Abstract
Invasive infections caused by drug-resistant bacteria are frequently responsible for fatal sepsis, morbidity and mortality rates. In this work, we propose a new methodology based on nanosecond high frequency electric field bursts, which enables successful eradication of bacteria in vivo. High frequency (15 kHz) 15–25 kV/cm 300–900 ns pulsing bursts were used separately and in combination with acetic acid (0.1–1%) to treat Pseudomonas aeruginosa in a murine model. Acetic acid 1% alone was effective resulting in almost 10-fold reduction of bacteria viability, however combination of nanosecond electric field and acetic acid 1% treatment was the most successful showing almost full eradication (0.01% survival compared to control) of the bacteria in the contaminated area. The short duration of the pulses (sub-microsecond) and high frequency (kHz range) of the burst enabled reduction of the muscle contractions to barely detectable level while the proposed applicators ensured predominantly topical treatment, without electroporation of deeper tissues. The results of our study have direct application for treatment of wounds and ulcers when chemical treatment is no longer effective.
Introduction
Invasive infections caused by drug-resistant bacteria are becoming increasingly prevalent and are associated with high morbidity and mortality rates, especially in patients with burn wounds1–4. The management of an infection in thermal injury presents challenges in terms of clinical diagnosis and rapid definition of effective antimicrobial therapy4,5. Topical antibiotics are effective in reducing such wound infections, however the absolute and long-term benefit is small6 since the accelerated evolution of bacterial resistance is frequently associated with a widespread use of antibiotics7,8. Therefore, with the emergence of multidrug-resistant strains, alternative strategies to control or prevent infections must be developed in parallel with novel antimicrobial agents9–11.
Novel chemical antimicrobial compounds (antimicrobial peptides, bioactive nanoparticles, etc.) are usually associated with the specific mechanism of biointeraction12–14. As a result, the microbial inactivation efficacy varies dependent on the biological object, while the high resistance is common in gram-negative bacteria15–17. One of the solutions is the application of alternative universal agents, such as acetic, ascorbic or other acids for pH manipulation and sterilization of the wound18,19. Acetic acid (AA) already proved to be efficient against bacteria biofilms and treatment of ulcers, however physiologically tolerable concentrations should be used20. To prevent corrosive effects of acid the concentration should be <10%, while up to 5% are considered harmless20–22. Since a trade-off between the treatment efficiency and patient-friendly procedure is required, the research and application of additional physical methods in combination with acidic treatment is performed23. In this work, we propose nanosecond range electroporation as an effective tool for biocontrol of surface infections.
Electroporation is a pulsed electric field (PEF) induced phenomenon of increased cell membrane permeability24,25, which has found a variety of applications in food processing industry (bacterial decontamination)26, biotechnology (protein extraction, transformation)27–29 and cancer treatment (tissue ablation, electrochemotherapy)30,31. It is an electric pulse-dependent methodology, thus both the positive and side effects of the treatment depend on the parameters of electric field and the structure of electrodes (applicators)32,33. In addition, bacteria are less susceptible to electroporation compared to mammalian cells (due to cell size, cell wall, internal structure)34. Therefore, the parameters of PEF that are required to inactivate the bacteria or pathogenic yeasts35–37 will likely to result in tissue ablation of the treated area, which is non-desirable and thus it was believed that electroporation is not suitable for wound sterilization. Nevertheless, recently Golberg et al. showed a concept of electroporation-assisted wound sterilization by means of microsecond electroporation38. The group used 5 kV/cm × 70 µs × 80 pulses delivered at repetition frequency of 1 Hz between parallel plate electrodes, which resulted in a successful treatment. However, the methodology still has considerable problems requiring solution: (1) positioning and the type of used electrodes significantly limit the application only to topical skin infections; (2) the skin is damaged by electroporation due to long and high amplitude PEF pulses; (3) the treatment is relatively long, which limits the possibilities of coverage of large surface area; (4) muscle contractions are severe when PEF of such intensity and duration is used.
Therefore, in this work we propose a new methodology based on nanosecond high frequency PEF bursts, which provides solution to all of the described problems. As a bacterial model, the gram-negative Pseudomonas aeruginosa was used.
Pseudomonas aeruginosa is one of the most frequently responsible bacteria for fatal sepsis in burn wound patients and is in the top of the list of the drug resistive bacteria, which have the highest threat to human health according to World Health Organization1,39,40. As a result, we present a new method and in vitro and in vivo data on successful eradication of this bacteria using nanosecond range electroporation separately and in combination with acetic acid.
Results
Proposed methodology
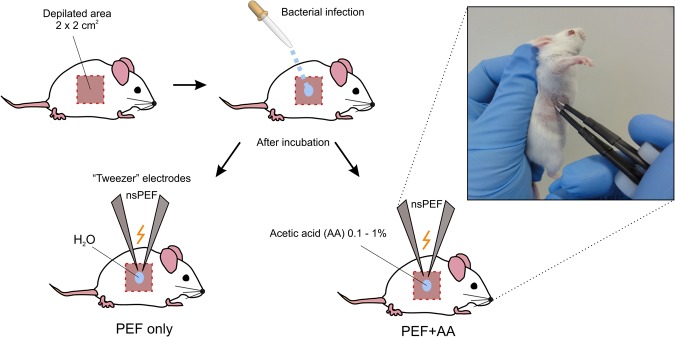
In this work, we propose a new methodology based on nanosecond high frequency PEF bursts for treatment of surface infections. The schematic of the proposed methodology is shown in Fig. 1.
Figure 1.
The schematic representation of proposed electroporation mediated methodology for biocontrol of surface infections, where PEF – pulsed electric field; nsPEF – high frequency nanosecond PEF bursts. Distilled water or low concentrations of acetic acid were used as an electrode-skin interface.
The basepoint is the short duration (nanosecond) of the electric field pulses (nsPEF), which combined with the high frequency (kHz range) of the burst allows to reduce the muscle contractions compared to available procedures. The “tweezer” electrodes and the topical delivery of low conductive liquid as an electrode-skin interface allows to ensure predominantly topical treatment, without electroporation of deeper tissues. The application of acetic acid enables additive and a more effective procedure, while the treatment being fast and flexible with capability to ensure a safety margin in pulse energy and/or acid concentration.
In vitro model
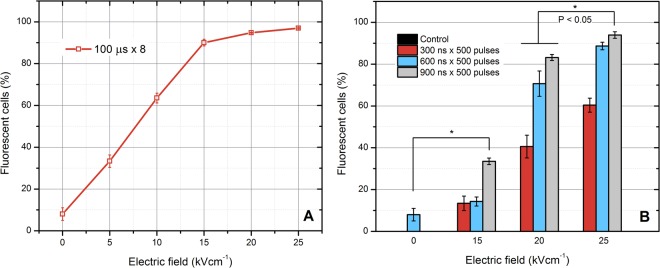
For selection of optimal parameters for the in vivo model, firstly we analyzed the dependence of bacteria permeabilization on PEF parameters in vitro. Bursts of nanosecond (300–900 ns) pulses of varied intensity (15–25 kV/cm) were delivered and the uptake of PI was evaluated using flow cytometry. Conventional microsecond electroporation (100 µs × 8) was used a reference. The results are summarized in Fig. 2.
Figure 2.
The dependence of P. aeruginosa permeabilization on the applied pulsed electric field parameters, where (A) microsecond range protocols; (B) nanosecond range protocols. The saturated permeabilization is achieved when the electric field amplitude exceeds 20 kV/cm. The 25 kV/cm, 900 ns protocol was defined as optimal due to high permeabilization rate of bacteria.
As it can be seen in Fig. 2A the P. aeruginosa requires PEF above 15 kV/cm to achieve high permeabilization rate. The result can be improved by increase of the number of pulses or pulse duration, however it is no applicable in the microsecond range due to possible severe muscle contractions. Also, the amplitude is already 10-fold higher compared to PEF, which is required to achieve high permeabilization in mammalian cells41.
Similar tendency was observed when bursts of nanosecond pulses were used (Fig. 2B). The saturated permeabilization was achieved only when the 25 kV/cm × 900 ns × 500 pulses protocol (P1) was applied. The permeabilization rate varied in a dose dependent manner. Therefore, the P1 protocol was selected as optimal (due to saturated permeabilization) and the number of pulses was doubled (25 kV/cm × 900 ns × 1000 – protocol P2) for further study to induce significant irreversible electroporation for inactivation of P. aeruginosa.
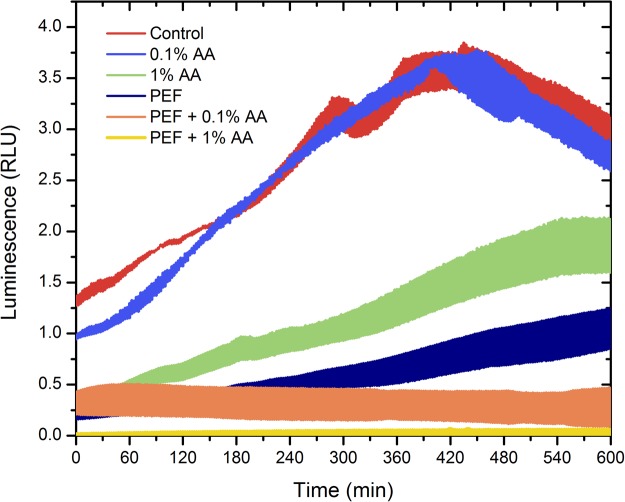
The inhibition efficacy of the P2 protocol was evaluated dynamically every 2.5 min for 10 h by measurement of the luminescence of the P. aeruginosa. The treatment was accompanied by addition of 0.1% and 1% AA during electroporation for determination of an additive effect. The results are summarized in Fig. 3.
Figure 3.
The dependence of P. aeruginosa luminescence on the applied treatment parameters, live bacteria luminesce; the width of colored area corresponds to standard deviation of data during selected treatment protocol; PEF – 25 kV/cm × 900 ns × 1000 pulses; AA – acetic acid. The 1% acetic acid combined with electroporation allowed complete inactivation of bacteria in vitro, which is unachievable if the treatments are used separately.
As it can be seen in Fig. 3. PEF itself (P2 protocol) reduced the luminescence of bacteria more than 5 times, however it was not sufficient to prevent further growth of P. aeruginosa and after 10 h the luminescence was comparable to the beginning of the experiment. Similar effect was observed when the 1% AA was used. The 0.1% AA had no effect on the luminescence of the P. aeruginosa due to insufficient concentration. However, in all experimental instances where combination of two procedures was used an additive effect (PEF + AA) was observed. The 1% acetic acid combined with electroporation allowed complete inactivation of bacteria in vitro, which was unachievable if the treatments were used separately. The width of colored area corresponds to standard deviation of data between independent experimental repetitions.
In vivo model
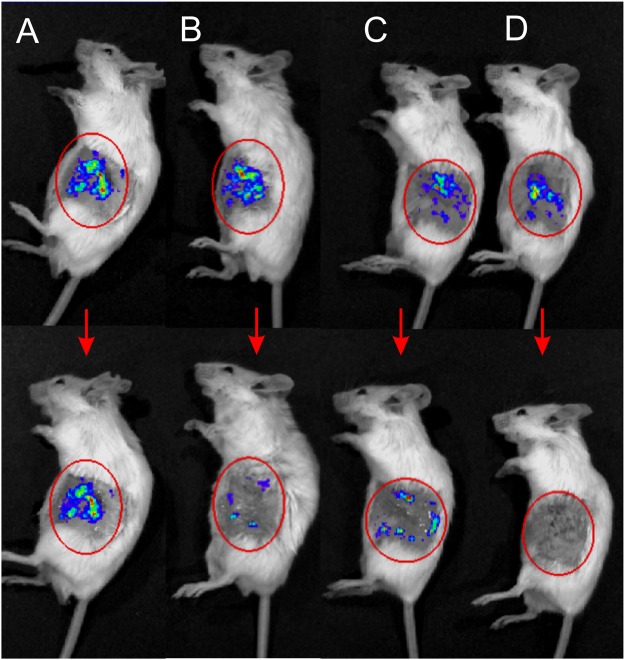
Based on the positive in vitro results, the methodology was tested in vivo. The exemplary images adequately representing the treatment efficiency using different protocols are shown in Fig. 4.
Figure 4.
The mice before treatment on the top and the same mice after the treatment on the bottom, where (A) treated with distilled water; (B) treated with distilled water and electroporation (25 kV/cm × 900 ns × 1000 pulses); (C) treated with acetic acid 1%; (D) treated with acetic acid 1% and electroporation. Combined treatment allows full eradication of bacteria.
Both the acetic acid and electroporation treatment reduced the viability of bacteria. The quantified results are summarized in Fig. 5.
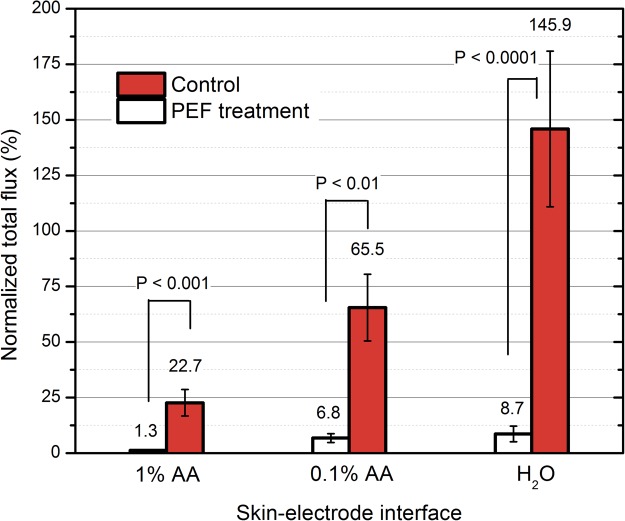
Figure 5.
The in vivo potential of acetic acid and electroporation for inactivation of P. aeruginosa. The combined treatment (AA 1% and PEF) results in up to full eradication of bacteria, where PEF: 25 kV/cm × 900 ns × 1000 pulses delivered at pulse repetition frequency of 15 kHz.
The luminescence (representing the extent of bacterial contamination) increased up to 46 ± 34% when the contaminated area was treated solely by distilled water. Acetic acid 1% alone was effective resulting in almost 10-fold reduction of P. aeruginosa luminescence, however we were also able to observe inhibition of P. aeruginosa when 0.1% AA was used, which was not the case in vitro. Both concentrations of acetic acid were used in vivo to highlight the fact that if the concentration of acetic acid is too low (i.e. 0.1%), fraction of bacteria will remain viable and potentially the infection can develop further.
The combination of PEF and acetic acid 1% treatment was the most successful resulting in almost full eradication (0.01% survival compared to control) of the bacteria in the contaminated area. However, the 0.1% AA + PEF treatment showed no statistically significant difference if compared to PEF only procedure. In all cases when PEF was used the muscle contractions were minimal due to high frequency and short duration of the burst.
Discussion
In this work, we have demonstrated that the application of nanosecond range PEF allows countering multiple limitations of micro-millisecond protocols and enables use of electroporation for biocontrol of surface infections. We have also used acetic acid (0.1, 1%), which showed a significant additive effect with PEF methodology.
Bacteria cell wall serves as an ultimate barrier against the environment, while application of PEF triggers permeabilization of plasma membrane and disruption of the cell wall integrity27,34. Therefore, possible mechanism of the effect presumably lies within the physical damage that is caused by PEF. The increased efficacy of the treatment with acetic acid can be attributed to the internal pH shock and high permeabilization of bacteria, which results in uptake of acid21,42. Also, generation of reactive oxygen species (ROS) is frequently associated with antimicrobial efficiency43. In mammalian cells, it is known that nanosecond range PEF itself can generate both extracellular (electrochemical) and intracellular ROS44 mediating lipid peroxidation. The oxidized bilayers are leaky and prone to spontaneous pore formation, which enables enhanced passage of ions and molecules across the cell membrane45,46. ROS can be also harmful for cell metabolism inducing oxidative stress, starting processes of apoptosis and mutating DNA and RNA47. All of these factors can contribute to the increased inhibition efficacy and PEF damage. However, taking into account that without electroporation it is the acetic acid molecule itself that kills bacteria (external pH is not that significant for cell survival)20, currently it is not possible to define the exact additive mechanism of AA with PEF against bacteria.
We have improved the currently available PEF wound sterilization concept38 significantly. The short duration of the pulses (sub-microsecond) and high frequency (kHz range) of the burst allowed reducing the muscle contractions to barely detectable level. The biggest concern, was the damage of healthy tissues, which may be occurring due extremely high PEF amplitudes. Nevertheless, the solution to use “tweezer” type electrodes with liquid skin/electrode interface ensured a predominantly topical procedure. After the treatment, we were able to observe PEF induced damage of epidermis, however the skin fully regenerated in less than one week. The treatment itself is fast (less than a second), while it is limited by technological platform that is available. Our facilities allowed to apply the burst to an effective area of 5–6 mm2, therefore coverage of 2 × 2 cm2 was a matter of several minutes. It is possible to improve the parameters by developing new electroporation systems and electrodes, which cover higher effective area.
Our proposed methodology should be also applicable against pathogenic yeast, fungi and gram-positive bacteria since they are more susceptible to electroporation47–50. The structure of gram-negative bacteria involves a two-layer cell wall and an outer membrane, which constitutes a higher challenge for successful permeabilization using PEF compared to other biological objects, which are associated with wound infections51,52. It implies, that the PEF amplitude and/or number and duration of the pulses can be reduced if used on other biological objects. Lastly, electroporation is a physical method, which is non-toxic and non-dependent on drug resistance, thus it makes the wound sterilization procedure universal and applicable without diagnostics of the pathogen causing the infection. It is particularly useful during intensive-care events when time and successful management of the wound are vital4,5.
Future works should involve optimization of the treatment parameters and determination of the optimal AA concentration and PEF parameters to ensure painless, fast and efficient treatment.
Material and Methods
Pulsed power setups
The 0–3 kV, 100 ns–1 ms square wave high voltage pulse generator was used for electroporation53. The setup generated pulsed electric field (0–25 kV/cm) using two applicators: (1) a commercially available 1 mm gap electroporation cuvette (Biorad, Hercules, USA) for in vitro experiments and (2) the “tweezer” type steel electrodes with 1.2 mm gap specifically developed for in vivo experiments (Refer to Fig. 1).
High frequency (15 kHz) 15–25 kV/cm 300–900 ns pulsing bursts were used. We have selected durations, which are short enough to minimize muscle contractions, however above the threshold when polarization time is insufficient to cause electroporation at selected PEF intensities. For in vitro experiments, the number of pulses was limited to 500, while a sequence of 1000 pulses was applied in vivo. Conventional 100 μs × 8 pulsing protocols (0–25 kV/cm) were used as a reference in vitro.
The waveform of the applied nanosecond pulses is shown in Fig. 6. The transient processes did not exceed more than 10% of the pulse amplitude, therefore the influence of transient processes was considered as negligible.
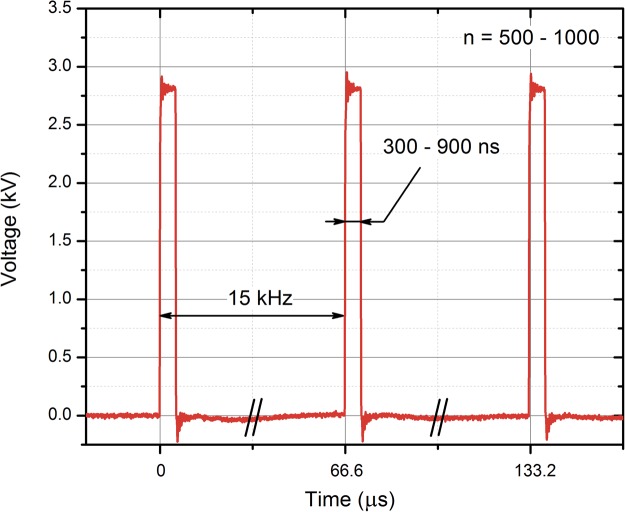
Figure 6.
The waveform of the applied electric pulses. The pulses have been measured using a DPO4034 oscilloscope (Tektronix, Beaverton, OR, United States), post-processed in OriginPro Software (OriginLab, Northampton, MA, United States); n – number of pulses.
Bacterial strain
P. aeruginosa ATCC27853 bacterial strain was transformed with plasmid pAKlux2 (a kind gift from Attila Karsi, Addgene plasmid #14080)54. This plasmid contains the luxCDABE operon made from luciferase (luxAB) and fatty acid reductase (lux CDE) genes. Therefore the live (but not dead) bacteria are bioluminescent. P. aeruginosa cells were grown overnight in liquid LB (Luria-Bertani) medium (10 g/l tryptone, 5 g/l yeast extract, 5 g/l NaCl) in the rotary shaker at 37 °C. 1 ml of the OD = 1 (600 nm; 1.5 × 109 cells) cell culture was transferred to 10 ml of the fresh LB medium and grown for 4 hours at the same conditions. Later the cells were washed 3 times with 1 M sorbitol and re-suspended in 1 M sorbitol at final concentration of 109.
Permeabilization assay
For analysis of cell permeabilization the propidium iodide (PI) (ThermoFisher Scientific Inc., USA) fluorescence dye was used. Just before electroporation, 63 μl of cell suspension were mixed with 7 μl of 300 μM PI to obtain final dye concentration of 30 μM. For PEF permeabilization, the 60 μl of the resultant suspension was transferred to 1 mm gap electroporation cuvette (Biorad, Hercules, USA). After electroporation, the cells were instantly transferred to 1.5 mL tubes (Eppendorf, Hamburg, Germany) and incubated for additional 10 min at room temperature followed by flow cytometric analysis (Amnis, Seattle, USA). The fluorescent cells (PI permeable) were gated as permeabilized in accordance with standard gating strategy used in electroporation studies55–57. After electroporation, a shift of spectra has been observed and the cells in the gate (which was defined based on the untreated control) have been interpreted as fluorescent positive (permeabilized), while the cells outside the gate as fluorescent negative (non-permeabilized). The number of the PI fluorescent cells in the untreated control did not exceed 10%. The darkfield (side scatter), brightfield and fluorescence images of each cell (total of 10000 cells for each unique set of parameters) were taken. At least three independent repetitions were performed. The examples of acquired fluorescence images are shown in Fig. 7.
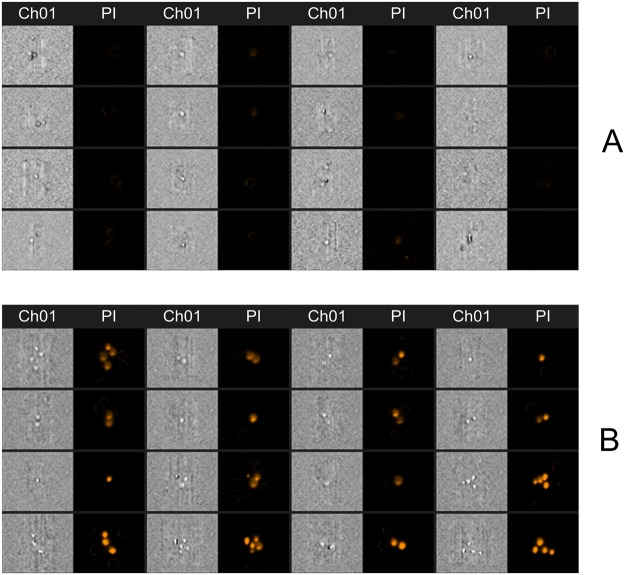
Figure 7.
The example of fluorescence photographs of propidium iodide negative (A) and propidium iodide positive (B) cells. For better perception and indication of permeabilization multiple cells are shown, however for quantitative analysis of cell permeabilization only single cells were used. Due to electroporation (B) the propidium iodide can successfully enter the cells, which is not the case in the untreated control (A). Ch01 – brightfield image; PI – fluorescence image (488 nm), taken using bandpass filter of 610–630 nm.
Only single cells were used in analysis. The permeabilization rates based on visual confirmation of PI electrotransfer in each sample were in agreement with percentages acquired after the definition of the gates.
In vivo model
In this study BALB/c mice were used. For experiments, the flanks of the animals were shaved, depilated using 8% Na2S × H2O and then rinsed with water. Subsequently, suspension of P. aeruginosa in PBS (OD = 8 at 600 nm), was inoculated onto the surface of skin using cotton swab. One hour later the mice were anesthetized by intraperitoneally injection of ketamine (80 mg/kg) and xylazine (10 mg/kg). After anesthesia was established, the mice were imaged with IVIS Spectrum (Caliper Life Sciences) using Living Image Software to confirm bacterial contamination. The animals with bacterial contamination were treated with 25 kV/cm 900 ns pulses (a total of 1000 pulses) at repetition frequency of 15 kHz. 150 µl of distilled water, 0.1% or 1% acetic acid was used as a skin-electrode interface. The “tweezer” type electrodes were applied (gap 1.2 mm) and a total of 70 ± 5 pulsing sequences were delivered to each mouse by accurately repositioning the electrodes to cover the whole treatment area. As a result, the treatment took less than 2–3 min and a total of 70000 ± 5000 pulses were delivered. The deviation in the pulsing number was influenced by the human factor since it was not always possible to accurately reposition the electrodes, therefore some overlapping occurred and more electrode repositions were required to cover the area. After the treatment mice were again analyzed with IVIS Spectrum.
The protocol was approved by the State Food and Veterinary Service (license Nr. G2–48) and the study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals. All applicable international, national and/or institutional ethical guidelines were followed.
Spectrophotometry
The luminescence of bacteria was evaluated using a Synergy 2 microplate reader and Gen5TM software (BioTek, USA). After electroporation the samples (120 μl) were distributed in to the wells of white 96-well flatbotom plate corresponding to different set of parameters. The luminescent signal was measured kinetically for a period of 10 h with 2.5 min intervals. The luminescence read time was 2 seconds. Beforehand, the changes in RLU (relative luminescence units) were compared to conventional clonogenic assay13 using several random data points and it was confirmed that bioluminescence assay is accurate to represent viability, i.e. CFU (colony forming units). The values of RLU were in good agreement with CFU with maximum deviation in the range of 10–15%.
Statistical analysis
One-way analysis of variance (ANOVA; P < 0.05) was used to compare different in vitro treatments. Tukey HSD multiple comparison test for evaluation of the difference was used when ANOVA indicated a statistically significant result (P < 0.05 was considered statistically significant). All experiments have been performed at least in triplicate and the treatment efficiency was expressed as mean ± standard deviation. For in vivo data, the Mann Whitney test was used. All data were post-processed in OriginPro software (OriginLab, Northhampton, MA, USA).
Acknowledgements
This work was supported by the Research Council of Lithuania Towards the Future Technologies Programme project No. LAT-02/2016. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Authors declare no conflict of interest.
Author Contributions
V.N., A.Z., I.G. conceived the experiments and methodology. V.N. and J.N. developed the experimental setup and applicators. I.G. supervised in vivo work, V.N., A.Z., I.G., E.P., R.C. have conducted in vivo experiments. V.N., A.P., J.S., S.M., E.L. have conducted microbiological experiments, flow cytometry and spectrophotometry. V.N., A.Z., I.G., J.N. interpreted the results. V.N., A.Z., I.G. have wrote the manuscript. All authors reviewed the manuscript and provided valuable comments on the work.
Data Availability
Derived data supporting the findings of this study are available from the corresponding author V.N. on request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Willyard C. Drug-resistant bacteria ranked. Nature. 2017;543:15. doi: 10.1038/nature.2017.21550. [DOI] [PubMed] [Google Scholar]
- 2.Fraimow H, Nahra R. Resistant gram-negative infections. Critical Care Clinics. 2013;29:895–921. doi: 10.1016/j.ccc.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Kaye KS, Pogue JM. Infections Caused by Resistant Gram-Negative Bacteria: Epidemiology and Management. Pharmacotherapy. 2015;35:949–962. doi: 10.1002/phar.1636. [DOI] [PubMed] [Google Scholar]
- 4.Azzopardi Ernest A., Azzopardi Elayne, Camilleri Liberato, Villapalos Jorge, Boyce Dean E., Dziewulski Peter, Dickson William A., Whitaker Iain S. Gram Negative Wound Infection in Hospitalised Adult Burn Patients-Systematic Review and Metanalysis- PLoS ONE. 2014;9(4):e95042. doi: 10.1371/journal.pone.0095042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner Keith H., Everett Jake, Trivedi Urvish, Rumbaugh Kendra P., Whiteley Marvin. Requirements for Pseudomonas aeruginosa Acute Burn and Chronic Surgical Wound Infection. PLoS Genetics. 2014;10(7):e1004518. doi: 10.1371/journal.pgen.1004518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tong Q-J, et al. A systematic review and meta-analysis on the use of prophylactic topical antibiotics for the prevention of uncomplicated wound infections. Infect. Drug Resist. 2018;11:417–425. doi: 10.2147/IDR.S151293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fair Richard J., Tor Yitzhak. Antibiotics and Bacterial Resistance in the 21st Century. Perspectives in Medicinal Chemistry. 2014;6:PMC.S14459. doi: 10.4137/PMC.S14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cefalu JE, Barrier KM, Davis AH. Wound Infections in Critical Care. Critical Care Nursing Clinics of North America. 2017;29:81–96. doi: 10.1016/j.cnc.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Everett J, et al. Arginine Is a Critical Substrate for the Pathogenesis of Pseudomonas aeruginosa in Burn Wound Infections. MBio. 2017;8:e02160–16. doi: 10.1128/mBio.02160-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasithevar M, Periakaruppan P, Muthupandian S, Mohan M. Antibacterial efficacy of silver nanoparticles against multi-drug resistant clinical isolates from post-surgical wound infections. Microb. Pathog. 2017;107:327–334. doi: 10.1016/j.micpath.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Bassetti M, et al. Preventive and therapeutic strategies in critically ill patients with highly resistant bacteria. Intensive Care Medicine. 2015;41:776–795. doi: 10.1007/s00134-015-3719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campion A, et al. Use of enhanced nisin derivatives in combination with food-grade oils or citric acid to control Cronobacter sakazakii and Escherichia coliO157:H7. Food Microbiol. 2017;65:254–263. doi: 10.1016/j.fm.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 13.Novickij, V. et al. Overcoming antimicrobial resistance in bacteria using bioactive magnetic nanoparticles and pulsed electromagnetic fields. Front. Microbiol. 8 (2018). [DOI] [PMC free article] [PubMed]
- 14.Dong ZY, et al. Antibacterial activity of silver nanoparticles against Staphylococcus warneri synthesized using endophytic bacteria by photo-irradiation. Front. Microbiol. 2017;8:1–8. doi: 10.3389/fmicb.2017.01090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gharsallaoui A, Oulahal N, Joly C, Degraeve P. Nisin as a Food Preservative: Part 1: Physicochemical Properties, Antimicrobial Activity, and Main Uses. Crit. Rev. Food Sci. Nutr. 2016;56:1262–1274. doi: 10.1080/10408398.2013.763765. [DOI] [PubMed] [Google Scholar]
- 16.Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: Clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clinical Microbiology Reviews. 2009;22:582–610. doi: 10.1128/CMR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar, P. et al. Molecular Insights into Antimicrobial Resistance Traits of Multidrug Resistant Enteric Pathogens isolated from India. Sci. Rep. 7 (2017). [DOI] [PMC free article] [PubMed]
- 18.Kumara DU, et al. Evaluation of bactericidal effect of three antiseptics on bacteria isolated from wounds. J Wound Care. 2015;24:5–10. doi: 10.12968/jowc.2015.24.1.5. [DOI] [PubMed] [Google Scholar]
- 19.Halstead Fenella D., Rauf Maryam, Moiemen Naiem S., Bamford Amy, Wearn Christopher M., Fraise Adam P., Lund Peter A., Oppenheim Beryl A., Webber Mark A. The Antibacterial Activity of Acetic Acid against Biofilm-Producing Pathogens of Relevance to Burns Patients. PLOS ONE. 2015;10(9):e0136190. doi: 10.1371/journal.pone.0136190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjarnsholt T, et al. Antibiofilm Properties of Acetic Acid. Adv. Wound Care. 2015;4:363–372. doi: 10.1089/wound.2014.0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madhusudhan VL. Efficacy of 1% acetic acid in the treatment of chronic wounds infected withPseudomonas aeruginosa: prospective randomised controlled clinical trial. International Wound Journal. 2015;13(6):1129–1136. doi: 10.1111/iwj.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagoba BS, Selkar SP, Wadher BJ, Gandhi RC. Acetic acid treatment of pseudomonal wound infections - A review. J. Infect. Public Health. 2013;6:410–415. doi: 10.1016/j.jiph.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Jeong HS, et al. Negative pressure wound therapy of chronically infected wounds using 1% acetic acid irrigation. Arch. Plast. Surg. 2015;42:59–67. doi: 10.5999/aps.2015.42.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsong TYY. Electroporation of cell membranes. Biophys. J. 1991;60:297–306. doi: 10.1016/S0006-3495(91)82054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gianulis EC, et al. Electroporation of mammalian cells by nanosecond electric field oscillations and its inhibition by the electric field reversal. Sci. Rep. 2015;5:13818. doi: 10.1038/srep13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahnič-Kalamiza S, Vorobiev E, Miklavčič D. Electroporation in Food Processing and Biorefinery. J. Membr. Biol. 2014;247:1279–1304. doi: 10.1007/s00232-014-9737-x. [DOI] [PubMed] [Google Scholar]
- 27.Haberl Meglic S, Marolt T, Miklavcic D. Protein Extraction by Means of Electroporation from E. coli with Preserved Viability. J. Membr. Biol. 2015;248:893–901. doi: 10.1007/s00232-015-9824-7. [DOI] [PubMed] [Google Scholar]
- 28.Yildirim S, Thompson MG, Jacobs AC, Zurawski DV, Kirkup BC. Evaluation of Parameters for High Efficiency Transformation of Acinetobacter baumannii. Sci. Rep. 2016;6:22110. doi: 10.1038/srep22110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haberl-Meglič S, Levičnik E, Luengo E, Raso J, Miklavčič D. The effect of temperature and bacterial growth phase on protein extraction by means of electroporation. Bioelectrochemistry. 2016;112:77–82. doi: 10.1016/j.bioelechem.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Golberg A, Bruinsma BG, Jaramillo M, Yarmush ML, Uygun BE. Rat liver regeneration following ablation with irreversible electroporation. Peer J. 2016;4:e1571. doi: 10.7717/peerj.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miklavcic D, Davalos RV. Electrochemotherapy (ECT) and irreversible electroporation (IRE) -advanced techniques for treating deep-seated tumors based on electroporation. Biomed. Eng. Online. 2015;14:I1. doi: 10.1186/1475-925X-14-S3-I1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langus J, Kranjc M, Kos B, Šuštar T, Miklavčič D. Dynamic finite-element model for efficient modelling of electric currents in electroporated tissue. Sci. Rep. 2016;6:26409. doi: 10.1038/srep26409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pucihar G, Krmelj J, Reberšek M, Napotnik TB, Miklavčič D. Equivalent pulse parameters for electroporation. IEEE Trans. Biomed. Eng. 2011;58:3279–3288. doi: 10.1109/TBME.2011.2167232. [DOI] [PubMed] [Google Scholar]
- 34.Pillet F, Formosa-Dague C, Baaziz H, Dague E, Rols M-P. Cell wall as a target for bacteria inactivation by pulsed electric fields. Sci. Rep. 2016;6:19778. doi: 10.1038/srep19778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novickij Vitalij, Lastauskienė Eglė, Švedienė Jurgita, Grainys Audrius, Staigvila Gediminas, Paškevičius Algimantas, Girkontaitė Irutė, Zinkevičienė Auksė, Markovskaja Svetlana, Novickij Jurij. Membrane Permeabilization of Pathogenic Yeast in Alternating Sub-microsecond Electromagnetic Fields in Combination with Conventional Electroporation. The Journal of Membrane Biology. 2017;251(2):189–195. doi: 10.1007/s00232-017-9951-4. [DOI] [PubMed] [Google Scholar]
- 36.Novickij Vitalij, Grainys Audrius, Švedienė Jurgita, Paškevičius Algimantas, Novickij Jurij. Controlled inactivation ofTrichophyton rubrumusing shaped electrical pulse bursts: Parametric analysis. Biotechnology Progress. 2016;32(4):1056–1060. doi: 10.1002/btpr.2276. [DOI] [PubMed] [Google Scholar]
- 37.Baah-Dwomoh A, Rolong A, Gatenholm P, Davalos RV. The feasibility of using irreversible electroporation to introduce pores in bacterial cellulose scaffolds for tissue engineering. Appl. Microbiol. Biotechnol. 2015;99:4785–4794. doi: 10.1007/s00253-015-6445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golberg A, et al. Pulsed electric fields for burn wound disinfection in a murine model. J. Burn Care Res. 2016;36:7–13. doi: 10.1097/BCR.0000000000000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rice, T. C., Seitz, A. P., Edwards, M. J., Gulbins, E. & Caldwell, C. C. Frontline Science: Sphingosine rescues burn-injured mice from pulmonary Pseudomonas aeruginosa infection. 100, 1233–1237 (2016). [DOI] [PMC free article] [PubMed]
- 40.Rybtke M, Hultqvist LD, Givskov M, Tolker-Nielsen T. Pseudomonas aeruginosa Biofilm Infections: Community Structure, Antimicrobial Tolerance and Immune Response. J. Mol. Biol. 2015;427:3628–3645. doi: 10.1016/j.jmb.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 41.Rems L, et al. Cell electrofusion using nanosecond electric pulses. Sci. Rep. 2013;3:3382. doi: 10.1038/srep03382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bitsch M, et al. Standardised method of surgical treatment of chronic leg ulcers. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2005;39:162–9. doi: 10.1080/02844310510006196. [DOI] [PubMed] [Google Scholar]
- 43.Gurunathan S, Han JW, Kwon DN, Kim JH. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res. Lett. 2014;9:1–17. doi: 10.1186/1556-276X-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pakhomova ON, et al. Oxidative effects of nanosecond pulsed electric field exposure in cells and cell-free media. Arch. Biochem. Biophys. 2012;527:55–64. doi: 10.1016/j.abb.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rems, L. & Miklavčič, D. Tutorial: Electroporation of cells in complex materials and tissue. J. Appl. Phys. 119 (2016).
- 46.Boonnoy P, Jarerattanachat V, Karttunen M, Wong-Ekkabut J. Bilayer Deformation, Pores, and Micellation Induced by Oxidized Lipids. J. Phys. Chem. Lett. 2015;6:4884–4888. doi: 10.1021/acs.jpclett.5b02405. [DOI] [PubMed] [Google Scholar]
- 47.Caprettini, V. et al. Soft electroporation for delivering molecules into tightly adherent mammalian cells through 3D hollow nanoelectrodes. Sci. Rep. 7 (2017). [DOI] [PMC free article] [PubMed]
- 48.Novickij, V. et al. Pulsed electric field-assisted sensitization of multidrug-resistant Candida albicans to antifungal drugs. Future Microbiol (2018). [DOI] [PubMed]
- 49.Ou QX, Nikolic-Jaric M, Gänzle M. Mechanisms of inactivation of Candida humilis and Saccharomyces cerevisiae by pulsed electric fields. Bioelectrochemistry. 2017;115:47–55. doi: 10.1016/j.bioelechem.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Li, D., Tang, Y., Lin, J. & Cai, W. Methods for genetic transformation of filamentous fungi. Microbial Cell Factories16 (2017). [DOI] [PMC free article] [PubMed]
- 51.James GA, et al. Biofilms in chronic wounds. Wound Repair Regen. 2008;16:37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 52.Howell-Jones RS, et al. A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. Journal of Antimicrobial Chemotherapy. 2005;55:143–149. doi: 10.1093/jac/dkh513. [DOI] [PubMed] [Google Scholar]
- 53.Novickij Vitalij, Grainys Audrius, Butkus Paulius, Tolvaišienė Sonata, Švedienė Jurgita, Paškevičius Algimantas, Novickij Jurij. High-frequency submicrosecond electroporator. Biotechnology & Biotechnological Equipment. 2016;30(3):607–613. doi: 10.1080/13102818.2016.1150792. [DOI] [Google Scholar]
- 54.Karsi A, Lawrence ML. Broad host range fluorescence and bioluminescence expression vectors for Gram-negative bacteria. Plasmid. 2007;57:286–295. doi: 10.1016/j.plasmid.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 55.Michie J, Janssens D, Cilliers J, Smit BJ, Böhm L. Assessment of electroporation by flow cytometry. Cytometry. 2000;41:96–101. doi: 10.1002/1097-0320(20001001)41:2<96::AID-CYTO3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 56.Novickij V, et al. Measurement of Transient Permeability of Sp2/0 MyelomaCells: Flow Cytometric Study. Meas. Sci. Rev. 2016;16:300–304. doi: 10.1515/msr-2016-0038. [DOI] [Google Scholar]
- 57.Novickij V, Ruzgys P, Grainys A, Šatkauskas S. High frequency electroporation efficiency is under control of membrane capacitive charging and voltage potential relaxation. Bioelectrochemistry. 2018;119:92–97. doi: 10.1016/j.bioelechem.2017.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Derived data supporting the findings of this study are available from the corresponding author V.N. on request.