Abstract
Two-pore channels (TPCs) are Ca2+-permeable endo-lysosomal ion channels subject to multi-modal regulation. They mediate their physiological effects through releasing Ca2+ from acidic organelles in response to cues such as the second messenger, NAADP. Here, we review emerging evidence linking TPCs to disease. We discuss how perturbing both local and global Ca2+ changes mediated by TPCs through chemical and/or molecular manipulations can induce or reverse disease phenotypes. We cover evidence from models of Parkinson's disease, non-alcoholic fatty liver disease, Ebola infection, cancer, cardiac dysfunction and diabetes. A need for more drugs targeting TPCs is identified.
Keywords: TPCN1, TPCN2, NAADP, Ca2+, Lysosomes
1. Introduction
Two-pore channels (TPCs) are ancient members of the voltage-gated ion channel superfamily [1]. Structurally, they are dimers with each subunit harbouring two similar ion channel domains connected by a cytoplasmic linker and each possessing six transmembrane helicies, [[2], [3], [4], [5]]. This duplicated domain architecture identifies TPCs as likely intermediates in the evolutionary transition from tetrameric one-domain channels (such as TRP channels) and monomeric four domain channels (such as voltage-gated Ca2+ channels) [6]. TPCs are unusual in that they reside on acidic organelles such as lysosomes [7,8] or the equivalent vacuoles in plants [9]. This is achieved through specific targeting motifs in their N-termini [10,11].
TPCs are thought to be non-selective cation channels permeable to Ca2+ [1]. They were originally functionally characterised as the long sought targets for the Ca2+ mobilizing messenger NAADP [7,8,12,13]. This second messenger, discovered in sea urchin eggs [14], has an established role in releasing Ca2+ from so called acidic Ca2+ stores [15,16]. NAADP regulates numerous functions from fertilisation in echinoderms [17,18] through to neuronal tasks such as neuronal differentiation [19] and neurotransmitter signalling [20] in mammalian cells. Subsequent studies have revealed regulation of TPCs by the endo-lysosomal lipid phosphatidylinositol 3,5 bisphosphate (PI(3,5)P2) [21,22]. However, not all studies concur, with several reports failing to demonstrate Ca2+ permeability and NAADP sensitivity of TPCs [21,23]. This may reflect the challenges associated with recording ion channels that do not reside on the cell surface and/or potential loss of accessory factors required for NAADP sensitivity [[24], [25], [26], [27]]. Certainly in intact cells, measurements of cytosolic Ca2+, albeit less direct than electrophysiology, affirm the role of TPCs in NAADP-mediated Ca2+ signalling [28,29].
The luminal concentration of Ca2+ in lysosomes is on par with that of the better characterised ER Ca2+ stores; ~500 μM [30]. However, lysosomes occupy a much smaller volume of the cell compared to the ER. This feature likely renders lysosomes better suited for generating localized Ca2+ signals. During signalling, local Ca2+ signals evoked by NAADP are thought to be amplified by Ca2+ release channels on the ER resulting in global Ca2+ signals that pervade the cell [31,32]. Such chatter during ‘evoked mode’ is probably underpinned by the process of Ca2+-induced Ca2+ release and facilitated by membrane contact sites between the organelles [33,34]. But growing evidence indicates that local Ca2+ signals act in their own – in ‘constitutive mode’ - to regulate cellular processes. Key here is the TPC interactome which reveals association with a number of proteins involved in membrane fusion, trafficking and organisation [26,35]. Such associations likely underpin roles for TPCs in regulating autophagy [36], endo-lysosomal morphology [26,37], retrograde transport from endosomes to the Golgi [38] and membrane contact site formation between late endosomes and the ER [39].
The pharmacology of TPCs is in its infancy. Indirect blockers include Ned-19 which is an NAADP antagonist discovered through ligand-based virtual screening [40]. Early work prior to the emergence of TPCs as NAADP targets, identified several L-type voltage-gated Ca2+ channel modulators as inhibitors of NAADP-mediated Ca2+ release [41]. These blockers did not affect NAADP binding suggesting a remote site of action likely on the target channel [42]. Consistent with this, recent docking analyses have identified a possible binding site within the TPC pore for L-type Ca2+ channel blockers [6]. This site is also predicted to accommodate local anaesthetics (Na+ channel blockers) which like Ca2+ channel blockers demonstrably inhibit endogenous NAADP-evoked Ca2+ signals and Ca2+ signals evoked by recombinant TPCs [6]. TPCs thus possess a ‘loose’ pharmacology relative to Ca2+ and Na+ channels. This suggests that core structural determinants underlying drug block were probably in place in a primordial two-domain channel early in the evolution of the voltage-gate ion channel superfamily. This is prior to the duplication events that led to extant four domain Ca2+ and Na+ channels and their subsequent divergence allowing them to distinguish Ca2+ and Na+ channel blockers [6].
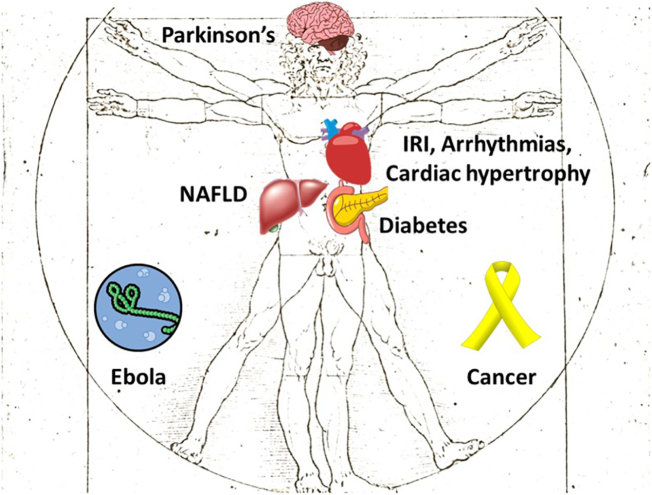
In this contribution, we review how chemical and molecular strategies are converging to uncover roles for TPCs in disease. These diseases affect a number of organs such as the brain, liver, heart and the pancreas. They include cancer and Ebola infection and possibly others (Fig. 1).
Fig. 1.
TPCs and disease. Schematic summarizing diseases associated with TPC dysfunction.
2. Parkinson's disease
Parkinson's disease is a common and disabling neurodegenerative disorder, characterised by a progressive decline in the control of voluntary movement. The neuropathological hallmark of Parkinson's is the selective death of dopaminergic neurons in the substantia nigra pars compacta and, in the surviving neurons, an accumulation of α-synuclein aggregates [43]. For many, the cause of the disease is unknown, however in around 10–15% of cases, it is associated with genetic mutations. TPCs have been implicated in Parkinson's mediated by mutation in the LRRK2 gene which represents the most common cause of familial disease.
LRRK2 encodes a large multi-domain enzyme and the prevalent G2019S mutation in Parkinson's affects its kinase domain enhancing catalytic activity [44]. It is unclear how this gain-of-function disrupts cellular function, but the endo-lysosomal system seems to be involved [45]. Indeed, several studies have shown that LRRK2 interacts with proteins on the endo-lysosomal compartment including TPCs [46] and several members of the Rab family [47] with which TPCs interact [26].
Hilfiker and colleagues identified autophagic defects in cells overexpressing wild-type and mutant (G2019S) LRRK2 [46]. These autophagic defects could be mimicked by treating cells with cell permeable NAADP and reversed through Ca2+ chelation, NAADP antagonism (with Ned-19) or by overexpressing a non-conducting TPC2 pore mutant [10,48]. This suggests TPC2 activity is enhanced by LRRK2. Whether LRRK2 directly phosphorylates TPCs is not known. Coupled with a recent study demonstrating that LRRK2 also associates with the voltage-gated Ca2+ channel CaV 2.1 and increases channel activity [49], hints that LRRK2 might have a common, hyper-activating action on Ca2+ channels.
LRRK2/TPC action was also studied in fibroblasts derived from Parkinson's patients with the G2019S LRRK2 mutation [37]. Using various markers, compartments of the endo-lysosomal system were demonstrably enlarged and clustered in LRRK2-Parkinson's fibroblasts relative to age matched controls. This was confirmed by other studies in neuronal cultures overexpressing mutant LRRK2 [50]. Morphology defects could be reversed by pharmacological inhibition of TPC regulators (NAADP, PI(3,5)P2 and the trafficking GTPase, Rab7) and molecular silencing of TPC2 (but not TPC1) [37]. Additionally, Ca2+ signals evoked by NAADP were enhanced in LRRK2- in Parkinson's. Thus, these results further support the hypothesis that pathological LRRK2 enhances TPC2 activity to cause dysfunction.
Although patients carrying mutations in LRRK2 present with a phenotype clinically and pathologically indistinguishable to idiopathic Parkinson's, it is possible that LRRK2 studies might not apply to the whole Parkinson's population. Interestingly, a recent transcriptomic meta-analysis of blood samples from sporadic Parkinson's patients found that TPCN2 was one of the top 20 genes with altered expression [51]. Additionally, endo-lysosomal morphology was disrupted and total lysosomal Ca2+ levels were reduced in fibroblasts from in Parkinson's patients with a mutation in GBA1 [52]. This gene encodes a lysosomal hydrolase and underlies the lysosomal storage disorder, Gaucher's disease. Mutations are also associated with an up to 20-fold increased risk of developing Parkinson's [53]. It is possible that lysosomal Ca2+ levels were depleted in GBA1-Parkinson's due to excessive TPC2 activity, however this has yet to be established. Intriguingly, a recent study demonstrated that Parkinson's is associated with multiple genes underlying other lysosomal storage diseases whereby almost half of the cohort analysed had 1 or more putative damaging mutations [54]. That lysosomal Ca2+, including NAADP-evoked signals [55], is disrupted in a quintessential lysosomal storage disorder (Niemann-Pick Disease Type C) identifies much scope for investigating TPC function in in Parkinson's.
In summary, pathogenic LRRK2 increases TPC2 functionality likely through a direct interaction, to disrupt autophagy and endo-lysosomal morphology. Furthermore, it is possible that TPC2 dysfunction features in other forms of Parkinson's.
3. Non-alcoholic fatty liver disease
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic disorder of the liver characterised by fat accumulation and fibrosis. NAADP is detectable in hepatocytes [56], liver homogenates bind NAADP [7] and expression of both TPC1 and TPC2 protein has been validated in the liver using knock-out samples [35,57]. Grimm et al. analysed TPC2 knock-out mice and reported several phenotypes consistent with NAFLD [35].
In mice fed with a Western style high cholesterol diet, the livers from the transgenic animals were discoloured and weighed more than those from wild type mice [35]. Liver cholesterol levels were also increased upon TPC2 knockout. This was associated with prevalent lipid droplets and fibrosis in the liver. The presence of gall stones was also noted in the knockout animals. Additionally, circulating levels of cholesterol and liver enzymes were increased, all pointing to cholesterol overload and liver damage. Consistent with this, synthetic enzymes for cholesterol ester synthesis were transcriptionally upregulated whereas those for cholesterol synthesis were down regulated. Taken together, these data point to a major role for TPC2 in cholesterol handling [35].
Mechanistic studies in mouse embryonic fibroblasts suggest that TPC2 knockout disturbs trafficking of LDL receptors [35]. LDL receptors mediate uptake of cholesterol through endocytosis, initiating delivery of cholesterol to the lysosome and beyond through both vesicular and non-vesicular means [58]. In TPC2 null cells, LDL appeared to accumulate in punctate structures [35]. Similar accumulation was seen with EGF which like LDL is internalised via receptor-mediated endocytosis and delivered to the lysosome [59]. Pulse chase experiments with EGF suggest a slowing of degradation [35]. However, neither the pH nor the proteolytic activity of lysosomes was altered pointing to compromised transport through the endocytic system. Indeed, EGF accumulated in Rab7 (late endosome/lysosome) positive structures leading the authors to speculate that a Ca2+-dependent block in fusion between late endosomes and lysosomes was the underlying cause. Consistent with this, the effects of TPC2 knockout on EGF distribution could be pheno-copied by buffering local Ca2+ changes with BAPTA and rescued by a wild-type TPC2 but not a non-conducting pore mutant [10,48]. Whether a similar mechanism is responsible for accumulation of LDL remains to be established but other studies suggest a modest slowing of PDGF receptor degradation upon PDGF stimulation in TPC2 (but not TPC1) knockout cells [60]. Again, this appears to be independent of changes in lysosomal pH [60]. Further work however is required to identify the exact ‘lesion’ within the endo-lysosomal system that TPC2 deletion causes given the accumulation of EGF in LAMP1 (predominantly lysosomal) as well as Rab7-positive structures [35].
In summary, de-regulated trafficking of receptors upon TPC2 knockout probably through disrupted local Ca2+ signalling provides a likely mechanism for liver dysfunction in NAFLD.
4. Ebola virus disease
The Ebola virus causes an often fatal haemorrhagic fever in humans exemplified by the 2014 outbreak in West Africa. Work by Davey and colleagues identified TPCs as potential drug targets for combatting Ebola infection [61].
Many viruses highjack the endocytic system to enter cells [62]. For Ebola, cleavage of the Ebolavirus glycoprotein by cysteine proteases within late endosomes/lysosomes is an essential step triggering binding to NPC1, subsequent fusion and escape of the viral core into the cytoplasm [63]. Sakurai et al. found that knock down or knockout of either TPC1 or TPC2 prevented Ebola infection in vitro [61]. Over-expression of non-conducting TPC2 pore mutant [10,48] also inhibited infection. So too did a number of voltage-gated Ca2+ channel blockers and Ned-19. The former included tetrandrine a plant alkaloid derived from a Chinese herb that is often used in traditional medicine [64]. Tetrandrine was particularly potent with an affinity in the nanomolar range. Importantly, tetrandrine proved efficacious in vivo in a mouse model of Ebola infection [61].
Mechanistically, tetrandrine blocked NAADP- and PI(3,5)P2-stimulated currents through TPC1 and TPC2, and NAADP-evoked Ca2+ signals live cells [61]. It also resulted in accumulation of virus particles in TPC2- and NPC1-positive compartments. This was associated with depletion of virus particles in TPC2-positive but NPC1-negative compartments. This led the authors to speculate that the virus leaves through the TPC2-only compartment because of the correlation between reduced presence of virus in this compartment and reduced infection [61]. However, one could argue that blocking TPCs would result in accumulation of virus in the compartment that it normally exits from i.e. the TPC2/NPC1-positive compartment. Simmons et al. found that viruses appeared to exit exclusively from NPC1-positive compartments and found no evidence for compartments that were TPC2-positive but NPC-negative [65]. One potential caveat in both studies [61,65] is the use of overexpressed TPC2 for assessing colocalization given the effect of TPC2 on perturbing endo-lysosomal morphology [26]. Unexplained at this point, is the mechanistic requirement for TPC1 in Ebola infection given its likely more proximal (endosomal) targeting within the endo-lysosomal system relative to TPC2 (lysosomal) [39,66]. The precise subcellular localisation of endogenous channels however is still unclear due to lack of appropriate antibodies and the ‘fluid’ nature of the endo-lysosomal system whereby different organelles are undergoing constant maturation and fusion. Further work is required to determine exactly how TPCs allow virus escape.
In summary, molecular and chemical targeting of TPCs provides a novel strategy for combatting Ebola uncovering a role for TPCs in viral entry probably through regulation of Ca2+-dependent fusion events.
5. Cancer
Cancer needs little introduction. It is a pervasive problem. Many cellular processes are subverted during carcinogenesis including the process of cell migration (underlying metastasis) and angiogenesis (underlying tumour vascularisation). TPC expression in cancer was originally characterised in SKBR3 (human breast cancer) and PC12 (rat pheochromocytoma) cells where TPC1 transcript levels were reported to be ~3–8 fold higher than TPC2 [8]. TPCs are also expressed in a number of other breast cancer lines [67].
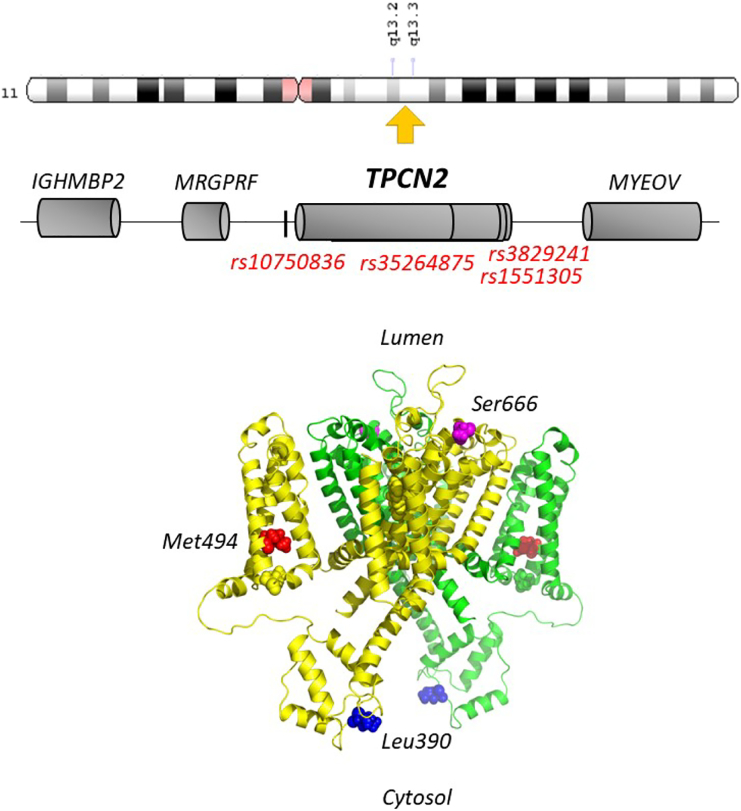
In humans, the genes for TPC1 and TPC2 are found on chromosome 12 (TPCN1, NCBI Gene ID: 53373) and 11 (TPCN2, NCBI Gene ID: 219931), respectively. The gene encoding TPC3, which is surprisingly absent from select mammals including the mouse, is a pseudogene in humans but likely functional in closely related primates [13,68]. The chromosomal region harbouring TPCN2 (11q13.2, Fig. 2) is often amplified in cancer and also contains the cyclin gene which is an established driver of oncogenesis [69]. Interestingly, increased expression of TPC2 and a number of other nearby genes in a proposed ‘cassette’ has been observed in oral squamous cell carcinoma cell lines and primary tumours [70,71]. Amongst the cassette, which may comprise two ‘cores’ [72], is TMEM16A which encodes a Ca2+-activated Cl− channel potentially pointing to this protein as an effector of de-regulated Ca2+ signals in cancer.
Fig. 2.
Structural features of TPC2. Schematic depicting TPCN2 and flanking genes (centre) on chromosome 11 (top). Approximate positions of single nucleotide variants are marked. A structural model of human TPC2 (bottom) highlighting the position of a pigmentation-linked human variant (red), a variant linked to diabetic traits in rat TPC2 (blue) and a proposed PKA phosphorylation site (magenta).
Increased expression of both TPC1 and TPC2 has been noted in several cell lines derived from bladder, blood and liver cancer relative to a malignant breast cancer line [73]. This was particularly striking for TPC1 which was elevated up to ~25-fold. Knockdown of TPC1 or TPC2 and antagonising NAADP action with, Ned-19 or inhibiting channel activity of TPCs with tetrandrine, were all found to inhibit migration of cancer lines. This points to TPCs as potential therapeutic targets in cancer [74] although increased expression of TPC2 is correlated with increased survival in bladder cancer [75]. Mechanistically, these manoeuvres were associated with enlarged lysosomal structures. Enlargement of endo-lysosomal structures by molecular or chemical interference of TPCs/NAADP has been reported in primary cultured fibroblasts although in this case, the effects were specific to TPC1 [39].
Inhibiting TPCs was also associated with reduced adhesion and reduced formation of the leading edge in cancer cell lines [73]. These findings translated to reduced metastatic tumour formation in an in vivo mouse model of metastasis although these experiments used a mammary cell reporter line. In this context, it is worth noting that the role of Ca2+ signalling from acidic organelles in the migration neural crest cells [76]. Neural crest cells are an embryonic population of cells born in the neural plate and which migrate through the embryo during early development [77]. Migration of these cells is often likened to metastasis. Knocking down an identified animal Ca2+-H+ exchanger in Xenopus, which is likely responsible for filling acidic organelles with Ca2+, was found to disrupt neural crest cell migration in vivo [78]. In vitro, this was also associated with reduced adhesion as evidenced by reduced dispersion and reduced focal adhesions. This could be recapitulated in wild type cells by the fast Ca2+ buffer, BAPTA but not the slower buffer, EGTA. These data point to local Ca2+ signals regulating the adhesion machinery [78]. Indeed, identified defects in integrin trafficking in mammalian cancer lines upon TPC knockdown was also ascribed to de-regulated local Ca2+ fluxes [73]. This is similar to the proposed mechanism underlying defective trafficking of LDL/EGF receptors upon TPC2 knockout [35]. Although it should be noted that trafficked cargo in TPC compromised cells accumulated in distinct organelles; early endosomes (integrins) [73] vs lysosomes/late endosomes (EGF receptors) [35].
Global Ca2+ signals deriving from acidic Ca2+ stores might also contribute to cancer. NAADP signalling has previously been implicated in agonist-evoked Ca2+ signalling in endothelial cells in response to cues such acetylcholine [79] and histamine [80]. Coupling of VEGF to NAADP/TPC signalling [81] is relevant in the context of angiogenesis - the process whereby endothelial cells give rise to new blood vessels. Here, endothelial cells breach the basement membrane and undergo migration and proliferation to form new vessel structures. This process is essential not only during development but also during cancer, where vascularisation feeds solid tumours. Work by Favia et al. found that TPCs were required for several aspects of VEGF-induced angiogenesis including migration of endothelial cells and tube formation in vitro [81]. Importantly, in vivo Matrigel assays showed that vascularisation by VEGF was inhibited by Ned-19 and in TPC2 but not in TPC1 knockout mice. TPC2 knockout mice however appeared to develop normally suggesting that compensatory changes maintain angiogenesis during development of these animals. Nevertheless, these studies highlight a role for TPCs in a process crucial for cancer progression.
Melanoma is a cancer that derives from pigmented melanocytes. A link between pigmentation and TPCs has been long appreciated through GWAS which correlated two non-synonymous variants in TPC2 with hair colour [82]. Overexpression of TPC2 (but not TPC1) induces pigmentation defects in Xenopus oocytes in a manner that requires interactivity with members of the Rab family of trafficking proteins [26]. And, a recent study suggested that both pigmentation variants in TPC2 result in a gain of channel function through either conformational changes within the pore (rs35264875, M484L) or reduced inhibition by mTOR (rs3829241, G734E) (Fig. 2) [83]. Collectively, these data link the level of TPC2 activity to pigmentation. But exactly how TPC2 regulates pigmentation is not clear at present. TPC2 localises to pigment bearing melanosomes (notably an acidic Ca2+ store) to potentially regulate melanin production through changes in luminal pH [84,85]. However, despite strong links between pigmentation and cancer there is still only suggestive genetic evidence linking TPC2 variants with melanoma which might be sex-specific [86].
In summary, cancer likely features changes in TPC expression and defects in both local and global TPC-mediated Ca2+ signalling. TPCs seem to be required for cellular processes that underpin cancer cell survival. And thus present themselves as viable therapeutic targets.
6. Cardiac dysfunction
The actions of NAADP in the heart have been long appreciated from early radioligand binding and flux assays using cardiac microsomes [87] through to more recent imaging studies resolving NAADP-evoked Ca2+ signals in individual live cells [88]. TPCs are demonstrably expressed in the heart possibly in a sex-specific manner whereby levels in female hearts were greater than male hearts [89]. TPCs have been implicated in ischemia reperfusion injury, arrhythmias and cardiac hypertrophy.
Interrupting blood supply to the heart causes ischemia and tissue injury. This can occur during myocardial infarction. Reperfusion is essential to restore oxygen and nutrients. But this process in itself exacerbates damage through opening of the mitochondrial transition pore. Ca2+ has long been considered an upstream culprit in ischemia-reperfusion injury but the underlying mechanisms are not well understood.
TPC1 and TPC2 levels are reportedly higher in left ventricular samples from ischemic as well as dilated hearts from patients suffering from heart failure [90]. Davidson et al. found that TPC1 knock-out mice showed reduced cardiac damage in an in vivo model of ischemia-reperfusion injury [91]. This effect was pheno-copied by blocking NAADP action with Ned-K, a novel Ned-19 analogue. In vitro, Ca2+ oscillations, and subsequent cell death of cardiomyocytes upon re-oxygenation following ischemia were inhibited by Ned-K and the lysosomotropic agent, GPN. The principle Ca2+ release channel in cardiomyocytes is the type 2 ryanodine receptor and previous studies have implicated this protein in reperfusion-induced Ca2+ oscillations [92]. This led to a model whereby activation of TPCs likely triggers Ca2+ release from the sarcoplasmic reticulum which is ultimately received by mitochondria resulting in MTP opening [91]. Knockdown of TPCs exacerbated cell death of cardiomyocytes during extended ischemia (no reperfusion) pointing to possible context-specific roles for TPCs in regulating cardiac viability [89].
How TPCs are activated during reperfusion remains to be established. NAADP levels decrease upon ischemia and increase upon reperfusion [91]. However, the absolute levels upon reperfusion are no higher than prior to ischemia. Thus, changes in NAADP levels per se are unlikely to trigger Ca2+ release through TPCs. One possibility is that TPCs become sensitised to NAADP during re-perfusion perhaps in response to oxidative stress, a known determinant of ischemia-reperfusion injury. Indeed, plant TPCs are reportedly responsive to oxidants [93].
Interestingly, extracellular NAADP appears to paradoxically protect hearts against ischemic injury [94,95]. Such effects were reversed by an antagonist of P2Y11 receptors or Ned-19 suggesting NAADP acts on the cell surface or intracellularly upon transport, respectively. Further mechanistic studies are warranted to relate these effects to TPCs.
Adrenaline plays a key role in the pathophysiology of the heart. Low level stimulation of β-adrenergic receptors acutely increases contractility (classically during the ‘fight or flight’ stress response) whereas more intense stimulation induces arrhythmias. Chronic stimulation induces hypertrophy. Accumulating evidence points to NAADP as a second messenger for adrenaline in the heart [[96], [97], [98]].
Work by Guse and colleagues provided evidence that the NAADP antagonist BZ194 [99] could prevent isoprenaline-induced spontaneous diastolic Ca2+ transients in electrically-paced cardiomyocytes and arrhythmias in vivo [88]. The arrhythmic Ca2+ transients were also prevented by bafilomycin indicating a requirement for acidic organelles and thus pointing to TPCs. Terrar and colleagues provided direct evidence for TPC2 in the actions of adrenaline [100]. In their study, isolated hearts from a TPC2 knock-out animals were less susceptible to arrhythmias upon ventricular burst pacing compared to wild type hearts. The hypertrophic response to isoprenaline in vivo was also modestly reduced upon TPC2 knock-out.
Mechanistically, these blunted responses were attributed to reduced action of adrenaline on the amplitude of evoked Ca2+ transients, effects that could be mimicked by Ned-19 and bafilomycin [100]. Bafilomycin however did not affect the amplitude of the Ca2+ transients in previous work [88]. Nevertheless, these analyses suggest that adrenaline action which is traditionally attributed to cyclic AMP-mediated phosphorylation of Ca2+ handling proteins such as voltage-gated Ca2+ channels, and more controversially ryanodine receptors, may be more complex. It is tempting to speculate that cyclic AMP-dependent protein kinase phosphorylates TPCs to augment their action. Indeed, evidence for positive regulation of TPC2 by PKA has recently been provided [101]. However, the proposed phosphosite (S666) appears to be situated at the start of the sixth trans-membrane region in domain II and therefore luminal (Fig. 2). This questions its physiological relevance. Downstream of TPC activation, Ca2+ may enhance Ca2+ release from the SR possibly through promoting Ca2+ uptake through a CamKinase II-dependent mechanism [100].
In sum, TPCs have emerged as common mediators of cardiac dysfunction in several scenarios of heart disease through disrupting global Ca2+ signalling. Chemical targeting of NAADP in vivo has proven beneficial.
7. Diabetes
Diabetes is a metabolic disorder characterised by hyper-glycemia resulting from either deficient secretion of insulin or insulin action. Functional NAADP effects in the insulin secreting beta cells of the endocrine pancreas has been long known [102] where it is considered a second messenger for glucose [103] and possibly insulin [104] and GLP-1 [105]. Several lines of evidence link TPCs to diabetes.
Insulin is a key anabolic hormone that controls blood sugar by promoting glucose uptake into peripheral tissues. It is secreted by beta cells in a Ca2+-dependent manner in response to glucose. The prevailing view is that this is due to inhibition of ATP-sensitive K+ channels, membrane depolarisation and activation of voltage-gated Ca2+ channels. However, it is increasingly appreciated that other mechanisms likely contribute to stimulus-secretion coupling [106]. Indeed, it has long been reported that glucose-induced Ca2+ signals can be inhibited by interfering with NAADP [40,103]. Consistent with this, isolated beta cells from mice lacking TPC1 or TPC2 show reduced glucose-evoked Ca2+ signals [125]. Double knockout mice however appear unperturbed [21] although TPC expression in these animals was not characterised [28].
Importantly, whole pancreata derived from TPC1 knock-out mice secrete less insulin in response to elevated glucose [125]. Moreover, in vivo studies of these animals showed that blood glucose levels were modestly increased in glucose tolerance tests. Reduced insulin secretion and elevated blood glucose are both key diabetic traits. Mice lacking TPC2 also show decreased insulin secretion in response to glucose challenge in vivo [107] although ex vivo studies using whole pancreata were less clear [125]. Glucose levels in TPC2 knockout mice in vivo were paradoxically either slightly reduced [107] or unchanged [125]. Beta-cell specific knockout of TPC2 did not affect glucose-evoked Ca2+ signalling, insulin secretion or glucose tolerance [108]. One possibility to explain these discordant results is that TPC knockout is compensated for in vivo, a perennial concern for transgenic mice studies. Here, a recent study focusing on alpha cells of the endocrine pancreas is potentially relevant. Alpha cells secrete glucagon which has the opposite effect to insulin on blood sugar levels. Knock-out of TPC2 but less so TPC1 was shown to reduce glucagon secretion [109]. Consequently, global TPC2 knock-out at least may mask its role in glucose homeostasis in vivo. Clearly further studies are required to relate loss of TPCs in single cells through organs to whole animal phenotypes particularly for TPC2.
In rats, TPC2 is present in a genomic locus on chromosome 1 that mapped previously to glucose intolerance [110]. Interestingly, it was the only gene in this locus (and 1 of 47 others genome wide) whose expression levels changed in glucose intolerant rats [107]. TPC2 levels negatively correlated with fasting glucose levels. Moreover, several variants in this locus associated with TPC expression levels. These analyses suggest that TPC2 expression may be causally related to glucose perturbances [107]. Interestingly, one of the variants resulted in a non-synonymous coding change in TPC2 itself (P350L). In mouse TPC1, for which the structure was recently resolved, the corresponding residue is leucine and located between the second E and F helicies that form an E-F like hand-like domain in the linker. In plants, this region binds Ca2+ but in mammalian TPCs, lack of Ca2+ coordinating residues likely renders this region Ca2+-insensitive. In human TPC2, the corresponding residue is again leucine like the rat TPC2 variant (Fig. 2). The coding change is predicted to be benign but its exposed nature may affect interactions between TPC2 and regulatory proteins in the cytosol.
A variant in the human TPCN2 gene (rs1551305) has also been implicated in diabetes. Fan et al. identified a variant within an intron toward the 3′ of the gene (Fig. 2) that associates with type 2 diabetes and reduced beta cell function [111]. It would be interesting to examine TPC2 levels in this cohort.
In sum, studies in both rodent models and humans support a role for TPCs in regulating global Ca2+ signals and endocrine pancreatic function. Knockout of TPCs in some but not all models recapitulates diabetic traits. And genetic studies further link TPCs to diabetes possibly through changes in TPC levels.
8. Other disorders
Mutation of presenilins (PS1 and PS2) underlie familial forms of Alzheimer's disease [112]. In cells lacking PS1 and PS2, lysosomal Ca2+ content is reduced [113] and this is associated with altered post-translational modification of TPCs (probably glycosylation) [114]. In PS1-knockout cells, NAADP-evoked Ca2+ release is reduced consistent with reduced store content but Ca2+ release by the TRPML agonist is paradoxically enhanced [115]. Intriguingly, the latter could be reversed by Ned-19 [115]. This warrants further work to better define the relationships between TPCs, TRPMLs and NAADP in Alzheimer's.
Further phenotypic analyses of TPC knockout mice have implicated TPC1 in infertility through a requirement for the acrosome reaction in sperm [116]. The Mendelian ratio of offspring was slightly affected upon TPC1 knockdown pointing to a sub-fertile phenotype. TPC2 has been implicated in skeletal muscle atrophy through proposed regulation of autophagic signalling [117]. This follows on from work demonstrating a requirement for NAADP/TPCs in autophagy [36,46]. But further corroborating evidence is required to causally link skeletal muscle function to autophagy/lysosomal function given the reported increase in lysosomal pH upon TPC2 knockout [117] in light of other studies where pH was unaffected [28,35,117]. Analyses of TPC1 and TPC2 double knockout mice suggests a role for TPCs in mature onset obesity [118]. But an effect on body weight was not noted in a previous analyses of TPC2 knockout mice even when fed on a cholesterol rich diet which induced major liver dysfunction [35].
Interestingly, a recent GWAS linked TPC2 to systemic lupus erythematosus [119], a chronic autoimmune disease. A variant (rs10750836) located upstream of the TPCN2 gene (Fig. 2) was found to correlate with reduced TPC2 expression levels in B cells from patients [119]. This effect was supported by reporter expression analyses. Functional studies of B cells upon TPC2 knockout/inhibition is eagerly awaited not least due to the essential role for lysosomal compartments in antigen processing.
In sum, TPCs might be involved in a number of additional disorders affecting the brain, reproductive system, skeletal muscle, adipose tissue and the immune system. But further supporting evidence is required.
9. Closing remarks
As discussed, NAADP antagonists have proven beneficial in correcting disease phenotypes in cellular and animal models of Parkinson's (Ned-K), cancer (Ned-19) and heart arrhythmia (BZ194), often mimicking the effects of TPC knockdown/knockout. They, thus present themselves as lead drugs for combatting TPC dysfunction. But because the effects of NAADP on TPCs is likely indirect, a clear mechanism of action is difficult to ascertain as NAADP-binding proteins continue to evade molecular identification.
An alternative (and complementary) approach to manipulate this pathway is to directly target TPCs. Success with tetrandrine in combatting Ebola infection is most encouraging in this respect although its pleiotropic actions should not be ignored [64]. Indeed, chemical targeting of Ca2+ channels is not without its challenges. Nevertheless, successful drugging of voltage-gated Ca2+ channels (with for example dihydropyridines) and ryanodine receptors (with dantrolene) in hypertension and malignant hyperthermia offer hope. In this context, emerging structures of TPCs [5,[120], [121], [122],126] should aid in the rational design of TPC blockers.
Finally, with growing evidence linking disorders of the pancreas and liver with loss of TPC function should focus attention on strategies to boost TPC activity. It is intriguing that tolbutamide, a diabetic drug which targets kATP channels to stimulate insulin secretion appears to require TPC2 for its activity [125]. Administration of NAADP itself is reportedly beneficial in diabetes [123] but exactly how NAADP enters cells requires work. A major hurdle at present is the lack of TPC agonists. Here, TRP mucolipins which like TPCs localise to the endo-lysosomal system to (de-)regulate Ca2+ dependent output, lead the way with the availability of both activators and inhibitors [124].
No doubt future advances will offer us new approaches to target TPCs in disease.
Transparency document
Transparency document.
Acknowledgements
We thank Christopher J Penny and Taufiq Rahman for assistance with structural modelling, and Margarida Ruas, Katie Holl and Leah Solberg Woods for assistance with genome analysis. Work in the laboratory is funded by the BBSRC and Parkinson's UK.
Footnotes
This article is part of a Special Issue entitled: Calcium signaling in health, disease and therapy edited by Geert Bultynck and Jan Parys.
The Transparency document associated with this article can be found, in online version.
References
- 1.Patel S. Function and dysfunction of two-pore channels. Sci. Signal. 2015;8:re7. doi: 10.1126/scisignal.aab3314. [DOI] [PubMed] [Google Scholar]
- 2.Hooper R., Churamani D., Brailoiu E., Taylor C.W., Patel S. Membrane topology of NAADP-sensitive two-pore channels and their regulation by N-linked glycosylation. J. Biol. Chem. 2011;286:9141–9149. doi: 10.1074/jbc.M110.189985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rietdorf K., Funnell T.M., Ruas M., Heinemann J., Parrington J., Galione A. Two-pore channels form homo- and heterodimers. J. Biol. Chem. 2011;286:37058–37062. doi: 10.1074/jbc.C111.289835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Churamani D., Hooper R., Brailoiu E., Patel S. Domain assembly of NAADP-gated two-pore channels. Biochem. J. 2012;441:317–323. doi: 10.1042/BJ20111617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel S., Penny C.J., Rahman T. Two-pore channels enter the atomic era. Structure of plant TPC revealed. Trends Biochem. Sci. 2016;41:475–477. doi: 10.1016/j.tibs.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahman T., Cai X., Brailoiu G.C., Abood M.E., Brailoiu E., Patel S. Two-pore channels provide insight into the evolution of voltage-gated Ca2+ and Na+ channels. Sci. Signal. 2014;7 doi: 10.1126/scisignal.2005450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calcraft P.J., Ruas M., Pan Z., Cheng X., Arredouani A., Hao X., Tang J., Rietdorf K., Teboul L., Chuang K.T., Lin P., Xiao R., Wang C., Zhu Y., Lin Y., Wyatt C.N., Parrington J., Ma J., Evans A.M., Galione A., Zhu M.X. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brailoiu E., Churamani D., Cai X., Schrlau M.G., Brailoiu G.C., Gao X., Hooper R., Boulware M.J., Dun N.J., Marchant J.S., Patel S. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J. Cell Biol. 2009;186:201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peiter E., Maathuis F.J., Mills L.N., Knight H., Pelloux J., Hetherington A.M., Sanders D. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature. 2005;434:404–408. doi: 10.1038/nature03381. [DOI] [PubMed] [Google Scholar]
- 10.Brailoiu E., Rahman T., Churamani D., Prole D.L., Brailoiu G.C., Hooper R., Taylor C.W., Patel S. An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals. J. Biol. Chem. 2010;285:38511–38516. doi: 10.1074/jbc.M110.162073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larisch N., Schulze C., Galione A., Dietrich P. An N-terminal dileucine motif directs two-pore channels to the tonoplast of plant cells. Traffic. 2012;13:1012–1022. doi: 10.1111/j.1600-0854.2012.01366.x. [DOI] [PubMed] [Google Scholar]
- 12.Zong X., Schieder M., Cuny H., Fenske S., Gruner C., Rotzer K., Griesbeck O., Harz H., Biel M., Wahl-Schott C. The two-pore channel TPCN2 mediates NAADP-dependent Ca2+-release from lysosomal stores. Pflugers Arch. 2009;458:891–899. doi: 10.1007/s00424-009-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brailoiu E., Hooper R., Cai X., Brailoiu G.C., Keebler M.V., Dun N.J., Marchant J.S., Patel S. An ancestral deuterostome family of two-pore channels mediate nicotinic acid adenine dinucleotide phosphate-dependent calcium release from acidic organelles. J. Biol. Chem. 2010;285:2897–2901. doi: 10.1074/jbc.C109.081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee H.C., Aarhus R. A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J. Biol. Chem. 1995;270:2152–2157. doi: 10.1074/jbc.270.5.2152. [DOI] [PubMed] [Google Scholar]
- 15.Patel S., Docampo R. Acidic calcium stores open for business: expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol. 2010;20:277–286. doi: 10.1016/j.tcb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel S., Muallem S. Acidic Ca2+ stores come to the fore. Cell Calcium. 2011;50:109–112. doi: 10.1016/j.ceca.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Lim D., Kyozuka K., Gragnaniello G., Carafoli E., Santella L. NAADP+ initiates the Ca2+ response during fertilization of starfish oocytes. FASEB J. 2001;15:2257–2267. doi: 10.1096/fj.01-0157com. [DOI] [PubMed] [Google Scholar]
- 18.Churchill G.C., O'Neil J.S., Masgrau R., Patel S., Thomas J.M., Genazzani A.A., Galione A. Sperm deliver a new messenger: NAADP. Curr. Biol. 2003;13:125–128. doi: 10.1016/s0960-9822(03)00002-2. [DOI] [PubMed] [Google Scholar]
- 19.Brailoiu E., Churamani D., Pandey V., Brailoiu G.C., Tuluc F., Patel S., Dun N.J. Messenger-specific role for NAADP in neuronal differentiation. J. Biol. Chem. 2006;281:15923–15928. doi: 10.1074/jbc.M602249200. [DOI] [PubMed] [Google Scholar]
- 20.Pandey V., Chuang C.C., Lewis A.M., Aley P., Brailoiu E., Dun N., Churchill G.C., Patel S. Recruitment of NAADP-sensitive acidic Ca2+ stores by glutamate. Biochem. J. 2009;422:503–512. doi: 10.1042/BJ20090194. [DOI] [PubMed] [Google Scholar]
- 21.Wang X., Zhang X., Dong X.P., Samie M., Li X., Cheng X., Goschka A., Shen D., Zhou Y., Harlow J., Zhu M.X., Clapham D.E., Ren D., Xu H. TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012;151:372–383. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jha A., Ahuja M., Patel S., Brailoiu E., Muallem S. Convergent regulation of the lysosomal two-pore channel-2 by Mg2+, NAADP, PI(3,5)P2 and multiple protein kinases. EMBO J. 2014;33:501–511. doi: 10.1002/embj.201387035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cang C., Bekele B., Ren D. The voltage-gated sodium channel TPC1 confers endolysosomal excitability. Nat. Chem. Biol. 2014;10:463–469. doi: 10.1038/nchembio.1522. [DOI] [PubMed] [Google Scholar]
- 24.Lin-Moshier Y., Walseth T.F., Churamani D., Davidson S.M., Slama J.T., Hooper R., Brailoiu E., Patel S., Marchant J.S. Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J. Biol. Chem. 2012;287:2296–2307. doi: 10.1074/jbc.M111.305813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walseth T.F., Lin-Moshier Y., Jain P., Ruas M., Parrington J., Galione A., Marchant J.S., Slama J.T. Photoaffinity labeling of high affinity nicotinic acid adenine dinucleotide 2′-phosphate (NAADP) proteins in sea urchin egg. J. Biol. Chem. 2012;287:2308–2315. doi: 10.1074/jbc.M111.306563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin-Moshier Y., Keebler M.V., Hooper R., Boulware M.J., Liu X., Churamani D., Abood M.E., Walseth T.F., Brailoiu E., Patel S., Marchant J.S. The two-pore channel (TPC) interactome unmasks isoform-specific roles for TPCs in endolysosomal morphology and cell pigmentation. PNAS. 2014;111:13087–13092. doi: 10.1073/pnas.1407004111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marchant J.S., Patel S. Questioning regulation of two-pore channels by NAADP. Messenger. 2013;2:113–119. doi: 10.1166/msr.2013.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruas M., Davis L.C., Chen C.C., Morgan A.J., Chuang K.T., Walseth T.F., Grimm C., Garnham C., Powell T., Platt N., Platt F.M., Biel M., Wahl-Schott C., Parrington J., Galione A. Expression of Ca2+-permeable two-pore channels rescues NAADP signalling in TPC-deficient cells. EMBO J. 2015;34:1743–1758. doi: 10.15252/embj.201490009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jentsch T.J., Hoegg-Beiler M.B., Vogt J. Departure gate of acidic Ca2+ confirmed. EMBO J. 2015;34:1737–1739. doi: 10.15252/embj.201591884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christensen K.A., Myers J.T., Swanson J.A. pH-dependent regulation of lysosomal calcium in macrophages. J. Cell Sci. 2002;115:599–607. doi: 10.1242/jcs.115.3.599. [DOI] [PubMed] [Google Scholar]
- 31.Cancela J.M., Churchill G.C., Galione A. Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature. 1999;398:74–76. doi: 10.1038/18032. [DOI] [PubMed] [Google Scholar]
- 32.Galione A., Morgan A.J., Arredouani A., Davis L.C., Rietdorf K., Ruas M., Parrington J. NAADP as an intracellular messenger regulating lysosomal calcium-release channels. Biochem. Soc. Trans. 2010;38:1424–1431. doi: 10.1042/BST0381424. [DOI] [PubMed] [Google Scholar]
- 33.Kilpatrick B.S., Eden E.R., Schapira A.H., Futter C.E., Patel S. Direct mobilisation of lysosomal Ca2+ triggers complex Ca2+ signals. J. Cell Sci. 2013;126:60–66. doi: 10.1242/jcs.118836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penny C.J., Kilpatrick B.S., Han J.M., Sneyd J., Patel S. A computational model of lysosome-ER Ca2+ microdomains. J. Cell Sci. 2014;127:2934–2943. doi: 10.1242/jcs.149047. [DOI] [PubMed] [Google Scholar]
- 35.Grimm C., Holdt L.M., Chen C.C., Hassan S., Muller C., Jors S., Cuny H., Kissing S., Schroder B., Butz E., Northoff B., Castonguay J., Luber C.A., Moser M., Spahn S., Lullmann-Rauch R., Fendel C., Klugbauer N., Griesbeck O., Haas A., Mann M., Bracher F., Teupser D., Saftig P., Biel M., Wahl-Schott C. High susceptibility to fatty liver disease in two-pore channel 2-deficient mice. Nat. Commun. 2014;5:4699. doi: 10.1038/ncomms5699. [DOI] [PubMed] [Google Scholar]
- 36.Pereira G.J., Hirata H., Fimia G.M., do Carmo L.G., Bincoletto C., Han S.W., Stilhano R.S., Ureshino R.P., Bloor-Young D., Churchill G., Piacentini M., Patel S., Smaili S.S. Nicotinic acid adenine dinucleotide phosphate (NAADP) regulates autophagy in cultured astrocytes. J. Biol. Chem. 2011;286:27875–27881. doi: 10.1074/jbc.C110.216580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hockey L.N., Kilpatrick B.S., Eden E.R., Lin-Moshier Y., Brailoiu G.C., Brailoiu E., Futter C., Schapira A.H., Marchant J.S., Patel S. Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition. J. Cell Sci. 2015;128:232–238. doi: 10.1242/jcs.164152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruas M., Rietdorf K., Arredouani A., Davis L.C., Lloyd-Evans E., Koegel H., Funnell T.M., Morgan A.J., Ward J.A., Watanabe K., Cheng X., Churchill G.C., Zhu M.X., Platt F.M., Wessel G.M., Parrington J., Galione A. Purified TPC isoforms form NAADP receptors with distinct roles for Ca2+ signaling and endolysosomal trafficking. Curr. Biol. 2010;20:703–709. doi: 10.1016/j.cub.2010.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kilpatrick B.S., Eden E.R., Hockey L.N., Yates E., Futter C.E., Patel S. An endosomal NAADP-sensitive two-pore Ca2+ channel regulates ER-endosome membrane contact sites to control growth factor signaling. Cell Rep. 2017;18:1636–1645. doi: 10.1016/j.celrep.2017.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naylor E., Arredouani A., Vasudevan S.R., Lewis A.M., Parkesh R., Mizote A., Rosen D., Thomas J.M., Izumi M., Ganesan A., Galione A., Churchill G.C. Identification of a chemical probe for NAADP by virtual screening. Nat. Chem. Biol. 2009;5:220–226. doi: 10.1038/nchembio.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Genazzani A.A., Empson R.M., Galione A. Unique inactivation properties of NAADP-sensitive Ca2+ release. J. Biol. Chem. 1996;271:1159911602. doi: 10.1074/jbc.271.20.11599. [DOI] [PubMed] [Google Scholar]
- 42.Genazzani A.A., Mezna M., Dickey D.M., Michelangeli F., Walseth T.F., Galione A. Pharmacological properties of the Ca2+-release mechanism sensitive to NAADP in the sea urchin egg. Br. J. Pharmacol. 1997;121:1489–1495. doi: 10.1038/sj.bjp.0701295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braak H., Del Tredici K., Rub U., de Vos R.A., Jansen Steur E.N., Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 44.West A.B., Moore D.J., Biskup S., Bugayenko A., Smith W.W., Ross C.A., Dawson V.L., Dawson T.M. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc. Natl. Acad. Sci. U. S. A. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alegre-Abarrategui J., Wade-Martins R. Parkinson disease, LRRK2 and the endocytic-autophagic pathway. Autophagy. 2009;5:1208–1210. doi: 10.4161/auto.5.8.9894. [DOI] [PubMed] [Google Scholar]
- 46.Gomez-Suaga P., Luzon-Toro B., Churamani D., Zhang L., Bloor-Young D., Patel S., Woodman P.G., Churchill G.C., Hilfiker S. Leucine-rich repeat kinase 2 regulates autophagy through a calcium-dependent pathway involving NAADP. Hum. Mol. Genet. 2012;21:511–525. doi: 10.1093/hmg/ddr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madero-Perez J., Fdez E., Fernandez B., Lara Ordonez A.J., Blanca Ramirez M., Romo Lozano M., Rivero-Rios P., Hilfiker S. Cellular effects mediated by pathogenic LRRK2: homing in on Rab-mediated processes. Biochem. Soc. Trans. 2017;45:147–154. doi: 10.1042/BST20160392. [DOI] [PubMed] [Google Scholar]
- 48.Penny C.J., Patel S. Poring over two-pore channel pore mutants. Messenger (Los Angel.) 2015;4:46–52. doi: 10.1166/msr.2015.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bedford C., Sears C., Perez-Carrion M., Piccoli G., Condliffe S.B. LRRK2 regulates voltage-gated calcium channel function. Front. Mol. Neurosci. 2016;9:35. doi: 10.3389/fnmol.2016.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henry A.G., Aghamohammadzadeh S., Samaroo H., Chen Y., Mou K., Needle E., Hirst W.D. Pathogenic LRRK2 mutations, through increased kinase activity, produce enlarged lysosomes with reduced degradative capacity and increase ATP13A2 expression. Hum. Mol. Genet. 2015;24:6013–6028. doi: 10.1093/hmg/ddv314. [DOI] [PubMed] [Google Scholar]
- 51.Santiago J.A., Littlefield A.M., Potashkin J.A. Integrative transcriptomic meta-analysis of Parkinson's disease and depression identifies NAMPT as a potential blood biomarker for de novo Parkinson's disease. Sci. Rep. 2016;6 doi: 10.1038/srep34579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kilpatrick B.S., Magalhaes J., Beavan M.S., McNeill A., Gegg M.E., Cleeter M.W., Bloor-Young D., Churchill G.C., Duchen M.R., Schapira A.H., Patel S. Endoplasmic reticulum and lysosomal Ca2+ stores are remodelled in GBA1-linked Parkinson disease patient fibroblasts. Cell Calcium. 2016;59:12–20. doi: 10.1016/j.ceca.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sidransky E., Lopez G. The link between the GBA gene and parkinsonism. Lancet Neurol. 2012;11:986–998. doi: 10.1016/S1474-4422(12)70190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robak L.A., Jansen I.E., van Rooij J., Uitterlinden A.G., Kraaij R., Jankovic J., Heutink P., Shulman J.M. Excessive burden of lysosomal storage disorder gene variants in Parkinson's disease. Brain. 2017;140:3191–3203. doi: 10.1093/brain/awx285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lloyd-Evans E., Morgan A.J., He X., Smith D.A., Elliot-Smith E., Sillence D.J., Churchill G.C., Schuchman E.H., Galione A., Platt F.M. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat. Med. 2008;14:1247–1255. doi: 10.1038/nm.1876. [DOI] [PubMed] [Google Scholar]
- 56.Churamani D., Carrey E.A., Dickinson G.D., Patel S. Determination of cellular nicotinic acid-adenine dinucleotide phosphate (NAADP) levels. Biochem. J. 2004;380:449–454. doi: 10.1042/BJ20031754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hooper R., Churamani D., Davidson S.M., Lin-Moshier Y., Walseth T.F., Patel S., Marchant J.S. TPC1 knockout knocks out TPC1. Mol. Cell. Biol. 2015;35:1882–1883. doi: 10.1128/MCB.00020-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldstein J.L., Brown M.S. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 2009;29:431–438. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tomas A., Futter C.E., Eden E.R. EGF receptor trafficking: consequences for signaling and cancer. Trends Cell Biol. 2014;24:26–34. doi: 10.1016/j.tcb.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruas M., Chuang K.T., Davis L.C., Al-Douri A., Tynan P.W., Tunn R., Teboul L., Galione A., Parrington J. TPC1 has two variant isoforms and their removal has different effects on endo-lysosomal functions compared to loss of TPC2. Mol. Cell. Biol. 2014;34:3981–3992. doi: 10.1128/MCB.00113-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakurai Y., Kolokolstov A.A., Chen C.C., Tidwell M.W., Bauta W.E., Klugbauer N., Grimm C., Wahl-Schott C., Biel M., Davey R.A. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science. 2015;347(6225) doi: 10.1126/science.1258758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith A.E., Helenius A. How viruses enter animal cells. Science. 2004;304:237–242. doi: 10.1126/science.1094823. [DOI] [PubMed] [Google Scholar]
- 63.Cote M., Misasi J., Ren T., Bruchez A., Lee K., Filone C.M., Hensley L., Li Q., Ory D., Chandran K., Cunningham J. Small molecule inhibitors reveal Niemann-Pick C1 is essential for Ebola virus infection. Nature. 2011;477:344–348. doi: 10.1038/nature10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhagya N., Chandrashekar K.R. Tetrandrine–a molecule of wide bioactivity. Phytochemistry. 2016;125:5–13. doi: 10.1016/j.phytochem.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 65.Simmons J.A., D'Souza R.S., Ruas M., Galione A., Casanova J.E., White J.M. Ebolavirus glycoprotein directs fusion through NPC1 + endolysosomes. J. Virol. 2016;90:605–610. doi: 10.1128/JVI.01828-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Castonguay J., Orth J.H.C., Muller T., Sleman F., Grimm C., Wahl-Schott C., Biel M., Mallmann R.T., Bildl W., Schulte U., Klugbauer N. The two-pore channel TPC1 is required for efficient protein processing through early and recycling endosomes. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-10607-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jahidin A.H., Stewart T.A., Thompson E.W., Roberts-Thomson S.J., Monteith G.R. Differential effects of two-pore channel protein 1 and 2 silencing in MDA-MB-468 breast cancer cells. Biochem. Biophys. Res. Commun. 2016;477:731–736. doi: 10.1016/j.bbrc.2016.06.127. [DOI] [PubMed] [Google Scholar]
- 68.Cai X., Patel S. Degeneration of an intracellular ion channel in the primate lineage by relaxation of selective constraints. Mol. Biol. Evol. 2010;27:2352–2359. doi: 10.1093/molbev/msq122. [DOI] [PubMed] [Google Scholar]
- 69.Wilkerson P.M., Reis-Filho J.S. The 11q13-q14 amplicon: clinicopathological correlations and potential drivers. Genes Chromosom. Cancer. 2013;52:333–355. doi: 10.1002/gcc.22037. [DOI] [PubMed] [Google Scholar]
- 70.Huang X., Godfrey T.E., Gooding W.E., McCarty K.S., Jr., Gollin S.M. Comprehensive genome and transcriptome analysis of the 11q13 amplicon in human oral cancer and synteny to the 7F5 amplicon in murine oral carcinoma. Genes Chromosom. Cancer. 2006;45:1058–1069. doi: 10.1002/gcc.20371. [DOI] [PubMed] [Google Scholar]
- 71.Xu C., Liu Y., Wang P., Fan W., Rue T.C., Upton M.P., Houck J.R., Lohavanichbutr P., Doody D.R., Futran N.D., Zhao L.P., Schwartz S.M., Chen C., Mendez E. Integrative analysis of DNA copy number and gene expression in metastatic oral squamous cell carcinoma identifies genes associated with poor survival. Mol. Cancer. 2010;9:143. doi: 10.1186/1476-4598-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sugahara K., Michikawa Y., Ishikawa K., Shoji Y., Iwakawa M., Shibahara T., Imai T. Combination effects of distinct cores in 11q13 amplification region on cervical lymph node metastasis of oral squamous cell carcinoma. Int. J. Oncol. 2011;39:761–769. doi: 10.3892/ijo.2011.1094. [DOI] [PubMed] [Google Scholar]
- 73.Nguyen O.N., Grimm C., Schneider L.S., Chao Y.K., Atzberger C., Bartel K., Watermann A., Ulrich M., Mayr D., Wahl-Schott C., Biel M., Vollmar A.M. Two-pore channel function is crucial for the migration of invasive cancer cells. Cancer Res. 2017;77:1427–1438. doi: 10.1158/0008-5472.CAN-16-0852. [DOI] [PubMed] [Google Scholar]
- 74.Grimm C., Bartel K., Vollmar A.M., Biel M. Endolysosomal cation channels and cancer-a link with great potential. Pharmaceuticals (Basel, Switz.) 2018;11 doi: 10.3390/ph11010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shivakumar M., Lee Y., Bang L., Garg T., Sohn K.A., Kim D. Identification of epigenetic interactions between miRNA and DNA methylation associated with gene expression as potential prognostic markers in bladder cancer. BMC Med. Genet. 2017;10:30. doi: 10.1186/s12920-017-0269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patel S. Ins and outs of Ca2+ transport by acidic organelles and cell migration. Commun. Integr. Biol. 2018;11 [Google Scholar]
- 77.Mayor R., Theveneau E. The neural crest. Development. 2013;140:2247–2251. doi: 10.1242/dev.091751. [DOI] [PubMed] [Google Scholar]
- 78.Melchionda M., Pittman J.K., Mayor R., Patel S. Ca2+/H+ exchange by acidic organelles regulates cell migration in vivo. J. Cell Biol. 2016;212:803–813. doi: 10.1083/jcb.201510019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brailoiu G.C., Gurzu B., Gao X., Parkesh R., Aley P.K., Trifa D.I., Galione A., Dun N.J., Madesh M., Patel S., Churchill G.C., Brailoiu E. Acidic NAADP-sensitive calcium stores in the endothelium: agonist-specific recruitment and role in regulating blood pressure. J. Biol. Chem. 2010;285:37133–37137. doi: 10.1074/jbc.C110.169763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Esposito B., Gambara G., Lewis A.M., Palombi F., D'Alessio A., Taylor L.X., Genazzani A.A., Ziparo E., Galione A., Churchill G.C., Filippini A. NAADP links histamine H1 receptors to secretion of von Willebrand factor in human endothelial cells. Blood. 2011;117:4968–4977. doi: 10.1182/blood-2010-02-266338. [DOI] [PubMed] [Google Scholar]
- 81.Favia A., Desideri M., Gambara G., D'Alessio A., Ruas M., Esposito B., Del B.D., Parrington J., Ziparo E., Palombi F., Galione A., Filippini A. VEGF-induced neoangiogenesis is mediated by NAADP and two-pore channel-2-dependent Ca2+ signaling. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E4706–E4715. doi: 10.1073/pnas.1406029111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sulem P., Gudbjartsson D.F., Stacey S.N., Helgason A., Rafnar T., Jakobsdottir M., Steinberg S., Gudjonsson S.A., Palsson A., Thorleifsson G., Palsson S., Sigurgeirsson B., Thorisdottir K., Ragnarsson R., Benediktsdottir K.R., Aben K.K., Vermeulen S.H., Goldstein A.M., Tucker M.A., Kiemeney L.A., Olafsson J.H., Gulcher J., Kong A., Thorsteinsdottir U., Stefansson K. Two newly identified genetic determinants of pigmentation in Europeans. Nat. Genet. 2008;40:835–837. doi: 10.1038/ng.160. [DOI] [PubMed] [Google Scholar]
- 83.Chao Y.K., Schludi V., Chen C.C., Butz E., Nguyen O.N.P., Muller M., Kruger J., Kammerbauer C., Ben-Johny M., Vollmar A.M., Berking C., Biel M., Wahl-Schott C.A., Grimm C. TPC2 polymorphisms associated with a hair pigmentation phenotype in humans result in gain of channel function by independent mechanisms. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E8595–e8602. doi: 10.1073/pnas.1705739114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ambrosio A.L., Boyle J.A., Aradi A.E., Christian K.A., Di Pietro S.M. TPC2 controls pigmentation by regulating melanosome pH and size. Proc. Natl. Acad. Sci. U. S. A. 2016;113:5622–5627. doi: 10.1073/pnas.1600108113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bellono N.W., Escobar I.E., Oancea E. A melanosomal two-pore sodium channel regulates pigmentation. Sci. Rep. 2016;6 doi: 10.1038/srep26570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kocarnik J.M., Park S.L., Han J., Dumitrescu L., Cheng I., Wilkens L.R., Schumacher F.R., Kolonel L., Carlson C.S., Crawford D.C., Goodloe R.J., Dilks H.H., Baker P., Richardson D., Matise T.C., Ambite J.L., Song F., Qureshi A.A., Zhang M., Duggan D., Hutter C., Hindorff L., Bush W.S., Kooperberg C., Le Marchand L., Peters U. Pleiotropic and sex-specific effects of cancer GWAS SNPs on melanoma risk in the population architecture using genomics and epidemiology (PAGE) study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bak J., Billington R.A., Timar G., Dutton A.C., Genazzani A.A. NAADP receptors are present and functional in the heart. Curr. Biol. 2001;11:987–990. doi: 10.1016/s0960-9822(01)00269-x. [DOI] [PubMed] [Google Scholar]
- 88.Nebel M., Schwoerer A.P., Warszta D., Siebrands C.C., Limbrock A.C., Swarbrick J.M., Fliegert R., Weber K., Bruhn S., Hohenegger M., Geisler A., Herich L., Schlegel S., Carrier L., Eschenhagen T., Potter B.V., Ehmke H., Guse A.H. Nicotinic acid adenine dinucleotide phosphate (NAADP)-mediated calcium signaling and arrhythmias in the heart evoked by beta-adrenergic stimulation. J. Biol. Chem. 2013;288:16017–16030. doi: 10.1074/jbc.M112.441246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garcia-Rua V., Feijoo-Bandin S., Rodriguez-Penas D., Mosquera-Leal A., Abu-Assi E., Beiras A., Maria Seoane L., Lear P., Parrington J., Portoles M., Rosello-Lleti E., Rivera M., Gualillo O., Parra V., Hill J.A., Rothermel B., Gonzalez-Juanatey J.R., Lago F. Endolysosomal two-pore channels regulate autophagy in cardiomyocytes. J. Physiol. 2016;594:3061–3077. doi: 10.1113/JP271332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garcia-Rua V., Otero M.F., Lear P.V., Rodriguez-Penas D., Feijoo-Bandin S., Noguera-Moreno T., Calaza M., Alvarez-Barredo M., Mosquera-Leal A., Parrington J., Brugada J., Portoles M., Rivera M., Gonzalez-Juanatey J.R., Lago F. Increased expression of fatty-acid and calcium metabolism genes in failing human heart. PLoS One. 2012;7 doi: 10.1371/journal.pone.0037505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Davidson S.M., Foote K., Kunuthur S., Gosain R., Tan N., Tyser R., Zhao Y.J., Graeff R., Ganesan A., Duchen M.R., Patel S., Yellon D.M. Inhibition of NAADP signalling on reperfusion protects the heart by preventing lethal calcium oscillations via two-pore channel 1 and opening of the mitochondrial permeability transition pore. Cardiovasc. Res. 2015;108:357–366. doi: 10.1093/cvr/cvv226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fauconnier J., Roberge S., Saint N., Lacampagne A. Type 2 ryanodine receptor: a novel therapeutic target in myocardial ischemia/reperfusion. Pharmacol. Ther. 2013;138:323–332. doi: 10.1016/j.pharmthera.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 93.Kawano T., Kadono T., Fumoto K., Lapeyrie F., Kuse M., Isobe M., Furuichi T., Muto S. Aluminum as a specific inhibitor of plant TPC1 Ca2+ channels. Biochem. Biophys. Res. Commun. 2004;324:40–45. doi: 10.1016/j.bbrc.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 94.Djerada Z., Peyret H., Dukic S., Millart H. Extracellular NAADP affords cardioprotection against ischemia and reperfusion injury and involves the P2Y11-like receptor. Biochem. Biophys. Res. Commun. 2013;434:428–433. doi: 10.1016/j.bbrc.2013.03.089. [DOI] [PubMed] [Google Scholar]
- 95.Khalaf A., Babiker F. Discrepancy in calcium release from the sarcoplasmic reticulum and intracellular acidic stores for the protection of the heart against ischemia/reperfusion injury. J. Physiol. Biochem. 2016;72:495–508. doi: 10.1007/s13105-016-0498-0. [DOI] [PubMed] [Google Scholar]
- 96.Macgregor A., Yamasaki M., Rakovic S., Sanders L., Parkesh R., Churchill G.C., Galione A., Terrar D.A. NAADP controls cross-talk between distinct Ca2+ stores in the heart. J. Biol. Chem. 2007;282:15302–15311. doi: 10.1074/jbc.M611167200. [DOI] [PubMed] [Google Scholar]
- 97.Lewis A.M., Aley P.K., Roomi A., Thomas J.M., Masgrau R., Garnham C., Shipman K., Paramore C., Bloor-Young D., Sanders L.E., Terrar D.A., Galione A., Churchill G.C. beta-Adrenergic receptor signaling increases NAADP and cADPR levels in the heart. Biochem. Biophys. Res. Commun. 2012;427:326–329. doi: 10.1016/j.bbrc.2012.09.054. [DOI] [PubMed] [Google Scholar]
- 98.Gul R., Park D.R., Shawl A.I., Im S.Y., Nam T.S., Lee S.H., Ko J.K., Jang K.Y., Kim D., Kim U.H. Nicotinic acid adenine dinucleotide phosphate (NAADP) and cyclic ADP-ribose (cADPR) mediate Ca2+ signaling in cardiac hypertrophy induced by beta-adrenergic stimulation. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dammermann W., Zhang B., Nebel M., Cordiglieri C., Odoardi F., Kirchberger T., Kawakami N., Dowden J., Schmid F., Dornmair K., Hohenegger M., Flugel A., Guse A.H., Potter B.V. NAADP-mediated Ca2+ signaling via type 1 ryanodine receptor in T cells revealed by a synthetic NAADP antagonist. Proc. Natl. Acad. Sci. U. S. A. 2009;106:10678–10683. doi: 10.1073/pnas.0809997106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Capel R.A., Bolton E.L., Lin W.K., Aston D., Wang Y., Liu W., Wang X., Burton R.A., Bloor-Young D., Shade K.T., Ruas M., Parrington J., Churchill G.C., Lei M., Galione A., Terrar D.A. Two-pore channels (TPC2s) and nicotinic acid adenine dinucleotide phosphate (NAADP) at lysosomal-sarcoplasmic reticular junctions contribute to acute and chronic beta-adrenoceptor signaling in the heart. J. Biol. Chem. 2015;290:30087–30098. doi: 10.1074/jbc.M115.684076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee C.S., Tong B.C., Cheng C.W., Hung H.C., Cheung K.H. Characterization of two-pore channel 2 by nuclear membrane electrophysiology. Sci. Rep. 2016;6 doi: 10.1038/srep20282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Patel S. NAADP on the up in pancreatic beta cells: a sweet message? BioEssays. 2003;25:430–433. doi: 10.1002/bies.10276. [DOI] [PubMed] [Google Scholar]
- 103.Masgrau R., Churchill G.C., Morgan A.J., Ashcroft S.J.H., Galione A. NAADP: a new second messenger for glucose-induced Ca2+ responses in clonal pancreatic β-cells. Curr. Biol. 2003;13:247–251. doi: 10.1016/s0960-9822(03)00041-1. [DOI] [PubMed] [Google Scholar]
- 104.Johnson J.D., Misler S. Nicotinic acid-adenine dinucleotide phosphate-sensitive calcium stores initiate insulin signaling in human beta cells. PNAS. 2002;99:14566–14571. doi: 10.1073/pnas.222099799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim B.J., Park K.H., Yim C.Y., Takasawa S., Okamoto H., Im M.J., Kim U.H. Generation of nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose by glucagon-like peptide-1 evokes Ca2+ signal that is essential for insulin secretion in mouse pancreatic islets. Diabetes. 2008;57:868–878. doi: 10.2337/db07-0443. [DOI] [PubMed] [Google Scholar]
- 106.Rutter G.A., Pullen T.J., Hodson D.J., Martinez-Sanchez A. Pancreatic beta-cell identity, glucose sensing and the control of insulin secretion. Biochem. J. 2015;466:203–218. doi: 10.1042/BJ20141384. [DOI] [PubMed] [Google Scholar]
- 107.Tsaih S.W., Holl K., Jia S., Kaldunski M., Tschannen M., He H., Andrae J.W., Li S.H., Stoddard A., Wiederhold A., Parrington J., Ruas da S.M., Galione A., Meigs J., Hoffmann R.G., Simpson P., Jacob H., Hessner M., Solberg Woods L.C. Identification of a novel gene for diabetic traits in rats, mice, and humans. Genetics. 2014;198:17–29. doi: 10.1534/genetics.114.162982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cane M.C., Parrington J., Rorsman P., Galione A., Rutter G.A. The two pore channel TPC2 is dispensable in pancreatic beta-cells for normal Ca(2)(+) dynamics and insulin secretion. Cell Calcium. 2016;59:32–40. doi: 10.1016/j.ceca.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hamilton A., Zhang Q., Salehi A., Willems M., Knudsen J.G., Ringgaard A.K., Chapman C.E., Gonzalez-Alvarez A., Surdo N.C., Zaccolo M., Basco D., Johnson P.R.V., Ramracheya R., Rutter G.A., Galione A., Rorsman P., Tarasov A.I. Adrenaline stimulates glucagon secretion by Tpc2-dependent ca(2+) mobilization from acidic stores in pancreatic alpha-cells. Diabetes. 2018;67:1128–1139. doi: 10.2337/db17-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Solberg Woods L.C., Holl K.L., Oreper D., Xie Y., Tsaih S.W., Valdar W. Fine-mapping diabetes-related traits, including insulin resistance, in heterogeneous stock rats. Physiol. Genomics. 2012;44:1013–1026. doi: 10.1152/physiolgenomics.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fan Y., Li X., Zhang Y., Fan X., Zhang N., Zheng H., Song Y., Shen C., Shen J., Ren F., Yang J. Genetic variants of TPCN2 associated with type 2 diabetes risk in the Chinese population. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sherrington R., Rogaev E.I., Liang Y., Rogaeva E.A., Levesque G., Ikeda M., Chi H., Lin C., Li G., Holman K., Tsuda T., Mar L., Foncin J.F., Bruni A.C., Montesi M.P., Sorbi S., Rainero I., Pinessi L., Nee L., Chumakov I., Pollen D., Brookes A., Sanseau P., Polinsky R.J., Wasco W., Da Silva H.A., Haines J.L., Perkicak-Vance M.A., Tanzi R.E., Roses A.D., Fraser P.E., Rommens J.M., St George-Hyslop P.H. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 113.Coen K., Flannagan R.S., Baron S., Carraro-Lacroix L.R., Wang D., Vermeire W., Michiels C., Munck S., Baert V., Sugita S., Wuytack F., Hiesinger P.R., Grinstein S., Annaert W. Lysosomal calcium homeostasis defects, not proton pump defects, cause endo-lysosomal dysfunction in PSEN-deficient cells. J. Cell Biol. 2012;198:23–25. doi: 10.1083/jcb.201201076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Neely Kayala K.M., Dickinson G.D., Minassian A., Walls K.C., Green K.N., Laferla F.M. Presenilin-null cells have altered two-pore calcium channel expression and lysosomal calcium: implications for lysosomal function. Brain Res. 2012;1489:8–16. doi: 10.1016/j.brainres.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee J.H., McBrayer M.K., Wolfe D.M., Haslett L.J., Kumar A., Sato Y., Lie P.P., Mohan P., Coffey E.E., Kompella U., Mitchell C.H., Lloyd-Evans E., Nixon R.A. Presenilin 1 maintains lysosomal Ca(2+) homeostasis via TRPML1 by regulating vATPase-mediated lysosome acidification. Cell Rep. 2015;12:1430–1444. doi: 10.1016/j.celrep.2015.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Arndt L., Castonguay J., Arlt E., Meyer D., Hassan S., Borth H., Zierler S., Wennemuth G., Breit A., Biel M., Wahl-Schott C., Gudermann T., Klugbauer N., Boekhoff I. NAADP and the two-pore channel protein 1 participate in the acrosome reaction in mammalian spermatozoa. Mol. Biol. Cell. 2014;25:948–964. doi: 10.1091/mbc.E13-09-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lin P.H., Duann P., Komazaki S., Park K.H., Li H., Sun M., Sermersheim M., Gumpper K., Parrington J., Galione A., Evans A.M., Zhu M.X., Ma J. Lysosomal two-pore channel subtype 2 (TPC2) regulates skeletal muscle autophagic signaling. J. Biol. Chem. 2015;290:3377–3389. doi: 10.1074/jbc.M114.608471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lear P.V., Gonzalez-Touceda D., Porteiro C.B., Viano P., Guymer V., Remzova E., Tunn R., Chalasani A., Garcia-Caballero T., Hargreaves I.P., Tynan P.W., Christian H.C., Nogueiras R., Parrington J., Dieguez C. Absence of intracellular ion channels TPC1 and TPC2 leads to mature-onset obesity in male mice, due to impaired lipid availability for thermogenesis in brown adipose tissue. Endocrinology. 2015;156:975–986. doi: 10.1210/en.2014-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wen L., Zhu C., Zhu Z., Yang C., Zheng X., Liu L., Zuo X., Sheng Y., Tang H., Liang B., Zhou Y., Li P., Zhu J., Ding Y., Chen G., Gao J., Tang L., Cheng Y., Sun J., Elango T., Kafle A., Yu R., Xue K., Zhang Y., Li F., Li Z., Guo J., Zhang X., Zhou C., Tang Y., Shen N., Wang M., Yu X., Liu S., Fan X., Gao M., Xiao F., Wang P., Wang Z., Zhang A., Zhou F., Sun L., Yang S., Xu J., Yin X., Cui Y., Zhang X. Exome-wide association study identifies four novel loci for systemic lupus erythematosus in Han Chinese population. Ann. Rheum. Dis. 2018;77:417. doi: 10.1136/annrheumdis-2017-211823. [DOI] [PubMed] [Google Scholar]
- 120.Guo J., Zeng W., Chen Q., Lee C., Chen L., Yang Y., Cang C., Ren D., Jiang Y. Structure of the voltage-gated two-pore channel TPC1 from Arabidopsis thaliana. Nature. 2016;531:196–201. doi: 10.1038/nature16446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kintzer A.F., Stroud R.M. Structure, inhibition and regulation of two-pore channel TPC1 from Arabidopsis thaliana. Nature. 2016;531:258–264. doi: 10.1038/nature17194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.She J., Guo J., Chen Q., Zeng W., Jiang Y., Bai X.C. Structural insights into the voltage and phospholipid activation of the mammalian TPC1 channel. Nature. 2018;556:130–134. doi: 10.1038/nature26139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park K.H., Kim B.J., Shawl A.I., Han M.K., Lee H.C., Kim U.H. Autocrine/paracrine function of nicotinic acid adenine dinucleotide phosphate (NAADP) for glucose homeostasis in pancreatic beta-cells and adipocytes. J. Biol. Chem. 2013;288:35548–35558. doi: 10.1074/jbc.M113.489278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Grimm C. Endolysosomal cation channels as therapeutic targets—pharmacology of TRPML channels. Messenger. 2016;5:30. [Google Scholar]
- 125.Arredouani A., Ruas M., Collins S.C., Parkesh R., Clough F., Pillinger T., Coltart G., Rietdorf K., Royle A., Johnson P., Braun M., Zhang Q., Sones W., Shimomura K., Morgan A.J., Lewis A.M., Chuang K.T., Tunn R., Gadea J., Teboul L., Heister P.M., Tynan P.W., Bellomo E.A., Rutter G.A., Rorsman P., Churchill G.C., Parrington J., Galione A. Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP) and Endolysosomal Two-pore Channels Modulate Membrane Excitability and Stimulus-Secretion Coupling in Mouse Pancreatic ß Cells. J. Biol. Chem. 2015;290(35):21376–21392. doi: 10.1074/jbc.M115.671248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Patel S. Two-pore channels open up. Nature. 2018;556(7699):130–134. doi: 10.1038/d41586-018-02783-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.