L (IQR 4.80–9.63 pmol/L), which was significant greater than the levels in patients with mild/moderate disease (p = 0.002, Fig. 1). CMR valve orifice area correlated with serum OPG levels (rs = −0.24, p = 0.011; Table 2). Following linear regression analysis, the severity of aortic stenosis had a significant effect on OPG levels (p = 0.0002). On multivariable analysis (adjusting for age, sex, presence of fibrosis, LVEF, NYHA, NT-proBNP, and CRP) severe AS remained significant, increasing serum OPG by 1.30 pmol/L (95% CI 0.002–2.60 pmol/L, p = 0.049). A further multivariable analysis was performed to exclude the effect of concomitant cardiovascular disease in patients with severe AS on serum OPG levels. Adjusting for CAD, diabetes, and hypertension, AS severity was an independent predictor of serum OPG (p = 0.008).
Figure 1.
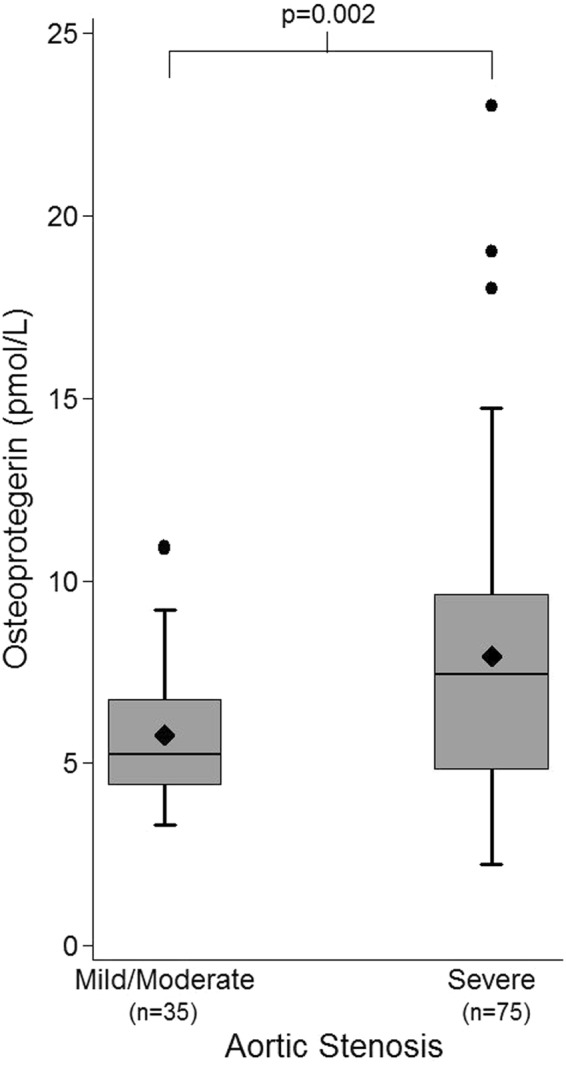
Box and Whisker Plots of Osteoprotegerin levels by Aortic Stenosis Severity. Patients with severe disease had higher serum OPG levels than those with mild/moderate disease (7.91 ± 3.82 pmol/L vs 5.76 ± 1.96 pmol/L, p = 0.002). Diamond represents the mean OPG level. Outlier data are included on the plots.
In order to investigate whether serum OPG levels could predict severe AS in patients, receiver operator curves were constructed, and the area under the ROC curve (AUC) was calculated. The AUC for AS severity was 0.69 (Supplementary Data, Fig. 1).
Low-Gradient Aortic Stenosis
Low-gradient AS, defined as a stroke volume (SV) index <35 ml/m2, was identified in 16 (14.5%) patients. Of these, 3 had mild/moderate AS, and the remaining 13 had severe AS. There was no difference in AS severity between patients with low-gradient AS and those without (p = 0.38). CMR identified no LV fibrosis in 7 patients, midwall pattern fibrosis in 6 patients, and chronic infarction pattern fibrosis in 3 patients. Serum OPG levels were similar between patients with low-gradient AS and those without (7.0 ± 2.9 pmol/L vs. 7.3 ± 3.6 pmol/L; p = 0.90).
Effect of Myocardial Fibrosis on OPG Levels
LGE % on CMR correlated with serum OPG (rs = 0.25, p = 0.01; Table 2). The median serum OPG for patients without myocardial fibrosis on CMR (n = 36) was 5.25 pmol/L (IQR 4.26–7.95 pmol/L). Patients with midwall fibrosis (n = 38) had a median serum OPG of 6.78 pmol/L (IQR 4.64–8.61 pmol/L), which was not significantly higher than those with no fibrosis (p = 0.12). Patients with a chronic infarction pattern of fibrosis had a median serum OPG of 6.97 pmol/L (IQR 5.43–11.12 pmol/L), which was significantly higher than those without fibrosis (p = 0.005) but not higher than those with midwall fibrosis (p = 0.27, Fig. 2). On linear regression analysis, ‘any fibrosis’ (midwall or infarction) had a significant effect on OPG levels (p = 0.01). On multivariable analysis (adjusting for age, sex, AS severity, & LVEF) however, a significant effect was no longer observed (p = 0.20). There was also no significant correlation between fibrosis and OPG levels when adjusting for CAD, diabetes, and hypertension (p = 0.11). Separating out midwall and chronic infarction patterns of fibrosis, linear regression analysis showed a significant effect of chronic infarction pattern on OPG levels (HR 2.21, 95% CI 0.57, 3.86; p = 0.009), but not of midwall fibrosis pattern (HR 1.00, 95% CI −0.36, 2.36; p = 0.15).
Figure 2.
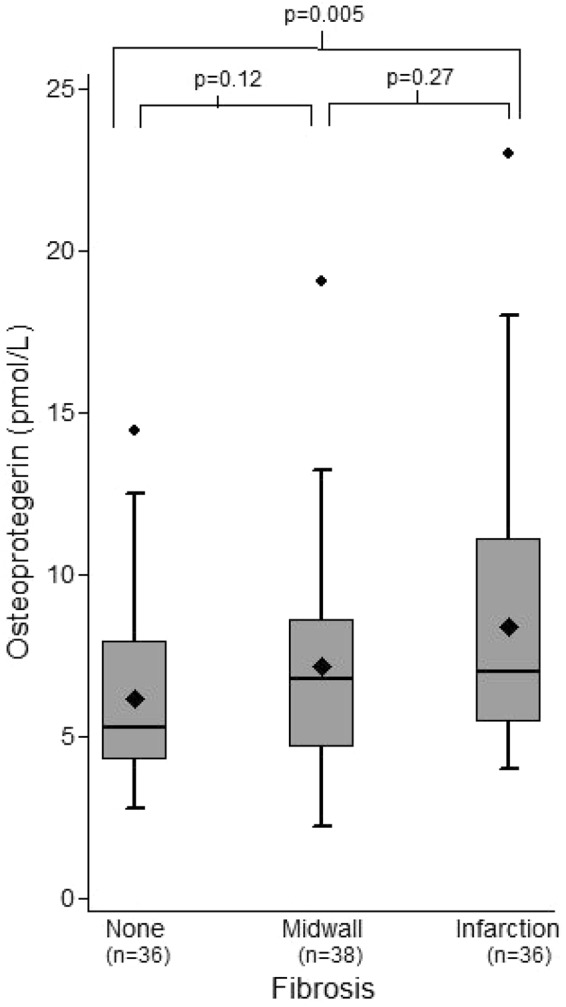
Box and Whisker Plots of Osteoprotegerin levels by pattern of Myocardial Fibrosis on CMR. Patients with midwall fibrosis on CMR did not have significantly higher levels of OPG compared to those without fibrosis (median 6.78 pmol/L [IQR 4.64–8.61] vs median 5.25 pmol/L [IQR 4.26–7.95 pmol/L], p = 0.12). There was no difference between those with midwall or chronic infarction pattern fibrosis (median 6.78 pmol/L [IQR 4.64–8.61 pmol/L] vs median 6.97 pmol/L [IQR 5.43–11.12 pmol/L], p = 0.27). However, OPG levels in patients with chronic infarction fibrosis were significantly higher than those without fibrosis (p = 0.005). Diamond represents the mean OPG level. Outlier data are included on the plots.
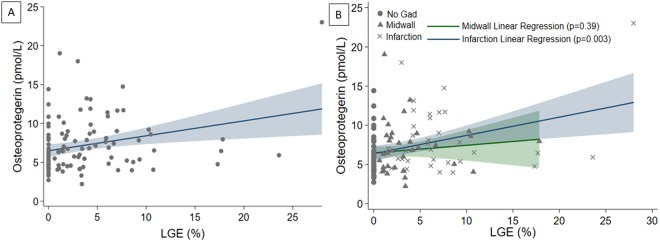
The effect of ‘any fibrosis’ on serum OPG per 1% increase in LGE on CMR was significant on univariable linear regression analysis (p = 0.005). On multivariable analysis, this remained significant, increasing serum OPG by 0.16 pmol/L (95% CI 0.03–0.30 pmol/L) per 1% increase in LGE (p = 0.02, Fig. 3A). Separating out patterns of fibrosis on CMR, there was no significant effect of midwall fibrosis on linear regression on serum OPG (p = 0.15) and this remained true on multivariable analysis (p = 0.19). However, there was a significant effect of chronic myocardial infarction pattern fibrosis univariable analysis (p = 0.009; Fig. 3B). On multivariable analysis, however, this effect only showed a strong trend (HR 2.11, 95% CI −0.10, 4.32; p = 0.061). Age (p = 0.004), LVEF (p = 0.035), and CRP (p = 0.017) each remained significant on the multivariable analysis.
Figure 3.
Effect of Extent of Fibrosis on OPG per %LGE on CMR. Panel (A) On multivariable linear regression analysis, ‘any’ fibrosis (per % increase in LGE on CMR) increased serum OPG by 0.16 pmol/L (95% CI 0.03–0.30 pmol/L, p = 0.02). Panel (B) Separating out patients based on pattern of fibrosis, midwall fibrosis had no effect on OPG levels (0.10 pmol/L, 95% CI −0.13–0.32, p = 0.39), whereas chronic infarction pattern fibrosis increased serum OPG by 0.19 pmol/L (95% CI 0.03–0.35 pmol/L) for every 1% increase in LGE on CMR (p = 0.02).
Serum OPG levels predicted LV myocardial fibrosis with an AUC of 0.65 (Supplementary Data, Fig. 2).
Left Ventricular Ejection Fraction and Serum OPG Levels
LV ejection fraction did not correlate with serum OPG levels (rs = −0.04, p = 0.67; Table 2). CMR-derived ejection fraction was not significantly different between patients with mild/moderate disease and those with severe disease (62 ± 14% vs 57 ± 17%, p = 0.11). Per 10% change, LV ejection fraction did not have a significant effect on serum OPG (p = 0.77). On multivariable analysis (adjusted for age, sex, AS severity, and presence of myocardial fibrosis), there was still no effect observed (p = 0.39, Fig. 4).
Figure 4.
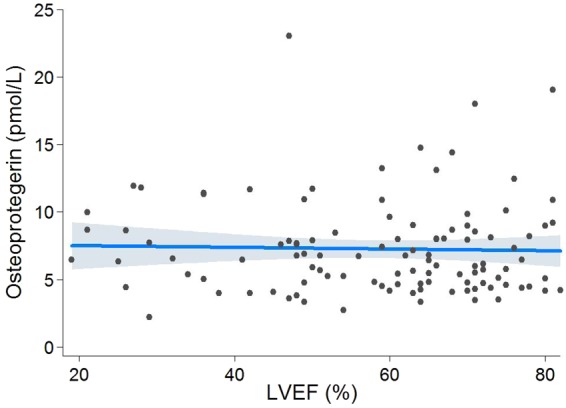
Effect of EF on OPG Levels. On multivariable linear regression analysis (adjusted for age, sex, AS severity, and presence of myocardial fibrosis), serum OPG levels remained unchanged per 10% change in LV ejection fraction (0.18 pmol/L, 95% CI −0.23–0.59 pmol/L, p = 0.39).
NT-proBNP and Serum OPG Levels
NT-proBNP correlated with serum OPG levels in patients (rs = 0.30, p = 0.002; Table 2). A total of 64 (58.2%) patients met the diagnostic criteria for the heart failure with preserved ejection fraction (HFpEF) syndrome, characterised by heart failure symptoms, LV EF ≥ 50%, and a serum NT-proBNP > 125 pg/ml36. Serum OPG levels did not differ significantly between patients with HFpEF and those without (7.1 ± 3.6 pmol/L vs. 7.3 ± 3.5 pmol/L; p = 0.74). Patients with HFpEF were not more likely to have severe AS (p = 0.49), nor either pattern of fibrosis on CMR (p = 0.31).
Effect of OPG on survival
There were 30 (27.3%) deaths over the median follow-up period of 1.9 years (IQR 1.2–2.7 years). Using the Cox proportional hazards method, serum OPG had a significant effect on survival on univariable analysis (p < 0.0001). On multivariable analysis (adjusted for age, sex, AVR/TAVR intervention, presence of myocardial fibrosis, NYHA class, CAD, diabetes, and hypertension), this effect remained statistically significant, with every 1 pmol/L increase in baseline serum OPG increasing the risk of death by 19% (HR 1.19, 95% CI 1.06–1.34; p = 0.004).
Discussion
This is the first study to investigate the association between myocardial fibrosis on CMR and serum OPG levels in patients with AS. There was no association between midwall fibrosis and serum OPG levels in our study. However, for patients with chronic infarction pattern fibrosis on CMR, there was an effect on serum OPG on univariable analysis (p = 0.009), but there was only a trend on multivariable analysis when accounting for age, sex, AS severity, LVEF, NYHA, NT-proBNP, and CRP (p = 0.061). We included patients with both mild/moderate and severe AS, and confirmed the findings of others that disease severity is independently associated with raised serum OPG22–24. The patient groups were also well-matched in terms of baseline characteristics and medical therapy. Specifically, statin therapy, which reduces inflammatory (TNFα mediated) OPG release by endothelial cells37, did not differ between groups. While LVEF did not appear to have a significant effect on serum OPG in the current study, only 15 patients (13.6%) had a LVEF ≤ 40%, complicating the assessment of the effect of concomitant heart failure on OPG in our patient cohort. On multivariable analysis, the raised serum OPG in patients with chronic ischaemic pattern fibrosis on CMR appeared to associate more with older age and higher levels of serum NT-proBNP and CRP. Importantly, we have also confirmed the association between higher serum OPG and poorer mid-term survival (median 1.9 years in the present study) in patients with AS23, demonstrating a 19% increase in the risk of death per 1 pmol/L rise in serum in OPG. This is clinically important, as it appears that the risk associated with OPG elevation is independent of LVEF and midwall myocardial fibrosis.
In addition to regulating bone calcification38, the RANKL/RANK/OPG pathway also plays a key role in immune function via modulation of dendritic cell function, and is upregulated in T lymphocytes during inflammation39. In addition to blockade of RANK, OPG also interacts with tumour necrosis factor related apoptosis inducing ligand (TRAIL), to block TRAIL-related apoptosis40. The RANKL/RANK/OPG axis is also active within the vasculature and has been shown to play a major role in pathological vascular calcification, in a manner analogous to bone turnover8, and is upregulated in accelerated atherosclerotic plaques16. OPG has been shown to induce vascular fibrosis in aortic samples of apolipoprotein E knockout (ApoE[−/−]) mice, via transforming growth factor-β1 (TGF-β1), which also mediates OPG release in response to angiotensin II in vascular smooth muscle cells41. A recent meta-analysis including a total of 26,442 participants from the general population demonstrated an association between raised baseline serum OPG and an increased risk of incident cardiovascular disease at a mean follow-up of 8.5 years, independent of age and clinically-relevant risk factors35.
Defining the role of OPG in patients with AS may therefore be complicated by the presence of concomitant cardiovascular disease. There also appears to be a difference between the actions of OPG in vascular and cardiac tissue in mouse models, as it has shown to be pro-fibrotic in the former41 and anti-fibrotic in the latter33. This may be explained by tissue-specific differences (e.g. in cellular autophagy), however, these effects are yet to be confirmed in humans. We have shown that severe AS is independently associated with raised serum OPG, and that patients with chronic ischaemia pattern fibrosis on CMR have raised OPG on univariate analysis. In patients with infarction, this is due to presence of coronary artery disease, but also due to a lower mean ejection fraction and higher serum CRP. In this way, LV dysfunction, systemic inflammation, and CAD are likely to mediate serum OPG in patients with infarction on CMR, in addition to the degree of calcification of the aortic valve. The effect of the severity of AS on serum OPG, as determined by aortic valve area on CMR, was shown to be independent of age, sex, fibrosis on CMR, LV EF, and NYHA. This suggests that progressive calcification of the valve itself is the cause of the raise in serum OPG. Of course, this is likely to be affected by progression to pressure overload induced heart failure, or potentially the presence of concomitant cardiovascular disease, such as CAD, HTN, diabetes, which can be associated with systemic inflammation and/or endothelial dysfunction16,42,43. Indeed, the severity of endothelial dysfunction in these conditions is heterogeneous and directly affected by medications such as Angiotensin II receptor blockers (ARB) and ACE-inhibitors44–46, which were used in 46.3% of patients in our cohort, but prescription rates were similar between severity groups. Serum OPG may present a further prognostic marker for patients with severe AS, and may aid in determining the ideal timing for surgical intervention, but does not predict the presence of midwall fibrosis on CMR.
Limitations
Our study was from a single centre and therefore subject referral bias, although we believe this was limited as our centre is a national referral centre with patients treated from across the country. In addition, our cohort contained predominantly Caucasian patients and therefore our results cannot be extrapolated to other ethnicities. Finally, we did not undertake T1-mapping with the CMR scanning, as it had not been validated in our institution at the time of recruiting our patients, and therefore we are unable to comment about the potential association between OPG and interstitial/diffuse fibrosis. Nonetheless, it is important to emphasise the predictive role of OPG in this cohort.
Conclusion
There was no association between serum OPG and the presence of myocardial midwall fibrosis in our study. However, higher OPG levels were seen in patients with chronic myocardial infarction compared to patients with no fibrosis, which was due to increased levels of serum markers of inflammation, LV dysfunction, and CAD. We have confirmed the findings of others that OPG levels increase with AS severity, and that increased serum OPG levels are associated with poorer survival at mid-term follow-up (median of 1.9 years), independent of the presence of midwall fibrosis on the CMR. These data suggest that the RANKL/RANK/OPG axis is unlikely to play a central role in myocardial midwall fibrosis in patients with aortic stenosis.
Electronic supplementary material
Author Contributions
Conceived and designed study: V.S.V., E.N., P.D.F., D.J.P., S.K.P. obtained and analysed data: B.L.L., B.H., A.L., A.A., T.M., S.S., N.A., W.L., J.D., D.A., C.E.R. Statistical Analysis: S.N. Contributed reagents/analysis tools: J.D. Wrote paper: B.L.L., V.S.V. All authors approved the manuscript.
Data Availability
Data can be obtained from the corresponding author and will be deposited in an open access repository upon acceptance.
Competing Interests
Prof. Pennell has received research funding from Siemens; has served as a consultant to Bayer; and is a director of and shareholder in CVIS. Dr. Prasad has received honoraria for talks from Bayer Schering. This project was supported by the Rosetrees Trust, the National Institute for Health Research (NIHR) Cardiovascular Biomedical Research Unit (CBRU) of Royal Brompton and Harefield NHS Trust and Imperial College London, and the British Heart Foundation through BHF CRTF (FS/17/21/32712) and BHF CRTF (FS/15/29/31492).
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Vassilios S. Vassiliou and Sanjay K. Prasad jointly supervised this work.
Contributor Information
Brian Halliday, Email: b.halliday@rbht.nhs.uk.
Vassilios S. Vassiliou, Email: V.Vassiliou@uea.ac.uk
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-32738-y.
References
- 1.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–3326. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien KD. Pathogenesis of calcific aortic valve disease: a disease process comes of age (and a good deal more) Arterioscler Thromb Vasc Biol. 2006;26:1721–1728. doi: 10.1161/01.ATV.0000227513.13697.ac. [DOI] [PubMed] [Google Scholar]
- 3.Nishimura RA, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70:252–289. doi: 10.1016/j.jacc.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner H, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 5.Hein S, et al. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–991. doi: 10.1161/01.CIR.0000051865.66123.B7. [DOI] [PubMed] [Google Scholar]
- 6.Babu-Narayan SV, et al. Late gadolinium enhancement cardiovascular magnetic resonance of the systemic right ventricle in adults with previous atrial redirection surgery for transposition of the great arteries. Circulation. 2005;111:2091–2098. doi: 10.1161/01.CIR.0000162463.61626.3B. [DOI] [PubMed] [Google Scholar]
- 7.Vassiliou VS, et al. Midwall Fibrosis and 5-Year Outcome in Moderate and Severe Aortic Stenosis. J Am Coll Cardiol. 2017;69:1755–1756. doi: 10.1016/j.jacc.2017.01.034. [DOI] [PubMed] [Google Scholar]
- 8.Collin-Osdoby P. Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ Res. 2004;95:1046–1057. doi: 10.1161/01.RES.0000149165.99974.12. [DOI] [PubMed] [Google Scholar]
- 9.Kaden JJ, et al. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulate aortic valve calcification. J Mol Cell Cardiol. 2004;36:57–66. doi: 10.1016/j.yjmcc.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Walsh MC, Choi Y. Biology of the TRANCE axis. Cytokine Growth Factor Rev. 2003;14:251–263. doi: 10.1016/S1359-6101(03)00027-3. [DOI] [PubMed] [Google Scholar]
- 11.Kong YY, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 12.Shetty R, et al. Expression of bone-regulatory proteins in human valve allografts. Heart. 2006;92:1303–1308. doi: 10.1136/hrt.2005.075903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pohjolainen V, et al. Noncollagenous bone matrix proteins as a part of calcific aortic valve disease regulation. Hum Pathol. 2008;39:1695–1701. doi: 10.1016/j.humpath.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Panizo S, et al. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ Res. 2009;104:1041–1048. doi: 10.1161/CIRCRESAHA.108.189001. [DOI] [PubMed] [Google Scholar]
- 15.Bucay N, et al. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhore CR, et al. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 17.Min H, et al. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med. 2000;192:463–474. doi: 10.1084/jem.192.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aoki A, et al. Association of serum osteoprotegerin with vascular calcification in patients with type 2 diabetes. Cardiovasc Diabetol. 2013;12:11. doi: 10.1186/1475-2840-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roysland R, et al. Prognostic value of osteoprotegerin in chronic heart failure: The GISSI-HF trial. Am Heart J. 2010;160:286–293. doi: 10.1016/j.ahj.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Ueland T, et al. Osteoprotegerin predicts progression of chronic heart failure: results from CORONA. Circ Heart Fail. 2011;4:145–152. doi: 10.1161/CIRCHEARTFAILURE.110.957332. [DOI] [PubMed] [Google Scholar]
- 21.Ueland T, et al. Dysregulated osteoprotegerin/RANK ligand/RANK axis in clinical and experimental heart failure. Circulation. 2005;111:2461–2468. doi: 10.1161/01.CIR.0000165119.62099.14. [DOI] [PubMed] [Google Scholar]
- 22.Ueland T, et al. Osteoprotegerin levels predict mortality in patients with symptomatic aortic stenosis. J Intern Med. 2011;270:452–460. doi: 10.1111/j.1365-2796.2011.02393.x. [DOI] [PubMed] [Google Scholar]
- 23.Dahl JS, et al. Relation of osteoprotegerin in severe aortic valve stenosis to postoperative outcome and left ventricular function. Am J Cardiol. 2013;112:1433–1438. doi: 10.1016/j.amjcard.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Helske S, et al. Increased circulating concentrations and augmented myocardial extraction of osteoprotegerin in heart failure due to left ventricular pressure overload. Eur J Heart Fail. 2007;9:357–363. doi: 10.1016/j.ejheart.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Poulsen SH, Sogaard P, Nielsen-Kudsk JE, Egeblad H. Recovery of left ventricular systolic longitudinal strain after valve replacement in aortic stenosis and relation to natriuretic peptides. J Am Soc Echocardiogr. 2007;20:877–884. doi: 10.1016/j.echo.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 26.Weidemann F, et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577–584. doi: 10.1161/CIRCULATIONAHA.108.847772. [DOI] [PubMed] [Google Scholar]
- 27.Ng AC, et al. Alterations in multidirectional myocardial functions in patients with aortic stenosis and preserved ejection fraction: a two-dimensional speckle tracking analysis. Eur Heart J. 2011;32:1542–1550. doi: 10.1093/eurheartj/ehr084. [DOI] [PubMed] [Google Scholar]
- 28.Dweck MR, et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271–1279. doi: 10.1016/j.jacc.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 29.Barone-Rochette G, et al. Prognostic significance of LGE by CMR in aortic stenosis patients undergoing valve replacement. J Am Coll Cardiol. 2014;64:144–154. doi: 10.1016/j.jacc.2014.02.612. [DOI] [PubMed] [Google Scholar]
- 30.Chin CWL, et al. Myocardial Fibrosis and Cardiac Decompensation in Aortic Stenosis. J Am Coll Cardiol Img. 2017;10:1320–1333. doi: 10.1016/j.jcmg.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicoletti A, Michel JB. Cardiac fibrosis and inflammation: interaction with hemodynamic and hormonal factors. Cardiovasc Res. 1999;41:532–543. doi: 10.1016/S0008-6363(98)00305-8. [DOI] [PubMed] [Google Scholar]
- 32.Saidenberg Kermanac’h N, Bessis N, Cohen-Solal M, De Vernejoul MC, Boissier MC. Osteoprotegerin and inflammation. Eur Cytokine Netw. 2002;13:144–153. [PubMed] [Google Scholar]
- 33.Feng W, et al. IL-17 induces myocardial fibrosis and enhances RANKL/OPG and MMP/TIMP signaling in isoproterenol-induced heart failure. Exp Mol Pathol. 2009;87:212–218. doi: 10.1016/j.yexmp.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Vassiliou VS, et al. Lipoprotein(a) in patients with aortic stenosis: Insights from cardiovascular magnetic resonance. PLoS One. 2017;12:e0181077. doi: 10.1371/journal.pone.0181077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tschiderer L, Willeit J, Schett G, Kiechl S, Willeit P. Osteoprotegerin concentration and risk of cardiovascular outcomes in nine general population studies: Literature-based meta-analysis involving 26,442 participants. PLoS One. 2017;12:e0183910. doi: 10.1371/journal.pone.0183910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ponikowski P, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 37.Ben-Tal Cohen E, et al. Statins decrease TNF-alpha-induced osteoprotegerin production by endothelial cells and smooth muscle cells in vitro. Biochem Pharmacol. 2007;73:77–83. doi: 10.1016/j.bcp.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Simonet WS, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/S0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 39.Wong BR, et al. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med. 1997;186:2075–2080. doi: 10.1084/jem.186.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pritzker LB, Scatena M, Giachelli CM. The role of osteoprotegerin and tumor necrosis factor-related apoptosis-inducing ligand in human microvascular endothelial cell survival. Mol Biol Cell. 2004;15:2834–2841. doi: 10.1091/mbc.e04-01-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toffoli B, et al. Osteoprotegerin promotes vascular fibrosis via a TGF-beta1 autocrine loop. Atherosclerosis. 2011;218:61–68. doi: 10.1016/j.atherosclerosis.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 42.Venuraju SM, Yerramasu A, Corder R, Lahiri A. Osteoprotegerin as a predictor of coronary artery disease and cardiovascular mortality and morbidity. J Am Coll Cardiol. 2010;55:2049–2061. doi: 10.1016/j.jacc.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Chong AY, et al. Endothelial dysfunction and damage in congestive heart failure: relation of flow-mediated dilation to circulating endothelial cells, plasma indexes of endothelial damage, and brain natriuretic peptide. Circulation. 2004;110:1794–1798. doi: 10.1161/01.CIR.0000143073.60937.50. [DOI] [PubMed] [Google Scholar]
- 44.Varin R, et al. Improvement of endothelial function by chronic angiotensin-converting enzyme inhibition in heart failure: role of nitric oxide, prostanoids, oxidant stress, and bradykinin. Circulation. 2000;102:351–356. doi: 10.1161/01.CIR.102.3.351. [DOI] [PubMed] [Google Scholar]
- 45.Rhee EJ, et al. Relationship of serum osteoprotegerin levels with coronary artery disease severity, left ventricular hypertrophy and C-reactive protein. Clin Sci (Lond) 2005;108:237–243. doi: 10.1042/CS20040255. [DOI] [PubMed] [Google Scholar]
- 46.Ueland T, et al. Prognostic value of osteoprotegerin in heart failure after acute myocardial infarction. J Am Coll Cardiol. 2004;44:1970–1976. doi: 10.1016/j.jacc.2004.06.076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be obtained from the corresponding author and will be deposited in an open access repository upon acceptance.