Abstract
Sea-island cotton (Gossypium barbadense) has drawn great attention in the textile industry for its comprehensive resistance and superior fiber properties. However, the mechanisms involved in fiber growth and development are unclear. As TCP transcription factors play important roles in plant growth and development, this study investigated the TCP family genes in G. barbadense (GbTCP). We identified 75 GbTCP genes, of which 68 had no introns. Phylogenetic analyses categorized the GbTCP transcription factors into 11 groups. Genomic analyses showed that 66 genes are located on 21 chromosomes. Phylogenetic analyses of G. arboreum, G. raimondii, G. hirsutum, G. barbadense, Theobroma cacao, Arabidopsis thaliana, Oryza sativa, Sorghum bicolor, and Zea mays, Picea abies, Sphagnum fallax and Physcomitrella patens, categorized 373 TCP genes into two classes (Classes I and II). By studying the structures of TCP genes in sea-island cotton, we identified genes from the same evolutionary branches that showed similar motif patterns. qRT-PCR results suggested that the GbTCPs had different expression patterns in fibers at various developmental stages of cotton, with several showing specific expression patterns during development. This report helps lay the foundation for future investigations of TCP functions and molecular mechanisms in sea-island cotton fiber development.
Introduction
TCP proteins are plant-specific transcription factors that play important regulatory roles in plant growth and development in a wide range of plants. TCP is an acronym of the earliest described family members: teosinte branched 1 in Zea mays, CYC in cycloidea, and proliferating cell factors 1 and 2 (PCF1 and PCF2) in Oryza sativa1. TCP family members contain a conserved TCP domain consisting of 55–59 amino acids; this region contains a basic secondary helix–loop–helix (bHLH domain) structure, which promotes DNA binding. In addition, TCP family members form homologous or allo-diploid interactions with other proteins through the bHLH domain1–3.
TCP transcription factor family members are categorized into two classes (Class I and II) according to their TCP domain. Class I, also known as the TCP-P family, includes PCF1 and PCF2; Class II, also known as the TCP-C family, includes CYC and TB1. The most striking difference between the two subfamilies is that Class I members are missing four amino acids in the basic region, whereas Class II members have a conserved polar-rich amino acid region of about 18 amino acids that forms a hydrophilic alpha-helix R domain1. In general, the main function of Class I TCP proteins is to promote cell proliferation in leaves, thereby regulating plant growth and development. Class II proteins play inhibitory roles in cell division in plant development, functioning as negative regulators of leaf growth and positive regulators of aging.
Doebley et al.4 reported that the maize domestication gene, TB1, inhibits the growth and development of lateral branches, whereas the loss of TB1 gene function causes increased lateral branches in maize. TB1 also contributes to the formation of female inflorescences in maize. The CYC gene is involved in regulating the asymmetry of petals and stamens in snapdragon5. PCF1 and PCF2 affect DNA replication and repair as well as chromosomal structure changes by binding to the promoter of the PCNA gene6. Many recent studies have investigated Class II TCP proteins. AtTCP2, AtTCP4, and AtTCP10 inhibit the development of leaves in Arabidopsis. AtTCP13 inhibits the development of leaves through the regulation of miR319 via the mitogen-activated protein kinase signaling pathway, thereby affecting cell division6. TCP members play various regulatory roles in cell elongation and petal symmetry7,8, regulation of the circadian clock, embryonic development, and seed germination.
TCP proteins are the only known family of transcription factors in plants, and they play antagonistic and synergistic roles in plant growth and development. As a class of ancient transcription factors, the TCP protein is widely present in multicellular algae and mosses; however, such species have fewer TCP family members9. By contrast, gymnosperms and angiosperms have numerous members of the TCP family due to gene duplication during evolution10–12. At present, TCPs have been identified in more than 20 species, including Arabidopsis thaliana13, O. sativa14, Populus euphratica15, Lycopersicon esculentum16, Citrullus lanatus17, Orchis italica18, Sorghum bicolor19, G. raimondii20, G. arboreum21, and G. hirsutum22. TCP family members are involved in plant growth and development, seed germination, jasmonic acid synthesis, and the regulation of leaf senescence23,24. In addition, they participate in the development of gametes25,26 and play important roles in circadian rhythm and defense responses27.
Cotton is an economically important crop, and its fibers are a source of natural textile materials. Two types of tetraploid cotton (AADD), G. hirsutum and G. barbadense, were formed ~1–2 million years ago. Owing to its slender fiber and high strength, G. barbadense has become an important raw material for high-grade and special cotton textiles. The textile industry is under intense global economic competitive pressure to produce high-quality cotton fibers and respond to consumer demand for pure cotton textiles. Therefore, improving fiber quality is an urgent task. G. barbadense fiber is an important resource material for studying cotton quality improvement, resistance inheritance, and heterosis utilization28. Genome-wide sequencing of diploid cotton was first completed in G. raimondii29 and G. arboreum30, followed by the recent sequencing of G. barbadense31. These sequences allow the identification of GbTCP genes at the whole-genome level.
TCP genes are bHLH transcription factors that have drawn great attention in recent years. These transcription factors are plant-specific, and they play a major role in the regulation of meristem growth and development. Although TCP transcription factors have been described in A. thaliana, further characterization is needed. Recent studies have shown that TCP proteins play important roles in the early development of cotton fiber. Overexpression of GhTCP14 in A. thaliana changes the level and distribution of auxin, thereby affecting root and epidermal cell initiation and elongation31. GbTCP15 silencing in cotton causes shorter fibers. In addition, this transcription factor plays a role in the regulation of jasmonic acid biosynthesis, reactive oxygen species, calcium channels, and ethylene signaling32. However, the molecular mechanisms involved in TCP gene function in sea-island cotton fiber development require further clarification. Moreover, it is urgent to identify the number of TCP genes, their protein structures, and physicochemical properties in sea-island cotton. This study identified the TCP family members in G. barbadense. In addition, we analyzed the physical and chemical properties and sequence characteristics of TCP proteins. We revealed the expression patterns of TCP genes at various developmental stages in G. barbadense. The information from this report will be useful for improving cotton fiber quality in the future.
Results
Identification of the TCP gene family in G. barbadense
Members of the TCP transcription family have a conserved domain called the TCP domain1. To identify the members of the TCP transcription factor family in G. barbadense, 115 protein sequences were searched in the database (Supplementary Dataset 6); we excluded proteins without the TCP domain, those containing deletions or non-full-length segments, and those with redundant sequences of the same genes. We identified 75 matched gene sequences that had TCP domains consisting of 54–58 amino acids. Based on the nomenclature of Arabidopsis genes, we named the 75 G. barbadense TCP genes GbTCP1–GbTCP75.
Further analyses of the 75 TCP genes in G. barbadense revealed that the base length of the coding region of this family ranged between 546 and 1647 bp, with a mean length of 1044 bp. The predicted amino acid length ranged between 181 and 548 amino acids, with a mean length of 347 amino acids. The isoelectric point of the amino acids ranged between 5.8 and 10.28, with a mean value of 8.15. The predicted molecular mass of the proteins ranged between 20 and 58 kDa, with a mean molecular mass of 37.49 kDa (Table 1).
Table 1.
TCP gene family in G. barbadense.
| GbTCP gene | Gen symbol | Length (aa) | MW (Da) | PI | Nucleotide length (bp) | Chr.Location |
|---|---|---|---|---|---|---|
| GbTCP1 | Gbscaffold3209.1.0 | 398 | 43667.39 | 9.12 | 1197 | At_Chr07:3209:117679..119246 (−strand) |
| GbTCP2 | Gbscaffold17189.2.0 | 410 | 45042.61 | 8.76 | 1233 | scaffold 17189:6809..8503 (−strand) |
| GbTCP3 | Gbscaffold13071.3.0 | 410 | 44931.16 | 6.75 | 1233 | scaffold 13071:90196..91428 (+strand) |
| GbTCP4 | Gbscaffold26927.3.0 | 410 | 44891.10 | 6.77 | 1233 | At_Chr05:26927:34102..37231 (−strand) |
| GbTCP5 | Gbscaffold9879.8.0 | 463 | 50146.82 | 7.01 | 1392 | Dt_Chr10:9879:133400..134985 (−strand) |
| GbTCP6 | Gbscaffold10110.20.0 | 448 | 48610.09 | 6.50 | 1347 | Dt_Chr01:10110:264271..267394 (+strand) |
| GbTCP7 | Gbscaffold14699.6.0 | 446 | 48341.77 | 6.52 | 1341 | At_Chr1:14699:173200..176290 (+strand) |
| GbTCP8 | Gbscaffold11947.1.0 | 401 | 43857.08 | 6.23 | 1206 | Dt_Chr05:11947:8102..9639 (+strand) |
| GbTCP9 | Gbscaffold3304.3.0 | 401 | 43770.03 | 6.34 | 1206 | At_Chr4:3304:50754..53349 (−strand) |
| GbTCP10 | Gbscaffold27504.10.0 | 326 | 36208.93 | 5.80 | 981 | Dt_Chr12:27504:134857..137301 (+strand) |
| GbTCP11 | Gbscaffold3032.9.0 | 325 | 36033.79 | 5.80 | 978 | At_Chr12:3032:97502..99904 (−strand) |
| GbTCP12 | Gbscaffold4415.8.0 | 300 | 31950.48 | 7.98 | 903 | Dt_Chr10:4415:151551..153362 (+strand) |
| GbTCP13 | Gbscaffold11062.6.0 | 257 | 26578.76 | 9.51 | 775 | Dt_Chr02:11062:196862..200965 (−strand) |
| GbTCP14 | Gbscaffold9836.1.0 | 258 | 26681.92 | 9.72 | 777 | At_Chr03:9836:7544..9624 (+strand) |
| GbTCP15 | Gbscaffold12374.5.0 | 255 | 26400.52 | 9.66 | 768 | At_Chr13:12375:93696..97512 (+strand) |
| GbTCP16 | Gbscaffold6022.10.0 | 256 | 26449.59 | 9.60 | 771 | scaffold:6022:132362..136420 (+strand) |
| GbTCP17 | Gbscaffold5204.13.0 | 243 | 25399.58 | 9.99 | 732 | Dt_Chr12:5204:275630..276729 (+strand) |
| GbTCP18 | Gbscaffold8038.3.0 | 243 | 25288.52 | 10.07 | 732 | At_Chr12:8038:28929..29660 (+strand) |
| GbTCP19 | Gbscaffold13479.8.0 | 487 | 51110.92 | 7.05 | 1464 | Dt_Chr04:13479:112305..114024 (−strand) |
| GbTCP20 | Gbscaffold13479.9.0 | 487 | 51114.90 | 7.05 | 1464 | Dt_Chr04:13479:115147..116733 (−strand) |
| GbTCP21 | Gbscaffold3670.7.0 | 487 | 50995.89 | 7.38 | 1464 | At_Chr04:3670:218204..220153 (+strand) |
| GbTCP22 | Gbscaffold6358.6.0 | 338 | 35465.42 | 9.08 | 1017 | scaffold:6358:38870..40002 (−strand) |
| GbTCP23 | Gbscaffold6358.4.0 | 318 | 33564.41 | 9.62 | 957 | scaffold:6358:28785..29751 (−strand) |
| GbTCP24 | Gbscaffold6358.5.0 | 292 | 30750.21 | 9.95 | 879 | scaffold:6358:30040..31731 (−strand) |
| GbTCP25 | Gbscaffold3763.19.0 | 292 | 30750.21 | 9.95 | 879 | At_Chr11:3763:159012..159890 (−strand) |
| GbTCP26 | Gbscaffold7158.20.0 | 345 | 36301.62 | 8.32 | 1038 | Dt_Chr08:7158:227528..229399 (−strand) |
| GbTCP27 | Gbscaffold3763.21.0 | 338 | 35365.29 | 9.08 | 1017 | At_Chr11:3763:164134..166435 (−strand) |
| GbTCP28 | Gbscaffold10005.2.0 | 369 | 39992.18 | 7.10 | 1110 | scaffold:10005:27378..29771 (+strand) |
| GbTCP29 | Gbscaffold10005.3.0 | 369 | 39933.15 | 7.10 | 1110 | scaffold:10005:41953..43384 (+strand) |
| GbTCP30 | Gbscaffold5915.15.0 | 300 | 31935.46 | 7.30 | 903 | At_Chr10:5915:172472..175178 (−strand) |
| GbTCP31 | Gbscaffold12010.14.0 | 501 | 55920.84 | 6.67 | 1506 | Dt_Chr12:12010:125213..126718 (−strand) |
| GbTCP32 | Gbscaffold6430.1.0 | 520 | 58034.18 | 6.56 | 1563 | At_Chr12:6430:32168..33784 (+strand) |
| GbTCP33 | Gbscaffold11914.1.0 | 309 | 34245.51 | 8.71 | 930 | At_Chr05:11914:2645..5844 (−strand) |
| GbTCP34 | Gbscaffold3271.1.0 | 311 | 34381.65 | 8.58 | 936 | Dt_Chr04:3271:52752..56167 (+strand) |
| GbTCP35 | Gbscaffold10591.2.0 | 181 | 20888.33 | 6.97 | 546 | At_Chr09:10591:50944..52832 (+strand) |
| GbTCP36 | Gbscaffold6973.22.0 | 395 | 42248.17 | 6.91 | 1188 | At_Chr11:6973:225102..226598 (+strand) |
| GbTCP37 | Gbscaffold8063.4.0 | 395 | 42218.14 | 6.91 | 1188 | Dt_Chr11:8063:48766..50556 (+strand) |
| GbTCP38 | Gbscaffold2445.12.0 | 409 | 43120.44 | 8.48 | 1230 | Dt_Chr07:2445:123806..125786 (+strand) |
| GbTCP39 | Gbscaffold3074.6.0 | 418 | 44438.78 | 8.60 | 1257 | At_Chr07:3075:53199..55192 (+strand) |
| GbTCP40 | Gbscaffold1228.13.0 | 406 | 44113.56 | 6.75 | 1221 | Dt_Chr12:1228:202373..204040 (+strand) |
| GbTCP41 | Gbscaffold12205.15.0 | 406 | 44171.60 | 6.62 | 1221 | Dt_Chr07:12205:408964..410321 (−strand) |
| GbTCP42 | Gbscaffold255.7.0 | 406 | 44071.52 | 6.75 | 1221 | At_Chr12:255:115731..117563 (−strand) |
| GbTCP43 | Gbscaffold1098.5.0 | 344 | 37567.58 | 8.85 | 1035 | Dt_Chr12:1098:91344..92648 (−strand) |
| GbTCP44 | Gbscaffold1098.6.0 | 344 | 37607.69 | 8.85 | 1035 | Dt_Chr12:1098:105220..106451 (−strand) |
| GbTCP45 | Gbscaffold11587.4.0 | 365 | 39732.14 | 9.26 | 1098 | Dt_Chr13:11587:92002..93910 (−strand) |
| GbTCP46 | Gbscaffold11587.3.0 | 351 | 38091.43 | 9.01 | 1056 | Dt_Chr13:11587:84998..86280 (−strand) |
| GbTCP47 | Gbscaffold2634.14.0 | 351 | 38091.43 | 9.01 | 1056 | At_ Chr13:2634:548185..549445 (+strand) |
| GbTCP48 | Gbscaffold2634.13.0 | 350 | 37945.38 | 9.43 | 1053 | At_ Chr13:2634:545016..546265 (+strand) |
| GbTCP49 | Gbscaffold2634.11.0 | 365 | 39572.10 | 9.14 | 1098 | At_ Chr13:2634:524590..526305 (+strand) |
| GbTCP50 | Gbscaffold4159.7.0 | 266 | 30312.08 | 7.78 | 801 | At_Chr07:4159:105843..107385 (+strand) |
| GbTCP51 | Gbscaffold24195.4.0 | 324 | 37158.25 | 8.67 | 975 | Dt_Chr11:24195:40530..42344 (+strand) |
| GbTCP52 | Gbscaffold3298.31.0 | 325 | 37548.66 | 9.05 | 978 | At_Chr11:3298:266275..267753 (−strand) |
| GbTCP53 | Gbscaffold10878.77.0 | 361 | 40868.45 | 7.76 | 1086 | Dt_Chr12:10878:675793..677225 (−strand) |
| GbTCP54 | Gbscaffold20071.10.0 | 354 | 40070.77 | 8.62 | 1065 | At_Chr12:20071:84673..85769 (−strand) |
| GbTCP55 | Gbscaffold8597.2.0 | 337 | 36405.77 | 6.34 | 1014 | Dt_Chr09:8597:1518..3614 (−strand) |
| GbTCP56 | Gbscaffold3493.31.0 | 341 | 36933.31 | 6.26 | 1026 | At_Chr09:3493:413695..414872 (−strand) |
| GbTCP57 | Gbscaffold3493.34.0 | 341 | 36933.31 | 6.26 | 1026 | At_Chr09:3493:426673..427826 (−strand) |
| GbTCP58 | Gbscaffold3276.14.0 | 384 | 41042.16 | 8.95 | 1155 | Scaffold:3276:404854..406503 (+strand) |
| GbTCP59 | Gbscaffold7552.1.0 | 312 | 33464.86 | 9.69 | 939 | At_Chr12:7552:5798..7141 (−strand) |
| GbTCP60 | Gbscaffold4907.6.0 | 300 | 31763.48 | 8.67 | 903 | Dt_Chr04:4907:280558..283567 (+strand) |
| GbTCP61 | Gbscaffold4907.5.0 | 300 | 31767.46 | 8.67 | 903 | Dt_Chr04:4907:278542..279485 (+strand) |
| GbTCP62 | Gbscaffold4840.4.0 | 298 | 31463.13 | 9.49 | 897 | At_Chr09:4840:398461..399357 (−strand) |
| GbTCP63 | Gbscaffold4840.3.0 | 298 | 31407.02 | 9.52 | 897 | At_Chr09:4840:394814..398315 (−strand) |
| GbTCP64 | Gbscaffold8136.6.0 | 304 | 32525.09 | 8.65 | 915 | Dt_Chr07:8136:112863..114487 (+strand) |
| GbTCP65 | Gbscaffold20098.3.0 | 294 | 31335.97 | 9.51 | 885 | Dt_Chr12:20098:60296..62034 (+strand) |
| GbTCP66 | Gbscaffold15344.21.0 | 211 | 22717.48 | 9.05 | 636 | At_Chr13:15344:175451..176086 (+strand) |
| GbTCP67 | Gbscaffold15344.20.0 | 196 | 21105.75 | 8.91 | 591 | At_Chr13:15344:173880..175470 (+strand) |
| GbTCP68 | Gbscaffold12564.5.0 | 196 | 21187.85 | 8.93 | 591 | Dt_Chr13:12564:37197..37911 (+strand) |
| GbTCP69 | Gbscaffold12564.6.0 | 196 | 21153.83 | 8.93 | 591 | Dt_Chr13:12564:41196..41900 (+strand) |
| GbTCP70 | Gbscaffold15617.7.0 | 548 | 57784.21 | 6.72 | 1647 | Dt_Chr01:15617:89104..91488 (+strand) |
| GbTCP71 | Gbscaffold7853.2.0 | 200 | 20978.22 | 10.28 | 603 | At_Chr05:7853:15611..16213 (−strand) |
| GbTCP72 | Gbscaffold34286.3.0 | 463 | 50230.89 | 7.01 | 1392 | At_Chr10:34286:47234..49758 (+strand) |
| GbTCP73 | Gbscaffold12073.3.0 | 435 | 48293.95 | 6.07 | 1308 | Dt_Chr04:12073:264571..267141 (+strand) |
| GbTCP74 | Gbscaffold7711.1.0 | 344 | 37621.73 | 8.53 | 1035 | At_Chr12:scaffold7711:2991..4722 (−strand) |
| GbTCP75 | Gbscaffold354.4.0 | 302 | 32016.81 | 9.81 | 909 | At_Chr12:scaffold354:306918..308284 (−strand) |
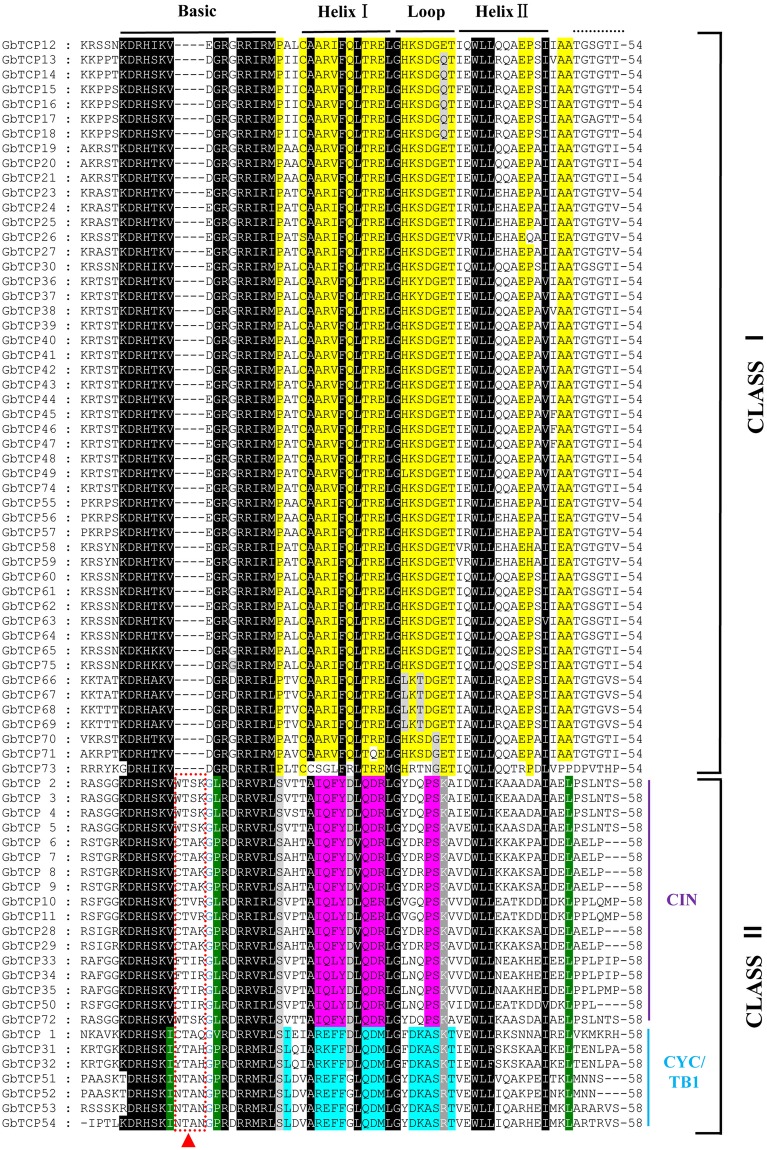
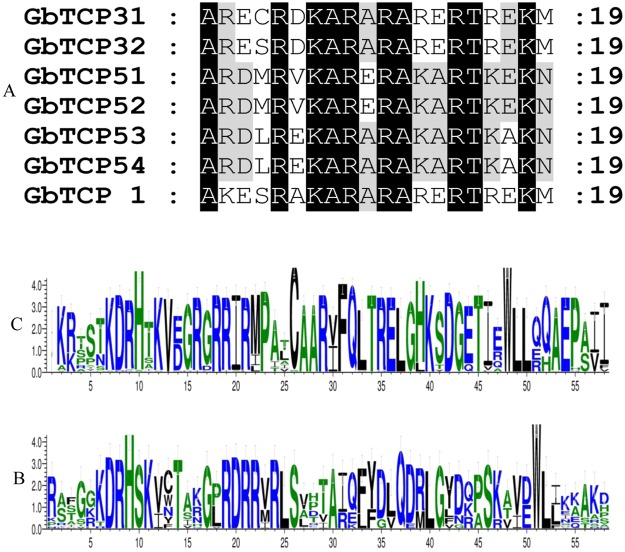
Analyses of the 75 TCP protein domains of sea-island cotton revealed a typical bHLH domains composed of 54 or 58 amino acids. Figure 1 depicts the entire TCP domain, showing that the conserved rate of the alkaline region was 93.3%, whereas the conserved rate of the ring region was only 62.2%. Based on the TCP domain classification by Cubas et al.1, the TCP family of sea-island cotton can be divided into Class I and II subfamilies. The most obvious difference between these classes is that Class I members are missing four amino acids in the alkaline region. In G. barbadense, 50 GbTCP members belong to the Class I subfamily, and 24 GbTCP members belong to the Class II subfamily. In addition to the bHLH structure, the Class II subfamily is subdivided into two branches: CYC/TB1 and CIN. The CYC/TB1 gene has a conserved R domain, which is rich in polar amino acids and forms a hydrophilic α-spiral1. Of the TCP genes in G. barbadense, seven are structurally classified as CYC/TB1 and their R domains consist of 19 amino acids (Fig. 2).
Figure 1.
Multiplex sequence alignment of 75 proteins in the GbTCP family. Each letter represents one amino acid, and the left column corresponds to the name of the gene. The black region indicates the highly conserved residues of the GbTCP family members, and the yellow region indicates residues conserved only in the class I subfamily. The purple region indicates residues conserved in the Class II subfamily of CIN-like proteins. Blue indicates the residues conserved in the CYC TB1 class of proteins. The top black bar represents the conserved domain in TCP proteins.
Figure 2.
(A) The R domain in CYC/TB1 class members of the GbTCP gene family; (B) a conserved motif in the Class I subfamily of the GbTCP gene family; (C) a conserved motif in the Class II subfamily of the GbTCP gene family.
Evolutionary analyses of the TCP transcription factor family
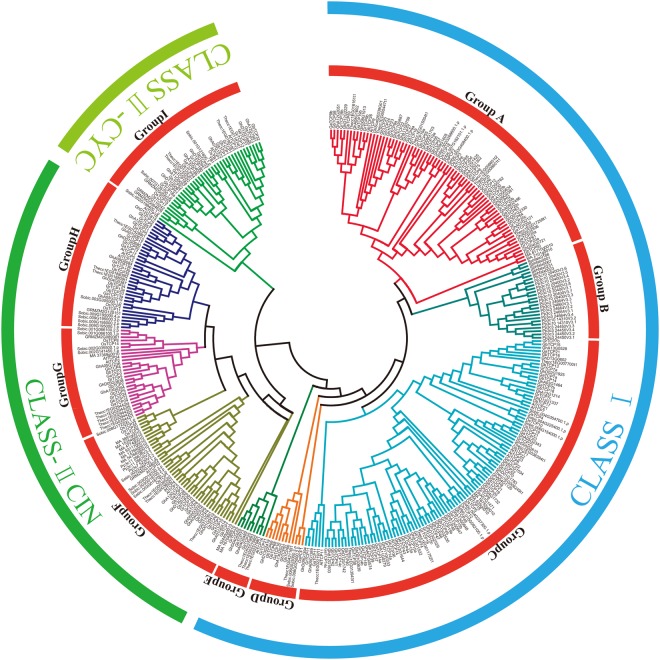
To further investigate the evolutionary relationship of the GbTCP transcription factor family in plants, we investigated the GbTCP sequences in G. arboreum, G. raimondii, G. barbadense, G. hirsutum, Theobroma cacao, A. thaliana, O. sativa, S. bicolor, and Zea mays, P. abies, S. fallax and Physcomitrella patens. These TCP protein sequences were used to construct a tree without roots. Neighbor-joining phylogenetic tree (Fig. 3) analyses divided the TCP transcription factor family into nine different subfamilies, denoted by the letters A–I. The TCP members in G. barbadense were heterogeneously distributed among nine subfamilies, and the C subgroup was the largest of all subfamilies, with 106 members and 26 GbTCP family members. The smallest branch is that the E subgroup has 9 members, and there is one member of the GbTCP family.
Figure 3.
Phylogenetic tree of TCP genes indicating that TCP genes can be clustered into nine groups. Phylogenetic tree of TCP proteins from Gossypium arboreum, G. raimondii, G. barbadense, Theobroma cacao, G. hirsutum, Arabidopsis thaliana, Oryza sativa, Sorghum bicolor, and Zea mays, Picea abies, Sphagnum fallax and Physcomitrella patens using the MEGA6.0 software neighbor-joining method, the JTT model, and a BootStrap set of 1000 repeats for building a rootless tree. The outer circle is marked in blue, green and dark green, which represent the Classes I, Classes I -CIN, Classes II -CYC.
We further classified the TCP proteins in the above plants according to the TCP domain classification methods described by Martíntrillo et al.3. We categorized 226 members in the A, B, C, D, and E subfamilies that belong to Class I TCP in structure, accounting for 60.5% of the total; 39 members of the I subfamily were structurally categorized into Class II-CYC. A total of 108 TCP members in the F, G, and H subgroups belong to the Class II-CIN category. Further analyses revealed that 51 GbTCP members belong to Class I, accounting for 68% of the total GbTCP family members, and 17 members belong to Class II-CIN, accounting for 23% of the total. Only seven GbTCP members were structurally classified as Class II-CYC, accounting for 9% of the total. Interestingly, except for the algae plant, the TCP genes of these plants were distributed in almost every branch.
Replication of chromosomes and duplication of genes
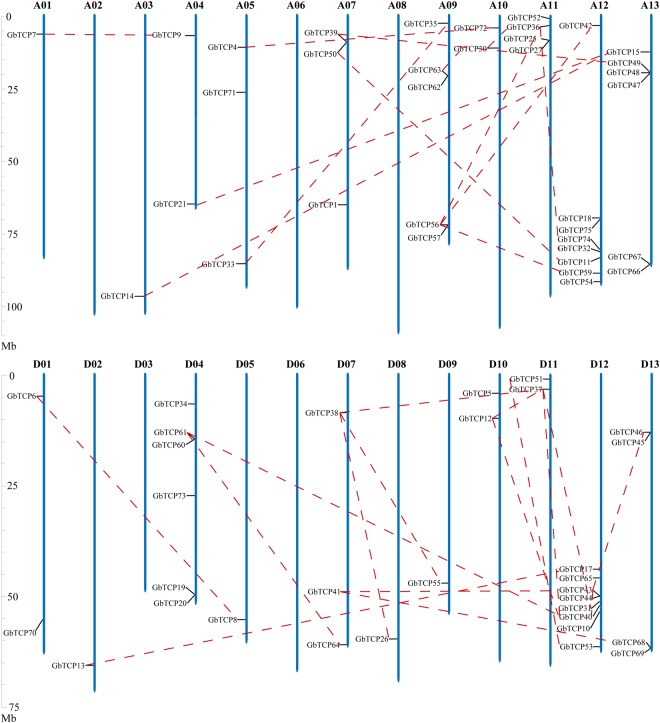
Analyses of the G. barbadense TCP gene distribution among the chromosomes showed that 66 GbTCP genes were widely distributed, but non-uniformly, on the G. barbadense chromosomes; the remaining 9 GbTCPs could not be mapped to any of the chromosomes (Fig. 4). There are no GbTCP genes on chromosomes A02, A06, A08, D03, and D06. The A01, A03, D02, D05, and A03 chromosomes each contain one TCP gene, whereas A04, A10, D01, D10, and D11 chromosomes each contain two GbTCP genes. D13 and A11 each contain four GbTCP genes, whereas chromosomes D04 and A13 each contain six. Most GbTCP genes are concentrated in the A09, A11, A12, A13, D04, D12, and D13 chromosomes. A12 and D12 chromosomes each contain eight GbTCP genes on both ends of the chromosomes.
Figure 4.
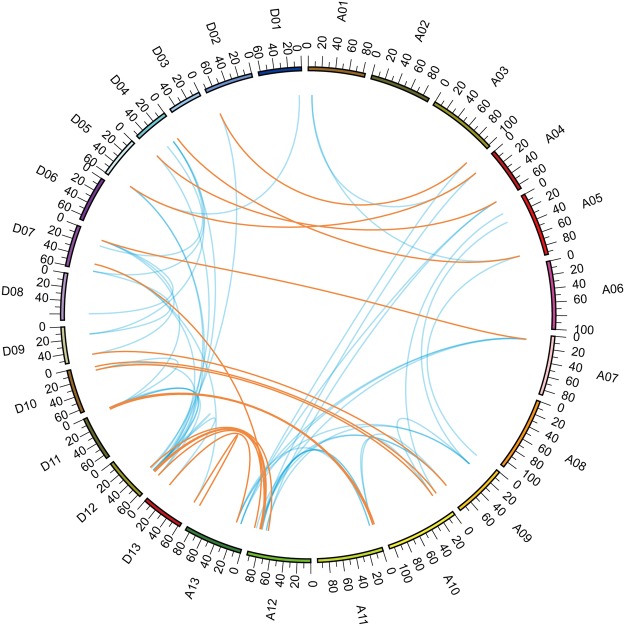
The physical locations of TCP genes on G. barbadense chromosomes. The red dotted lines link the paralogs TCP genes. The scale is in megabases, Mb.
In the sea-island cotton TCP gene family, seven genes belong to Class II-CYC, and they all have R domains. GbTCP1 is located on chromosome A7, GbTCP52 is on chromosome A11, GbTCP32 and GbTCP54 are on chromosome A12, GbTCP51 is on chromosome D11, and GbTCP31 and GbTCP53 are located on chromosome D12. These R domain-containing genes show similar distributions on their respective chromosomes, as they are each located near the two ends of the chromosome arms.
Collinearity analyses of gene duplication in the TCP gene family of G. barbadense were performed as described by Cannon et al.33. The results revealed that 20 members of the TCP gene family formed 10 tandem replication group pairs, accounting for ~27% of the entire GbTCP family. Two pairs of tandem replication genes were detected on chromosome 9 (GbTCP62/GbTCP63 and GbTCP56/GbTCP57) and chromosome 13 (GbTCP47/GbTCP48 and GbTCP66/GbTCP67). One tandem replicating genome pair (GbTCP 25/GbTCP 27) was located on chromosome 11 of group A. Lastly, there were two tandem replicating genome pairs on chromosome 4 (GbTCP60/GbTCP61 and GbTCP19/GbTCP20) and chromosome 13 (GbTCP45/GbTCP46 and GbTCP68/GbTCP69), and one tandem duplication gene pair (GbTCP43/GbTCP44) on chromosome 12 of group D.
The MCScanX software was used to analyze gene replication and collinearity in the genome segment of the TCP gene family. We identified 42 pairs of TCP genes that have a collinear relationship, some of which are involved in multiple gene duplication events. As shown in Fig. 5, significant collinearity was detected in most TCP members of G. barbadense. The complex linear relationship indicates that some TCP genes are involved in multiple gene duplication events. This explains why tetraploid cotton has more TCP genes than diploid cotton. In addition, it indicates that TCP gene duplication occurred during the evolution of tetraploid G. barbadense. We also identified 39 orthologous genes that make up 21 pairs of segment copy pairs (Supplementary Dataset 5), a phenomenon that occurs in orthologous genes. The genes involved are GbTCP21/(GbTCP19 and GbTCP20) and GbTCP67/(GbTCP68 and GbTCP69). In addition, GbTCP56, GbTCP61, GbTCP63, GbTCP43, GbTCP25, and GbTCP19 are involved in both tandem replication and replication of chromosomes. In total, the genes involved in the replication of G. barbadense include 81.3% of the GbTCP family genes. This indicates that tandem replication and fragment replication play important roles in the expansion of GbTCP family genes in G. barbadense.
Figure 5.
Correspondence between homologous genes in the TCP family of sea-island cotton. The Circos plot shows the relative positional relationship of the TCP genes in sea-island cotton, where each colored band represents a chromosome of G. barbadense; The ends of the orange lines are oriented toward the orthologous genes from the At and Dt sub-genomes. The ends of the blue lines point toward paralog pairs derived from segmental duplication.
Using the ratio of nonsynonymous substitutions (Ka) to synonymous (Ks) substitutions, we studied the evolutionary selection pressures of TCP genes in G. barbadense. The results showed that the Ka/Ks ratio of 29 out of 39 paralogs was less than 1 (Supplementary Dataset 4), which indicates that the TCP family of genes in G. barbadense tend to be purified after chromosomal segment replication.
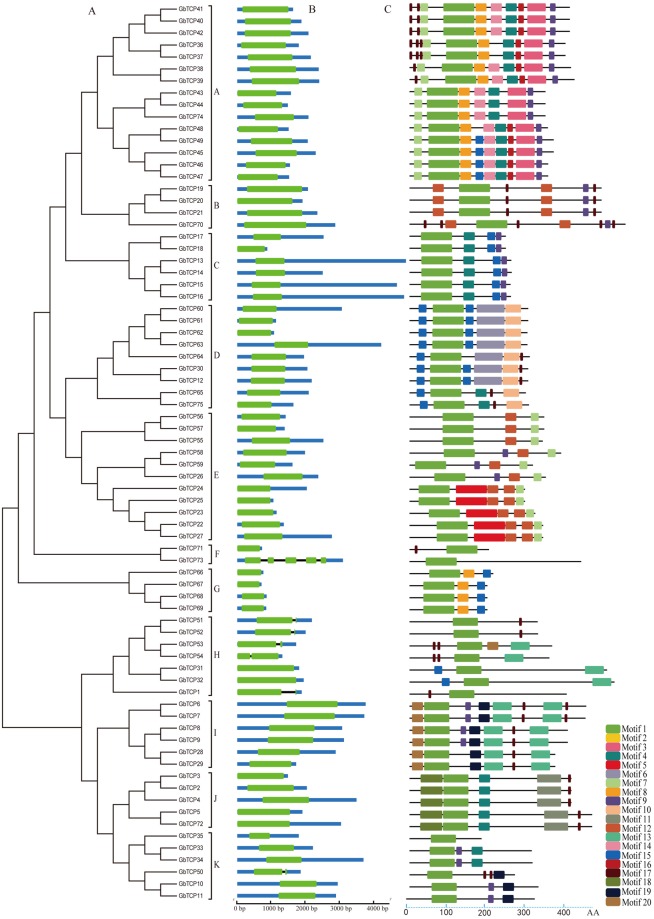
Using the GbTCP family of protein sequences, a neighbor-joining phylogenetic tree was constructed, dividing the GbTCP family member into 11 subfamilies, as shown in Fig. 6A. Next, we used the CDS sequence of the TCP family of G. barbadense and the gene DNA sequences to further study the intron and exon structures of TCP genes (Fig. 6B). As shown in Fig. 6B, 68 of the 75 GbTCP genes of G. barbadense had no introns, accounting for 90% of the total. The remaining seven GbTCP genes all contained introns; four in GbTCP73 and one each in GbTCP50, GbTCP51, GbTCP52, GbTCP53, GbTCP54, and GbTCP1. Compared to several other subfamilies, the two GbTCP genes in subfamily F vary widely in exon length and number of introns. In the GbTCP gene family of G. barbadense, most of the GbTCP genes in the same subgroup have similar patterns in their exon length and intron number. For example, the GbTCP genes in subfamilies A, B, C, D, E, G, I, and J have no introns; F and K subfamily members have one intron. Analyses of 11 subfamilies of the GbTCP gene family in G. barbadense revealed that genes generated by repetition have similar gene structure, which suggests that these genes are derived from a common ancestor.
Figure 6.
(A) Multiple alignment of 75 full-length GbTCP proteins using Clustal 2.0. The phylogenetic tree was constructed using MEGA 6.0 software and linked by the neighbor-joining method with 1000 bootstrap repeats. (B) Exon/intron distribution in the GbTCP family genes. Green lines represent exons, and blue lines represent untranslated regions; a scale (bottom) to estimate the size of exons and introns is provided. (C) Base sequence diagram of 75 GbTCP family proteins. Each colored box represents a motif of the protein, and the lower scale is used to estimate the size of the protein.
A conserved motif analysis of 75 GbTCP protein sequences in G. barbadense using the MEME program predicted 20 motifs (Fig. 6C); Subsequently by use the program InterProScan to annotate these motifs. The results show that the only identity that can be identified in the database is the conserved TCP domain (motif1). However, all 75 proteins have a common motif 1. Further analyses within the same subfamily showed that most of the GbTCP members have essentially the same motif composition, but there is a large difference in motif composition among various subfamilies. While the functions of the other 18 sequences are unknown, the conserved motif in the same subfamily of TCP proteins is strikingly similar, which indicates that the structure of GbTCP protein is significantly conserved in a specific subfamily. Interestingly, the subfractions of the F subfamily are spaced far apart, with a low degree of conservation, and a relatively small number of conserved motifs. Compared to other subfamilies, the C and G subfamilies have shorter TCP protein sequences, the motif composition is relatively conservative, and the sequences are significantly reduced. In addition, certain motifs exist only in certain subfamilies. For example, the H subfamily GbTCP genes belong to the TCP–CYC class, but the motif composition in the H subgroup varies greatly from that in other subgroups.
Analyses of TCP gene expression in G. barbadense fibers
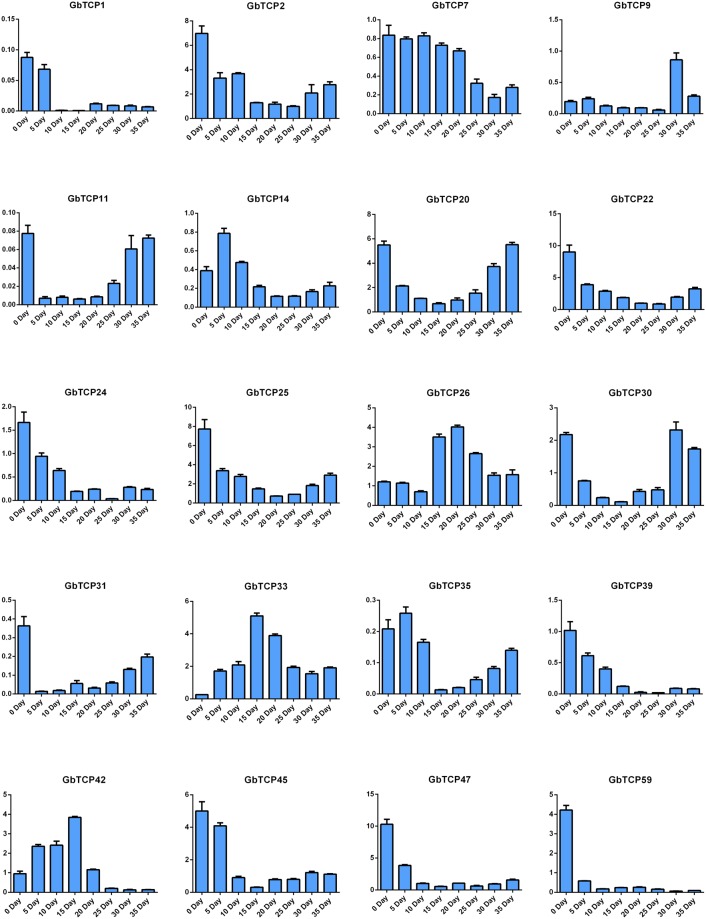
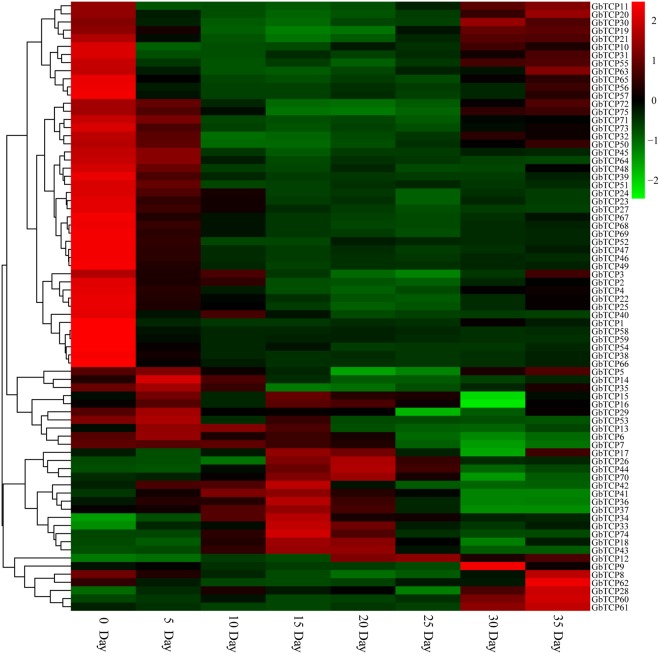
Specific primers were designed to amplify 75 genes in the G. barbadense TCP family. The expression levels of G. barbadense (Xinhai 21) TCP genes were analyzed using quantitative real time (qRT)-polymerase chain reaction (PCR) analyses of fiber samples on day 0 (flowering day) and days 5, 10, 15, 20, 25, 30, and 35, with UBQ7 used as the reference gene. As shown in Figs 7 and 8, 48 genes were highly expressed in ovules on day 0 in the initial stage of G. barbadense fiber development. The GbTCP17, GbTCP26, GbTCP44, GbTCP70, GbTCP42, GbTCP41, GbTCP36, GbTCP37, GbTCP34, GbTCP33, GbTCP74, GbTCP18, and GbTCP43 genes were highly expressed in fibers on day 15 during the elongation stage of fiber development. GbTCP12, GbTCP26, GbTCP44, and GbTCP70 genes were highly expressed in the secondary wall synthesis stage of fiber development. In addition, high expression levels of 16 GbTCP genes were detected at the maturation stage of fiber development. For example, GbTCP9 was highly expressed in fiber on day 30, and GbTCP8, GbTCP62, GbTCP28, GbTCP60, and GbTCP61 genes were highly expressed in fiber on day 35. No significant differences in expression were detected in GbTCP1, GbTCP2, GbTCP3, GbTCP31, or GbTC39 genes throughout the fiber development period. Furthermore, several GbTCP genes showed low expression levels throughout the development period. The varied expression patterns in GbTCP genes suggest functional differences in GbTCP genes during fiber development.
Figure 7.
Expression of 20 GbTCP genes in various stages of sea-island cotton fiber development. The X-axis represents fiber samples from different growth periods, and the Y-axis represents the relative expression level of GbTCP gene. Error bars represent the standard deviation of three replicates.
Figure 8.
Heat map of the expression patterns of 75 GbTCP genes in various fiber growth stages; expression profile data of GbTCP gene on days 0, 5, 10, 15, 20, 25, 30, and 35 by quantitative real-time (qRT) polymerase chain reaction (PCR). Expression values are log2-transformed. The expression levels are represented by the color bar.
The gene expression patterns in each branch of the sea-island cotton TCP gene family differ from that in other branches throughout the fiber development period. For example, similar expression patterns were detected in six GbTCP genes (GbTCP1, GbTCP31, GbTCP32, GbTCP51, GbTCP52, GbTCP53, and GbTCP54) belonging to the Class II CYC/TBI subfamily. These genes showed high expression only in the ovules on day 0, and low expression during the other periods of fiber development. Analyses of the expression characteristics of 17 genes in the CIN subfamily showed that 12 TCP genes had higher expression in the mature stages of fiber development. High expression of GbTCP6, GbTCP7, GbTCP33, GbTCP34, and GbTCP35 was consistently detected during days 5–20 in cotton fiber development; this period is critical for fibrous cell primary wall elongation. We speculate these five genes may be involved in the fiber cell growth of the primary wall.
We found that Class I TCP gene expression levels differ from Class II gene expression levels in G. barbadense. GbTCP Class I genes have more obvious expression specificity, with expression patterns indicating constitutive expression. For example, GbTCP17, GbTCP26, GbTCP36, GbTCP37, GbTCP41, GbTCP42, GbTCP44, and GbTCP70 all have higher expression in G. barbadense, whereas GbTCP40, GbTCP58, and GbTCP59 genes are expressed only in the ovule. Most of the genes were expressed throughout the entire period, which suggests that some sea-island cotton Class I-TCP genes may be involved in the development of cotton fiber.
Expression of GbTCPs in Xinhai 25 and Ashmon
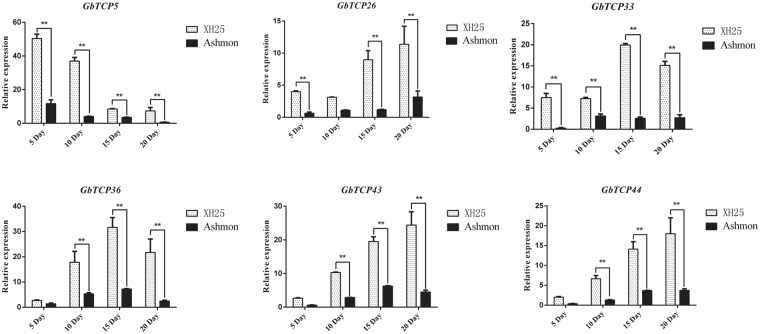
To further study the expression characteristics of GbTCPs in the elongation and synthesis of secondary walls of cotton fiber, we analyzed fiber quality data from the previous 3 years (Supplementary Table 1). Two varieties of cotton with special fiber qualities were selected: Xinhai 25 (longer fibers) and Ashmon (shorter fibers). The fibers from days 10, 15, and 20 were used to study the expression of six GbTCP genes (GbTCP5, GbTCP26, GbTCP33, GbTCP36, GbTCP43, and GbTCP44). As shown in Fig. 9, higher expression levels of six genes were detected in the long fibers of Xinhai 25 than in the short fibers of Ashmon. According to the gene expression trend, made the following classification. It was found that the expression trend of the GbTCP5 gene was different from the other 5 genes. The expression of GbTCP5 gene was highest in the fiber of 5–10 Day of two cotton varieties, and the expression amount in 5 Day fiber was 6 times as much as that of 15 Day. These findings indicate that GbTCP5 genes are involved in the initiation stage of cotton fiber development. GbTCP33 and GbTCP36 expression increased continuously in fibers from days 5 to 15, peaking on day 15; these findings suggest the involvement of these two genes in the elongation stage of fiber development. The expressions of GbTCP26, GbTCP43, and GbTCP44 showed an increasing trend from days 5 to 20, with the highest expression detected on day 20, which suggests the involvement of these three genes in the secondary wall synthesis phase of development.
Figure 9.
Comparison of the expression of six genes in XH25 and Ashmon. The expression of TCP genes in various fiber developmental stages of Xinhai 25 and Ashmon was analyzed by qRT-PCR; Significant differences between xinhai25 and Ashmon were determined by Student t-test. *Significant differences in Xinhai 25 and Ashmon gene expression levels (P < 0.05); **Greater significant differences in gene expression levels between Xinhai 25 and Ashmon (P < 0.01); Error bars represent SD for three independent experiments. The Y-axis represents the relative expression of genes.
On this basis, we studied the expression of these six genes in the upland cotton Xinluzhong 36. The results showed that these 6 genes reached their peak values during the 15-day period of fiber development in upland cotton, indicating that these genes play a role in the elongation of the fiber of upland cotton, as shown in the (Fig. 9). In addition, we found that the expression trends of GhTCP15a-D (GbTCP43) and GhTCP15a-A (GbTCP44) in 15 days-20 days were different from those in sea-island cotton. The remaining GhTCP6a-A (GbTCP5), GhTCP19b-A (GbTCP26), GhTCP13a-A (GbTCP33) and GhTCP14a-D (GbTCP36) genes have the same expression pattern in upland cotton and sea-island cotton. At the same time, the expression of these four genes in sea-island cotton was much greater than that of upland cotton. Pearson correlation coefficient was used to detect the correlation between genes in different Gossypium species. It was found that these genes were positively correlated in Xinhai 25 and Xinluzhong 36.
Discussion
The TCP gene family is a class of specific transcription factors that play an important role in plant growth and development by regulating the expression of downstream target genes. However, no systematic studies on the TCP gene family in G. barbadense have been reported. The TCP gene family in sea-island cotton has many members with complex functions. Moreover, these members can have great genetic differences, displaying various complex, competitive, and interactive relationships. TCP genes in G. barbadense and A. thaliana have several differences. First, the number of TCP genes in G. barbadense is much higher than that in A. thaliana, which contains 37 more TCP genes than G. raimondii20 and 39 more than G. arboreum21.This finding suggests that the evolution of cotton included diploid to tetraploid gene-gain events. Our results indicate that, compared to the TCP gene family in rice and Arabidopsis, the cotton TCP gene family has obvious gene duplication, as evidenced by the great number of genes in the GbTCP family. The phylogenetic tree has numerous and dense branches, but no obvious periphery branches, which indicates that the GbTCP family is fairly conserved. We found that the amino acid lengths in the TCP family members of sea-island cotton differ considerably, which indicates that the origin and evolution of the TCP family of sea-island cottons may be complicated. Furthermore, this variation in length might contribute to the multiple biological functions of TCP genes.
It was found that 7 GbTCP genes in G. barbadense have a introns, one more than G. raimondii20, three more than G. arboreum21, and three fewer than G. hirsutum22. This discrepancy may contribute to the more desirable fiber of the sea-island cotton compared to that from other cotton genera. The distribution of introns and exons and the distribution of amino acid motifs revealed that each gene within a subgroup had a similar structure, and that these highly similar sequences are associated with tandem duplication and segmental duplication caused during the evolution of the genome. These phenomena play an important role in the process of genome rearrangement and expansion, as well as in the diversity of gene function and amplification of the gene family34.
G. arboreum is the chromosomal ancestor of the tetraploid sea-island cotton group D, and the G. raimondii is the ancestor of group A chromosomes. Studying the multiple replication events of the GbTCP gene family will further our understanding of the polyploid formation process in the cotton genome. Large chromosome duplication fragments and tandem duplication of chromosomes play an important role in the amplification of the GbTCP gene family precisely because the GbTCP family of genes tends to be purified after chromosomal segment replication. Therefore, the GbTCP genes are conserved and thus participate in a variety of cotton physiological activities. Structurally, the 10 pairs of tandem duplication genes in sea-island cotton belong to the Class I family. The expression data of different fiber stages revealed these pairs of genes amplified by the same ancestor have the same expression pattern, which indicates that these genes may play similar roles in the evolutionary process.
A previous study reported that a GbTCP transcription factor in G. barbadense can elongate fibers and root hairs by regulating the metabolism of jasmonic acid and activating downstream genes that control fiber development and root hair elongation35. Wang et al. found that GhTCP14 is specifically expressed primarily in the initial stages of fiber development when overexpressed in A. thaliana. GhTCP14 overexpression changes the distribution of auxin, and influences the expression levels of auxin-related genes, such as AUX1, PIN2, and IAA3. These findings indicate that GhTCP14 regulates cotton fiber development by directly regulating auxin32.
In this study, the starting point for fiber development occurred on days 0–5, and fiber development extended on days 10–20. In line with those results, GbTCP36 (GbTCP) and GbTCP44 (GhTCP14) were continuously expressed in the initiation and elongation stages of G. barbadense fiber development. The expression of GbTCP36 peaked on day 15 whereas GbTCP44 peaked on day 20, which indicates that these two genes play an important role in the development of cotton fiber initiation and elongation. GbTCP5 is involved in the initial stage of fiber development in XH25 and Ashmon. GbTCP26, GbTCP43, and GbTCP44 are involved in secondary wall synthesis in fiber development. GbTCP33 and GbTCP36 may be involved in fiber elongation and secondary wall synthesis. The expression of structural genes belonging to class A, including GbTCP37, GbTCP41, GbTCP42, GbTCP43, and GbTCP74 (Fig. 6A), show expression characteristics consistent with expression levels reported in G. hirsutum22. However, in the present study, these genes sustained expression in fiber on days 5–20, peaking on day 15. Therefore, G. barbadense fiber development extends over a long period, which suggests that these genes play an important role in the elongation of G. barbadense fiber.
TCP genes play an important role as plant-specific transcription factors in plant growth and development. These genes not only regulate cell growth, proliferation, and differentiation they also affect the growth of lateral branches, flowers, and other organs. Studies in Arabidopsis have revealed that TCP proteins within the same subfamily share a common motif and have similar functions. Expression of the AtTCP2, AtTCP3, AtTCP4, AtTCP10, and AtTCP24 genes in the CIN subfamily of A. thaliana is regulated by miR319-mediated post-transcriptional regulation, playing an inhibitory role in cell division during leaf development36. The AtTCP14 and AtTCP15 genes, which are structural members of the Class I family, are involved in regulating internode development and leaf shape in A. thaliana, thereby controlling the development of leaves and the proliferation of young internode cells37.
The expression characteristics of the GbTCP family in sea-island cotton Xinhai 21 indicated that numerous genes in the GbTCP family are involved in the physiological process of cotton fiber development, and the expression of genes differ in various stages of fiber development. However, genes in the same subfamily showed similar expression trends. For example, GbTCP genes overexpressed in fibers on days 0–5 may be involved in the differentiation and protuberance of fibroblasts. Genes that express significant amounts in fibers on days 10–25 may cooperate with other genes during primary wall elongation and secondary wall thickening. GbTCP genes with high expression on days 30–35 may aid cell dehydration and promote fiber maturation.
This study was the first to identify 75 GbTCP gene family members in G. barbadense and to investigate their role in cotton fiber development. Our results provide a foundation for future functional studies to determine the molecular mechanisms of TCP genes in the development of cotton fiber.
Conclusion
We used bioinformatics tools to analyze a genome-wide database to identify 75 TCP genes in G. barbadense. The GbTCP genes are divided into two subfamilies (Class I and II) according to their structural characteristics; 51 TCP genes belong to Class I, and 24 TCP genes belong to Class II. Chromosomal mapping showed that only 66 of the 75 TCP genes were heterogeneously distributed on 21 chromosomes. Analyses of the collinear relationship among GbTCP genes revealed significant collinear relationships in 81.3% of the TCP genes in G. barbadense. The complex linear relationship indicated that several TCP genes are involved in multiple gene duplication events. Structural analyses of GbTCP genes in G. barbadense cotton showed that 68 genes had no introns, and most of the GbTCP genes in the same subgroup had similar patterns of exon length, intron number, and conserved motifs. qRT-PCR analyses of GbTCP gene expression characteristics in the fiber revealed varying expression levels according to the period of fiber development. We identified several genes that are highly expressed in the elongation stage and secondary wall synthesis stage of fiber development, which suggests that GbTCP genes may play an important role in the physiological process of cotton fiber development.
Materials and Methods
Materials and growth conditions
G. hirsutum Xin lu zhong 36, G. barbadense Xinhai 21, Xinhai 25, and Ashmon were obtained from the Xinjiang Agricultural University, Agronomy Courtyard Key Laboratory of Agricultural Biological Technology. The plants were planted on April 12, 2016, at the Xinjiang Alar City Xinjiang Academy of Agricultural Science Experimental Station. The annual sunshine hours in this location ranged from 2556 to 2991 h, and the average frost-free period was 200 days or more; these conditions were suitable for growth of sea-island cotton. After planting, the crop was maintained under normal field management. Ovules were obtained on the first flowering day, which was marked day 0. Fibers were sampled in duplicate on days 5, 10, 15, 20, 25, 30, and 35. The cotton fibers were placed in liquid nitrogen immediately after sampling for later use.
Identification of TCP gene family members
The complete genome sequence of sea-island cotton was downloaded from Washington State University in the United States cotton genome database (https://www.cottongen.org/) and the Hua Zhong Agricultural University Center for cotton genetic improvement (http://cotton.cropdb.org/cotton/). Then, we built a local Blast database separately by downloading the TCP hidden Markov model (PF03634) from the Pfam database (http://pfam.xfam.org/) and used it as a BLAST query. The search was performed using HMMER 3.0, and the results obtained were searched using SMART (http://smart.embl-heidelberg.de/) and ExPASy-PROSITE (http://www.expasy.org/) online tools to make structural predictions of the searched proteins. We manually deleted sequences with no TCP domains and non-full-length genes. All remaining TCP amino acid sequences were predicted for molecular weight and isoelectric point using the online ProtParam tool (http://www.expasy.org/protparam/).
Sequence alignment and phylogenetic analyses
The 24 TCP protein sequences of A. thaliana were downloaded from the Arabidopsis resource database TAIR (http://arabidopsis.org/). The TCP protein sequences of T. cacao, O. sativa, and G. hirsutum were from the plant transcription factor database (http://planttfdb.cbi.pku.edu.cn/). The TCP protein sequences of G. raimondii and G. arboreum were from the cotton genome database (https://www.cottongen.org/). We performed multiple sequence alignment of the TCP protein sequences from the above seven plants using ClustalX38. We used NJ MEGA 6.0 software39 and JTT model for these analyses and the results were a root-free evolutionary tree (execution parameters: BootStrap method 1000; Poisson model: pairwise deletion). To validate the NJ tree, the maximum likelihood (ML) method was also used. The bootstrap method was used with 1,000 replicates.
Chromosomal location and gene duplication
The chromosomal location information of each TCP gene was downloaded from the sea-island cotton database (http://cotton.cropdb.org/). The 75 genes of the TCP family were located on 26 chromosomes of G. barbadense, as determined using the MapInspect2.2 software. All the protein sequences of sea-island cotton were included in a local database using Basic Local Alignment Search Tool (BLAST). The entire protein sequences were used as queries to search the above-mentioned database with an e-value of 1e−5. The blastp result was analyzed by MCScanX40 to produce the collinearity blocks across the whole genome. We used the Circos tool to visualize the chromosomal repeat fragment information and the chromosomal locations of the TCP genes41. The replacement rates of synonymous (Ks) and nonsynonymous (Ka) mutations were calculated as described previously42. These rates were used to identify DNA polymorphisms using DnaSPV5.0 software43, and the Ka/Ks ratio was analyzed to assess the selection pressure of each gene. In general, Ka/Ks > 1 indicates a positive selection effect, Ka/Ks < 1 indicates a purification option, and Ka/Ks = 1 indicates a neutral selection44. We use the formula T = Ks/2r to calculate the date of the replication event, where “r” is the neutral substitution rate. The neutral replacement rate used in the current study is 2.6 × 10−9 45.
Gene structure and analyses of conserved motifs
The cDNA sequences and intron/exon lengths in TCP genes were obtained from the G. barbadense database, and the GbTCP gene family was analyzed online using the Gene Structure Display Server (http://gsds.cbi.pku.edu.cn/)46. Conserved motifs were predicted in the G. barbadense TCP family of proteins using the MEME website (http://meme-suite.org/index.html)47. The parameters were set to: “any” motif repeat number, 6 to 100 motif width, and maximum number of motifs: 20. In addition, we use the InterProScan4 program to annotate the identified motif48.
RNA extraction and qRT-PCR
Total RNA was extracted from G. barbadense fiber tissues on days 0, 5, 10, 15, 20, 25, 30, and 35 using an RNAprep Pure Plant Kit (Tiangen, http://www.tiangen.com). RNAs were processed to remove genomic DNA. A Colibri Microvolume Spectrometer (Titertek-Berthold, http://www.titertek-berthold.com) was used to assess the concentration and quality of RNA. First-strand cDNA was synthesized using a Transcriptor First Strand cDNA Synthesis Kit (Thermo Scientific, http://www.Thermo.com) with 2 µg total RNA. According to the sequences of 75 genes from the TCP of the G. barbadense, 75 primer pairs (Supplementary Dataset 2) were designed using Primer Express 3.0.1. The annealing temperature was between 58 °C and 60 °C for qRT-PCR. The G. barbadense UBQ7 gene was used as a reference gene, using 20 μL per reaction. The reactions included 1.5 μL cDNA, 10 μL 2 × PowerUpTMSYBRTM Green Master Mix (Applied Biosystems, USA), 0.4 μL each of upstream and downstream primers, and 7.7 μL RNase-Free ddH2O. Two replicate samples of each period were subjected to three biological replicates using an ABI 7500 Fast Real-Time PCR instrument (Applied Biosystems, USA). Amplification parameters were as follows: activation at 50 °C for 2 min, pre-denaturation at 95 °C for 2 min, denaturation at 95 °C for 15 s, and annealing at 60 °C for 1 min for 40 cycles. The data were quantitatively analyzed using the 2−△△CT method49 and were processed using Microsoft Excel 2010 software.
Electronic supplementary material
Acknowledgements
This work was supported by the key research and development tasks of Xinjiang (2016B01001–1). We would like to thank the reviewers for their comments and suggestion.
Author Contributions
K.Z., Z.Y.N. and Q.J.C. conceived and designed the experiment, K.Z. and Z.Y.N. performed most of the research. Y.S.C. and Z.E.Y. performed some gene expression analysis. K.Z., Z.Y.N., Y.Y.Q. and G.Q.S. analyzed the data, wrote and revised the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kai Zheng and Zhiyong Ni contributed equally.
Contributor Information
Guoqing Sun, Email: sunguoqing02@caas.cn.
Quanjia Chen, Email: chqjia@126.com.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-32626-5.
References
- 1.Cubas P, Lauter N, Doebley J, Coen E. The TCP domain: a motif found in proteins regulating plant growth and development. The Plant Journal. 1999;18(2):215–222. doi: 10.1046/j.1365-313X.1999.00444.x. [DOI] [PubMed] [Google Scholar]
- 2.Kosugi S, Ohashi Y. PCF1 and PCF2 specifically bind to cis elements in the rice proliferating cell nuclear antigen gene. The Plant Cell. 1997;9(9):1607–1619. doi: 10.1105/tpc.9.9.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martíntrillo M, Cubas P. TCP genes: a family snapshot ten years later. Trends in Plant Science. 2010;15.1:31–39. doi: 10.1016/j.tplants.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Doebley J, Stec A, Hubbard L. The evolution of apical dominance in maize. Nature. 1997;386(6624):485. doi: 10.1038/386485a0. [DOI] [PubMed] [Google Scholar]
- 5.Luo D, Carpenter R, Vincent C, Copsey L, Coen E. Origin of floral asymmetry in Antirrhinum. Nature. 1996;383(6603):794. doi: 10.1038/383794a0. [DOI] [PubMed] [Google Scholar]
- 6.Palatnik JF, et al. Control of leaf morphogenesis by microRNAs. Nature. 2003;425(6955):257. doi: 10.1038/nature01958. [DOI] [PubMed] [Google Scholar]
- 7.Costa MMR, Fox S, Hanna AI, Baxter C, Coen E. Evolution of regulatory interactions controlling floral asymmetry. Development. 2005;132(22):5093–5101. doi: 10.1242/dev.02085. [DOI] [PubMed] [Google Scholar]
- 8.Broholm SK, et al. TCP domain transcription factor controls flower type specification along the radial axis of the Gerbera (Asteraceae) inflorescence. Proceedings of the National Academy of Sciences. 2008;105(26):9117–9122. doi: 10.1073/pnas.0801359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navaud O, Dabos P, Carnus E, Tremousaygue D, Hervé C. TCP transcription factors predate the emergence of land plants. Journal of molecular evolution. 2007;65(1):23–33. doi: 10.1007/s00239-006-0174-z. [DOI] [PubMed] [Google Scholar]
- 10.Reeves PA, Olmstead RG. Evolution of the TCP gene family in Asteridae: cladistic and network approaches to understanding regulatory gene family diversification and its impact on morphological evolution. Molecular Biology and Evolution. 2003;20(12):1997–2009. doi: 10.1093/molbev/msg211. [DOI] [PubMed] [Google Scholar]
- 11.Kölsch A, Gleissberg S. Diversification of CYCLOIDEA‐like TCP Genes in the Basal Eudicot Families Fumariaceae and Papaveraceae s. str. Plant Biology. 2006;8(5):680–687. doi: 10.1055/s-2006-924286. [DOI] [PubMed] [Google Scholar]
- 12.Citerne HL, Luo D, Pennington RT, Coen E, Cronk QC. A phylogenomic investigation of CYCLOIDEA-like TCP genes in the Leguminosae. Plant Physiology. 2003;131(3):1042–1053. doi: 10.1104/pp.102.016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S. The Arabidopsis thaliana TCP transcription factors: a broadening horizon beyond development. Plant signaling & behavior. 2015;10(7):e1044192. doi: 10.1080/15592324.2015.1044192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao X, Ma H, Wang J, Zhang D. Genome‐Wide Comparative Analysis and Expression Pattern of TCP Gene Families in Arabidopsis thaliana and Oryza sativa. Journal of Integrative Plant Biology. 2007;49(6):885–897. doi: 10.1111/j.1744-7909.2007.00509.x. [DOI] [Google Scholar]
- 15.Ma X, et al. Genome-wide Identification of TCP family transcription factors from populus euphratica and their involvement in leaf shape regulation. Scientific reports. 2016;6:32795. doi: 10.1038/srep32795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parapunova V, et al. Identification, cloning and characterization of the tomato TCP transcription factor family. BMC plant biology. 2014;14(1):157. doi: 10.1186/1471-2229-14-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi P, et al. Genome-wide identification and expression analysis of the ClTCP transcription factors in Citrullus lanatus. BMC plant biology. 2016;16(1):85. doi: 10.1186/s12870-016-0765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Paolo S, Gaudio L, Aceto S. Analysis of the TCP genes expressed in the inflorescence of the orchid Orchis italica. Scientific reports. 2015;5:16265. doi: 10.1038/srep16265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francis A, et al. Comparative phylogenomic analysis provides insights into TCP gene functions in Sorghum. Scientific reports. 2016;6:38488. doi: 10.1038/srep38488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma J, et al. Genome-wide identification and expression analysis of TCP transcription factors in Gossypium raimondii. Scientific reports. 2014;4:6645. doi: 10.1038/srep06645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma J, et al. Comprehensive analysis of TCP transcription factors and their expression during cotton (Gossypium arboreum) fiber early development. Scientific reports. 2016;6:21535. doi: 10.1038/srep21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, et al. Genome-wide identification and characterization of TCP transcription factor genes in upland cotton (Gossypium hirsutum) Scientific Reports. 2017;7(1):10118. doi: 10.1038/s41598-017-10609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schommer C, et al. Control of jasmonate biosynthesis and senescence by miR319 targets. PLoS biology. 2008;6(9):e230. doi: 10.1371/journal.pbio.0060230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hervé C, et al. In vivo interference with attcp20 function induces severe plant growth alterations and deregulates the expression of many genes important for development. Plant Physiology. 2009;149(3):1462. doi: 10.1104/pp.108.126136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagnussat GC, et al. Genetic and molecular identification of genes required for female gametophyte development and function in arabidopsis. Development. 2005;132(3):603. doi: 10.1242/dev.01595. [DOI] [PubMed] [Google Scholar]
- 26.Takeda T, et al. Rna interference of the arabidopsis putative transcription factor tcp16 gene results in abortion of early pollen development. Plant Molecular Biology. 2006;61(1–2):165–177. doi: 10.1007/s11103-006-6265-9. [DOI] [PubMed] [Google Scholar]
- 27.Prunedapaz JL, Breton G, Para A, Kay SA. A functional genomics approach reveals che as a component of the arabidopsis circadian clock. Science. 2009;323(5920):1481. doi: 10.1126/science.1167206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alghazi Y, Bourot S, Arioli T, Dennis ES, Llewellyn DJ. Transcript profiling during fiber development identifies pathways in secondary metabolism and cell wall structure that may contribute to cotton fiber quality. Plant & Cell Physiology. 2009;50(7):1364–1381. doi: 10.1093/pcp/pcp084. [DOI] [PubMed] [Google Scholar]
- 29.Wang K, et al. The draft genome of a diploid cotton Gossypium raimondii. Nature genetics. 2012;44(10):1098. doi: 10.1038/ng.2371. [DOI] [PubMed] [Google Scholar]
- 30.Li F, et al. Genome sequence of the cultivated cotton Gossypium arboreum. Nature genetics. 2014;46(6):567. doi: 10.1038/ng.2987. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, et al. Gossypium barbadense genome sequence provides insight into the evolution of extra-long staple fiber and specialized metabolites. Scientific Reports. 2015;5(14139):14139. doi: 10.1038/srep14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang MY, et al. The cotton transcription factor TCP14 functions in auxin-mediated epidermal cell differentiation and elongation. Plant physiology. 2013;162(3):1669–1680. doi: 10.1104/pp.113.215673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cannon SB, Mitra A, Baumgarten A, Young ND, May G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC plant biology. 2004;4(1):10. doi: 10.1186/1471-2229-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu W, Hu G, Han B. Genome-wide survey and expression profiling of heat shock proteins and heat shock factors revealed overlapped and stress specific response under abiotic stresses in rice. Plant Science. 2009;176(4):583–590. doi: 10.1016/j.plantsci.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Hao J, et al. GbTCP, a cotton TCP transcription factor, confers fibre elongation and root hair development by a complex regulating system. Journal of Experimental Botany. 2012;63(17):6267. doi: 10.1093/jxb/ers278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mao Y, et al. MicroRNA319a-targeted Brassica rapa ssp. pekinensis TCP genes modulate head shape in chinese cabbage by differential cell division arrest in leaf regions. Plant physiology. 2014;164(2):710–720. doi: 10.1104/pp.113.228007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kieffer M, Master V, Waites R, Davies B. TCP14 and TCP15 affect internode length and leaf shape in Arabidopsis. The Plant Journal. 2011;68(1):147–158. doi: 10.1111/j.1365-313X.2011.04674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The clustal_x windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research. 1997;25(25):4876–82. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. Mega6: molecular evolutionary genetics analysis version 6.0. Molecular Biology & Evolution. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic acids research. 2012;40(7):e49–e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krzywinski M, et al. Circos: an information aesthetic for comparative genomics. Genome Research. 2009;19(9):1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu R, et al. Comprehensive analysis of NAC domain transcription factor gene family in Populus trichocarpa. BMC plant biology. 2010;10(1):145. doi: 10.1186/1471-2229-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Librado P, Rozas J. DnaSPv5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25(11):1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 44.Yang X, Tuskan GA. Divergence of the Dof gene families in poplar, Arabidopsis, and rice suggests multiple modes of gene evolution after duplication. Plant physiology. 2006;142(3):820–830. doi: 10.1104/pp.106.083642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang T, et al. Sequencing of allotetraploid cotton (Gossypium hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nature biotechnology. 2015;33(5):531. doi: 10.1038/nbt.3207. [DOI] [PubMed] [Google Scholar]
- 46.Hu B, et al. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2014;31(8):1296–1297. doi: 10.1093/bioinformatics/btu817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bailey TL, et al. MEME SUITE: tools for motif discovery and searching. Nucleic acids research. 2009;37(suppl_2):W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quevillon, E. et al. Interproscan: protein domains identifier. Nucleic Acids Research, 33 (Web Server issue), 116–20 (2005). [DOI] [PMC free article] [PubMed]
- 49.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.